1Dustin M. Graham and 2,3Kwoon Y. Wong
1Nature Publishing Group, New York, NY.
2Department of Ophthalmology & Visual Sciences, Department of Molecular, Cellular & Developmental Biology, University of Michigan, Ann Arbor, MI 48105.
3Correspondence: kwoon@umich.edu
1. Introduction.
For the greater part of 150 years it was assumed that the mammalian retina contained only two classes of photoreceptors, rods and cones. However, a flurry of studies in the late 1990s and early 2000s demonstrated the existence of a third class of mammalian photoreceptors that differs greatly from rods and cones. It utilizes a different photopigment, is much less sensitive to light, responds to light far more slowly, and has far lower spatial resolution, characteristics that fit with its primary function of signaling ambient light levels (irradiance) to the brain. Most surprisingly, these photoreceptors are retinal ganglion cells (RGCs), and thus, have the unique ability to communicate directly with higher visual centers of the brain. These intrinsically photosensitive retinal ganglion cells (ipRGCs) are a rare subpopulation of ganglion cells (<5%) whose primary role is to signal light for largely subconscious, non-image-forming visual reflexes, such as pupillary constriction, neuroendocrine regulation, and synchronizing daily (“circadian”) physiological rhythms to the light/dark cycle (“circadian photoentrainment”). Recent research has revealed additional roles for ipRGCs in conscious visual perception, including brightness discrimination and contrast detection. Many visual behaviors under ipRGC control are remarkably tonic, and require prolonged integration of ambient light levels. The unique properties of ipRGCs make them well suited for regulating such behaviors.
2. History and discovery.
As a graduate student in 1923, Clyde Keeler made a number of interesting observations from mice that had severe outer retinal degeneration (Keeler, 1928;Van Gelder, 2008). Though they lacked most of their rod and cone photoreceptors, and were considered functionally blind, these mice were still able to constrict their pupils in response to light (Keeler, 1927), leading Keeler to deduce that some other photoreceptive cells must be lurking in the retina. But an alternative, more parsimonious explanation for these pupil reflexes was that they were driven by the small number of surviving cone photoreceptors, and so the existence of non-rod non-cone photoreceptors remained uncertain. Seven decades later, Russell Foster and colleagues engineered mice that lacked all rods and cones, which displayed not only robust pupillary light reflexes, but also maintained the ability to shift their circadian rhythms in accordance with shifting light cycles, and to suppress nocturnal pineal melatonin secretion in response to light pulses (Freedman, 1999; Lucas, 1999). These processes disappeared, however, when animals had their eyes removed, strongly supporting Keeler’s prediction of a novel ocular photoreceptor (Klein, 1972). But it wasn’t until the discovery of a new light-sensitive molecule (or opsin), from an unlikely source, that Keeler’s prediction would prove true.
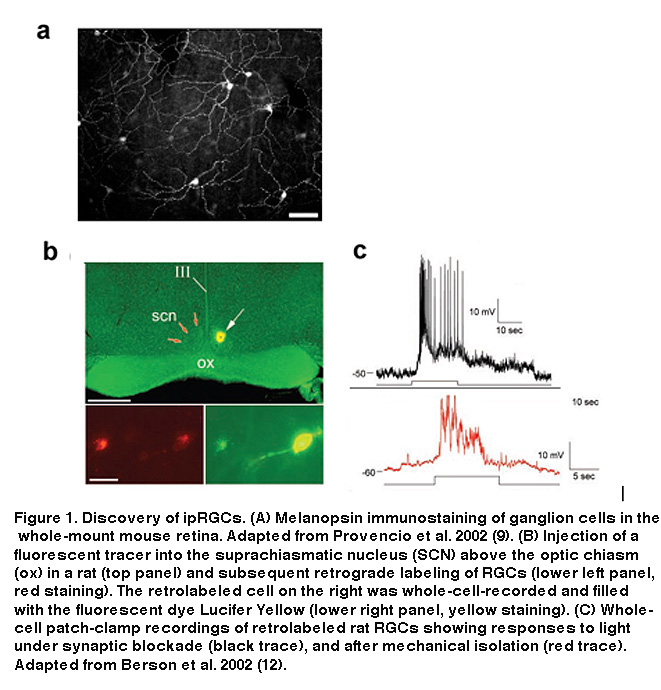
Iggy Provencio and colleagues, studying the photosensitive skin cells (“dermal melanophores”) of frogs, cloned a novel opsin-like molecule which they proposed was responsible for the redistribution of skin pigment in response to light (Provencio, 1998). Orthologs of this putative opsin, which Provencio et al. called melanopsin, were also found to be expressed in a small subset of RGCs in mouse (Figure 1A) and human retinas (Provencio, 1998; Provencio, 2000; Provencio, 2002), and additional work found that melanopsin-containing cells send their axons to the suprachiasmatic nucleus (SCN), the site of the mammalian central circadian clock (Gooley, 2001; Hannibal, 2002).
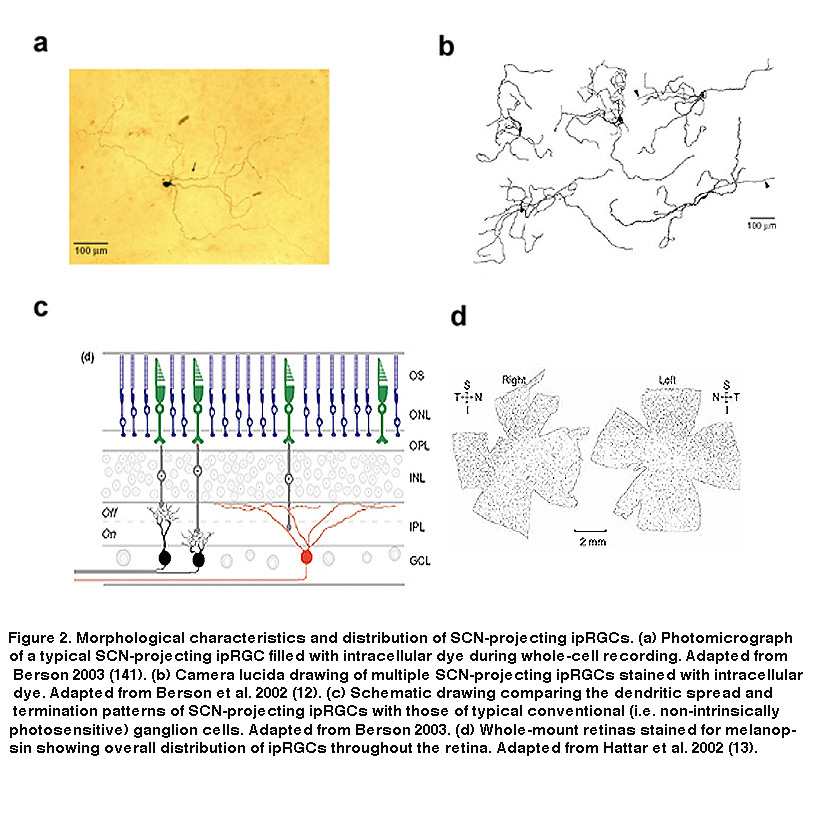
These findings suggested that these RGCs could be the mysterious third photoreceptor type Keeler had predicted almost 80 years earlier. They express a putative opsin, potentially allowing them to directly respond to light, and they send axon projections to a brain target known to be involved in two of the visual reflexes observable in rodless/coneless mice, i.e. circadian photoentrainment and photic suppression of pineal melatonin release. However, definitive proof that these melanopsin-containing, SCN-projecting RGCs could directly sense light was still lacking. Combining tracer injections into the SCN with electrophysiology, David Berson and colleagues tested the hypothesis that SCN-projecting RGCs are photoreceptive. They made whole-cell recordings from RGCs retrogradely labeled by the tracers (Figure 1B), and found that these cells were indeed able to respond to light in the presence of a cocktail of drugs that eliminated all rod and cone signaling in the retina (Figure 1C, top) (Berson, 2002). Even after mechanical isolation from the rest of the retina, these cells were still photosensitive (Figure 1C, bottom), leaving to rest all doubts that the labeled RGCs were true photoreceptors (Berson, 2002). Immunohistochemistry performed after whole-cell recording confirmed the presence of melanopsin in these SCN-projecting cells (Hattar, 2002). The morphology and distribution of these SCN-projecting ipRGCs are illustrated in Figure 2.
3. Melanopsin.
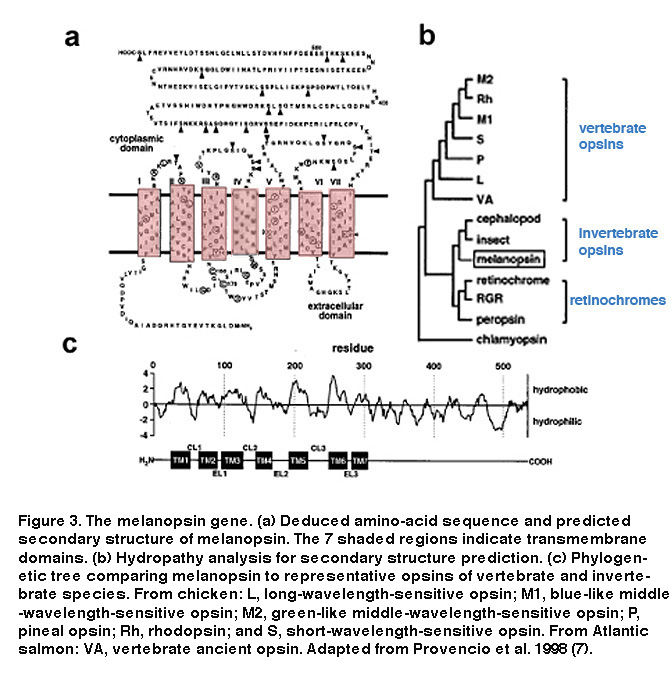
We now know that the ability of ipRGCs to respond directly to light is indeed due to their expression of melanopsin. The melanopsin gene (opn4) has been found in many mammalian species, e.g. mice, monkeys, and humans (Provencio, 2000). Hydrophobicity analysis of melanospin’s amino-acid sequence predicted a 7-transmembrane structure, common to all G protein-coupled receptors (Figure 3A, B) (Provencio, 1998). Interestingly, melanopsin shares higher homology with the rhabdomeric opsins (r-opsins) of invertebrate photoreceptors than with the ciliary opsins (c-opsins) of vertebrate photoreceptors, suggesting that melanopsin could signal light through a different phototransduction mechanism than that used in vertebrate rods and cones (Figure 3C) (Provencio, 1998) (see “Phototransduction” below).
Although the first studies of ipRGCs implicated melanopsin as the photopigment in these cells, a role for a group of blue-light-absorbing flavoproteins known as cryptochromes could not be initially ruled out (Berson, 2007). Cryptochromes function as photopigments in plants and invertebrate animals, and early studies favored their functioning as retinal photopigments in the mammalian circadian system (Kavakli, 2002; Van Gelder, 2002). However, subsequent work provided overwhelming evidence that melanopsin is the photopigment in ipRGCs. When the melanopsin gene was deleted via transgenic techniques in mice (“melanopsin-knockout mice”), RGCs retrogradely labeled from the SCN no longer could respond directly to light (Lucas, 2003). In addition, animals missing the melanopsin gene showed deficiencies in multiple visual reflexes such as pupillary constriction and photoentrainment (Panda, 2003; Panda, 2002; Ruby, 2002; Lucas, 2003; Hattar, 2003). Despite this evidence, there were still controversies regarding melanopsin’s ability to function as a photopigment. For example, it remained possible that melanopsin is not the ipRGC photopigment, but is instead an isomerase essential for the proper functioning of the ipRGC photopigment. These doubts were firmly extinguished with a series of experiments whereby the melanopsin gene was heterologously expressed in multiple cell types that are normally insensitive to light. When made to express melanopsin, these cells were able to robustly respond to light, indicating melanopsin’s ability to function as a bona fide photopigment (Melyan, 2005; Panda, 2005; Qiu, 2005).
4. Phototransduction
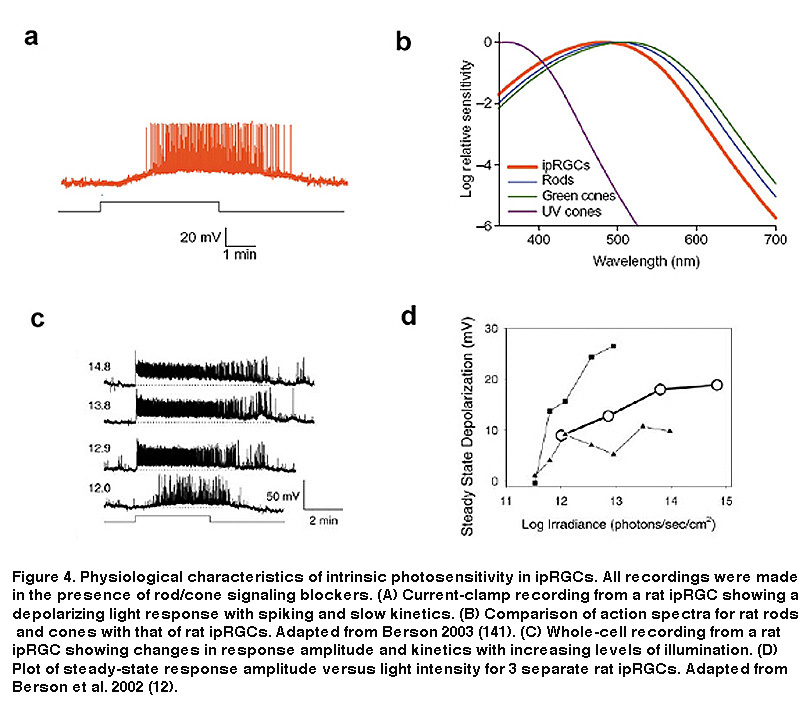
As seen in Figure 4, the intrinsic light response of ipRGCs differs dramatically from those of rods and cones. Most notable, is the difference in response polarity: the direct photoresponse of ipRGCs is depolarizing, whereas rods and cones hyperpolarize to light. In addition, ipRGCs are much less sensitive to light than the classical photoreceptors, and signal with far slower kinetics (Figure 4A) (Berson, 2002; Do, 2009). IpRGCs are also capable of very long-lasting light responses, faithfully encoding stimulus energy over relatively long periods of time (Figure 4C). This feature sets ipRGCs apart from all other mammalian RGCs, which cannot stably represent ambient light levels in this fashion (Wong, 2007; Wong, 2012; Barlow, 1969). Another remarkable feature of ipRGC physiology is the ability of their dendrites (which contain melanopsin) to respond directly to light (Berson, 2002). Combined with their large overlapping dendritic fields, the entire ipRGC population creates what Provencio and colleagues called a “photoreceptive net” (Provencio, 2002. These characteristics of ipRGCs are no doubt tailored to their role in signaling diffuse ambient light levels over many hours for tonic, non-image-forming visual behaviors such as photoentrainment and pupillary reflex. A further distinction of ipRGCs is their action spectrum (i.e. wavelength-sensitivity function), due to the utilization of melanopsin as their photopigment. IpRGC photoreception is most sensitive to light at around 480 nm (Berson, 2002; Dacey, 2005; Tu, 2005), significantly different (≥20 nm) from the best wavelengths for stimulating rods and cones (Figure 4B).
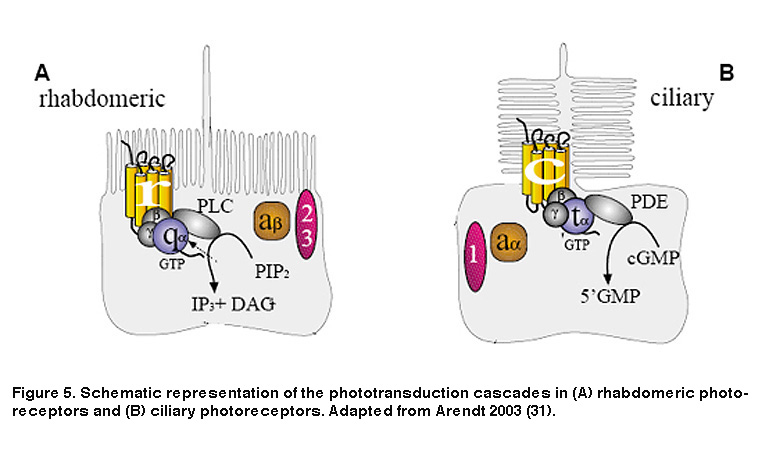
As noted earlier, animal photoreceptors come in two basic flavors: rhabdomeric (found mainly in invertebrates), and ciliary (vertebrate rods and cones being most notable). A defining feature of rhabdomeric and ciliary photoreceptors is the distinct biochemical cascades both cell classes use to transduce light energy into an electrical signal the brain can interpret. Rods and cones use a cascade involving cyclic guanosine monophosphate (cGMP) as a second messenger (Figure 5B) (Arendt, 2003; Fu, 2007). The response to light is a decrease in cGMP levels which closes cGMP-gated cation channels, causing the plasma membrane to hyperpolarize. This is in stark contrast to rhabdomeric photoreceptors which use a phosphoinositide signaling cascade involving the enzyme phospholipase C (PLC) and breakdown of the membrane lipid phosphatidylinositol bisphosphate (PIP2) (Figure 5A), leading to the opening of cation channels and membrane depolarization (Hardie, 2001; Hardie, 2001). In addition, although both phototransduction cascades are G protein-mediated, the specific G proteins required are different. Ciliary photoreceptors require transducin, a member of the Gi/o family of G proteins, whereas rhabdomeric photoreceptors use a G protein of the Gq/11 family (Figure 5).
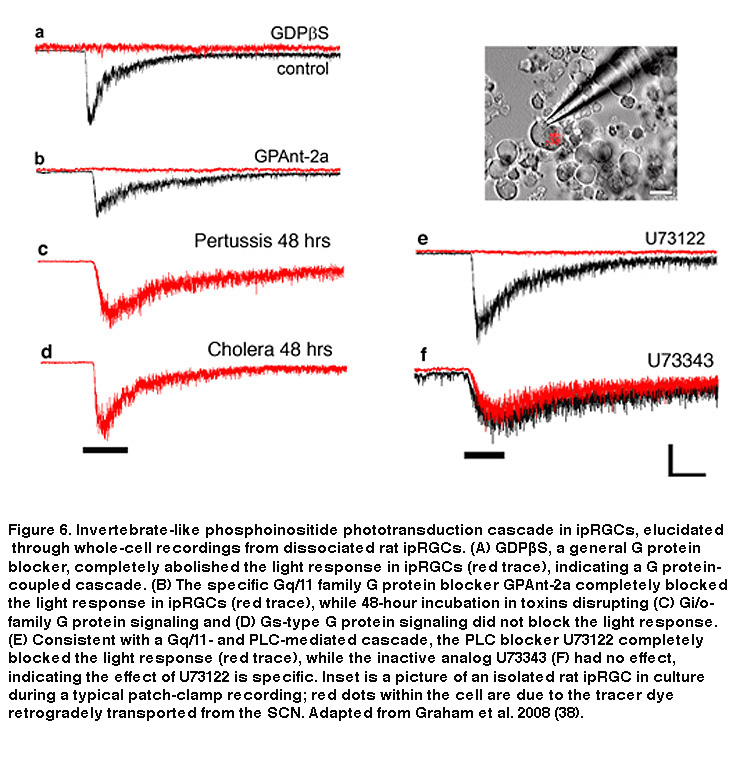
Just like rod and cone photopigments, melanopsin uses 11-cis-retinal as the light-absorbing chromophore, and light-induced isomerization of 11-cis-retinal to all-trans-retinal is the first step in the phototransduction process (Walker, 2008; Fu, 2005). However, melanopsin’s relatively high homology with invertebrate opsins, and the depolarizing intrinsic light response of ipRGCs, suggest these neurons may use a rhabdomeric phototransduction cascade distinct from the rod and cone ciliary cascade. Early pharmacological studies of ipRGCs could not confirm this hypothesis, most likely due to the whole-mount retina preparation employed. The combination of photosensitive ipRGC dendrites buried deep within the inner plexiform layer (IPL, where synapses are formed among bipolar, amacrine and ganglion cells) and a glial membrane sheath covering the retinal surface, created a significant diffusion barrier for pharmacological agents, especially hydrophobic drugs commonly used to study transduction mechanisms. To overcome this hurdle, David Berson and colleagues recorded from mechanically dissociated ipRGC somas to facilitate pharmacological manipulation. Isolated ipRGCs survive remarkably well in culture, generating robust light responses for up to 6 days. Using this system, Berson et al. showed that ipRGC phototransduction utilizes a rhabdomeric-like phosphoinositide cascade, requiring a member (or possibly members) of the Gq/11 family of G proteins and the effector enzyme phospholipase C (PLC) (Figure 6) (Graham, 2008) (but see (Chew, 2014)). In addition, the presence of specific Gq/11 and PLC isoforms was confirmed in ipRGCs using single-cell RT-PCR and immunocytochemistry, consistent with the pharmacological findings (Graham, 2008). Genetically knocking out the β4 isoform of PLC nearly eliminated the intrinsic light response of ipRGCs (Xue, 2011).
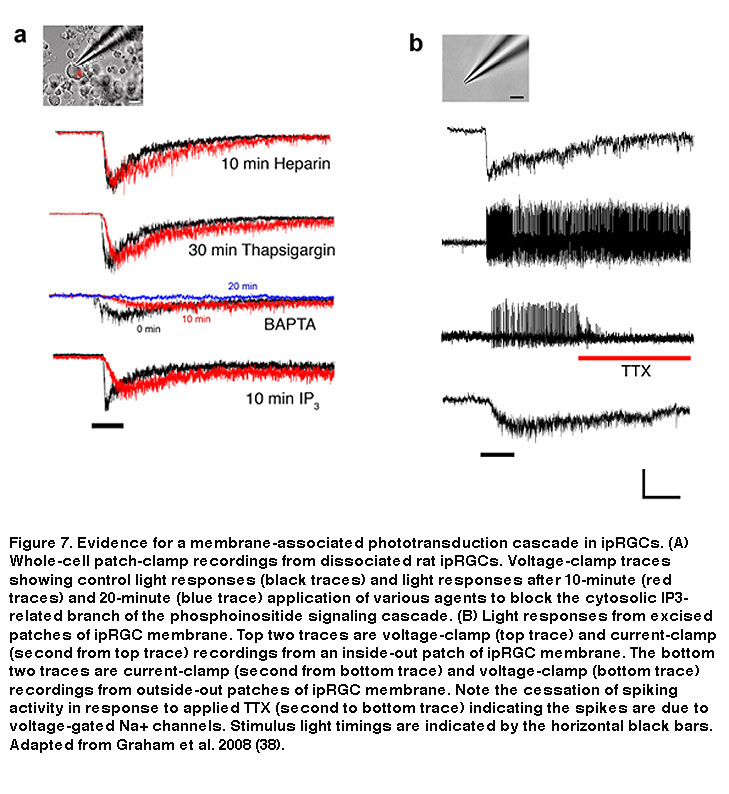
In rhabdomeric photoreceptors, breakdown of PIP2 by PLC generates two by-products: a membrane-bound component called diacylglycerol (DAG), and inositol-triphosphate (IP3), which is free to move about the cytosol (Hardie, 2001). DAG can be broken down within the membrane into polyunsaturated fatty acids (PUFAs), which have been shown to directly open Drosophila photoreceptors’ Transient Receptor Potential (TRP) channels (Chyb, 1999. IP3, on the other hand, can cause release of Ca2+ from intracellular stores, which can lead to the opening of so-called “store-operated channels” in the plasma membrane. In Drosophila photoreceptors the downstream mechanism linking PIP2 breakdown to TRP channel opening is still unclear, although a membrane-associated pathway is likely (Acharya, 1997). In ipRGCs, the light-gated cation channels are also TRP channels, specifically, TRPM6 and TRPM7 (Xue, 2011), and data strongly suggest that the cytosolic IP3 pathway is not required for phototransduction, similar to Drosophila photoreceptors (Figure 7) (Graham, 2008). Intracellular application of high concentrations of IP3 neither induced a current, nor did it occlude light responses in ipRGCs (Figure 7A), although it did modulate certain response properties, suggesting a non-essential secondary role for IP3 and intracellular Ca2+. Likewise, ipRGC phototransduction survived blockade of IP3 receptors with intracellular heparin, or intracellular Ca2+ store depletion using thapsigargin (Figure 7A). Only after extended exposure to very high (10mM) concentrations of the general Ca2+ chelator BAPTA, does one see significant attenuation of phototransduction (Graham, 2008), although this is probably a side effect of clamping resting intracellular Ca2+ levels so low that enzymes such as PLC are no longer able to function (Hardie, 2005). The most compelling evidence that ipRGC phototransduction does not require the cytosolic IP3 branch is that excised patches of ipRGC membrane (both outside-out and inside-out) remained autonomously photosensitive (Figure 7B), ruling out a vital role for highly diffusible cytosolic components (Graham, 2008). Based on these findings, all the necessary components for ipRGC phototransduction appear to be within, or tightly coupled to, the plasma membrane.
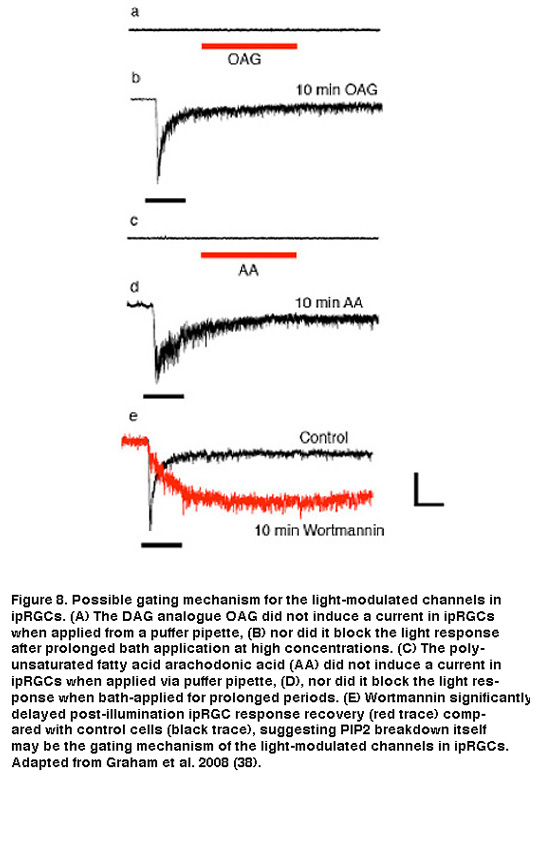
The exact channel-gating mechanism downstream of PLC activation is still not clear. Application of DAG or PUFAs, either to isolated ipRGC somas or to excised patches of ipRGC membrane, failed to evoke a current or to block light responses (Figure 8A-D). An alternative hypothesis, and one in line with a membrane-associated transduction cascade, is that the breakdown of PIP2 itself (as opposed to production of DAG or PUFAs) is the critical signal that opens the light-gated TRP channels in ipRGCs. There is evidence that PIP2 can either open or close a variety of ion channels, including the light-gated channels in Drosophila photoreceptors (Hardie, 2003; Suh, 2006). As a preliminary test of this hypothesis, Berson et al. pharmacologically interfered with PIP2 synthesis using wortmannin. This drug inhibits phosphoinositide 4-kinase (PI4-K), the synthetic enzyme for phosphatidylinositol 4-phosphate (PIP), which is an essential precursor of PIP2. According to the hypothesis, wortmannin should slow the termination of the photocurrent at light offset by delaying the restoration of resting levels of PIP2 and, hence, the closure of the light-gated channels. Indeed, when wortmannin was included in the recording pipette solution, response shutoff was dramatically delayed (Figure 8E). Further and more direct evidence, however, is needed to resolve the gating mechanism of the light-modulated channels in ipRGCs.
Following light exposure, all-trans-retinal in a rod, cone or ipRGC photopigment must be isomerized back to 11-cis-retinal to make the photopigment photoexcitable again (Hubbard, 1958). For rhodopsin, this re-isomerization requires the adjacent retinal pigment epithelium (RPE), while cone pigments rely on Müller glial cells in addition to the RPE (Wang, 2011). In contrast, photoexcited melanopsin appears to retain all-trans-retinal and isomerize it to 11-cis-retinal upon subsequent photon absorption, in a process called photoreversal (Mure, 2007; Emanuel, 2015; Koyanagi, 2005; Davies, 2011; Matsuyama, 2012). Thus, melanopsin can function as both photopigment and photoisomerase, similar to some invertebrate photopigments, and ipRGCs remain directly light-sensitive when RPE enzymes that synthesize 11-cis-retinal are genetically or pharmacologically inhibited (Doyle, 2006; Tu, 2006). Nevertheless, without these enzymes, the melanopsin-based light response of ipRGCs is attenuated, suggesting that melanopsin function depends on the RPE to some extent (Fu, 2005; Zhao, 2016).
5. Synaptic Input
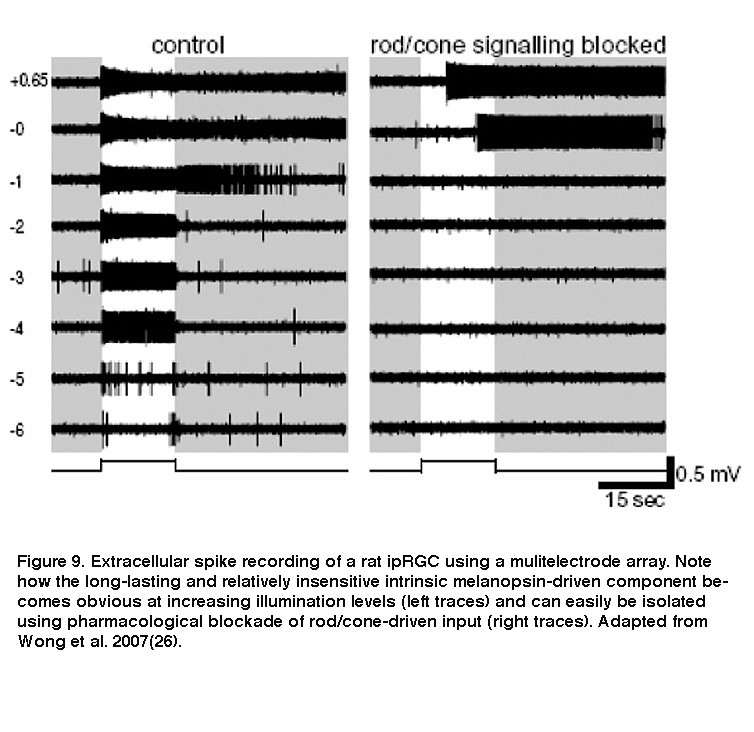
Although ipRGCs can function as photoreceptors, they nevertheless receive intraretinal synaptic input from rod/cone-driven circuits. Electron microscopy revealed synaptic contacts made by amacrine and bipolar cells onto melanopsin-immunopositive processes in the IPL (Belenky, 2003). Thus, ipRGCs respond to light not only directly through melanopsin, but also indirectly through synaptically mediated input from rods and cones (Dacey, 2005; Perez-Leon, 2006; Wong, 2007; Zhao, 2014; Weng, 2013). Both the intrinsic light response and the extrinsic, rod/cone-driven light response are remarkably tonic and can signal light continuously for at least 10 hours (Wong, 2012). Moreover, the receptive fields for both response components are coextensive with each ipRGC’s dendritic field (Wong, 2007). However, these components are drastically different in two important ways. Firstly, whereas the intrinsic photoresponse is very sluggish, the extrinsic response is as rapid as the light responses of conventional RGCs (Figure 9) (Wong, 2007). The intrinsic response integrates photons over many seconds, while the extrinsic response enables ipRGCs to track rapid changes in light intensity (Wong, 2007; Do, 2009). Secondly, the intensity thresholds for rod input, cone input and melanopsin phototransduction are vastly different, roughly 7 log photons cm-2 s-1, 9 log photons cm-2 s-1 and 11 log photons cm-2 s-1, respectively (Dacey, 2005; Zhao, 2014). The different dynamic ranges of synaptic input and intrinsic photoreception enable ipRGCs to encode light intensities spanning at least 9 orders of magnitude (Dacey, 2005).
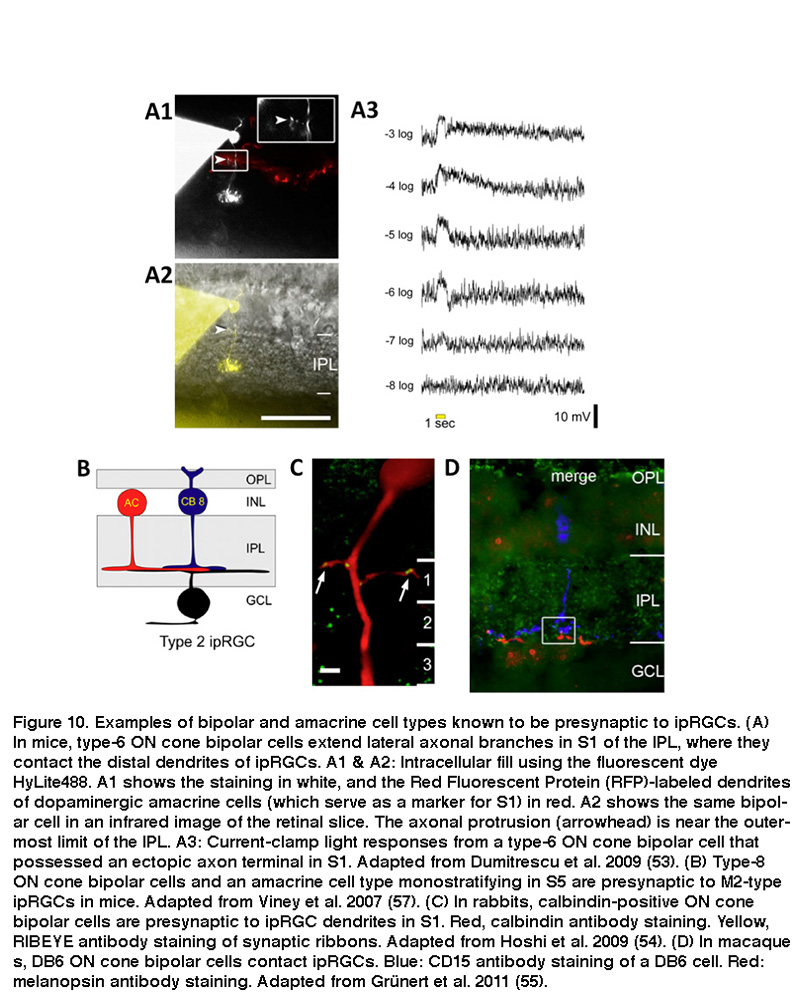
Using whole-cell voltage-clamp recordings combined with pharmacology, investigators demonstrated that SCN-projecting ipRGCs have spontaneous inhibitory and excitatory synaptic inputs in the dark, and that they express receptors for glutamate, GABA and glycine (Perez-Leon, 2006; Wong, 2007). Light stimulation increases both excitatory glutamatergic input and inhibitory GABA/glycinergic input, which are presumably mediated by bipolar and amacrine cells, respectively (Wong, 2007). Both inputs evoke primarily ON light responses, although comparatively weak OFF responses can also be seen under some conditions (Wong, 2007; Schmidt, 2010; Zhao, 2014). This predominating ON-channel input was surprising because SCN-projecting ipRGCs’ dendrites are found mainly in the distal half of the IPL, which had been assumed for decades to be the “OFF” sublamina where RGCs receive input only from OFF bipolar cells but not ON bipolars (Nelson, 1978; Famiglietti, 1976). ON bipolar cells use two unconventional strategies to release glutamate onto the dendrites of SCN-projecting mouse ipRGCs in the “OFF” sublamina. Some ON bipolar cells’ axons extend lateral protrusions that contain synaptic vesicles (Figure 10A), whereas others possess en passant (in passing) synaptic vesicles within their axonal shafts (Dumitrescu, 2009). Distally stratifying ipRGCs are also present in rabbit, marmoset and macaque retinas, and they likewise receive unconventional ON bipolar input in the “OFF” sublamina (Figure 10C) (Hoshi, 2009; Grunert, 2011).
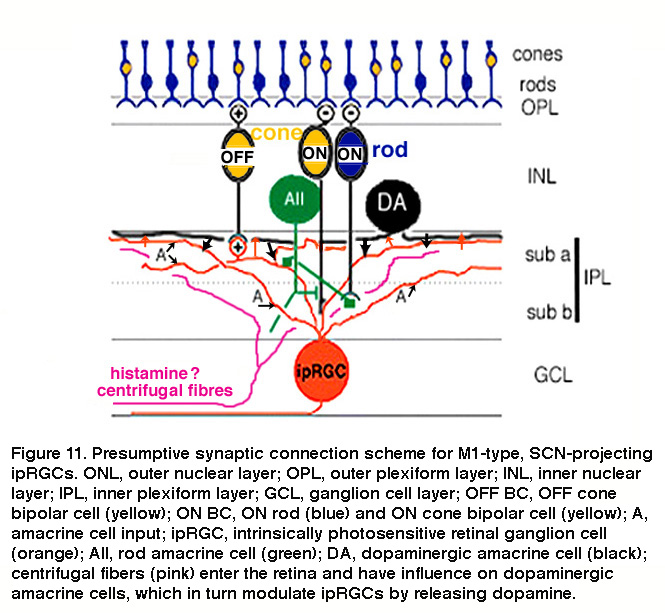
The mammalian retina contains about a dozen types of bipolar cells and over 30 types of amacrine cells (Kolb, 1981; Masland, 2011) (and see webvision chapter on “roles of amacrine cells”). In mice, the ON bipolar cells that use axonal side branches to contact “OFF”-sublamina ipRGC dendrites appear to be type-6 cone bipolar cells (Figure 10A) (Dumitrescu, 2009). Transsynaptic viral tracing has shown that these “OFF”-sublamina ipRGC dendrites are also directly postsynaptic from dopaminergic amacrine cells which costratify with these ipRGC dendrites (Viney, 2007). As will be discussed in detail in “Adaptation, neuromodulation, and circadian control” below, ipRGCs receive modulatory input from dopaminergic amacrine cells (Vugler, 2007; Van Hook, 2012). Transsynaptic viral tracing also revealed that proximally stratifying mouse ipRGCs with dendrites in the “ON” sublamina (see “Morphological and physiological diversity” below) are directly postsynaptic from type-8 ON cone bipolar cells and another type of monostratified amacrine cell (Figure 10B) (Viney, 2007). In rabbits and primates, distally stratifying ipRGCs are contacted by a type of calbindin-positive ON cone bipolar cell (Figure 10C) (Hoshi, 2009) and DB6 ON cone bipolars (Figure 10D) (Grunert, 2011), respectively. Besides receiving direct ON cone bipolar input, ipRGCs also receive ON rod bipolar input, albeit polysynaptically just like for all other mammalian RGCs (Weng, 2013; Grunert, 2011). Specifically, ipRGCs receive ON rod bipolar input through AII amacrine cells making gap junctions onto ON cone bipolars, which then synapse on ipRGCs. Another putative pathway that affects ipRGCs may be added by centrifugal fibers entering the retina. These centrifugal fibers are known to be histaminergic (Gastinger, 1999) and they have an effect on dopaminergic amacrine cells in mouse retina through H1 receptors (Frazao, 2011). The synaptic input to “OFF”-stratifying ipRGCs is summarized in Figure 11.
6. Intraretinal Synaptic Output
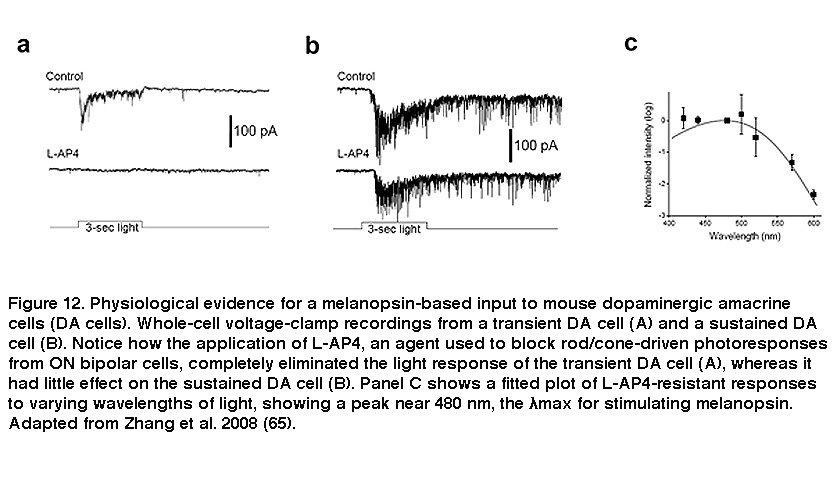
Besides receiving intraretinal synaptic input, ipRGCs mediate intraretinal synaptic output. For many decades, RGCs were thought to function strictly as retinal output neurons, signaling only to higher visual centers of the brain. But there is electron microscopy evidence that RGC dendrites are presynaptic in catfish (Sakai, 1986), and more recent work by Douglas McMahon and coauthors yielded evidence that ipRGCs signal to a subpopulation of dopaminergic amacrine cells (DA cells). Dopamine released by DA cells diffuses throughout the retina and reshapes the functional properties of retinal circuits according to current lighting conditions and time of day, and thus allows the retina to be a more dynamic system (Dowling, 2012). DA cells come in three flavors showing transient, sustained and null responses to light (Zhang, 2007). The light responses of the transient DA cells were eliminated by L-AP4, which blocks rod/cone signaling to ON bipolar cells (Figure 12A). By contrast, the sustained DA cells remained light-responsive in the presence of L-AP4 (Figure 12B), or in rod/cone degenerate retinas (Zhang, 2008). In the presence of L-AP4, the sustained DA cells’ light responses peaked at a wavelength near 480nm (Figure 12C), matching very well with a melanopsin-based input (Zhang, 2008; Zhang, 2007). In melanopsin-knockout mice, blocking ON bipolar input with L-AP4 abolished all DA cells’ light responses, confirming that the L-AP4-resistant photoresponse originates from ipRGCs (Zhang, 2012).
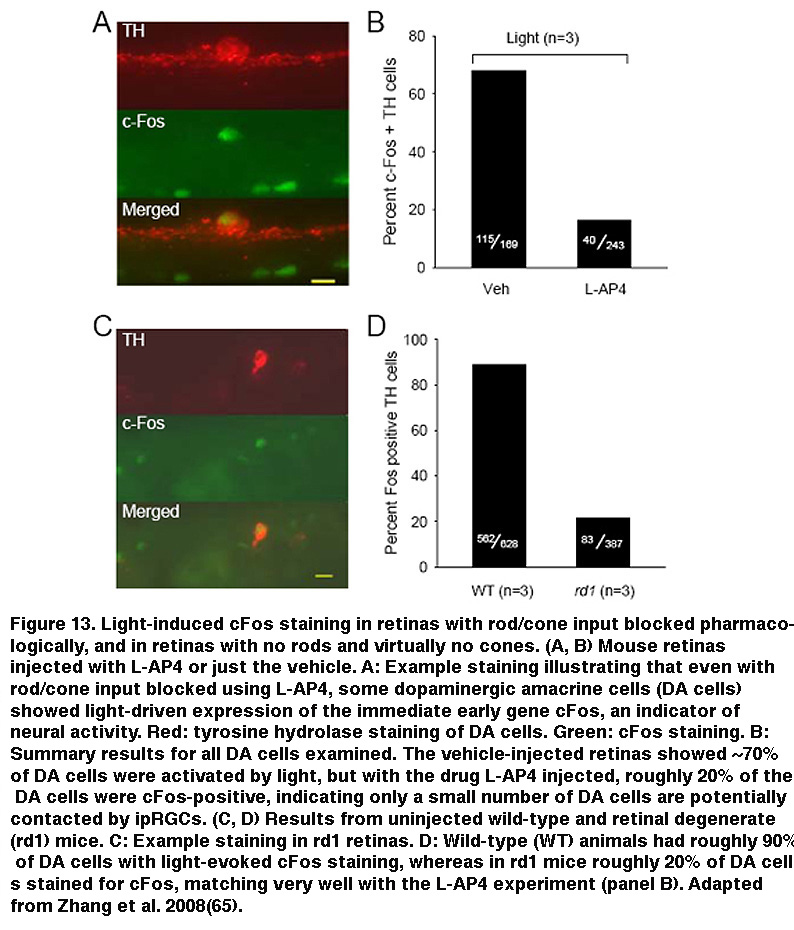
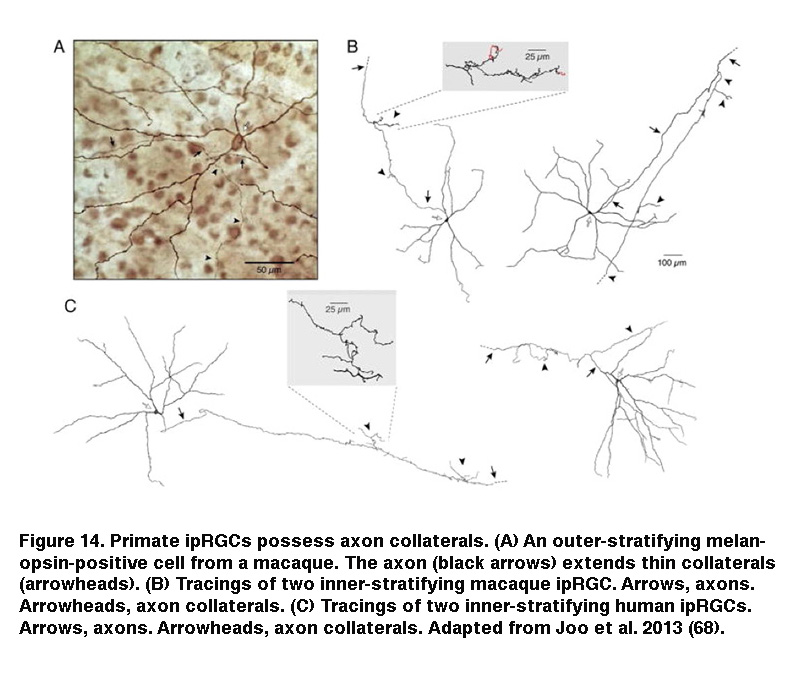
In addition, staining for cFos, an immediate early gene used to mark activated neurons, showed that roughly the same percentage of DA cells were activated by sustained light in rod/cone degenerate retinas as retinas injected with L-AP4 (Figure 13) (Zhang, 2008). Knocking out melanopsin suppressed light-induced dopamine release (Dkhissi-Benyahya, 2013. These observations strongly suggest that sustained DA cells receive input from ipRGCs. Both DA cells and SCN-projecting ipRGCs have dendrites stratifying in the outermost sublayer of the IPL, raising the possibility that these ipRGC dendrites signal directly to the DA dendrites (Vugler, 2007; Viney, 2007). Another plausible transmission route has been suggested by the observation that some ipRGCs extend axon collaterals toward the IPL (Figure 14) (Joo, 2013). In support of the latter possibility, blocking voltage-gated Na+ channels (which are present in RGC axons) with tetrodotoxin suppressed ipRGC signaling to DA cells (Prigge, 2016). Antagonizing AMPA/kainate receptors also disrupted ipRGC output to DA cells (Zhang, 2012), suggesting ipRGCs signal to DA cells by releasing glutamate in a Na+ spike-dependent manner.
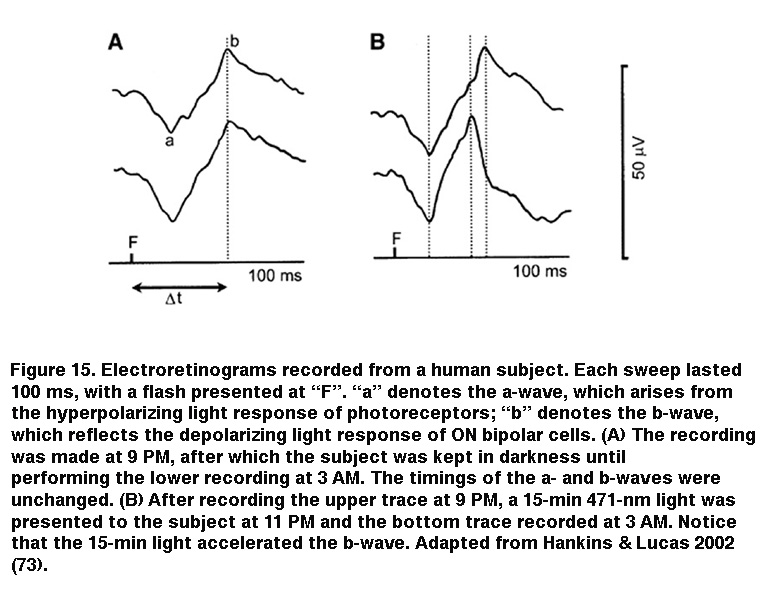
IpRGC signaling to sustained DA cells can account for the light-induced secretion of dopamine in rat retinas with severe rod/cone degeneration (Morgan, 1980; Vugler, 2007) (but see (Cameron, 2009)). Because dopamine is known to play myriad modulatory roles throughout the retina, intraretinal output from ipRGCs to DA cells could influence image-forming retinal circuits, and several studies have reinforced this possibility. Using a non-invasive field-potential recording technique called the electroretinogram, Mark Hankins and Rob Lucas found that prolonged nocturnal light exposure altered the photoresponse kinetics of ON bipolar cells in human subjects (Figure 15). This effect exhibited a remarkably long integration time and was most optimally induced by 483 nm light, implicating a melanopsin-based mechanism (Hankins, 2002). The same researchers later observed diurnal rhythms in the amplitude and kinetics of the mouse electroretinogram, which were abolished upon knocking out melanopsin (Barnard, 2006). Additional evidence for ipRGC regulation of retinal function came from a study by Ouria Dkhissi-Benyahya et al. examining clock gene expression in rods and cones. In wild-type mice, the expression of these clock genes (which are thought to drive circadian rhythms within the retina) exhibits a daily rhythm, but when melanopsin was knocked out, this rhythm was dampened substantially (Dkhissi-Benyahya, 2013).
Ca2+ imaging suggested an additional potential pathway for intraretinal signaling by ipRGCs. Under normal physiological conditions, about 3% of ganglion cell layer (GCL) cells in rodless coneless mouse retinas showed increases in Ca2+ dye signals in response to light, but in the presence of the gap junction blocker carbenoxolone, this percentage dropped to ~1%, leading the authors to propose that ipRGCs propagate their light responses to amacrine cells in the GCL (“displaced amacrine cells”) via gap junctions (Sekaran, 2003). But this conclusion was challenged by subsequent research showing that carbenoxolone has various non-specific effects, and can abolish light-driven increases in Ca2+ signals even in mechanically dissociated ipRGCs (Bramley, 2011). Nonetheless, tracer dye coupling between ipRGCs and displaced amacrines had been observed (Muller, 2010), and so gap-junction-mediated intraretinal signaling by ipRGCs remained a possibility. A recent study showed that rat ipRGCs indeed transmit their melanopsin-based light responses to displaced amacrine cells (Reifler, 2015). Whole-cell recordings were made from >2,000 randomly targeted displaced amacrine cells to search for cells exhibiting spiking, sustained ON light responses, and 154 such cells were encountered. Surprisingly, all of them were still photosensitive during bath application of L-AP4, DNQX and D-AP5, which fully block rod/cone signaling to the inner retina. In the presence of these drugs, all 154 amacrines gave sluggish depolarizing light responses resembling ipRGCs’ intrinsic light responses, but they lost all photosensitivity after the addition of the gap junction blocker meclofenamic acid, indicating that ipRGCs signal to these amacrines via gap junctions (Reifler, 2015).
7. Morphological and Physiological Diversity
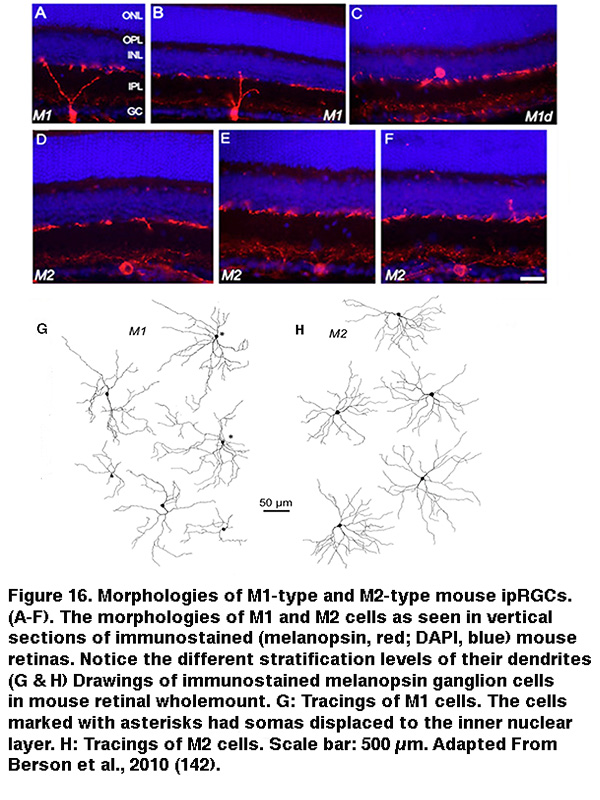
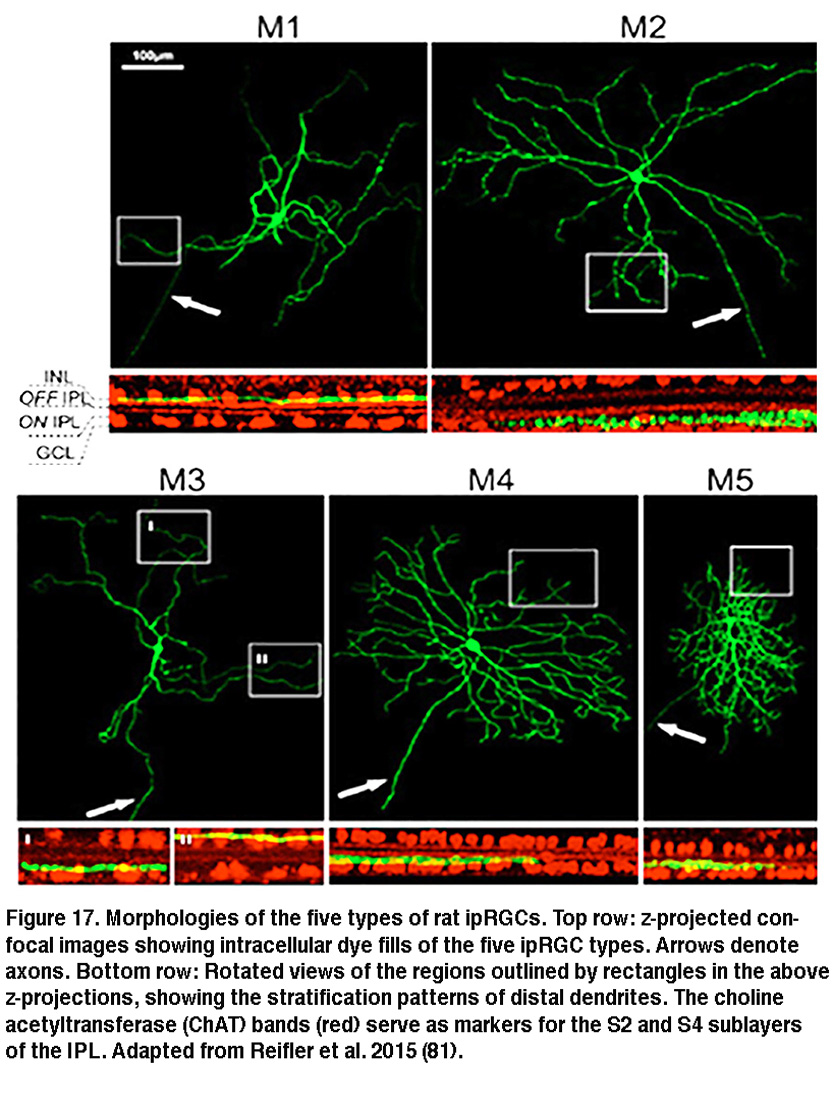
The SCN-projecting rat and mouse ipRGCs appear to be morphologically homogeneous, suggesting they constitute a single cell type (but see (Baver, 2008)). All such cells have somas medium in size among GCL neurons and sparse dendrites terminating in the outermost sublayer (“S1”) of the IPL, corresponding to the main plexus of melanopsin-immunoreactive dendrites (Figure 16A-C, G) (Berson, 2002; Hannibal, 2002; Hattar, 2002). However, a number of studies from the early 2000s implicated the existence of multiple types of ipRGCs. For example, there is an additional plexus of melanopsin-positive dendrites in the innermost sublayer (“S5”) of the IPL (Provencio, 2002). Ca2+ imaging and “blind” multielectrode-array extracellular spike recording also revealed diverse melanopsin-mediated light responses in the GCL (Sekaran, 2003; Tu, 2005). Using various fluorescent labeling techniques, subsequent work revealed the existence of at least five morphological types of melanopsin-expressing ipRGCs in the mouse retina. The SCN-projecting type originally described by David Berson is now called “M1”, whereas the new types are called M2, M3, M4 and M5 (Viney, 2007; Schmidt, 2008; Ecker, 2010). Examples of mouse M2 cells are shown in Figure 16D-F, H. By whole-cell-recording from a large sample of randomly selected RGCs, all five morphological types of ipRGCs were also found in the rat (Reifler, 2015); representative examples are shown in Figure 17. Among these ipRGC types, M1 is the only one that stratifies exclusively in S1 of the IPL. M3 is the only bistratified type, extending dendrites toward both S1 and S5. The remaining ipRGC types are all monostratified in S5, with M2 having relatively sparse dendrites, M4 having denser and radiate dendrites similar to the previously described alpha cell (Estevez, 2012; Schmidt, 2014), and M5 having the densest dendrites covering the smallest fields (Figure 17).
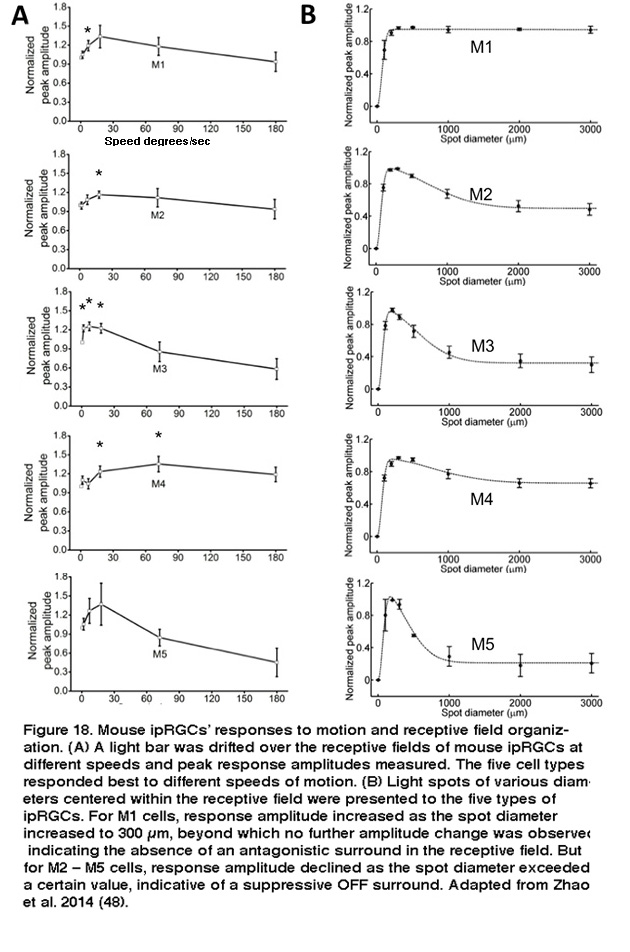
The five types of ipRGCs exhibit diverse physiological properties, suggesting they perform different functions. In both mouse and rat, the five ipRGC types spike spontaneously at different frequencies (Reifler, 2015; Zhao, 2014). The various types of mouse ipRGCs also differ significantly in various basic biophysical properties such as membrane resistance, membrane capacitance, Na+ spike waveform, Na+ spike threshold, and voltage-gated Ca2+ and K+ currents (Schmidt, 2009; Hu, 2013). Under dark-adapted conditions, the five mouse ipRGC types respond fairly similarly to full-field light, generating sustained depolarizing responses that last for the duration of the light (Zhao, 2014). Under light-adapted conditions, however, M1’s and M2’s cone-driven full-field light responses are significantly different, with the former’s responses being far more transient and much lower in amplitude (Schmidt, 2010). Diversity was also observed in the various cell types’ responses to moving lights, and to light spots of different sizes, even under dark-adapted conditions. All five cell types responded more strongly to moving lights than to static lights; however, different cell types appeared tuned to different speeds of motion (Figure 18A).
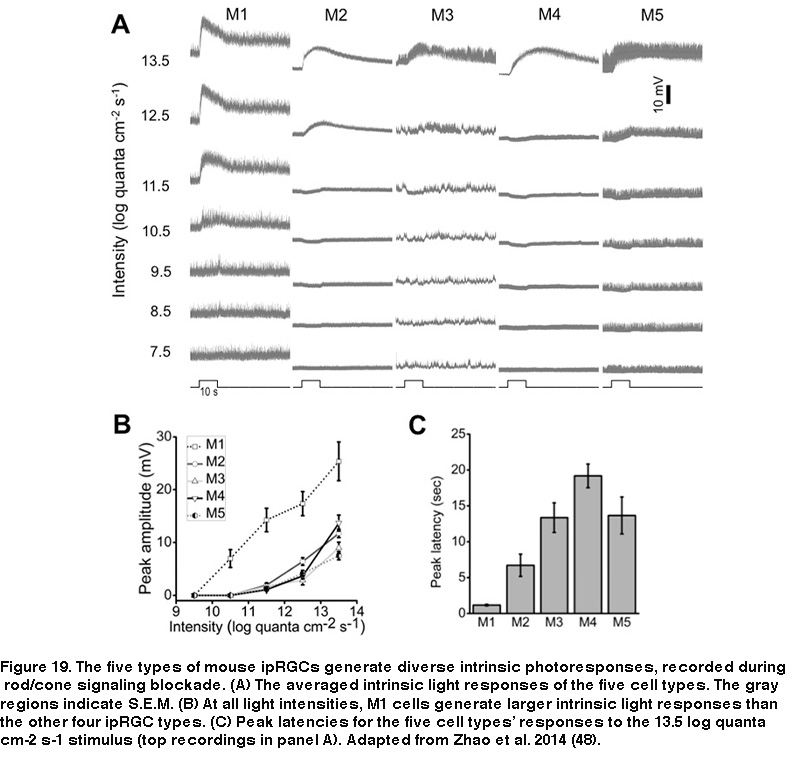
Furthermore, whereas M1’s receptive field consists of just an ON-center region, the non-M1 ipRGCs’ receptive fields display ON-center/OFF-surround antagonism, so that a large light spot illuminating the entire receptive field induces a weaker response than a smaller spot covering just the center region (Figure 18B) (Zhao, 2014). Under conditions blocking rod/cone signaling, the five types of rat and mouse ipRGCs generate melanopsin-based light responses that differ in kinetics, amplitude, and threshold, with M1 cells’ intrinsic photoresponses being the fastest, largest, and most sensitive (Figure 19) (Reifler, 2015; Zhao, 2014; Ecker, 2010; Schmidt, 2009; Estevez, 2012).
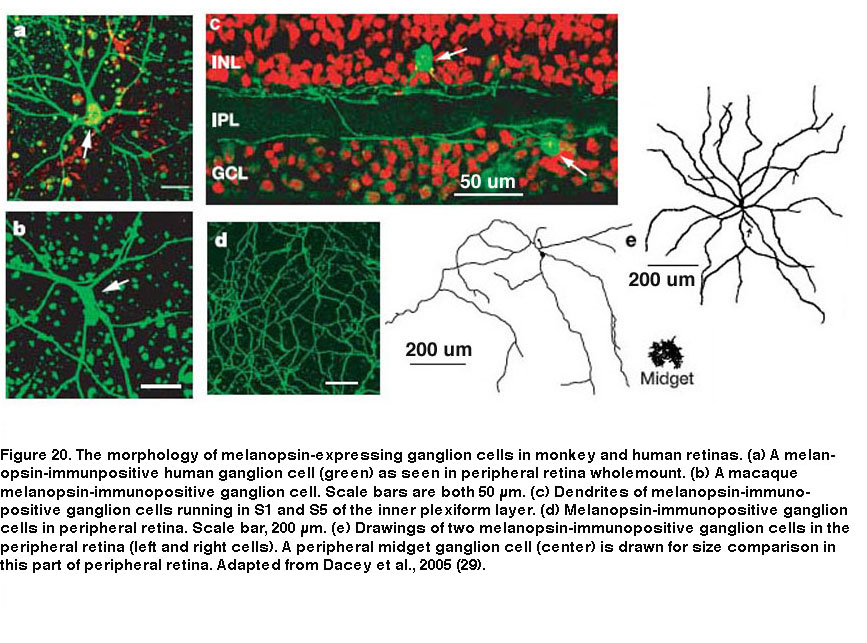
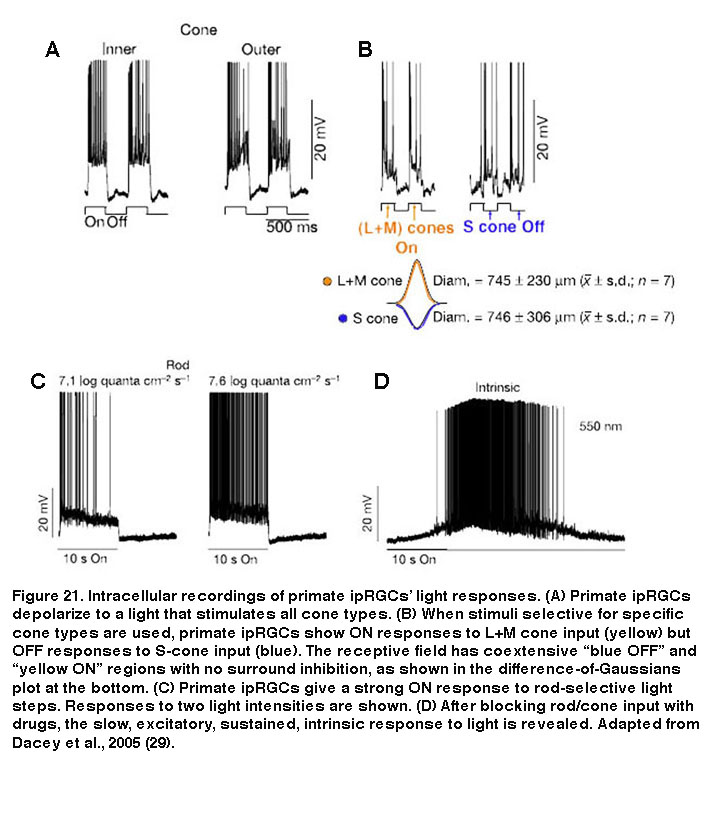
Besides mice and rats, many other species that have been examined also possess multiple morphological and/or physiological types of ipRGCs, although it is unknown whether these other species also have five ipRGC types. In primates including humans, only two morphological types of melanopsin-expressing RGCs have been documented (Figure 20). Both cell types have giant somas and sparse, monostratifying dendrites. The outer-stratifying type stratifies in S1 whereas the inner-stratifying type stratifies in S5, and so they could be homologous to the M1 and M2 types found in rodents (Dacey, 2005; Jusuf, 2007; Grunert, 2011; Hannibal, 2014; Neumann, 2011). The dendritic fields of the outer-stratifying cells tile the retina with little overlap, but they overlap substantially with the dendritic fields of the inner-stratifying cells, suggesting they constitute two distinct cell types (Jusuf, 2007). The receptive fields of both outer- and inner-stratifying primate ipRGCs consist only of an ON-center region, and both cell types appear to generate remarkably similar rod-driven, cone-driven and melanopsin-mediated light responses (Figure 21) (Dacey, 2005). Moreover, both types project to the lateral geniculate nucleus and olivary pretectal nucleus (see “Central Projections” below for further information) (Dacey, 2005; Dacey, 2003). Thus, functional differences between the outer- and inner-stratifying primate ipRGCs remain elusive, although there is anatomical evidence that they receive different synaptic inputs (Jusuf, 2007; Neumann, 2011).
8. Central Projections
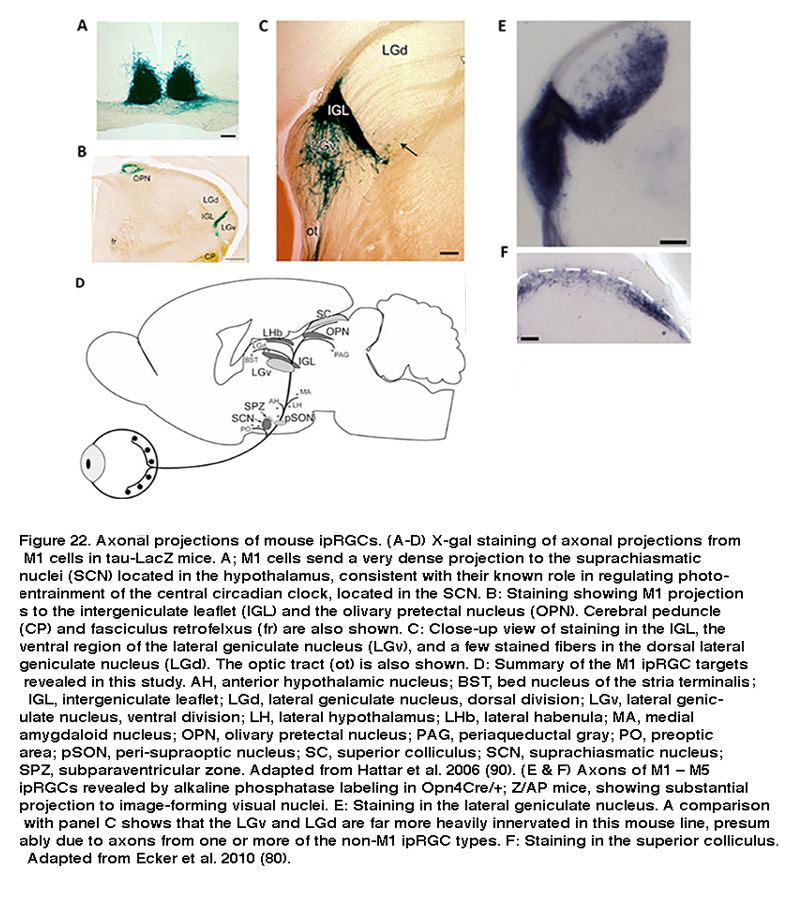
Using a transgenic mouse line whose ipRGCs express the marker enzyme β-galactosidase, Samer Hattar et al. visualized the axons of M1-type ipRGCs and characterized their projections throughout the brain. As expected for cells that regulate non-image-forming visual behaviors, Hattar and colleagues found M1-type ipRGCs sent dense projections to the SCN, the intergeniculate leaflet (IGL), and the olivary pretectal nucleus (OPN) (Figure 22A–C) (Hattar, 2006). The SCN and IGL are parts of the circadian system, while the OPN mediates the pupillary light reflex. In addition to these expected sites, the same study discovered a number of other central targets. Within the hypothalamus, M1 cells sent axonal fibers to the ventral portion of the subparaventricular zone, a region controlling the autonomic nervous system. Rostrolateral to the SCN, a number of scattered fibers reach the lateral and ventrolateral preoptic areas, which influence release of reproductive hormones from the pituitary (Hattar, 2006). The brain targets identified in this study are summarized in Figure 22D. These central projections suggest that M1 cells regulate a diverse array of non-image forming visual functions.
More recently, using a more sensitive Cre-based melanopsin reporter mouse line to label all five ipRGC types, Hattar and colleagues detected pronounced ipRGC projection to two additional visual nuclei: the dorsal division of the lateral geniculate nucleus (LGN), which is the primary thalamic relay site of retinal input to the visual cortex, and the superior colliculus (SC), a sensorimotor nucleus that detects novel visual stimuli (Figure 22E, F). Retrograde and anterograde labeling has shown that primate ipRGCs also project to both the LGN and SC (Dacey, 2005; Hannibal, 2014). These findings suggest that in addition to their well-known role in regulating subconscious visual reflexes, ipRGCs may contribute to conscious visual perception, a possibility that has received strong support from electrophysiological and behavioral studies (see “Behavioral aspects of ipRGC function” below).
9. Adaptation, Neuromodulation and Circadian Control
IpRGC photosensitivity displays various forms of plasticity. The gain of melanopsin phototransduction is influenced by background light level and prior lighting history. IpRGCs’ light responses are also modulated by neuromodulators released endogenously within the retina. Moreover, these neurons’ photoresponse amplitude depends on the time of day, suggesting it is under circadian control. All three aspects of ipRGC plasticity are considered in this section.
The image-forming visual system can operate over a wide spectrum of ambient light intensities covering more than ten orders of magnitude. While much of this range is due to the existence of two separate photoreceptor systems (rods and cones) that operate over different light intensity ranges, some of the capacity is due to the ability of both rods and cones to change their sensitivity to light, a process called adaptation (Perlman, 1998). When presented with a constant background light, rods and cones generate an initial peak response which relaxes during the steady illumination, indicating desensitization to the light over time. This desensitization allows the photoreceptors to increase their dynamic range, so they can respond to light intensities that would otherwise be saturating. This is termed “light adaptation”. Conversely, when placed back into a dark environment, rods and cones gradually regain their sensitivity, a process called “dark adaptation”. Both light and dark adaptation are a direct consequence of changes in the phototransduction cascade. While rods have a relatively limited ability to adapt to background illumination, saturating at high light levels, cones exhibit an almost infinite capacity to adapt to light levels (Fain, 2001; Knox, 2006).
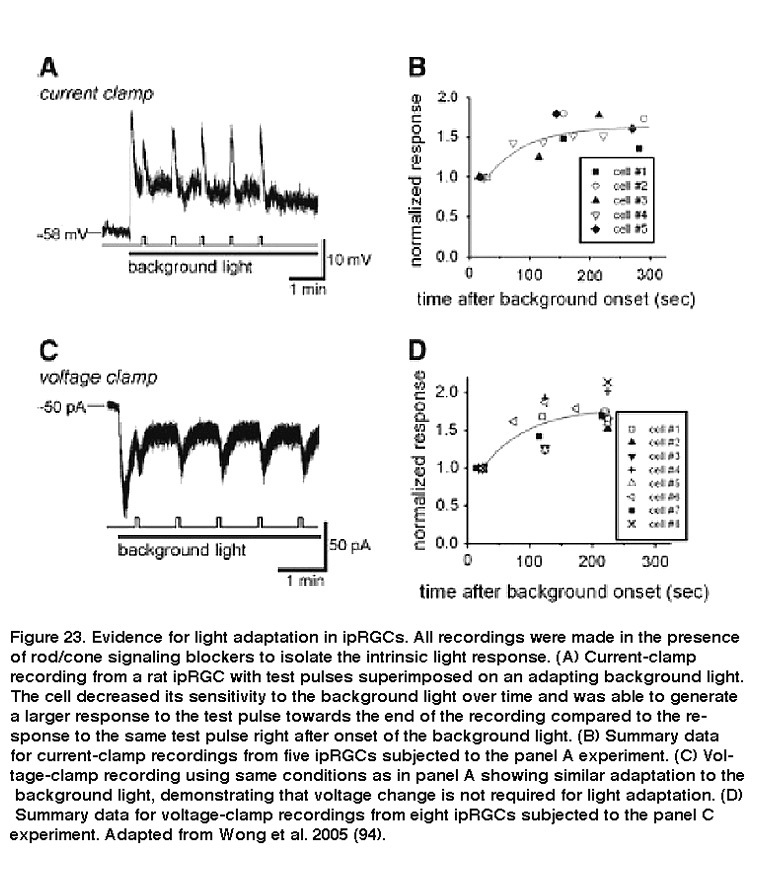
Due to their role in stable representations of absolute light levels, and their unusually tonic response properties, it was initially not clear whether ipRGCs’ intrinsic photosensitivity would display similar adaptation mechanisms as rods and cones. David Berson and colleagues found that when ipRGCs were exposed to a nearly saturating background light, these cells gradually became desensitized, while responses to a brighter pulse of light on top of the background light became larger over time (Figure 23).
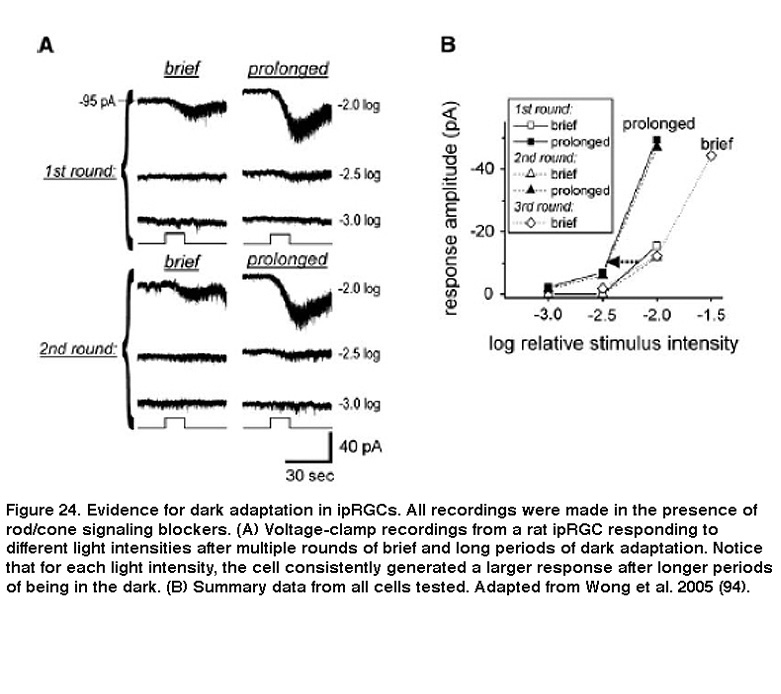
Background light also accelerated photoresponse kinetics, so that the response to a light pulse peaked faster, and also decayed faster toward to the baseline after cessation of the pulse. These are hallmarks of light adaptation. Furthermore, when previously light-adapted ipRGCs were left in the dark for increasing periods of time, their responses to the same light stimulus intensity increased, a clear indication of dark adaptation (Figure 24) (Wong, 2005). Although Wong et al.2005 (Wong, 2005) found that ipRGCs light-adapt and dark-adapt much slower than do the classical photoreceptors, subsequent work revealed two important similarities between ipRGC and rod/cone adaptations: for all three photoreceptor classes, the flash sensitivity vs. background intensity relationship can be described by the Weber-Fechner law, and light adaptation is at least partly mediated by Ca2+ (Do, 2013). These similarities are somewhat surprising, considering that ipRGCs use a phototransduction cascade that differs drastically from rods’ and cones’ (see the “Phototransduction” section above).
In addition to the above acute adaptational effects on ipRGCs’ intrinsic photosensitivity, prolonged exposure to light or darkness significantly modulates melanopsin mRNA levels. In rats maintained in constant darkness, melanopsin expression rose progressively for at least 5 days, whereas those kept in constant light became nearly completely devoid of melanopsin mRNA after 7 days (Hannibal, 2005).
As mentioned earlier in the “Intraretinal synaptic output” section, dopamine is an important neuromodulator that has widespread influences on retinal function. Thus, not surprisingly, it has been shown to modulate ipRGCs. Dopamine and an agonist for D1-class dopamine receptors were found to acutely attenuate the intrinsic light responses of dissociated ipRGCs (Van Hook, 2012). Moreover, prolonged application of a D2-class dopamine agonist enhanced the mRNA levels of melanopsin and pituitary adenylyl cyclase-activating peptide (PACAP), a peptide present in ipRGCs (Sakamoto, 2005). Adenosine, which appears to rise at night and during prolonged darkness (Ribelayga, 2005), has been reported to shorten the duration of ipRGCs’ intrinsic photoresponses, but unlike dopamine, it does not significantly reduce the peak amplitude of these responses (Sodhi, 2014. Melatonin, which is also released primarily at night, has been reported to modulate the rod/cone-driven light responses of M4-type rat ipRGCs (Pack, 2015). Finally, there is indirect evidence that cholecystokinin released from amacrine cells modulates retinal input to the central circadian clock (Shimazoe, 2008).
IpRGCs are well known to mediate photoentrainment of the central circadian clock (see “Behavioral aspects of ipRGC function below”). But there is evidence that these RGCs are themselves under circadian regulation. In both light/dark (“LD”) and constant dark (“DD”) conditions, melanopsin mRNA levels in rat retinas display a daily rhythm. This rhythm disappeared in rod/cone degenerate rats, suggesting it is driven by the retinal circadian clock rather than the SCN’s central circadian clock {Sakamoto, 2004 #138}. Multielectrode-array extracellular recording indeed demonstrated a statistically significant increase in the amplitude of rat ipRGCs’ intrinsic photoresponse early in the subjective night compared to all the other circadian times examined (Weng, 2009), although this increase was much smaller than the amplitude of the circadian rhythm in melanopsin mRNA expression (Sakamoto, 2004). The synaptically driven light response of M4 ipRGCs also exhibits circadian variation (Pack, 2015).
10. Behavioral Aspects of ipRGC Function
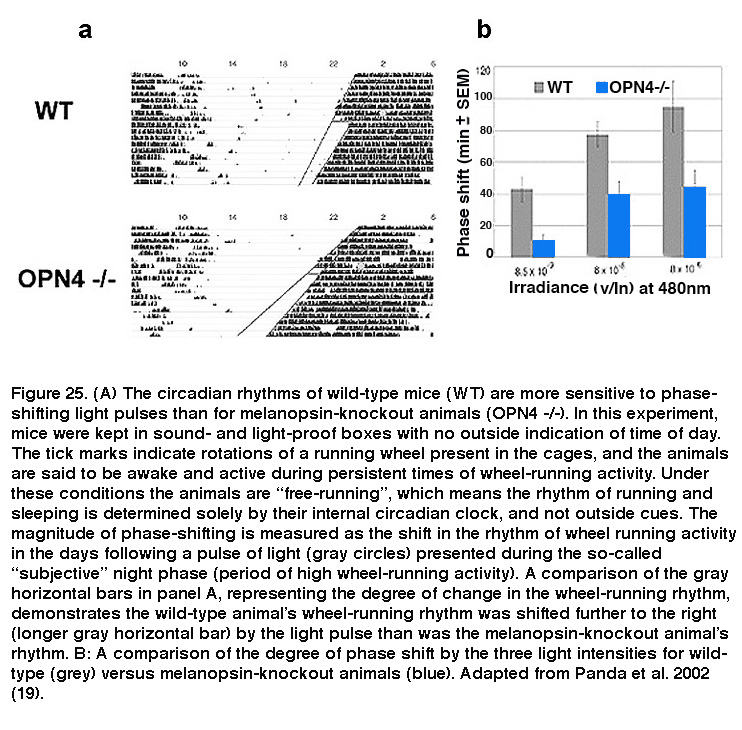
Daily rhythms in mammalian physiology and behavior, collectively called circadian rhythms, are controlled by a tiny cluster of cells in the SCN of the hypothalamus. SCN neurons possess a transcription/translation-based molecular clock that allows them to autonomously regulate activity patterns in near 24-hour rhythms, which ultimately lead to daily changes in behaviors such as sleep/wake cycles and rhythms in core body temperature. However, the circadian clock is not tuned perfectly to 24 hours, and in order to adjust to changes in the animals’ environmental light/dark phases, the SCN must be reset periodically so that circadian rhythms are synchronized (or “entrained”) to the light/dark cycle. Light is by far the most potent circadian entrainment cue, and ipRGCs are the primary cells that carry this signal since eliminating these neurons abolished photoentrainment in mice (Hatori, 2008; Goz, 2008; Guler, 2008). Melanopsin-knockout animals have relatively normal circadian rhythms, and do not display any overt dysfunction in their ability to entrain to light. However, light pulses induced significantly smaller phase shifts in these animals’ circadian rhythms (Figure 25), revealing a crucial role for melanopsin signaling (Panda, 2002; Ruby, 2002). On the other hand, rods are essential for photoentrainment to dim light (Altimus, 2010; Lall, 2010), whereas cones are important for proper responses of the photoentrainment system to short-duration light pulses (Dkhissi-Benyahya, 2007; Dollet, 2010).
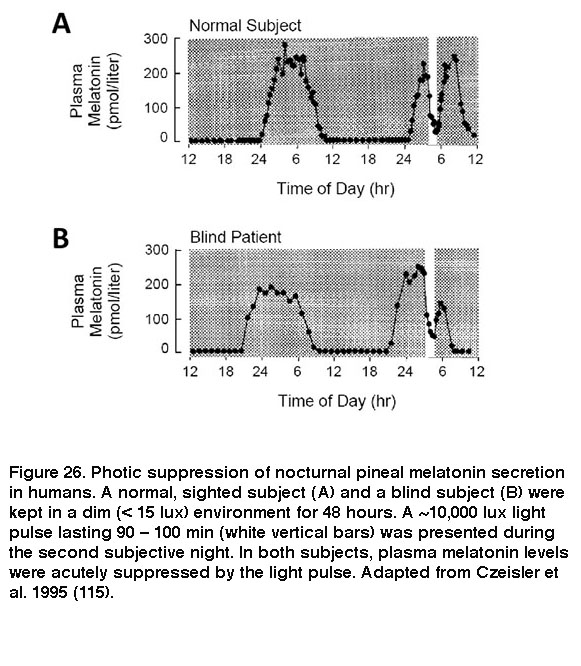
The SCN signals through various neural circuits to coordinate circadian rhythms throughout the body. In one of these circuits, the SCN regulates secretion of the sleep-promoting hormone melatonin from the pineal gland into the bloodstream: SCN > paraventricular nucleus of the hypothalamus > intermediolateral nucleus of the spinal cord > superior cervical ganglion à pineal gland (Larsen, 1998). As a result of this SCN regulation, melatonin is released from the pineal only during subjective night. Upon exposure to sufficiently intense light, however, ipRGC input acutely suppresses this release of melatonin (Brainard, 2001; Thapan, 2001; Vartanian, 2015). Because ipRGCs remain photosensitive in the absence of rods and cones, rodless coneless mice and some blind patients continue to exhibit this non-image-forming visual response (Figure 26) (Lucas, 1999; Czeisler, 1995; Zaidi, 2007).
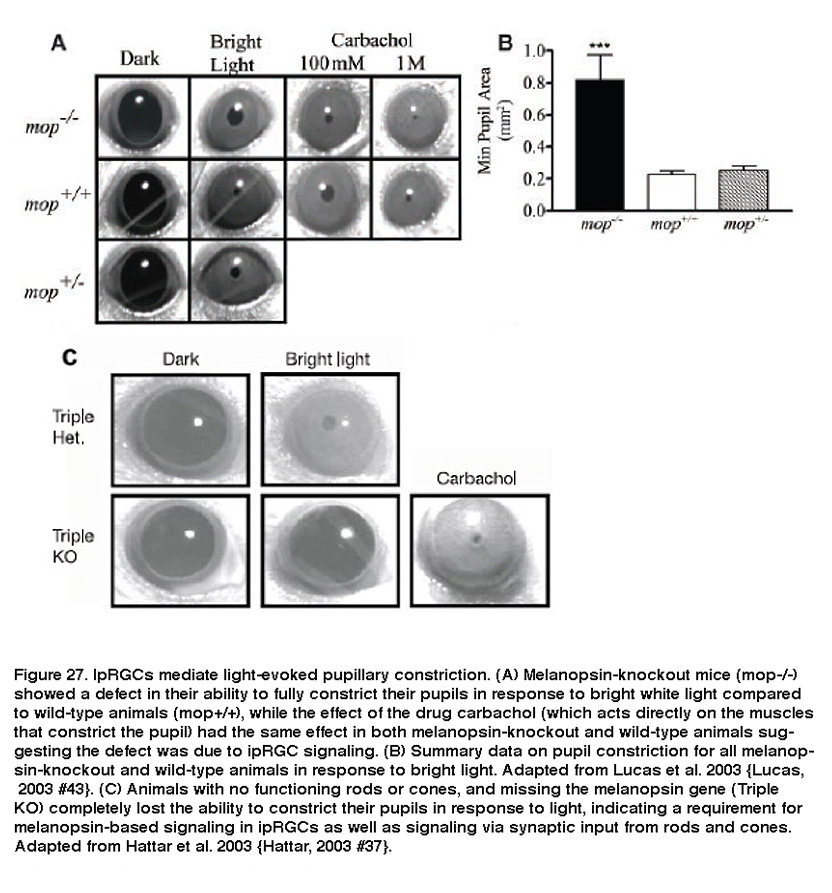
IpRGCs are also responsible for light-evoked constriction of the pupil. Projections of ipRGC axons to the OPN, the retino-recipient site responsible for the pupillary light reflex, make them likely candidates for regulating this response. Indeed, melanopsin-knockout animals had diminished pupillary light reflexes at high irradiance levels (Figure 27A, B) (Lucas, 2003). In addition, melanopsin-knockout mice without functional rods or cones were completely unable to constrict their pupils in response to light (Figure 27C) (Hattar, 2003; Panda, 2003). Furthermore, killing ipRGCs using various methods dramatically attenuated the pupillary light reflex (Guler, 2008; Hatori, 2008).
Numerous additional non-image-forming photic responses have been reported to be at least partly mediated by ipRGCs. These include acute suppression of the nocturnal locomotor activity of mice (“negative masking”) (Mrosovsky, 2003), light-induction of sleep (“photosomnolence”) (Lupi, 2008; Morin, 2011; Altimus, 2008; Tsai, 2009) and maintenance of light-induced sleep in mice (Muindi, 2013), modulation of cognitive performance in humans (Vandewalle, 2007; Vandewalle, 2013), enhancement of alertness in humans (Viola, 2008), antidepressant effect in rats (Iyilikci, 2009) and humans (Meesters, 2011), light-induced exacerbation of headache in humans (Noseda, 2010), photophobia and light avoidance in mice (Johnson, 2010; Matynia, 2012), light-dependent relaxation of blood vessels (Sikka, 2014), and stimulation of the secretion of follicle-stimulating hormone (FSH) in women (Danilenko, 2015).
In addition, there is emerging evidence that melanopsin contributes to visual perception, which is probably mediated by ipRGC projection to the LGN and/or SC (Dacey, 2005; Brown, 2010; Ecker, 2010; Estevez, 2012; Zhao, 2014). Some blind patients with severe outer retinal degeneration but relatively normal ipRGCs possess a rudimentary ability to detect the presence of intense blue light (Zaidi, 2007), and fully sighted humans as well as mice appear to depend partly on melanopsin for brightness discrimination (Brown, 2012). Mice lacking rod/cone photoreception (but with intact melanopsin photoreception) were able to distinguish a computer screen displaying black and white stripes from a uniform gray screen of an equal mean intensity, suggesting melanopsin is sufficient for a certain degree of pattern vision (Ecker, 2010). There is also preliminary psychophysical evidence that melanopsin directly contributes to color vision in humans, challenging the trichromatic theory (Horiguchi, 2013). Most recently, mice lacking melanopsin were shown to have behavioral deficits in contrast sensitivity (Schmidt, 2014).
IpRGCs are likely to perform additional image-forming visual functions because under conditions preserving synaptic input, primate ipRGCs receive color-opponent (blue OFF, yellow ON) input from cones (Figure 21B), implicating a capacity for color discrimination (Dacey, 2005). Furthermore, with the exception of M1 cells, all the other four types of mouse ipRGCs have receptive fields with antagonistic ON-center, OFF-surround regions (Figure 18B), suggesting these cells perform spatial analysis (Estevez, 2012; Zhao, 2014). Finally, the five types of mouse ipRGCs are tuned to different speeds of motion (Figure 18A), raising the possibility that they contribute to motion analysis (Zhao, 2014).
11. Roles in Development
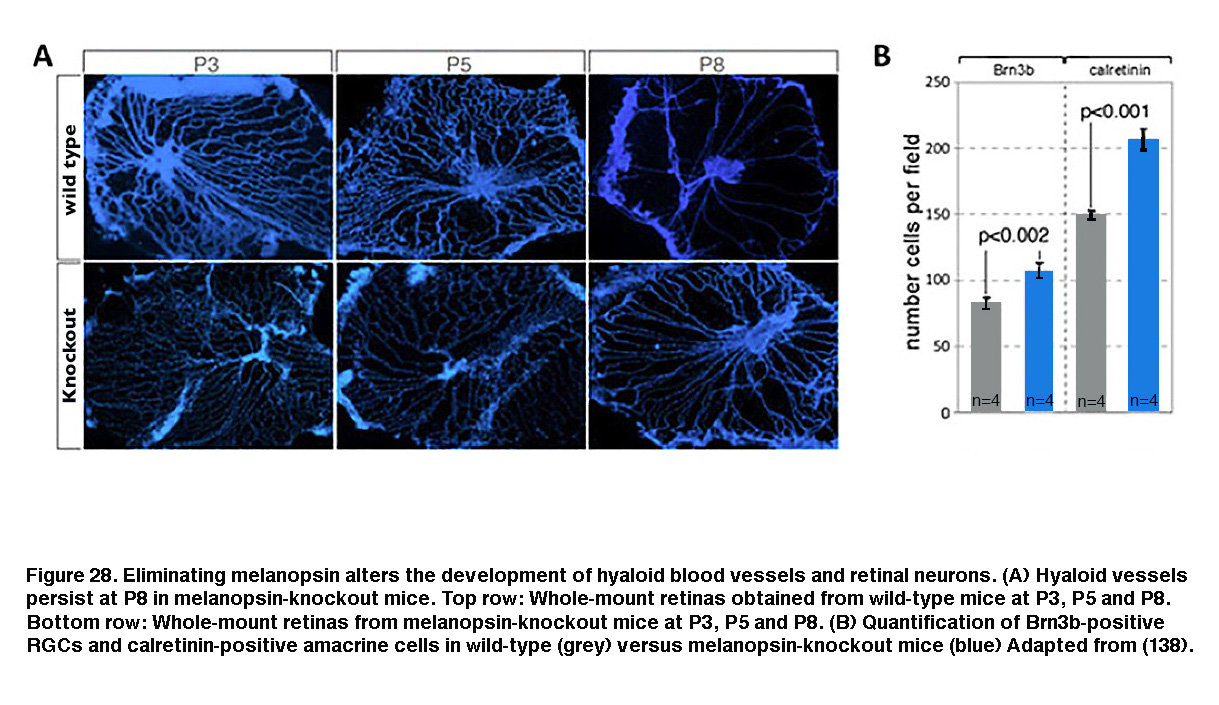
Melanopsin expression begins before birth (Tarttelin, 2003) and ipRGCs become photosensitive earlier than either rods or cones (Sekaran, 2005; Tu, 2005). One function for this early emergence of melanopsin-based photosensitivity is negative phototaxis, which causes mouse pups as young as P6 (postnatal day 6) to turn away from bright light (Johnson, 2010). Melanopsin has also been shown to regulate the early development of retinal neurons, retinal blood vessels and retinogeniculate circuits. In wild-type mice reared under normal light/dark conditions, embryonic hyaloid vasculature regresses and the retinal vasculature starts to form during early postnatal days. But in melanopsin-knockout mice or mice dark-reared from late gestation, the regression of the hyaloid vasculature was delayed and the retinal vasculature overgrew (Figure 28A, bottom row). Furthermore, the number of RGCs and amacrine cells increased (Figure 28B, blue). These observations suggest that during late gestation, melanopsin-mediated light detection activates a signaling pathway that suppresses the number of GCL neurons and regulates the timing of vasculature development (Rao, 2013).
As mentioned in the “Intraretinal synaptic output” section above, ipRGCs signal not only to the brain but also within the retina. Intraretinal signaling by ipRGCs is already apparent by P4. Besides participating in ion regulation (Berkowitz, 2010), such early signaling also controls the temporal properties of retinal waves. Retinal waves are spontaneous bursts of action potentials that propagate across RGCs during early postnatal development, and they contribute to the refinement of retinogeniculate projections. In wild-type mice, light increases the duration of each burst of spikes, and afferent axons from ipsilateral and contralateral retinas terminate in largely distinct parts of the dorsal LGN. In melanopsin-knockout mice, however, this effect of light on burst duration was absent, and ipsilateral and contralateral retinal inputs to the dorsal LGN became less segregated (Renna, 2011.
12. Conclusions
IpRGCs are mammalian photoreceptors whose morphological and physiological characteristics seem well suited for their primary role as light detectors for non-image forming visual reflexes. Because they were discovered so recently, many mysteries remain, and an untold number of functions for this rare and special type of ganglion cell should not be overlooked. Their invertebrate-like phototransduction cascade makes them unique among known vertebrate photoreceptors, and provides a window into possible mechanisms of the evolution of the retina. In addition to their intrinsic melanopsin-driven photosensitivity, ipRGCs receive rod and cone synaptic input and thus may provide the brain with different information in series, separated by complex spatial and temporal dynamics. Although they drive a number of tonic behaviors, requiring accurate representation of ambient light levels of long periods of time, ipRGCs can adapt to both light and darkness. They have an ability to communicate back to the retina, possibly changing the functional properties of image-forming retinal circuitry. Emerging evidence suggests that ipRGCs can also directly contribute to conscious visual perception. Finally, these RGCs regulate the early development of the retina and the retinogeniculate pathway. As the years catch up with this relatively young field of ganglion-cell photoreceptors, it is beginning to look like many RGC types are photoreceptive and perform diverse functional roles.
References.
Acharya JK, Jalink K. et al. InsP3 receptor is essential for growth and differentiation but not for vision in Drosophila. Neuron. 1997;18(6):881–7. [PubMed]
Altimus CM, Guler AD, Alam NM, Arman AC, Prusky GT, Sampath AP, and Hattar S. Rod photoreceptors drive circadian photoentrainment across a wide range of light intensities. Nat Neurosci 13: 1107-1112, 2010. [PubMed]
Altimus CM, Guler AD, Villa KL, McNeill DS, Legates TA, and Hattar S. Rods-cones and melanopsin detect light and dark to modulate sleep independent of image formation. Proc Natl Acad Sci U S A 105: 19998-20003, 2008. [PubMed]
Arendt D. Evolution of eyes and photoreceptor cell types. Int J Dev Biol. 2003;47(7-8):563–71. [PubMed]
Barlow HB, Levick WR. Three factors limiting the reliable detection of light by retinal ganglion cells of the cat. J Physiol. 1969;200(1):1–24. [PubMed]
Barnard AR, Hattar S, Hankins MW, and Lucas RJ. Melanopsin regulates visual processing in the mouse retina. Curr Biol 16: 389-395, 2006. [PubMed]
Baver SB, Pickard GE, Sollars PJ, and Pickard GE. Two types of melanopsin retinal ganglion cell differentially innervate the hypothalamic suprachiasmatic nucleus and the olivary pretectal nucleus. Eur J Neurosci 27: 1763-1770, 2008. [PubMed]
Belenky MA, Smeraski CA. et al. Melanopsin retinal ganglion cells receive bipolar and amacrine cell synapses. J Comp Neurol. 2003;460(3):380–93. [PubMed]
Berkowitz BA, Roberts R, and Bissig D. Light-dependant intraretinal ion regulation by melanopsin in young awake and free moving mice evaluated with manganese-enhanced MRI. Mol Vis 16: 1776-1780, 2010. [PubMed]
Berson DM, Dunn FA. et al. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295(5557):1070–3. [PubMed]
Berson DM. Phototransduction in ganglion-cell photoreceptors. Pflugers Arch. 2007;454(5):849–55. [PubMed]
Berson, DM, Castrucci AM and Provencio I (2010) Morphology and mosaics of melanopsin-expressing retinal ganglion cell types in mice. J Comp. Neurol. 518, 2405-2522. [PubMed]
Brainard GC, Hanifin JP, Greeson JM, Byrne B, Glickman G, Gerner E, and Rollag MD. Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. J Neurosci 21: 6405-6412, 2001. [PubMed]
Bramley JR, Wiles EM, Sollars PJ, and Pickard GE. Carbenoxolone blocks the light-evoked rise in intracellular calcium in isolated melanopsin ganglion cell photoreceptors. PLoS One 6: e22721, 2011. [PubMed]
Brown TM, Gias C, Hatori M, Keding SR, Semo M, Coffey PJ, Gigg J, Piggins HD, Panda S, and Lucas RJ. Melanopsin contributions to irradiance coding in the thalamo-cortical visual system. PLoS Biol 8: e1000558, 2010. [PubMed]
Brown TM, Tsujimura S, Allen AE, Wynne J, Bedford R, Vickery G, Vugler A, and Lucas RJ. Melanopsin-based brightness discrimination in mice and humans. Curr Biol 22: 1134-1141, 2012. [PubMed]
Cameron MA, Pozdeyev N, Vugler AA, Cooper H, Iuvone PM, and Lucas RJ. Light regulation of retinal dopamine that is independent of melanopsin phototransduction. Eur J Neurosci 29: 761-767, 2009. [PubMed]
Chew KS, Schmidt TM, Rupp AC, Kofuji P, and Trimarchi JM. Loss of gq/11 genes does not abolish melanopsin phototransduction. PLoS One 9: e98356, 2014. [PubMed]
Chyb S, Raghu P. et al. Polyunsaturated fatty acids activate the Drosophila light-sensitive channels TRP and TRPL. Nature. 1999;397(6716):255–9. [PubMed]
Czeisler CA, Shanahan TL, Klerman EB, Martens H, Brotman DJ, Emens JS, Klein T, and Rizzo JF, 3rd. Suppression of melatonin secretion in some blind patients by exposure to bright light. N Engl J Med 332: 6-11, 1995. [PubMed]
Dacey DM, Liao HW. et al. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature.2005;433(7027):749–54. [PubMed]
Dacey DM, Peterson BB, Robinson FR, and Gamlin PD. Fireworks in the primate retina: in vitro photodynamics reveals diverse LGN-projecting ganglion cell types. Neuron 37: 15-27, 2003. [PubMed]
Danilenko KV, and Sergeeva OY. Immediate effect of blue-enhanced light on reproductive hormones in women. Neuro Endocrinol Lett 36: 2015. [PubMed]
Dkhissi-Benyahya O, Coutanson C, Knoblauch K, Lahouaoui H, Leviel V, Rey C, Bennis M, and Cooper HM. The absence of melanopsin alters retinal clock function and dopamine regulation by light. Cell Mol Life Sci 70: 3435-3447, 2013. [PubMed]
Dkhissi-Benyahya O, Gronfier C, De Vanssay W, Flamant F, and Cooper HM. Modeling the role of mid-wavelength cones in circadian responses to light. Neuron 53: 677-687, 2007. [PubMed]
Do MT, Kang SH, Xue T, Zhong H, Liao HW, Bergles DE, and Yau KW. Photon capture and signalling by melanopsin retinal ganglion cells. Nature 457: 281-287, 2009. [PubMed]
Do MT, and Yau KW. Adaptation to steady light by intrinsically photosensitive retinal ganglion cells. Proc Natl Acad Sci U S A 110: 7470-7475, 2013. [PubMed]
Dollet A, Albrecht U, Cooper HM, and Dkhissi-Benyahya O. Cones are required for normal temporal responses to light of phase shifts and clock gene expression. Chronobiol Int 27: 768-781, 2010. [PubMed]
Dowling JE. The Retina: An Approachable Part of the Brain. Cambridge (MA): The Belknap Press of Harvard University Press; 1987.
Dumitrescu ON, Pucci FG, Wong KY, and Berson DM. Ectopic retinal ON bipolar cell synapses in the OFF inner plexiform layer: contacts with dopaminergic amacrine cells and melanopsin ganglion cells. J Comp Neurol 517: 226-244, 2009. [PubMed]
Ecker JL, Dumitrescu ON, Wong KY, Alum NM, Chen SK, LGates, T, renna JM, Prusky GT, Berson DM and Hattar S (2010) Melanopsin-expressing retinal ganglion-cell photoreceptors: cellular diversity and role in pattern vision. Neuron 67, 49-60. [PubMed]
Emanuel AJ, Do MT. Melanopsin twistability for sustained and broadband phototransduction. Neuron. Mar 4;85(5):1043-55, 2015. [PubMed]
Estevez ME, Fogerson PM, Ilardi MC, Borghuis BG, Chan E, Weng S, Auferkorte ON, Demb JB, and Berson DM. Form and function of the M4 cell, an intrinsically photosensitive retinal ganglion cell type contributing to geniculocortical vision. J Neurosci 32: 13608-13620, 2012. [PubMed]
Fain GL, Matthews HR. et al. Adaptation in vertebrate photoreceptors. Physiol Rev. 2001;81(1):117–151. [PubMed]
Famiglietti EV, Jr., and Kolb H. Structural basis for ON-and OFF-center responses in retinal ganglion cells. Science 194: 193-195, 1976. [PubMed]
Frazao R, MacMahon DG, Schunack W, Data P, Heidelberger R and Marshak DW (2011) Histamine elevates free intracellular calcium in mouse retinal dopaminergic cells via H-1 receptors. Invest. Ophthalmol. Vis. Sci. 52, 3083-3088.
Freedman MS, Lucas RJ. et al. Regulation of mammalian circadian behavior by non-rod, non-cone, ocular photoreceptors. Science. 1999;284(5413):502–4.[PubMed]
Fu Y, Yau KW. Phototransduction in mouse rods and cones. Pflugers Arch. 2007;454(5):805–19. [PubMed]
Fu Y, Zhong H, Wang MH, Luo DG, Liao HW, Maeda H, Hattar S, Frishman LJ, and Yau KW. Intrinsically photosensitive retinal ganglion cells detect light with a vitamin A-based photopigment, melanopsin. Proc Natl Acad Sci U S A 102: 10339-10344, 2005. [PubMed]
Gastinger, M.J., O’Brian, J.J., Larsen, J.N.J. and Marshak, D.W. (1999) Histamine immunoreactive axons in the macaque retina. Invest. Ophthal. Vis. Sci. 40, 487-495.
Gooley JJ, Lu J. et al. Melanopsin in cells of origin of the retinohypothalamic tract. Nat Neurosci. 2001;4(12):1165. [PubMed]
Goz D, Studholme K, Lappi DA, Rollag MD, Provencio I, and Morin LP. Targeted destruction of photosensitive retinal ganglion cells with a saporin conjugate alters the effects of light on mouse circadian rhythms. PLoS One 3: e3153, 2008. [PubMed]
Graham DM, Wong KY. et al. Melanopsin Ganglion Cells Use a Membrane-associated Rhabdomeric Phototransduction Cascade. J Neurophysiol.2008;99(5):2522–32. [PubMed]
Grunert U, Jusuf PR, Lee SC, and Nguyen DT. Bipolar input to melanopsin containing ganglion cells in primate retina. Vis Neurosci 28: 39-50, 2011. [PubMed]
Guler AD, Ecker JL, Lall GS, Haq S, Altimus CM, Liao HW, Barnard AR, Cahill H, Badea TC, Zhao H, Hankins MW, Berson DM, Lucas RJ, Yau KW, and Hattar S. Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature 453: 102-105, 2008. [PubMed]
Hankins MW, and Lucas RJ. The primary visual pathway in humans is regulated according to long-term light exposure through the action of a nonclassical photopigment. Curr Biol 12: 191-198, 2002. [PubMed]
Hannibal J, Georg B, Hindersson P, and Fahrenkrug J. Light and darkness regulate melanopsin in the retinal ganglion cells of the albino Wistar rat. J Mol Neurosci 27: 147-155, 2005. [PubMed]
Hannibal J, Hindersson P. et al. The photopigment melanopsin is exclusively present in pituitary adenylate cyclase-activating polypeptide-containing retinal ganglion cells of the retinohypothalamic tract. J Neurosci. 2002;22(1):RC191. [PubMed]
Hannibal J, Kankipati L, Strang CE, Peterson BB, Dacey D, and Gamlin PD. Central projections of intrinsically photosensitive retinal ganglion cells in the macaque monkey. J Comp Neurol 522: 2231-2248, 2014. [PubMed]
Hardie RC. Phototransduction in Drosophila melanogaster. J Exp Biol. 2001;204(Pt 20):3403–9. [PubMed]
Hardie RC, Raghu P. Visual transduction in Drosophila. Nature. 2001;413(6852):186–93. [PubMed]
Hardie RC. TRP channels in Drosophila photoreceptors: the lipid connection. Cell Calcium. 2003;33(5-6):385–93. [PubMed]
Hardie RC. Inhibition of phospholipase C activity in Drosophila photoreceptors by 1,2-bis(2-aminophenoxy)ethane N,N,N’,N’-tetraacetic acid (BAPTA) and di-bromo BAPTA. Cell Calcium. 2005;38(6):547–56. [PubMed]
Hatori M, Le H, Vollmers C, Keding SR, Tanaka N, Buch T, Waisman A, Schmedt C, Jegla T, and Panda S. Inducible ablation of melanopsin-expressing retinal ganglion cells reveals their central role in non-image forming visual responses. PLoS One 3: e2451, 2008. [PubMed]
Hattar S, Liao HW. et al. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002;295(5557):1065–70.[PubMed]
Hattar S, Lucas RJ. et al. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424(6944):76–81. [PubMed]
Hattar S, Kumar M. et al. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J Comp Neurol. 2006;497(3):326–49. [PubMed]
Horiguchi H, Winawer J, Dougherty RF, and Wandell BA. Human trichromacy revisited. Proc Natl Acad Sci U S A 110: E260-269, 2013. [PubMed]
Hoshi H, Liu WL, Massey SC, and Mills SL. ON inputs to the OFF layer: bipolar cells that break the stratification rules of the retina. J Neurosci 29: 8875-8883, 2009. [PubMed]
Hu C, Hill DD, and Wong KY. Intrinsic physiological properties of the five types of mouse ganglion-cell photoreceptors. J Neurophysiol 109: 1876-1889, 2013. [PubMed]
Iyilikci O, Aydin E, and Canbeyli R. Blue but not red light stimulation in the dark has antidepressant effect in behavioral despair. Behav Brain Res 203: 65-68, 2009. [PubMed]
Johnson J, Wu V, Donovan M, Majumdar S, Renteria RC, Porco T, Van Gelder RN, and Copenhagen DR. Melanopsin-dependent light avoidance in neonatal mice. Proc Natl Acad Sci U S A 107: 17374-17378, 2010. [PubMed]
Joo HR, Peterson BB, Dacey DM, Hattar S, and Chen SK. Recurrent axon collaterals of intrinsically photosensitive retinal ganglion cells. Vis Neurosci 30: 175-182, 2013. [PubMed]
Jusuf PR, Lee SC, Hannibal J, and Grunert U. Characterization and synaptic connectivity of melanopsin-containing ganglion cells in the primate retina. Eur J Neurosci 26: 2906-2921, 2007. [PubMed]
Kavakli IH, Sancar A. Circadian photoreception in humans and mice. Mol Interv. 2002;2(8):484–92. [PubMed]
Keeler, C.E. (1927). Iris movements in blind mice. Am. J. Physiol. 81, 107–112.
Keeler C. “Normal and “Rodless” Retinae of the House Mouse with Respect to the Electromotive Force Generated through Stimulation by Light. Proc Natl Acad Sci U S A. 1928;14(6):477–484. [PubMed]
Klein D, Weller J. Rapid Light-Induced Decrease in Pineal Serotonin N-Acetyltransferase Activity. Science. 1972;177(4048):532–533. [PubMed]
Knox BE, Solessio E. Shedding light on cones. J Gen Physiol. 2006;127(4):355–8. [PubMed]
Lall GS, Revell VL, Momiji H, Al Enezi J, Altimus CM, Guler AD, Aguilar C, Cameron MA, Allender S, Hankins MW, and Lucas RJ. Distinct contributions of rod, cone, and melanopsin photoreceptors to encoding irradiance. Neuron 66: 417-428, 2010. [PubMed]
Larsen PJ, Enquist LW, and Card JP. Characterization of the multisynaptic neuronal control of the rat pineal gland using viral transneuronal tracing. Eur J Neurosci 10: 128-145, 1998. [PubMed]
Lucas RJ, Freedman MS. et al. Regulation of the mammalian pineal by non-rod, non-cone, ocular photoreceptors. Science. 1999;284(5413):505–7. [PubMed]
Lucas RJ, Hattar S. et al. Diminished pupillary light reflex at high irradiances in melanopsin-knockout mice. Science. 2003;299(5604):245–7. [PubMed]
Lupi D, Oster H, Thompson S, and Foster RG. The acute light-induction of sleep is mediated by OPN4-based photoreception. Nat Neurosci 11: 1068-1073, 2008. [PubMed]
Masland RH. Cell populations of the retina: the Proctor lecture. Invest Ophthalmol Vis Sci 52: 4581-4591, 2011. [PubMed]
Matynia A, Parikh S, Chen B, Kim P, McNeill DS, Nusinowitz S, Evans C, and Gorin MB. Intrinsically photosensitive retinal ganglion cells are the primary but not exclusive circuit for light aversion. Exp Eye Res 105: 60-69, 2012. [PubMed]
Meesters Y, Dekker V, Schlangen LJ, Bos EH, and Ruiter MJ. Low-intensity blue-enriched white light (750 lux) and standard bright light (10,000 lux) are equally effective in treating SAD. A randomized controlled study. BMC Psychiatry 11: 17, 2011. [PubMed]
Melyan Z, Tarttelin EE, Bellingham J, Lucas RJ, and Hankins MW. Addition of human melanopsin renders mammalian cells photoresponsive. Nature 433: 741-745, 2005. [PubMed]
Morgan WW, and Kamp CW. Dopaminergic amacrine neurons of rat retinas with photoreceptor degeneration continue to respond to light. Life Sci 26: 1619-1626, 1980. [PubMed]
Morin LP, and Studholme KM. Separation of function for classical and ganglion cell photoreceptors with respect to circadian rhythm entrainment and induction of photosomnolence. Neuroscience 199: 213-224, 2011. [PubMed]
Mrosovsky N, and Hattar S. Impaired masking responses to light in melanopsin-knockout mice. Chronobiol Int 20: 989-999, 2003. [PubMed]
Muindi F, Zeitzer JM, Colas D, and Heller HC. The acute effects of light on murine sleep during the dark phase: importance of melanopsin for maintenance of light-induced sleep. Eur J Neurosci 37: 1727-1736, 2013. [PubMed]
Muller LP, Do MT, Yau KW, He S, and Baldridge WH. Tracer coupling of intrinsically photosensitive retinal ganglion cells to amacrine cells in the mouse retina. J Comp Neurol 518: 4813-4824, 2010. [PubMed]
Nelson R, Famiglietti EV, Jr., and Kolb H. Intracellular staining reveals different levels of stratification for on- and off-center ganglion cells in cat retina. J Neurophysiol 41: 472-483, 1978. [PubMed]
Neumann S, Haverkamp S, and Auferkorte ON. Intrinsically photosensitive ganglion cells of the primate retina express distinct combinations of inhibitory neurotransmitter receptors. Neuroscience 199: 24-31, 2011. [PubMed]
Noseda R, Kainz V, Jakubowski M, Gooley JJ, Saper CB, Digre K, and Burstein R. A neural mechanism for exacerbation of headache by light. Nat Neurosci 13: 239-245, 2010. [PubMed]
Pack W, Hill DD, and Wong KY. Melatonin modulates M4-type ganglion-cell photoreceptors. Neuroscience 303: 178-188, 2015. [PubMed]
Panda S, Sato TK. et al. Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting. Science. 2002;298(5601):2213–6. [PubMed]
Panda S, Provencio I. et al. Melanopsin is required for non-image-forming photic responses in blind mice. Science. 2003;301(5632):525–7. [PubMed]
Panda S, Nayak SK. et al. Illumination of the melanopsin signaling pathway. Science. 2005;307(5709):600–4. [PubMed]
Perez-Leon JA, Warren EJ. et al. Synaptic inputs to retinal ganglion cells that set the circadian clock. Eur J Neurosci. 2006;24(4):1117–23. [PubMed]
Perlman I, and Normann RA. Light adaptation and sensitivity controlling mechanisms in vertebrate photoreceptors. Prog Retin Eye Res 17: 523-563, 1998. [PubMed]
Prigge CL, and Zhang D. Pre- and post-synaptic mechanisms of signal transmission from ganglion cell photoreceptors to dopaminergic amacrine neurons. In: Association for Research in Vision and Ophthalmology, 2015.
Prigge CL, Yeh PT, Liou NF, Lee CC, You SF, Liu LL, McNeill DS, Chew KS, Hattar S, Chen SK, Zhang DQ. M1 ipRGCs Influence Visual Function through Retrograde Signaling in the Retina. J Neurosci Jul 6;36(27):7184-97, 2016. [PubMed]
Provencio I, Jiang G. et al. Melanopsin: An opsin in melanophores, brain, and eye. Proc Natl Acad Sci U S A. 1998;95(1):340–5. [PubMed]
Provencio I, Rodriguez IR. et al. A novel human opsin in the inner retina. J Neurosci. 2000;20(2):600–5. [PubMed]
Provencio I, Rollag MD. et al. Photoreceptive net in the mammalian retina. This mesh of cells may explain how some blind mice can still tell day from night. Nature.2002;415(6871):493. [PubMed]
Qiu X, Kumbalasiri T. et al. Induction of photosensitivity by heterologous expression of melanopsin. Nature. 2005;433(7027):745–9. [PubMed]
Rao S, Chun C, Fan J, Kofron JM, Yang MB, Hegde RS, Ferrara N, Copenhagen DR, and Lang RA. A direct and melanopsin-dependent fetal light response regulates mouse eye development. Nature 494: 243-246, 2013. [PubMed]
Reifler AN, Chervenak AP, Dolikian ME, Benenati BA, Meyers BS, Demertzis ZD, Lynch AM, Li BY, Wachter RD, Abufarha FS, Dulka EA, Pack W, Zhao X, and Wong KY. The rat retina has five types of ganglion-cell photoreceptors. Exp Eye Res 130: 17-28, 2015. [PubMed]
Reifer AN, Chervenak AP, Dolikian ME, Benenati BA, Li BY, Wachter RD, Lynch AM, Demertzis ZD, Meyers BS, Abufarha FS, Jaeckel ER, Flannery MP, Wong KY. AII spiking, sustained ON displaced amacrine ells receive gap-junction input from milano-sin ganglion cells. Current Biol. Nov 2;25(21):2763-73, 2015. [PubMed]
Renna JM, Weng S, and Berson DM. Light acts through melanopsin to alter retinal waves and segregation of retinogeniculate afferents. Nat Neurosci 14: 827-829, 2011. [PubMed]
Ribelayga C, and Mangel SC. A circadian clock and light/dark adaptation differentially regulate adenosine in the mammalian retina. J Neurosci 25: 215-222, 2005. [PubMed]
Ruby NF, Brennan TJ. et al. Role of melanopsin in circadian responses to light. Science. 2002;298(5601):2211–3. [PubMed]
Sakai HM, Naka K, and Dowling JE. Ganglion cell dendrites are presynaptic in catfish retina. Nature 319: 495-497, 1986. [PubMed]
Sakamoto K, Liu C, Kasamatsu M, Pozdeyev NV, Iuvone PM, and Tosini G. Dopamine regulates melanopsin mRNA expression in intrinsically photosensitive retinal ganglion cells. The European journal of neuroscience 22: 3129-3136, 2005. [PubMed]
Sakamoto K, Liu C, and Tosini G. Classical photoreceptors regulate melanopsin mRNA levels in the rat retina. J Neurosci 24: 9693-9697, 2004. [PubMed]
Schmidt TM, Alam NM, Chen S, Kofuji P, Li W, Prusky GT, and Hattar S. A role for melanopsin in alpha retinal ganglion cells and contrast detection. Neuron 82: 781-788, 2014. [PubMed]
Schmidt TM, and Kofuji P. Differential cone pathway influence on intrinsically photosensitive retinal ganglion cell subtypes. J Neurosci 30: 16262-16271, 2010. [PubMed]
Schmidt TM, and Kofuji P. Functional and morphological differences among intrinsically photosensitive retinal ganglion cells. J Neurosci 29: 476-482, 2009. [PubMed]
Schmidt TM, Taniguchi K, and Kofuji P. Intrinsic and extrinsic light responses in melanopsin-expressing ganglion cells during mouse development. J Neurophysiol 100: 371-384, 2008. [PubMed]
Sekaran S, Foster RG. et al. Calcium imaging reveals a network of intrinsically light-sensitive inner-retinal neurons. Curr Biol. 2003;13(15):1290–8. [PubMed]
Sekaran S, Lupi D, Jones SL, Sheely CJ, Hattar S, Yau KW, Lucas RJ, Foster RG, and Hankins MW. Melanopsin-dependent photoreception provides earliest light detection in the mammalian retina. Curr Biol 15: 1099-1107, 2005. [PubMed]
Shimazoe T, Morita M, Ogiwara S, Kojiya T, Goto J, Kamakura M, Moriya T, Shinohara K, Takiguchi S, Kono A, Miyasaka K, Funakoshi A, and Ikeda M. Cholecystokinin-A receptors regulate photic input pathways to the circadian clock. FASEB J 22: 1479-1490, 2008. [PubMed]
Sikka G, Hussmann GP, Pandey D, Cao S, Hori D, Park JT, Steppan J, Kim JH, Barodka V, Myers AC, Santhanam L, Nyhan D, Halushka MK, Koehler RC, Snyder SH, Shimoda LA, and Berkowitz DE. Melanopsin mediates light-dependent relaxation in blood vessels. Proc Natl Acad Sci U S A 111: 17977-17982, 2014. [PubMed]
Sodhi P, and Hartwick AT. Adenosine modulates light responses of rat retinal ganglion cell photoreceptors througha cAMP-mediated pathway. J Physiol 592: 4201-4220, 2014. [PubMed]
Suh BC, Inoue T. et al. Rapid chemically induced changes of PtdIns(4,5)P2 gate KCNQ ion channels. Science. 2006;314(5804):1454–7. [PubMed]
Tarttelin EE, Bellingham J, Bibb LC, Foster RG, Hankins MW, Gregory-Evans K, Gregory-Evans CY, Wells DJ, and Lucas RJ. Expression of opsin genes early in ocular development of humans and mice. Exp Eye Res 76: 393-396, 2003. [PubMed]
Thapan K, Arendt J, and Skene DJ. An action spectrum for melatonin suppression: evidence for a novel non-rod, non-cone photoreceptor system in humans. J Physiol 535: 261-267, 2001. [PubMed]
Tsai JW, Hannibal J, Hagiwara G, Colas D, Ruppert E, Ruby NF, Heller HC, Franken P, and Bourgin P. Melanopsin as a sleep modulator: circadian gating of the direct effects of light on sleep and altered sleep homeostasis in Opn4(-/-) mice. PLoS Biol 7: e1000125, 2009. [PubMed]
Tu DC, Zhang D. et al. Physiologic diversity and development of intrinsically photosensitive retinal ganglion cells. Neuron. 2005;48(6):987–99. [PubMed]
Van Gelder RN, Gibler TM. et al. Pleiotropic effects of cryptochromes 1 and 2 on free-running and light-entrained murine circadian rhythms. J Neurogenet.2002;16(3):181–203. [PubMed]
Van Gelder R N. Non-Visual Photoreception: Sensing Light without Sight. Curr Biol. 2008;18(1):R38–R39. [PubMed]
Van Hook MJ, Wong KY, and Berson DM. Dopaminergic modulation of ganglion-cell photoreceptors in rat. Eur J Neurosci 35: 507-518, 2012. [PubMed]
Vandewalle G, Collignon O, Hull JT, Daneault V, Albouy G, Lepore F, Phillips C, Doyon J, Czeisler CA, Dumont M, Lockley SW, and Carrier J. Blue light stimulates cognitive brain activity in visually blind individuals. J Cogn Neurosci 25: 2072-2085, 2013. [PubMed]
Vandewalle G, Gais S, Schabus M, Balteau E, Carrier J, Darsaud A, Sterpenich V, Albouy G, Dijk DJ, and Maquet P. Wavelength-dependent modulation of brain responses to a working memory task by daytime light exposure. Cereb Cortex 17: 2788-2795, 2007. [PubMed]
Vartanian GV, Li BY, Chervenak AP, Walch OJ, Pack W, Ala-Laurila P, and Wong KY. Melatonin Suppression by Light in Humans Is More Sensitive Than Previously Reported. J Biol Rhythms 30: 351-354, 2015. [PubMed]
Viney TJ, Balint K. et al. Local retinal circuits of melanopsin-containing ganglion cells identified by transsynaptic viral tracing. Curr Biol. 2007;17(11):981–8.[PubMed]
Viola AU, James LM, Schlangen LJ, and Dijk DJ. Blue-enriched white light in the workplace improves self-reported alertness, performance and sleep quality. Scand J Work Environ Health 34: 297-306, 2008. [PubMed]
Vugler AA, Redgrave P, Hewson-Stoate NJ, Greenwood J, and Coffey PJ. Constant illumination causes spatially discrete dopamine depletion in the normal and degenerate retina. J Chem Neuroanat 33: 9-22, 2007. [PubMed]
Vugler AA, Redgrave P. et al. Dopamine neurones form a discrete plexus with melanopsin cells in normal and degenerating retina. Exp Neurol. 2007;205(1):26–35. [PubMed]
Walker MT, Brown RL, Cronin TW, and Robinson PR. Photochemistry of retinal chromophore in mouse melanopsin. Proc Natl Acad Sci U S A 105: 8861-8865, 2008. [PubMed]
Weng S, Estevez ME, and Berson DM. Mouse ganglion-cell photoreceptors are driven by the most sensitive rod pathway and by both types of cones. PLoS One 8: e66480, 2013. [PubMed]
Weng S, Wong KY, and Berson DM. Circadian modulation of melanopsin-driven light response in rat ganglion-cell photoreceptors. J Biol Rhythms 24: 391-402, 2009. [PubMed]
Wong KY, Dunn FA. et al. Photoreceptor adaptation in intrinsically photosensitive retinal ganglion cells. Neuron. 2005;48(6):1001–10. [PubMed]
Wong KY, Dunn FA. et al. Synaptic influences on rat ganglion-cell photoreceptors. J Physiol. 2007;582(Pt 1):279–96. [PubMed]
Wong KY. A retinal ganglion cell that can signal irradiance continuously for 10 hours. J Neurosci 32: 11478-11485, 2012. [PubMed]
Xue T, Do MT, Riccio A, Jiang Z, Hsieh J, Wang HC, Merbs SL, Welsbie DS, Yoshioka T, Weissgerber P, Stolz S, Flockerzi V, Freichel M, Simon MI, Clapham DE, and Yau KW. Melanopsin signalling in mammalian iris and retina. Nature 479: 67-73, 2011. [PubMed]
Zaidi FH, Hull JT, Peirson SN, Wulff K, Aeschbach D, Gooley JJ, Brainard GC, Gregory-Evans K, Rizzo JF, 3rd, Czeisler CA, Foster RG, Moseley MJ, and Lockley SW. Short-wavelength light sensitivity of circadian, pupillary, and visual awareness in humans lacking an outer retina. Curr Biol 17: 2122-2128, 2007. [PubMed]
Zhang DQ, Belenky MA, Sollars PJ, Pickard GE, and McMahon DG. Melanopsin mediates retrograde visual signaling in the retina. PLoS One 7: e42647, 2012. [PubMed]
Zhang DQ, Wong KY, Sollars PJ, Berson DM, Pickard GE, and McMahon DG. Intraretinal signaling by ganglion cell photoreceptors to dopaminergic amacrine neurons. Proc Natl Acad Sci U S A 105: 14181-14186, 2008. [PubMed]
Zhang DQ, Zhou TR. et al. Functional heterogeneity of retinal dopaminergic neurons underlying their multiple roles in vision. J Neurosci. 2007;27(3):692–9.[PubMed]
Zhao X, Stafford BK, Godin AL, King WM, and Wong KY. Photoresponse diversity among the five types of intrinsically photosensitive retinal ganglion cells. J Physiol 592: 1619-1636, 2014. [PubMed]
Zhao X, Pack, W, Khan NW, Wong KY. Prolonged Inner Retinal Photoreception Depends on the Visual Retinoid Cycle. J Neurosci. Apr 13;36(15): 4209-17, 2016. [PubMed]
Updated November 20, 2016
The authors
Dr. Kwoon Wong grew up in Hong Kong, when it was still a British colony. His undergraduate major at the University of Texas at Austin was biochemistry, and as he started his PhD career at Harvard he was convinced that he wanted to study cancer biology. But an immensely satisfying rotation in Prof. John Dowling’s lab lured Kwoon into the world of neurophysiology, and he has been studying the visual system ever since. For his PhD thesis, he studied glutamate receptors and transporters at the photoreceptor-bipolar cell synapse in fish retinas. Then, at the 2002 Society for Neuroscience conference, he saw Prof. David Berson’s fascinating symposium presentation on melanopsin-expressing ganglion cells, a novel class of retinal photoreceptors involved in non-image-forming visual behaviors. Awestruck by this talk, Kwoon decided to do his postdoctoral work with Prof. Berson at Brown. During Kwoon’s six years in the Berson lab, he investigated melanopsin ganglion cells’ light responses and synaptic circuits. Since Jan 2010, he has been an Assistant Professor at the University of Michigan, where he has continued to study these exotic ganglion cells.
Dr. Dustin M. Graham was born and raised in Pleasanton, California and received his Ph.D. in Neuroscience from Brown University. He began his research career at Santa Clara University in the lab of Dr. David Tauck, studying neural pathways of learning and memory in the pond snail Lymnaea stagnalis. His interests turned to the retina while working with Dr. Ralph Nelson at the National Institutes of Health. There he helped develop a rapid labeling technique to delineate morphological subtypes of retinal neurons in zebrafish. As a graduate student, Dustin studied mammalian circadian rhythms and the newly discovered melanopsin ganglion cell activity in Dr. David Berson’s lab. He focused on the phototransduction cascade in ipRGCs, and developed a dissociation and culturing procedure to identify and record light responses from isolated ipRGCs. Dustin has since completed research fellowships in the Department of Psychology, University of Virginia, and in the Department of Neurobiology & Behavior, Stony Brook University, NY. He is currently an editor in the Nature Publishing Group, New York, NY.