Stuart Trenholm1 and Gautam B. Awatramani2
1Assistant Professor, Montreal Neurological Institute, McGill University, Montreal, Canada: stuart.trenholm@mcgill.ca
2Associate Professor, Department of Biology, University of Victoria, Victoria, Canada: gautam@uvic.ca
Abstract
Gap junctions are recognized in the electron microscope as dense starchy areas of opposed membrane between two cells. Small tracer molecules such as Neurobiotin pass through the gap junction pore, marking interconnected cells. Electrical signals pass through these junctions, extending the region of neuronal data collection, modifying the dynamic properties of the signal, and synchronizing impulse generation. In the retina, circuits comprised of many neuronal types transform information within the visual environment into a series of highly processed, simplified spike trains that are relayed to higher visual areas. Retinal signal processing relies on intricate interactions between specific cell types, which are mediated by both chemical and electrical synapses. In the field, great strides have been made in understanding the diversity of retinal cell types, of which there are likely at least 100 unique types, and how they connect to form cell-type-specific circuits. Here, we provide an overview of electrical coupling in the retina and outline the varied roles that gap junctions play in processing information in healthy and diseased states.
Introduction
The retina is built from approximately 100 distinct neuronal cell types, which reside in specific retinal layers and connect with one another to form intricate microcircuits (1-5). These circuits serve to extract principle features of the visual world, which get parsed into distinct visual channels via different retinal ganglion cell types. These differentially processed visual signals are then relayed out of the retina to a large number of higher visual areas (6, 7). Interestingly, microcircuits in the retina are connected through both chemical synapses and bi-directional electrical synapses formed by gap junctions. The rich interactions between electrical and chemical synapses enable circuits to operate in highly flexible and dynamic ways. Moreover, as the strength of gap junctions can be modified over a variety of timescales, they can reconfigure retinal microcircuits on both a millisecond time-scale as well as over the course of the day/night cycle. In addition, gap junctions exert their effects over a range of spatial scales, synchronizing activity at the sub-cellular level as well as across wide swaths of the retina. Given these diverse roles for gap junctions in retinal signal processing, it is not surprising that electrical synapses also play many roles in disease states. Finally, despite the advances outlined here regarding our understanding of the various roles for gap junctions in retinal signaling, it is likely that we are still only scratching the surface in identifying the full repertoire of their functional roles.
Gap junctions in the retina and how they affect receptive field size
The vertebrate retina contains five principal neuron classes: photoreceptors (rods and cones), horizontal cells, bipolar cells, amacrine cells and ganglion cells. For each class of neuron, we describe electrical and chemical synaptic connectivity patterns (Figure 1). For electrical synapses, when known, we outline the connexin subtypes involved in forming gap junctions and describe their biophysical properties. Also, whenever possible, we describe the strength of gap junctions between pairs of neurons, represented as either the junctional conductance or the coupling coefficient (i.e. the change in membrane potential of a post-junctional cell divided by the change in the membrane potential in a pre-junctional cell when then membrane potential of the pre-junctional cell is altered).
Despite the characterizations of coupling strength that we will outline below, it is important to keep in mind that the conductance through a gap junction is not static, and changes to the conductance can have significant effects on the roles that electrical synapses play in neuronal circuits. For instance, modifying the strength of gap junction coupling can significantly alter the receptive field size of retinal neurons by modifying the strength and extent of their excitatory receptive field surround. For many neurons in the retina, light adaptation or circadian rhythm can modulate the strength of electrical coupling and thus alter receptive field sizes. Changes to coupling strength could also modify other roles for gap junctions in retinal circuits. While molecular mechanisms underlying changes in coupling strength have been studied in detail (8), in the following section we focus on the functional outcomes of modifications in coupling strength on receptive field size.
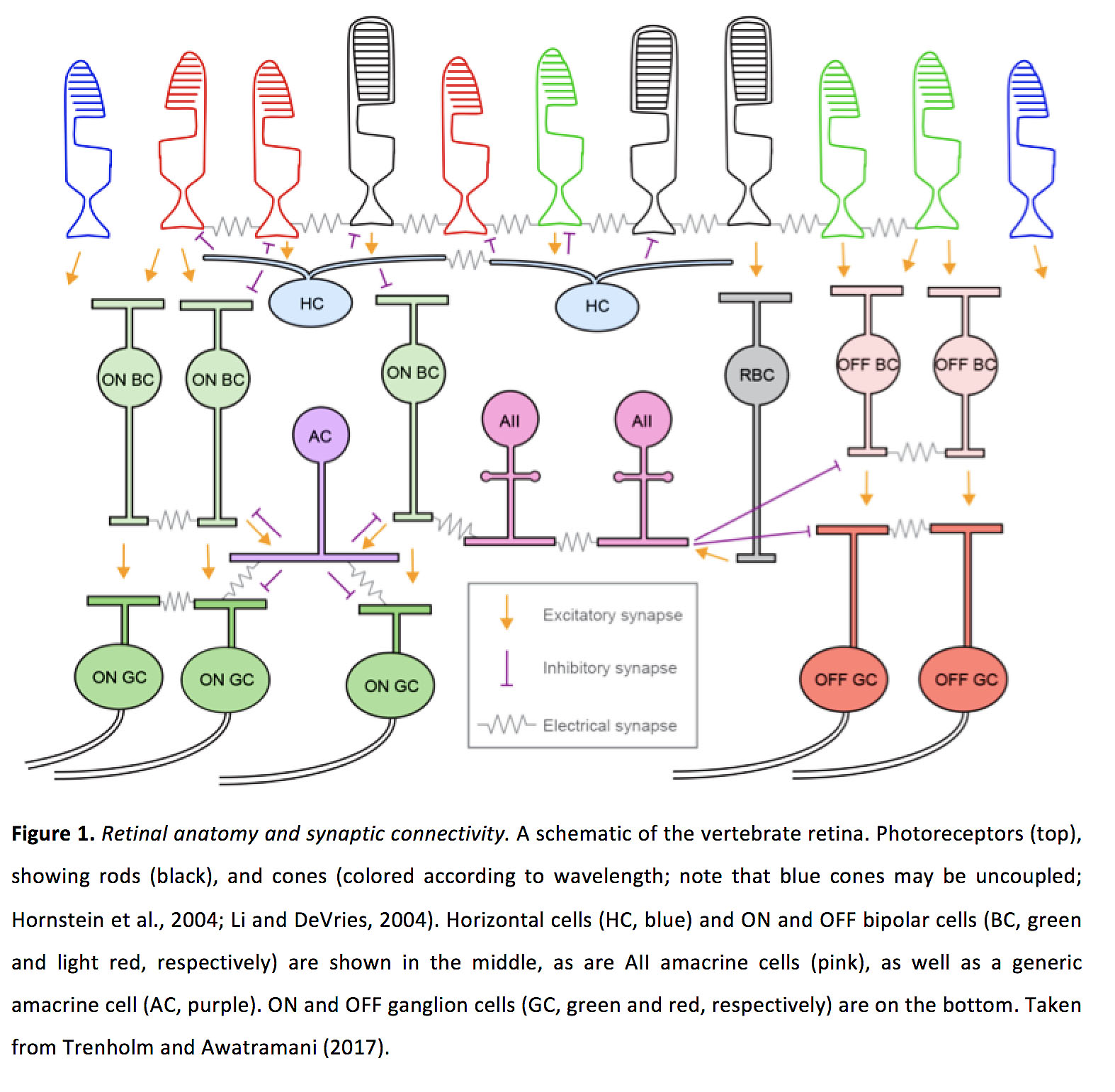
Figure 1. Retinal anatomy and synaptic connectivity. A schematic of the vertebrate retina. Photoreceptors (top), showing rods (black), and cones, colored according to wavelength. Note that blue cones may be uncoupled (14, 27). Horizontal cells (HC, blue), ON bipolar cells (ON BC, green), OFF bipolar cells (OFF BC, light red), and rod bipolar cells (RBC, gray) are shown in the middle, as are AII amacrine cells (pink), as well as a generic amacrine cell (AC, purple). ON and OFF ganglion cells (ON GC, green; OFF GC red) are on the bottom. Taken from Trenholm and Awatramani (2017) (197).
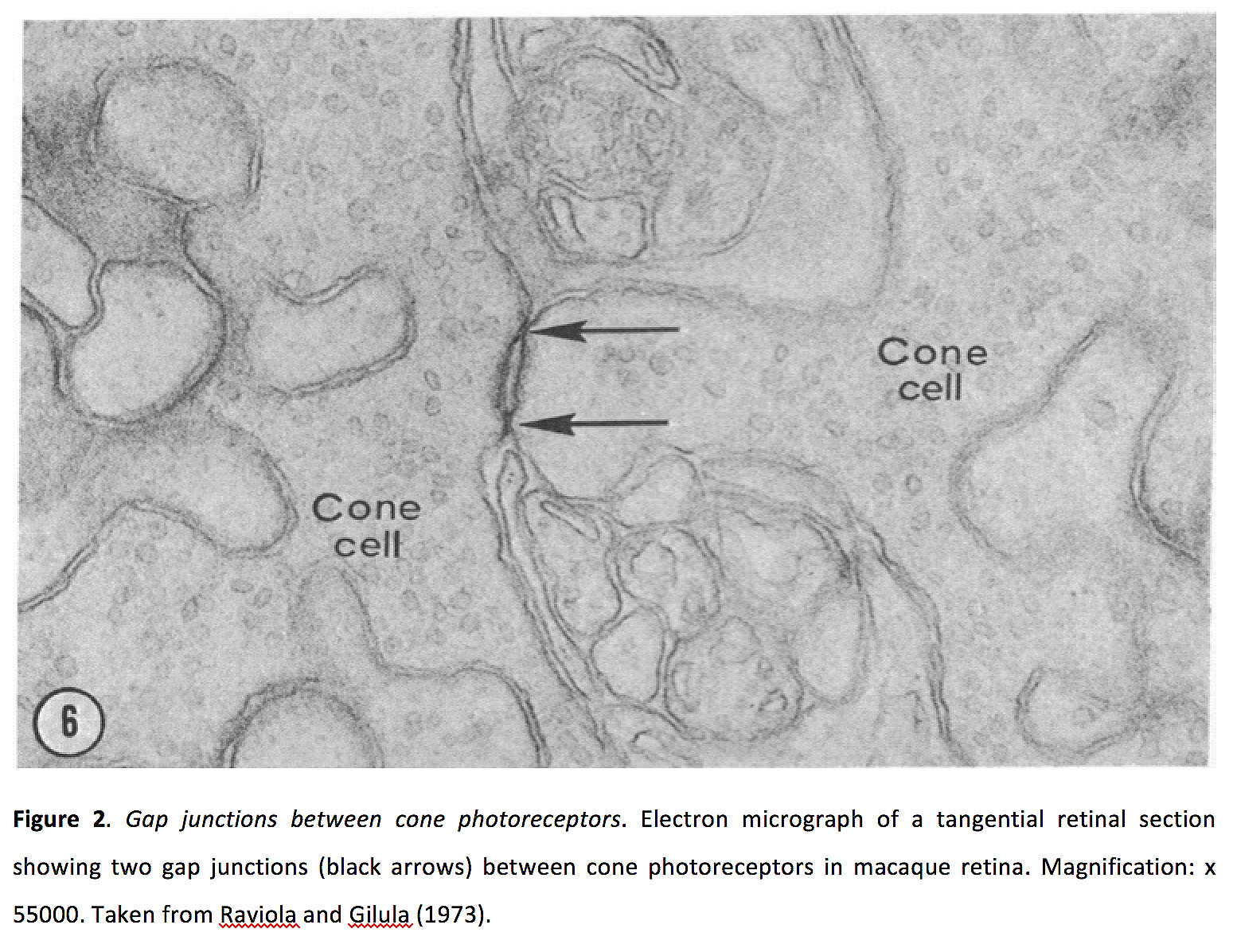 Figure 2. Gap junctions between cone photoreceptors. Electron micrograph of a tangential retinal section showing two gap junctions (black arrows) between cone photoreceptors in macaque retina. Magnification: x 55000. Taken from Raviola and Gilula (1973) (16).
Figure 2. Gap junctions between cone photoreceptors. Electron micrograph of a tangential retinal section showing two gap junctions (black arrows) between cone photoreceptors in macaque retina. Magnification: x 55000. Taken from Raviola and Gilula (1973) (16).
Photoreceptors
Vision starts in rod and cone photoreceptors, as photons are converted into an electro-chemical signal (See Webvision chapter Phototransduction in Rods and Cones). Photoreceptors make glutamatergic synapses with horizontal and bipolar cells (ionotropic sign conserving synapses with horizontal cells and OFF bipolar cells; metabotropic sing-inverting synapses with ON bipolar cells). Photoreceptors also form both homologous (i.e. cone-cone and rod-rod) and heterologous (rod-cone) electrical synapses (Figure 2) (9-17).
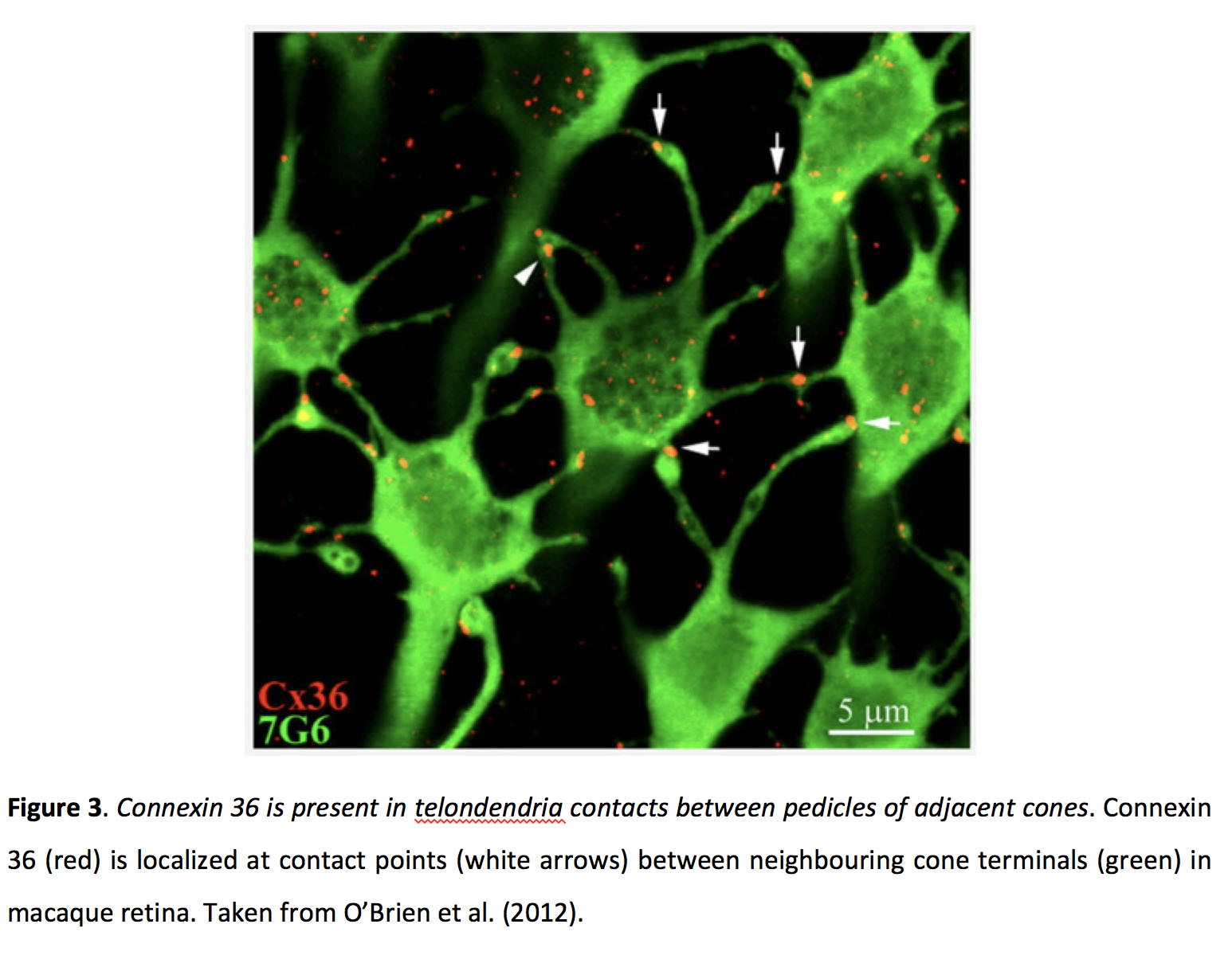 Figure 3. Connexin 36 is present in telodendria contacts between pedicles of adjacent cones. Connexin 36 (Cx36, red) is localized at contact points (white arrows) between neighbouring cone terminals (7G6, green, cone arrestin antibody) in macaque retina. Taken from O’Brien et al. (2012) (21).
Figure 3. Connexin 36 is present in telodendria contacts between pedicles of adjacent cones. Connexin 36 (Cx36, red) is localized at contact points (white arrows) between neighbouring cone terminals (7G6, green, cone arrestin antibody) in macaque retina. Taken from O’Brien et al. (2012) (21).
Cone gap junctions contain connexin 36 (Cx36) in mammals (Figure 3) (18-21) and Cx35 in zebrafish (22), while the connexin involved in rod electrical synapses has not been definitively identified (22-24), and coupling between rods and cones appears to rely on Cx36 (25). Photoreceptor gap junctions are localized around the axon terminal of cones (11, 16, 26-28).
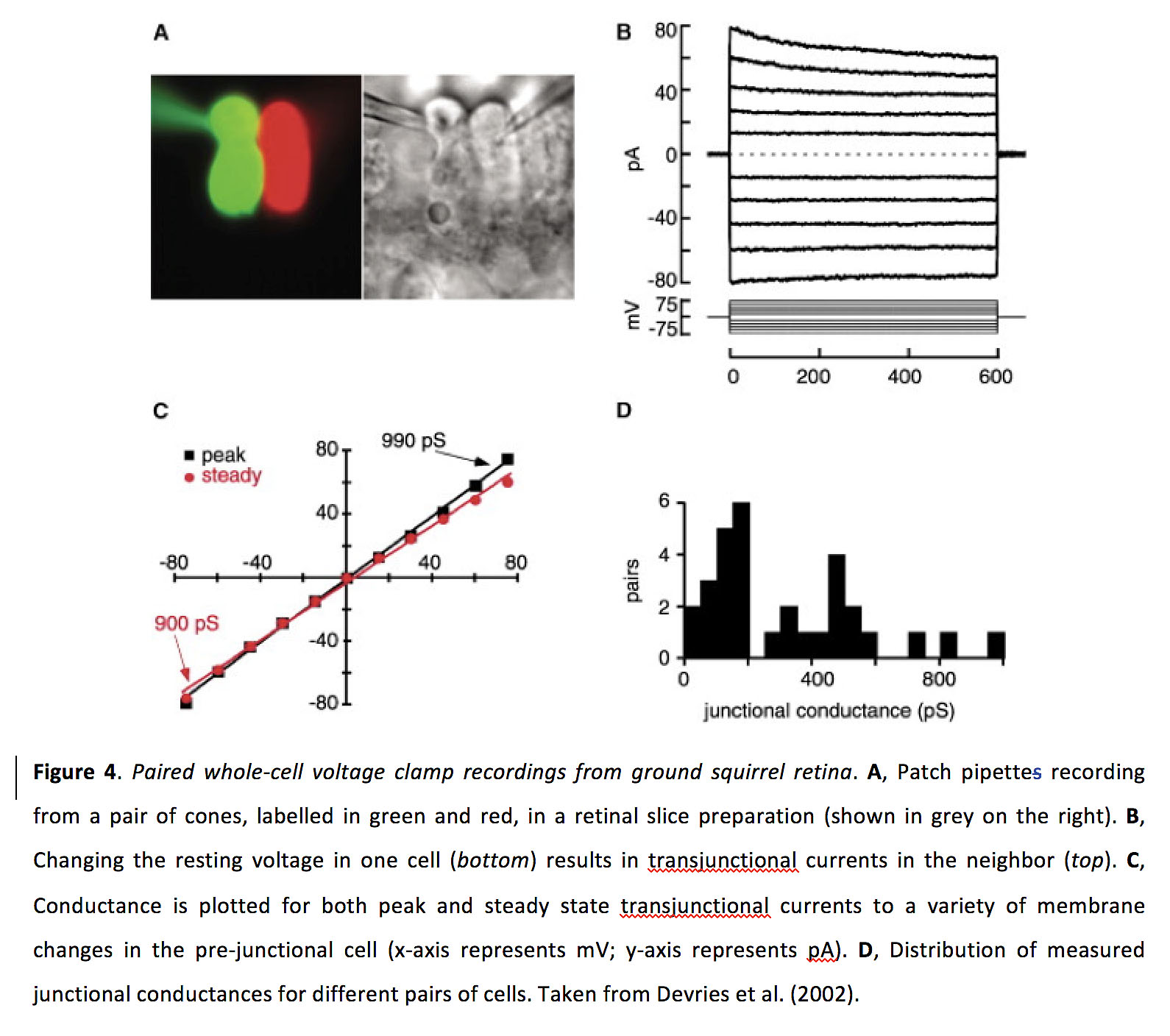 Figure 4. Paired whole-cell voltage clamp recordings from ground squirrel retina. A, Patch pipette recording from a pair of cones, labelled in green and red, in a retinal slice preparation (shown in grey on the right). B, Changing the resting voltage in one cell (bottom) results in transjunctional currents in the neighbor (top). C, Conductance is plotted for both peak and steady state transjunctional currents to a variety of membrane changes in the pre-junctional cell (x-axis represents mV; y-axis represents pA). D, Distribution of measured junctional conductances for different pairs of cells. Taken from Devries et al. (2002) (12).
Figure 4. Paired whole-cell voltage clamp recordings from ground squirrel retina. A, Patch pipette recording from a pair of cones, labelled in green and red, in a retinal slice preparation (shown in grey on the right). B, Changing the resting voltage in one cell (bottom) results in transjunctional currents in the neighbor (top). C, Conductance is plotted for both peak and steady state transjunctional currents to a variety of membrane changes in the pre-junctional cell (x-axis represents mV; y-axis represents pA). D, Distribution of measured junctional conductances for different pairs of cells. Taken from Devries et al. (2002) (12).
Based on physiological recordings, it appears that homologous photoreceptor coupling is stronger than heterologous coupling. The junctional conductance of cone-cone and rod-rod gap junctions appears to be bidirectionally symmetrical and in the range of 200-800 pS (Figure 4) (12, 14, 15, 27, 29), with a coupling coefficient of around 0.15 (14, 30). The junctional conductance of rod-cone gap junctions has been reported to be bidirectionally symmetrical and in the range of 40-200 pS (31), with a coupling coefficient of around 0.04 (32). From tracer dye experiments where the dye is injected into cones, it appears that being coupled to rods is more common than being coupled to only cones or to both rods and cones, and some cones may be uncoupled (13). In contrast, from tracer dye experiments where the dye is injected into rods, rod-rod coupling seems to be more common than rod-cone coupling and some rods may be uncoupled (15).
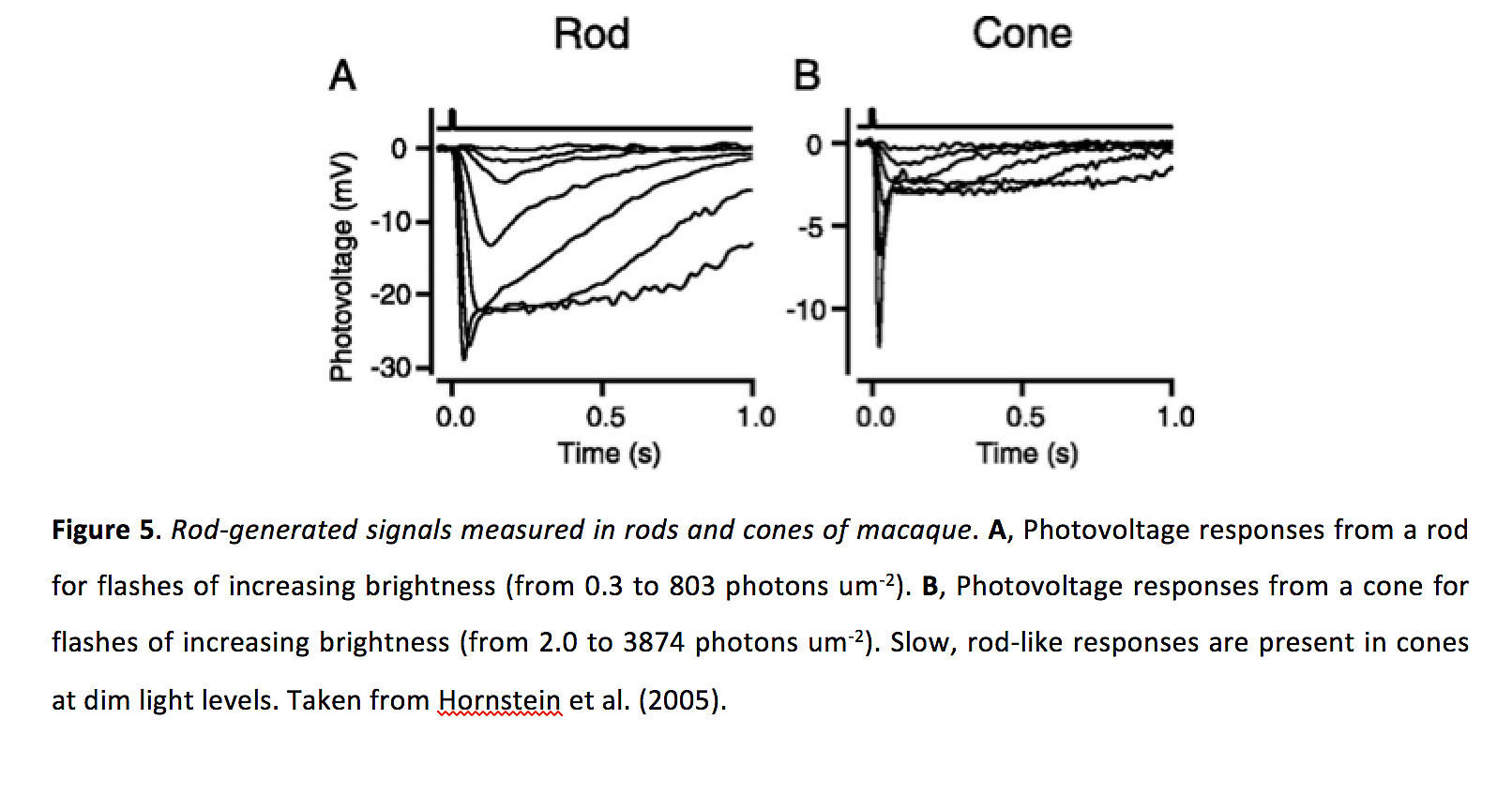 Figure 5. Rod-generated signals measured in rods and cones of macaque. A, Photovoltage responses from a rod for flashes of increasing brightness (from 0.3 to 803 photons um-2). B, Photovoltage responses from a cone for flashes of increasing brightness (from 2.0 to 3874 photons um-2). Slow, rod-like responses are present in cones at dim light levels. The stimulating wavelength is 500 nm. Taken from Hornstein et al. (2005) (13).
Figure 5. Rod-generated signals measured in rods and cones of macaque. A, Photovoltage responses from a rod for flashes of increasing brightness (from 0.3 to 803 photons um-2). B, Photovoltage responses from a cone for flashes of increasing brightness (from 2.0 to 3874 photons um-2). Slow, rod-like responses are present in cones at dim light levels. The stimulating wavelength is 500 nm. Taken from Hornstein et al. (2005) (13).
Circadian rhythm, and not light adaptation, appears to be the main controller for altering the strength of electrical coupling in photoreceptors in fish and mouse retinae (33). Psychophysical experiments in humans have indicated that cone coupling appears to be unaffected by light adaptation, but effects of circadian rhythm have not been tested (12). In contrast, light adaptation, not circadian rhythm, appears to modulate electrical coupling strength between salamander photoreceptors (31). Altering the strength of gap junctions can modify the receptive field size of photoreceptors. For instance, cones have larger receptive fields during states in which rods and cones are more strongly electrically coupled (33). Similarly, rod receptive fields are also expected to be larger when they are more strongly coupled, and coupling decreases trial-to-trial variability in their responses to dim flashes (15). Finally, heterotypic gap junctions allow cone responses to pass into rods (34), and rod responses to pass into cones (Figure 5) (13, 35-37), meaning that increases in electrical coupling should extend the range of light intensities over which a given rod or cone can respond, and at least for cones such coupling could result in increased reliability of threshold responses.
Horizontal cells
Horizontals receive glutamatergic inputs from photoreceptors, and in turn provide negative feedback to photoreceptors and possibly feedforward inhibition to bipolar cells (See Webvision chapter S-potentials and Horizontal cells). The mechanisms of feedback interactions are still actively being studied (38). Additionally, horizontal cells are densely interconnected with gap junctions (Figure 6) (26, 39).
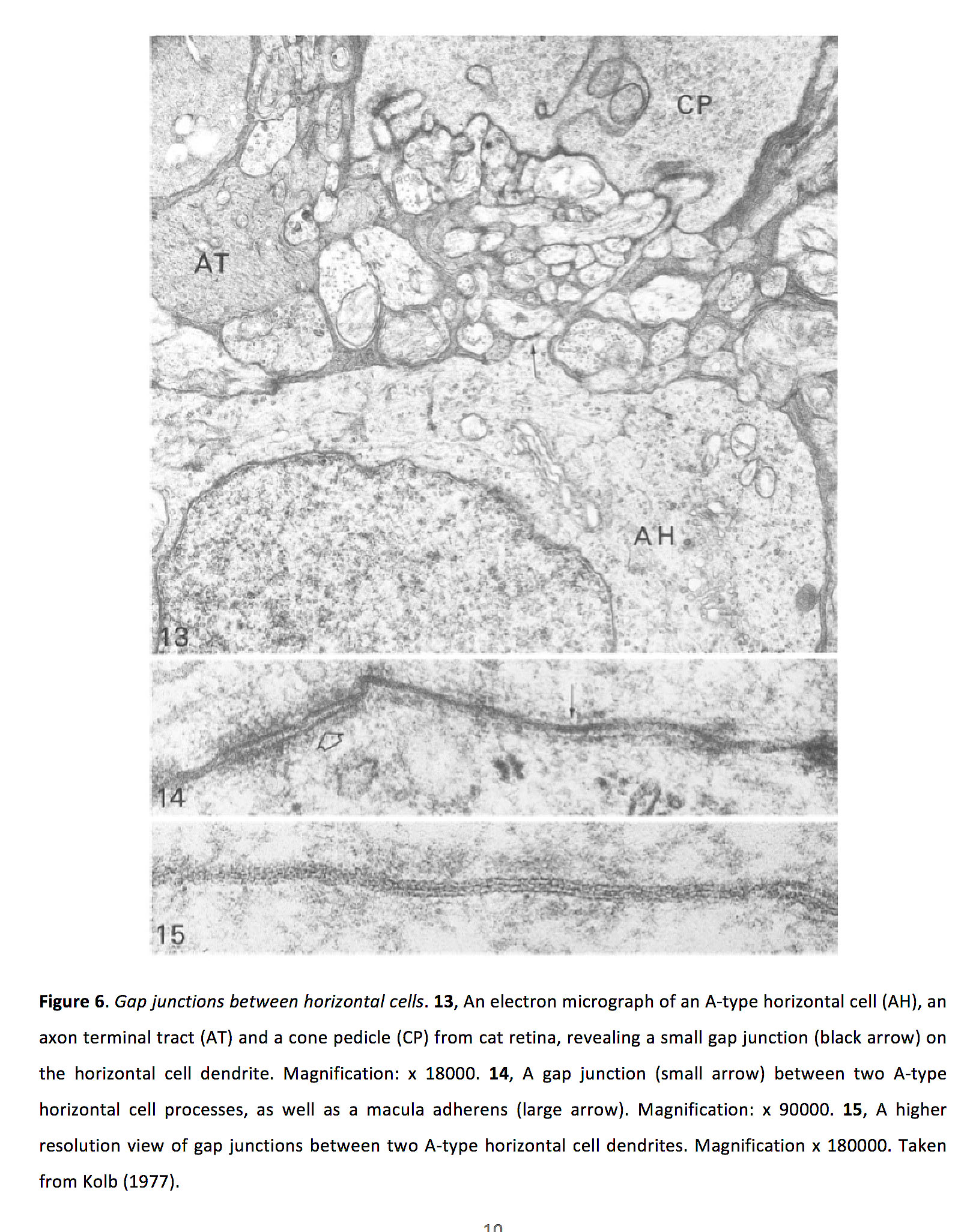 Figure 6. Gap junctions between horizontal cells. 13, An electron micrograph of an A-type horizontal cell (AH), an axon terminal tract (AT) and a cone pedicle (CP) from cat retina, revealing a small gap junction (black arrow) on the horizontal cell dendrite. Magnification: x 18000. 14, A gap junction (small arrow) between two A-type horizontal cell processes, as well as a macula adherens (large arrow). Magnification: x 90000. 15, A higher resolution view of gap junctions between two A-type horizontal cell dendrites. Magnification x 180000. Taken from Kolb (1977) (26).
Figure 6. Gap junctions between horizontal cells. 13, An electron micrograph of an A-type horizontal cell (AH), an axon terminal tract (AT) and a cone pedicle (CP) from cat retina, revealing a small gap junction (black arrow) on the horizontal cell dendrite. Magnification: x 18000. 14, A gap junction (small arrow) between two A-type horizontal cell processes, as well as a macula adherens (large arrow). Magnification: x 90000. 15, A higher resolution view of gap junctions between two A-type horizontal cell dendrites. Magnification x 180000. Taken from Kolb (1977) (26).
Horizontal cell gap junctions can be either axoaxonic or dendrodendritic. In mouse, Cx57 is present in both types of gap junctions (Figure 7) (40, 41), while Cx50 is localized in axonal gap junctions (42). In rabbit A-type horizontal cells, Cx50 localizes in dendritic gap junctions (43), and Cx57 is found in axonal gap junctions in B-type horizontal cells (44). In zebrafish, horizontal cell gap junctions appear to be comprised of Cx52.6, 52.9 and 55.5 (45, 46).
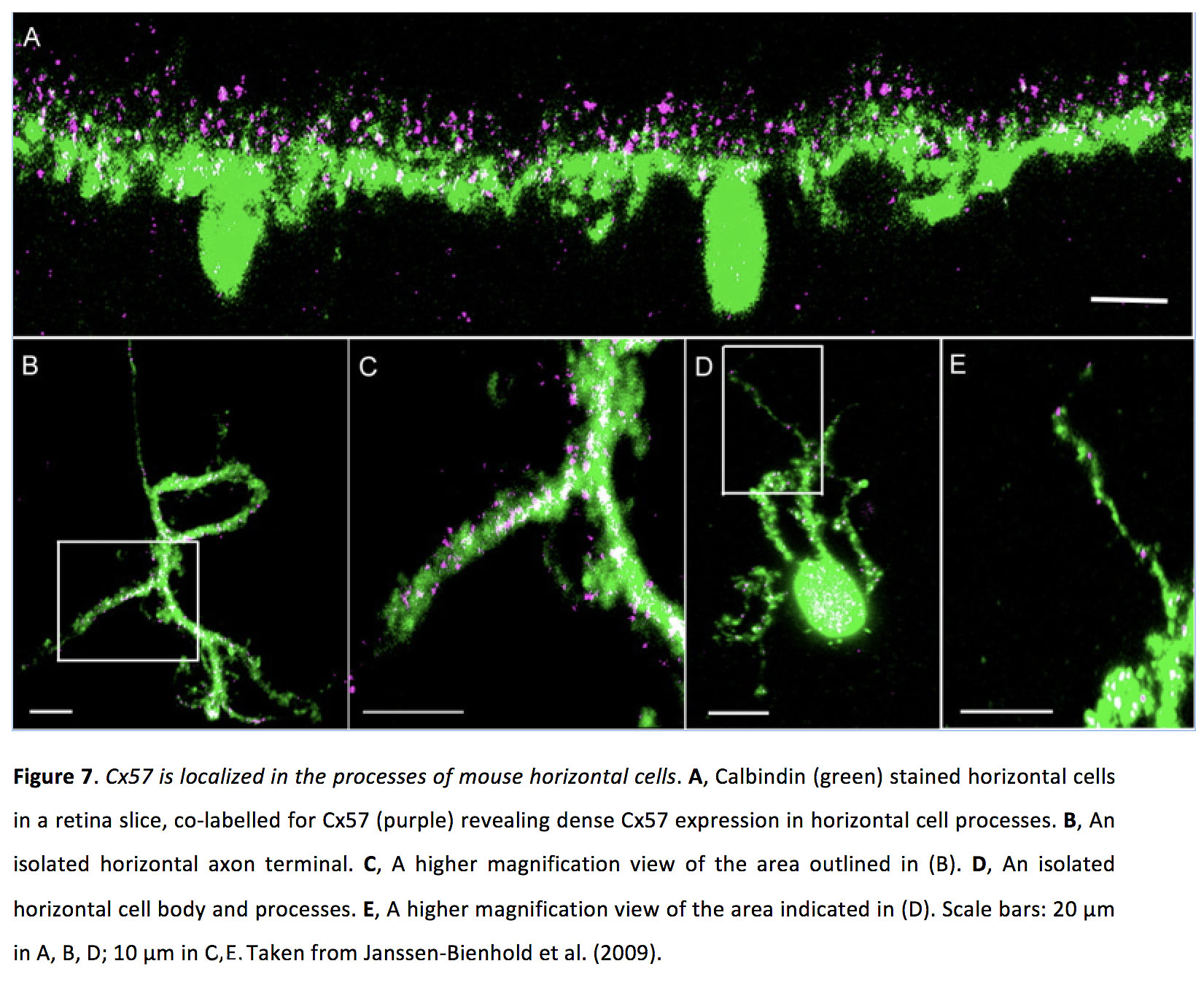 Figure 7. Cx57 is localized in the processes of mouse horizontal cells. A, Calbindin (green) stained horizontal cells in a retina slice, co-labelled for Cx57 (purple) revealing dense Cx57 expression in horizontal cell processes. B, An isolated horizontal axon terminal. C, A higher magnification view of the area outlined in (B). D, An isolated horizontal cell body and processes. E, A higher magnification view of the area indicated in (D). Scale bars: 20 µm in A, B, D; 10 µm in C, E. Taken from Janssen-Bienhold et al. (2009) (41).
Figure 7. Cx57 is localized in the processes of mouse horizontal cells. A, Calbindin (green) stained horizontal cells in a retina slice, co-labelled for Cx57 (purple) revealing dense Cx57 expression in horizontal cell processes. B, An isolated horizontal axon terminal. C, A higher magnification view of the area outlined in (B). D, An isolated horizontal cell body and processes. E, A higher magnification view of the area indicated in (D). Scale bars: 20 µm in A, B, D; 10 µm in C, E. Taken from Janssen-Bienhold et al. (2009) (41).
Coupling between horizontal cells appears to be very strong, with a bidirectional and symmetrical junctional conductance of > 1ns and with a coupling coefficient of 0.5 or greater in cultured horizontal cells (47-50). From remarkable tracer dye experiments, where dye is injected into a single horizontal cell, it has been found that dye can spread into more than 1000 surrounding horizontal cells extending across a distance of over 1 mm (Figure 8) (51).
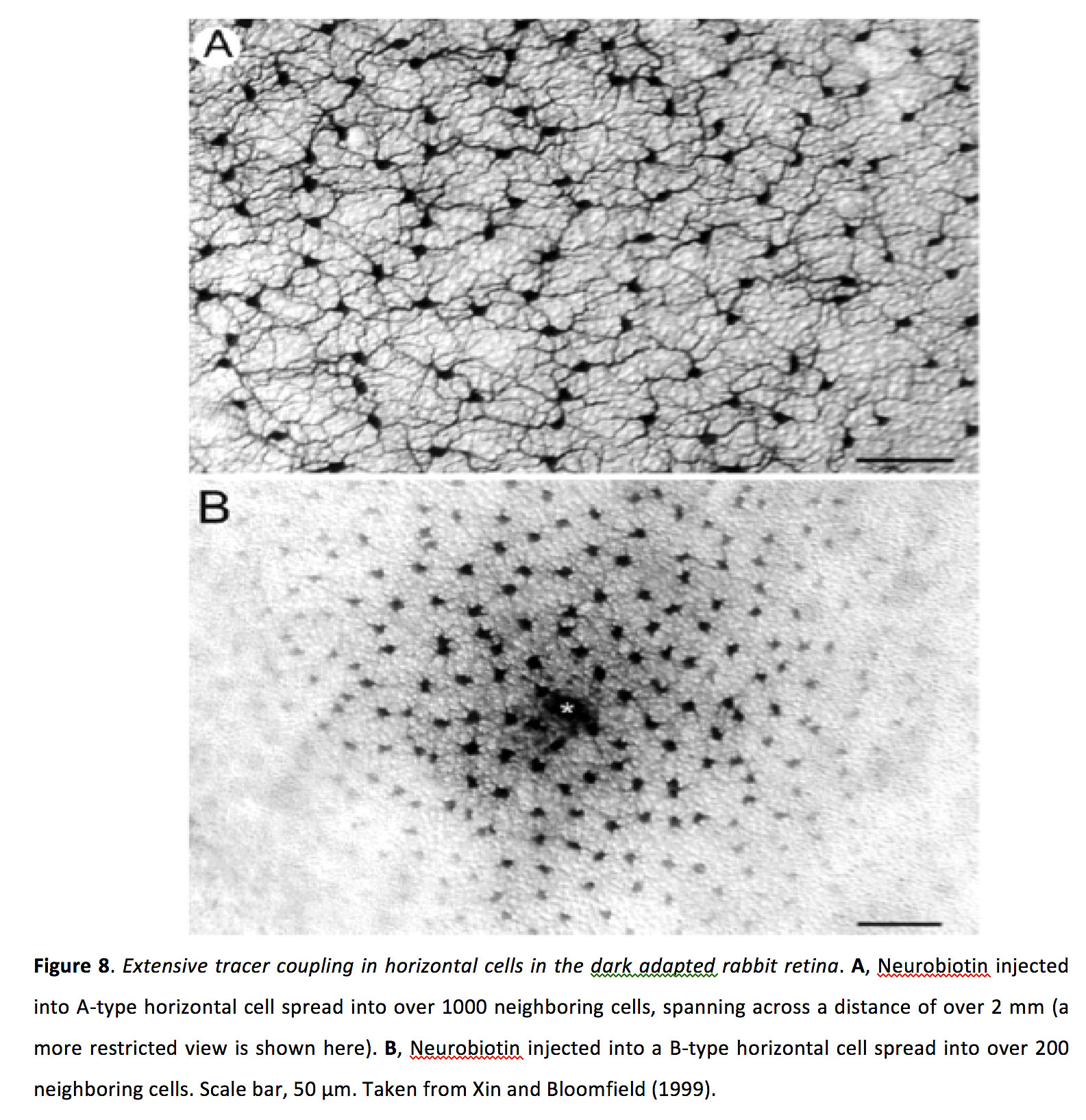 Figure 8. Extensive tracer coupling in horizontal cells in the dark-adapted rabbit retina. A, Neurobiotin injected into A-type horizontal cell spread into over 1000 neighboring cells, spanning across a distance of over 2 mm (a more restricted view is shown here). B, Neurobiotin injected into a B-type horizontal cell spread into over 200 neighboring cells. Scale bar, 50 µm. Taken from Xin and Bloomfield (1999) (51).
Figure 8. Extensive tracer coupling in horizontal cells in the dark-adapted rabbit retina. A, Neurobiotin injected into A-type horizontal cell spread into over 1000 neighboring cells, spanning across a distance of over 2 mm (a more restricted view is shown here). B, Neurobiotin injected into a B-type horizontal cell spread into over 200 neighboring cells. Scale bar, 50 µm. Taken from Xin and Bloomfield (1999) (51).
In horizontal cells, light adaptation appears to be the main factor controlling the strength of gap junction coupling (52, 53). Horizontal cell coupling has been shown to exhibit a tri-phasic relationship depending on luminance levels: tracer coupling experiments show minimal coupling in the dark adapted or “dark-suppressed” state; tracer coupling is greatly increased in medium light levels, during the so-called “light-sensitized” state; tracer coupling is very weak during the light adapted state (Figure 9) (51).
 Figure 9. Triphasic adaptation of horizontal cell tracer coupling. Injecting Lucifer yellow dye into a single H1 horizontal cell in goldfish retina results in little tracer coupling in dark adapted (“Dark Suppressed”) retina (A), extensive tracer coupling in the “Light Sensitized” state (B), and little if any tracer coupling in the “Light Adapted” state (C). Taken from Baldridge (2001) (198).
Figure 9. Triphasic adaptation of horizontal cell tracer coupling. Injecting Lucifer yellow dye into a single H1 horizontal cell in goldfish retina results in little tracer coupling in dark adapted (“Dark Suppressed”) retina (A), extensive tracer coupling in the “Light Sensitized” state (B), and little if any tracer coupling in the “Light Adapted” state (C). Taken from Baldridge (2001) (198).
Horizontal cell coupling seems to strongly affect receptive field size (54), with receptive fields being approximately three times larger in the light-sensitized state (51). Consistent with this finding, knocking out Cx57 in mice decreases the size of horizontal cell receptive fields (Figure 10) (55).
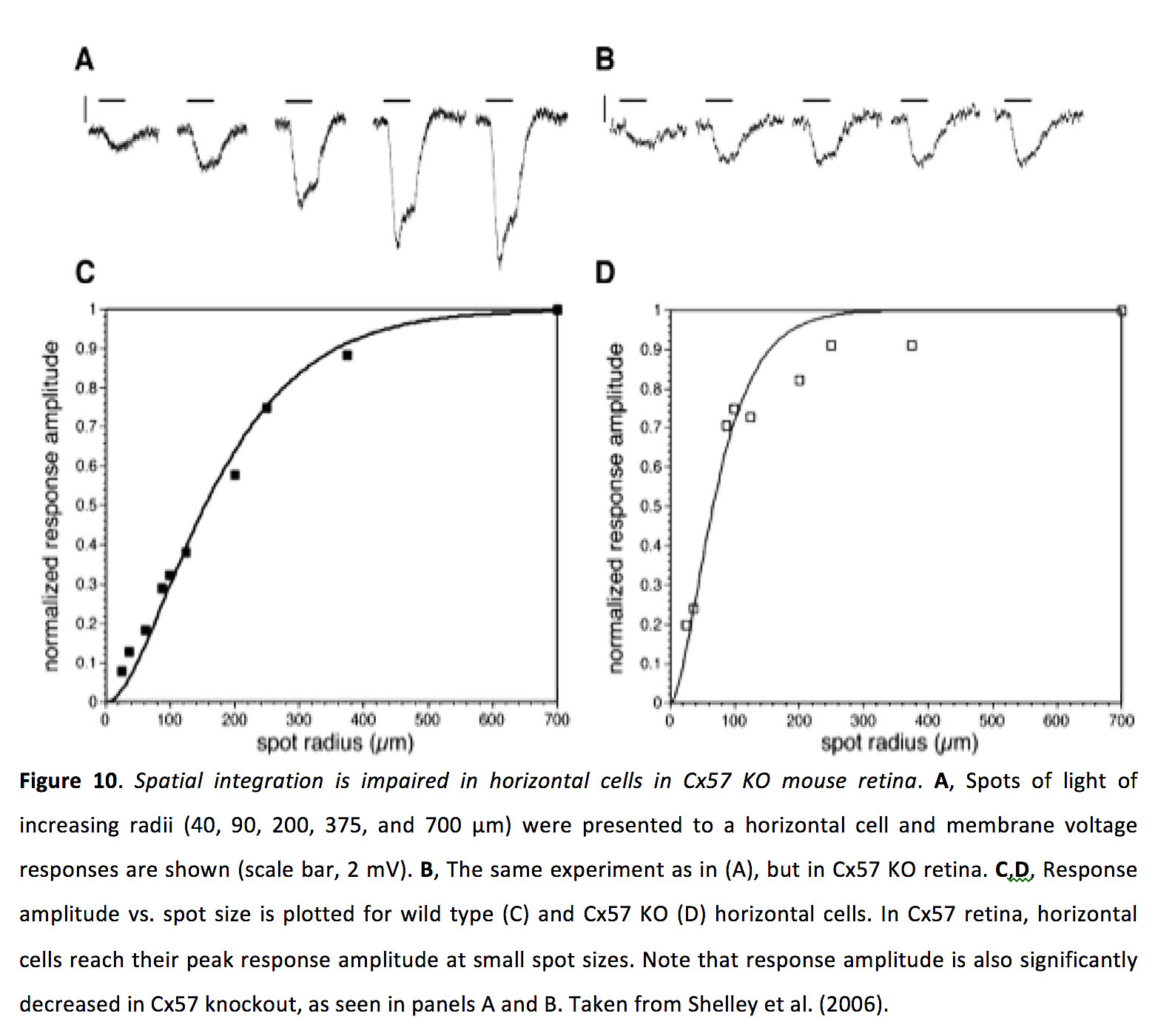 Figure 10. Spatial integration is impaired in horizontal cells in Cx57 KO mouse retina. A, Spots of light of increasing radii (40, 90, 200, 375, and 700 µm) were presented to a horizontal cell and membrane voltage responses are shown (scale bar, 2 mV). B, The same experiment as in (A), but in Cx57 KO retina. C,D, Response amplitude vs. spot size is plotted for wild type (C) and Cx57 KO (D) horizontal cells. In Cx57 retina, horizontal cells reach their peak response amplitude at small spot sizes. Note that response amplitude is also significantly decreased in Cx57 knockout, as seen in panels A and B. Taken from Shelley et al. (2006) (55).
Figure 10. Spatial integration is impaired in horizontal cells in Cx57 KO mouse retina. A, Spots of light of increasing radii (40, 90, 200, 375, and 700 µm) were presented to a horizontal cell and membrane voltage responses are shown (scale bar, 2 mV). B, The same experiment as in (A), but in Cx57 KO retina. C,D, Response amplitude vs. spot size is plotted for wild type (C) and Cx57 KO (D) horizontal cells. In Cx57 retina, horizontal cells reach their peak response amplitude at small spot sizes. Note that response amplitude is also significantly decreased in Cx57 knockout, as seen in panels A and B. Taken from Shelley et al. (2006) (55).
As horizontal cells provide negative feedback to photoreceptors, it has been posited that they might be responsible for generating inhibitory surround receptive fields in downstream ganglion cells (56, 57) in which case modifying horizontal cell coupling strength would modify ganglion cell receptive field size. However, the effect of horizontal cell feedback on ganglion cell surround responses remains uncertain, and at least in some cases horizontal cells do not drive significant surround responses in ganglion cells (58-60). Additionally, despite reducing horizontal cell receptive field size, knocking out Cx57 has little effect on ganglion cell spatial tuning and on visual acuity in mice (61). In contrast, horizontal cells might play a role in temporal tuning of ganglion cells. For instance, it has been suggested that strong electrical coupling might functionally remove horizontal cells from the retinal circuit, by effectively shunting their input current from photoreceptors. In such a situation, modifying coupling strength could switch the retina from a mode of short integration time in daylight conditions to a mode of long integration time in nightlight conditions (62). In another study, horizontal cell feedback to photoreceptors was shown to differentially affect transient and/or sustained portions of ganglion cell responses (58), and this could provide another mechanism whereby changes in horizontal cell coupling could affect temporal tuning in ganglion cells.
Bipolar cells
Bipolar cells, of which there are at least 10 types (63), pass photoreceptor signals onto amacrine and ganglion cells at glutamatergic synapses (See Webvision chapter Bipolar Cell Pathways in the Vertebrate Retina). Additionally, bipolar cells can be electrically coupled to other bipolar cells and amacrine cells. Most commonly, bipolar cells are coupled to AII amacrine cells (Figure 11) (64), though recent work shows that certain ON cone bipolar cells form gap junctions with non-AII amacrine cells (59, 65). See Webvision chapter on AII amacrine cells.
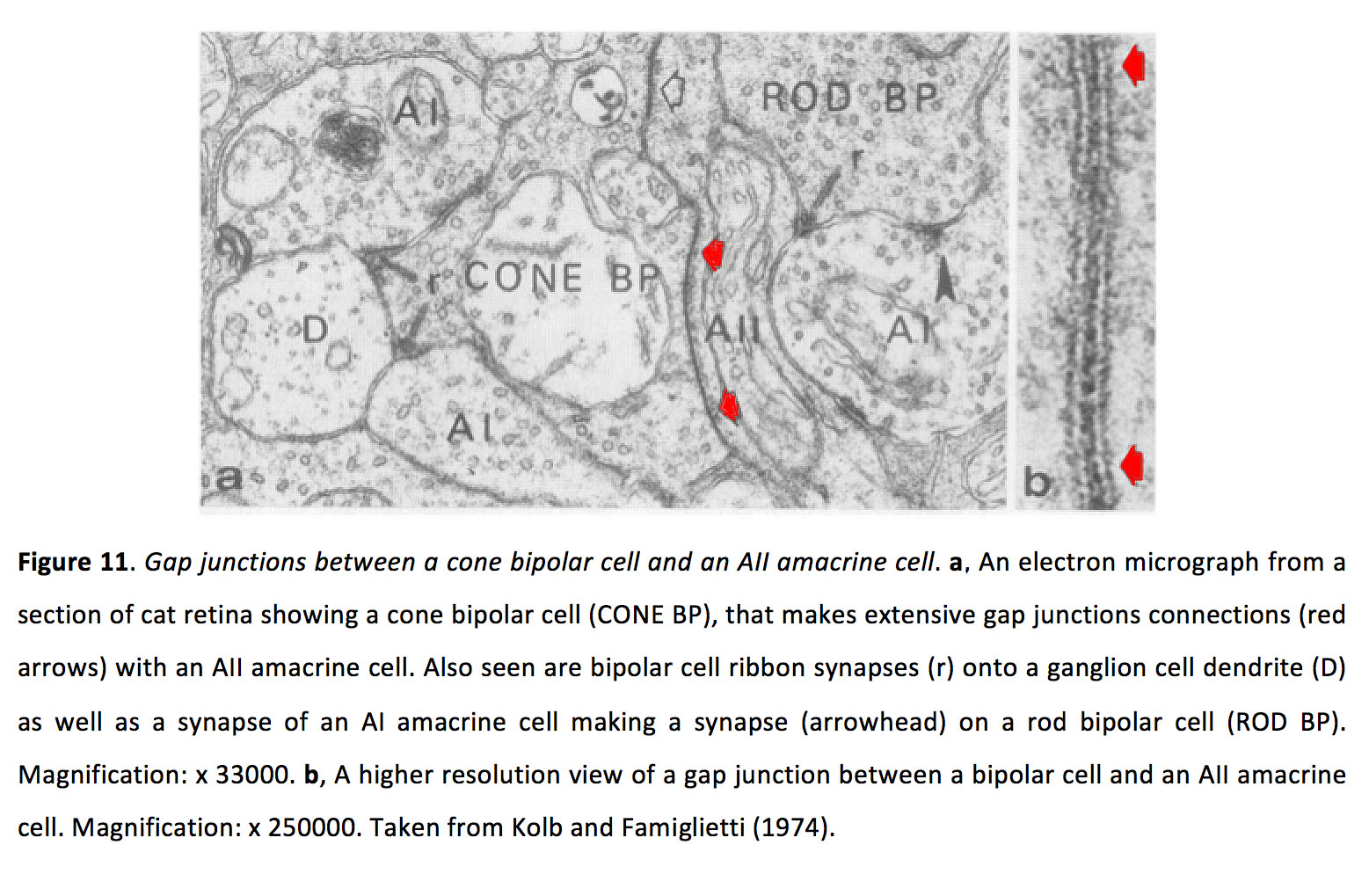 Figure 11. Gap junctions between a cone bipolar cell and an AII amacrine cell. a An electron micrograph from a section of cat retina showing a cone bipolar cell (CONE BP), that makes extensive gap junctions connections (red arrows) with an AII amacrine cell. Also seen are bipolar cell ribbon synapses (r) onto a ganglion cell dendrite (D) as well as a synapse of an AI amacrine cell making a synapse (arrowhead) on a rod bipolar cell (ROD BP). AI is also known as A17. Magnification: x 33000. b A higher resolution view of a gap junction between a bipolar cell and an AII amacrine cell. Magnification: x 250000. Taken from Kolb and Famiglietti (1974) (64).
Figure 11. Gap junctions between a cone bipolar cell and an AII amacrine cell. a An electron micrograph from a section of cat retina showing a cone bipolar cell (CONE BP), that makes extensive gap junctions connections (red arrows) with an AII amacrine cell. Also seen are bipolar cell ribbon synapses (r) onto a ganglion cell dendrite (D) as well as a synapse of an AI amacrine cell making a synapse (arrowhead) on a rod bipolar cell (ROD BP). AI is also known as A17. Magnification: x 33000. b A higher resolution view of a gap junction between a bipolar cell and an AII amacrine cell. Magnification: x 250000. Taken from Kolb and Famiglietti (1974) (64).
Gap junctions between bipolar cells can be localized in dendrites (66) or axon terminals (67, 68), though gap junctions between ON cone bipolar cells and AII amacrine cells are localized in the bipolar cell axon terminal (69). In mouse retina, bipolar cell gap junctions contain either Cx36 or Cx45 (Figure 12) (18, 70-73).
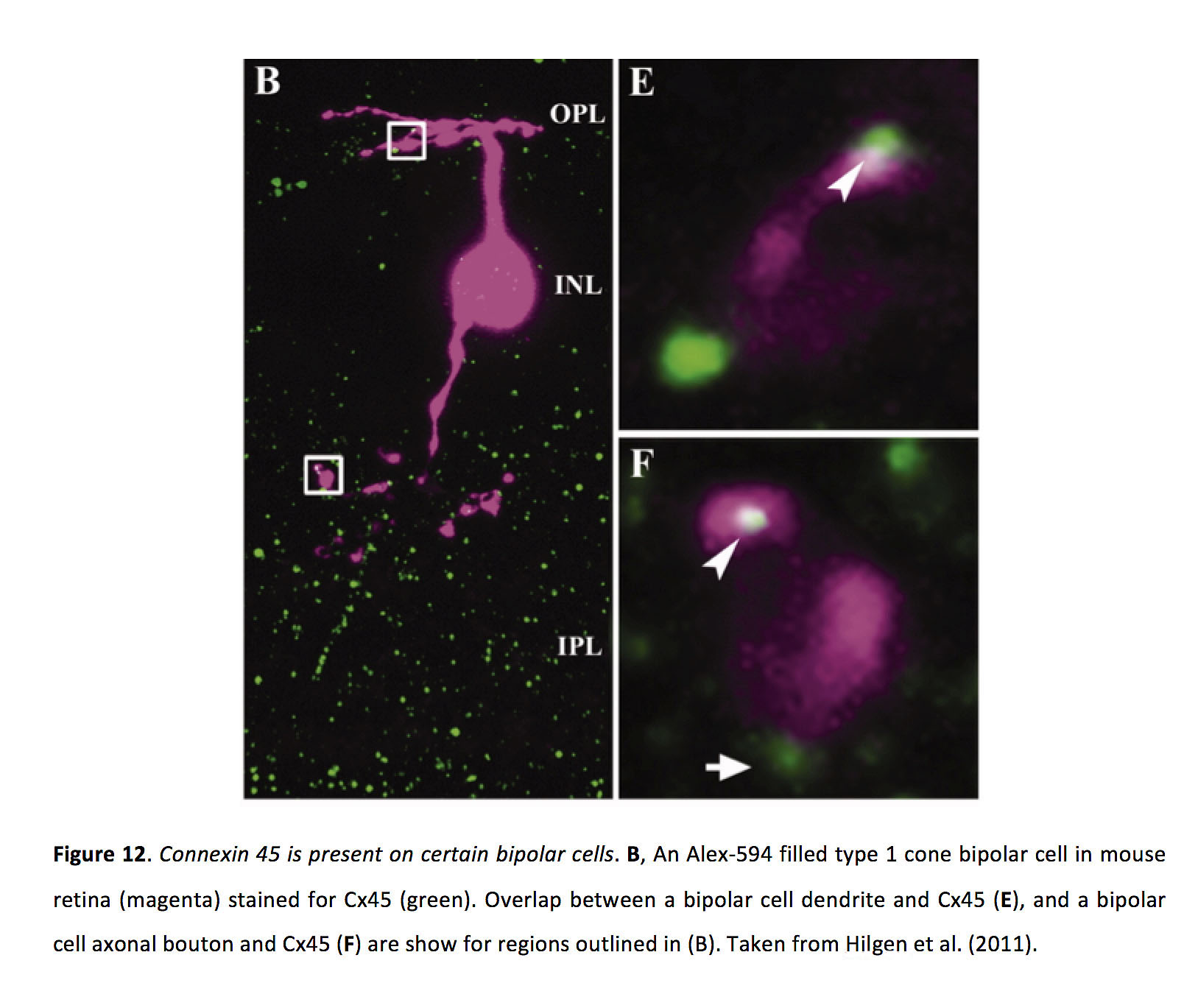 Figure 12. Connexin 45 is present on certain bipolar cells. B, An Alexa-594 filled type 1 cone bipolar cell in mouse retina (magenta) stained for Cx45 (green). Overlap between a bipolar cell dendrite and Cx45 (E), and a bipolar cell axonal bouton and Cx45 (F) are show for regions outlined in (B). Taken from Hilgen et al. (2011) (72).
Figure 12. Connexin 45 is present on certain bipolar cells. B, An Alexa-594 filled type 1 cone bipolar cell in mouse retina (magenta) stained for Cx45 (green). Overlap between a bipolar cell dendrite and Cx45 (E), and a bipolar cell axonal bouton and Cx45 (F) are show for regions outlined in (B). Taken from Hilgen et al. (2011) (72).
Bipolar cell to bipolar cell gap junctions are bidirectional and symmetrical, with a junctional conductance of around 1 nS and a coupling coefficient of around 0.3 (Figure 13) (66, 74-77).
ON cone bipolar cells form gap junctions with AII amacrine cells that are bidirectional but asymmetric, though this measured asymmetry is likely an artifact of differences in input resistance. Between ON cone bipolar cells and AII amacrine cells the junctional conductance is approximately 1 nS, with a coupling coefficient between 0.3-0.6 (Figure 14) (64, 70, 78-80).
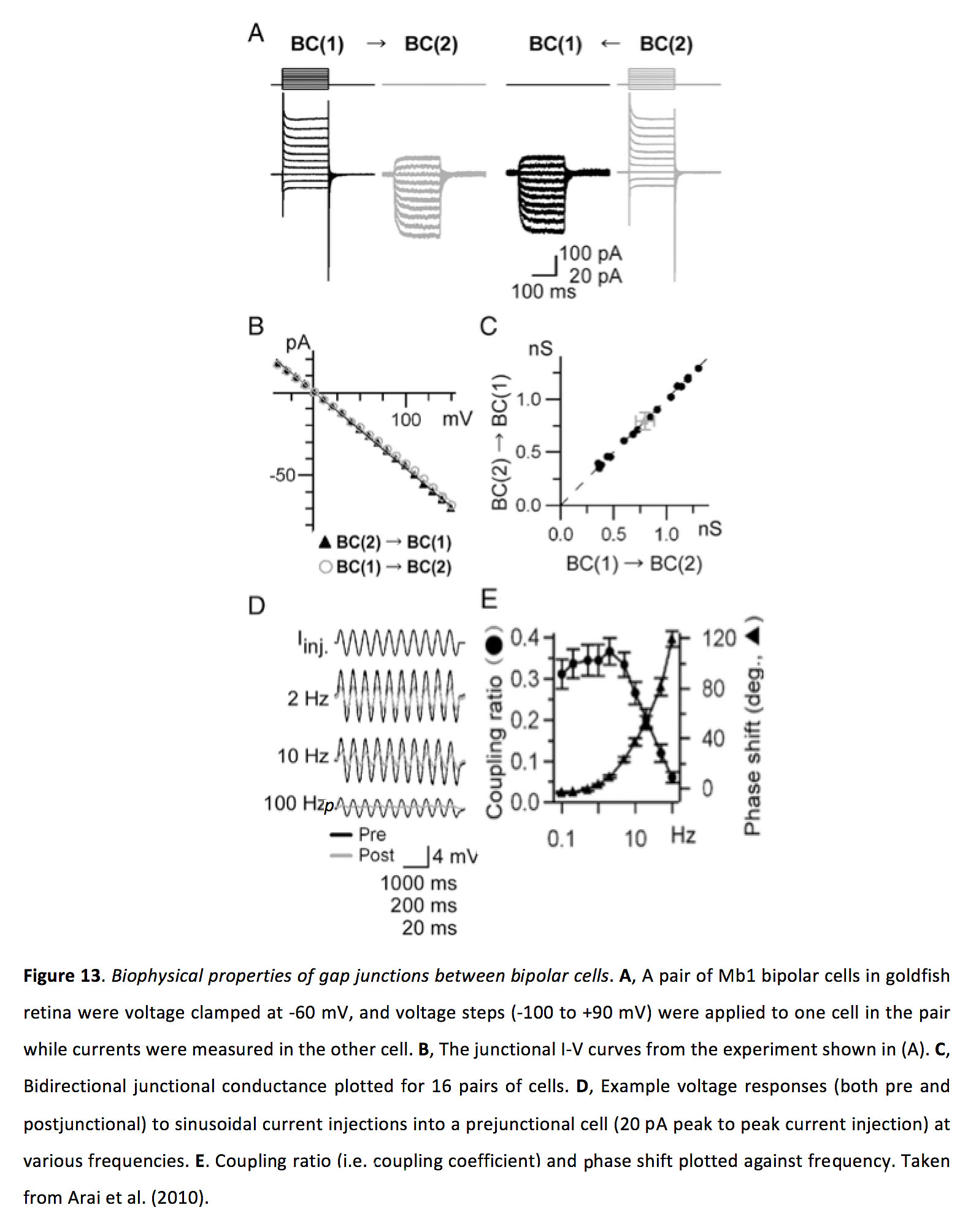 Figure 13. Biophysical properties of gap junctions between bipolar cells. A, A pair of Mb1 bipolar cells in goldfish retina were voltage clamped at -60 mV, and voltage steps (-100 to +90 mV) were applied to one cell in the pair while currents were measured in the other cell. B, The junctional I-V curves from the experiment shown in (A). C, Bidirectional junctional conductance plotted for 16 pairs of cells. D, Example voltage responses (both pre and postjunctional) to sinusoidal current injections into a prejunctional cell (20 pA peak to peak current injection) at various frequencies. E, Coupling ratio (i.e. coupling coefficient) and phase shift plotted against frequency. Taken from Arai et al. (2010) (66).
Figure 13. Biophysical properties of gap junctions between bipolar cells. A, A pair of Mb1 bipolar cells in goldfish retina were voltage clamped at -60 mV, and voltage steps (-100 to +90 mV) were applied to one cell in the pair while currents were measured in the other cell. B, The junctional I-V curves from the experiment shown in (A). C, Bidirectional junctional conductance plotted for 16 pairs of cells. D, Example voltage responses (both pre and postjunctional) to sinusoidal current injections into a prejunctional cell (20 pA peak to peak current injection) at various frequencies. E, Coupling ratio (i.e. coupling coefficient) and phase shift plotted against frequency. Taken from Arai et al. (2010) (66).
The gap junction conductance between electrically connected bipolar cells increases in the light adapted state, at least as shown in goldfish for Mb-1 bipolar cells (66). While it has been suggested that bipolar cell coupling can result in increased receptive field sizes, which some studies have found to be significantly larger than dendritic field sizes (74, 75, 81), but also see (82), to our knowledge the effect of changing light adaptation state on bipolar cell coupling and receptive field size has never been directly tested. Furthermore, since previous work has shown that light adaptation can produce antagonistic surround receptive fields in bipolar cells (83), such surround inhibition would be expected to counteract excitatory surround responses provided via gap junction inputs from neighbouring cells, thus limiting the ability of gap junction coupling to expand receptive field size. As such, while bipolar cell gap junctions appear to enhance their ability to integrate spatially separated stimuli and preferentially enhance their signaling of low contrast stimuli (84), further study is required to define the effect of light adaptation on the strength of gap junction coupling for bipolar cells, and for examining whether such changes effectively modify receptive field size.
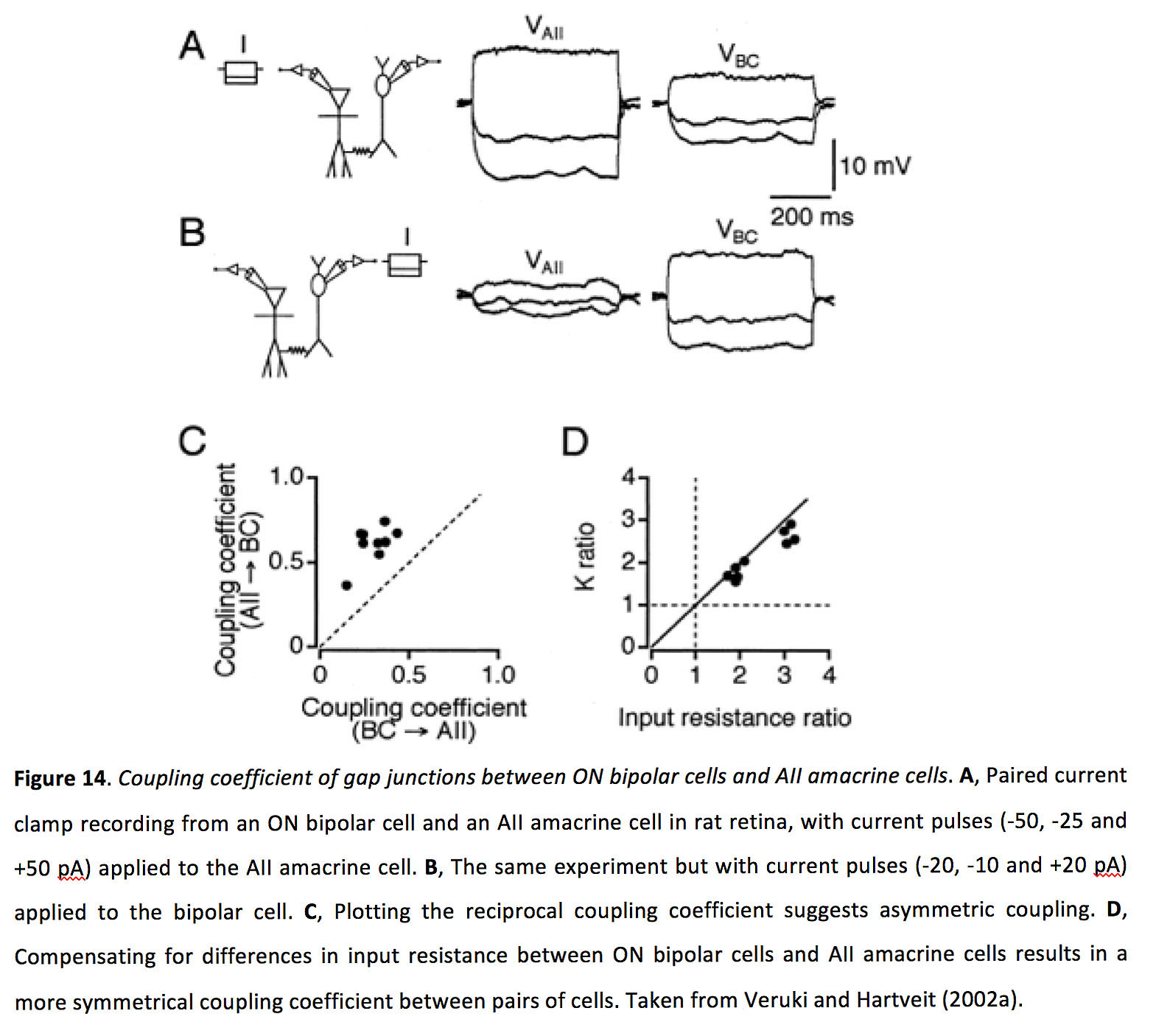 Figure 14. Coupling coefficient of gap junctions between ON bipolar cells and AII amacrine cells. A, Paired current clamp recording from an ON bipolar cell and an AII amacrine cell in rat retina, with current pulses (-50, -25 and +50 pA) applied to the AII amacrine cell. B, The same experiment but with current pulses (-20, -10 and +20 pA) applied to the bipolar cell. C, Plotting the reciprocal coupling coefficient suggests asymmetric coupling. D, Compensating for differences in input resistance between ON bipolar cells and AII amacrine cells results in a more symmetrical coupling coefficient between pairs of cells. Taken from Veruki and Hartveit (2002a) (199).
Figure 14. Coupling coefficient of gap junctions between ON bipolar cells and AII amacrine cells. A, Paired current clamp recording from an ON bipolar cell and an AII amacrine cell in rat retina, with current pulses (-50, -25 and +50 pA) applied to the AII amacrine cell. B, The same experiment but with current pulses (-20, -10 and +20 pA) applied to the bipolar cell. C, Plotting the reciprocal coupling coefficient suggests asymmetric coupling. D, Compensating for differences in input resistance between ON bipolar cells and AII amacrine cells results in a more symmetrical coupling coefficient between pairs of cells. Taken from Veruki and Hartveit (2002a) (199).
Amacrine cells
Amacrine cells, of which there are more than 45 types (5, 85), provide inhibition, via GABA or glycine release, to bipolar cells, other amacrine cells and ganglion cells. Some amacrine cells can also co-release acetylcholine (86-88), dopamine (89), or glutamate (90). Many amacrine cells form homologous gap junctions (78, 79, 91-93), and heterologous gap junctions with bipolar cells – as described above – and ganglion cells (94, 95). AII amacrine cells form gap junctions with one another in their dendritic arbors (Figure 15) (69). It seems likely that other amacrine cell types also make dendrodendritic gap junctions with amacrine and ganglion cells, as this is often the only location where their processes overlap.
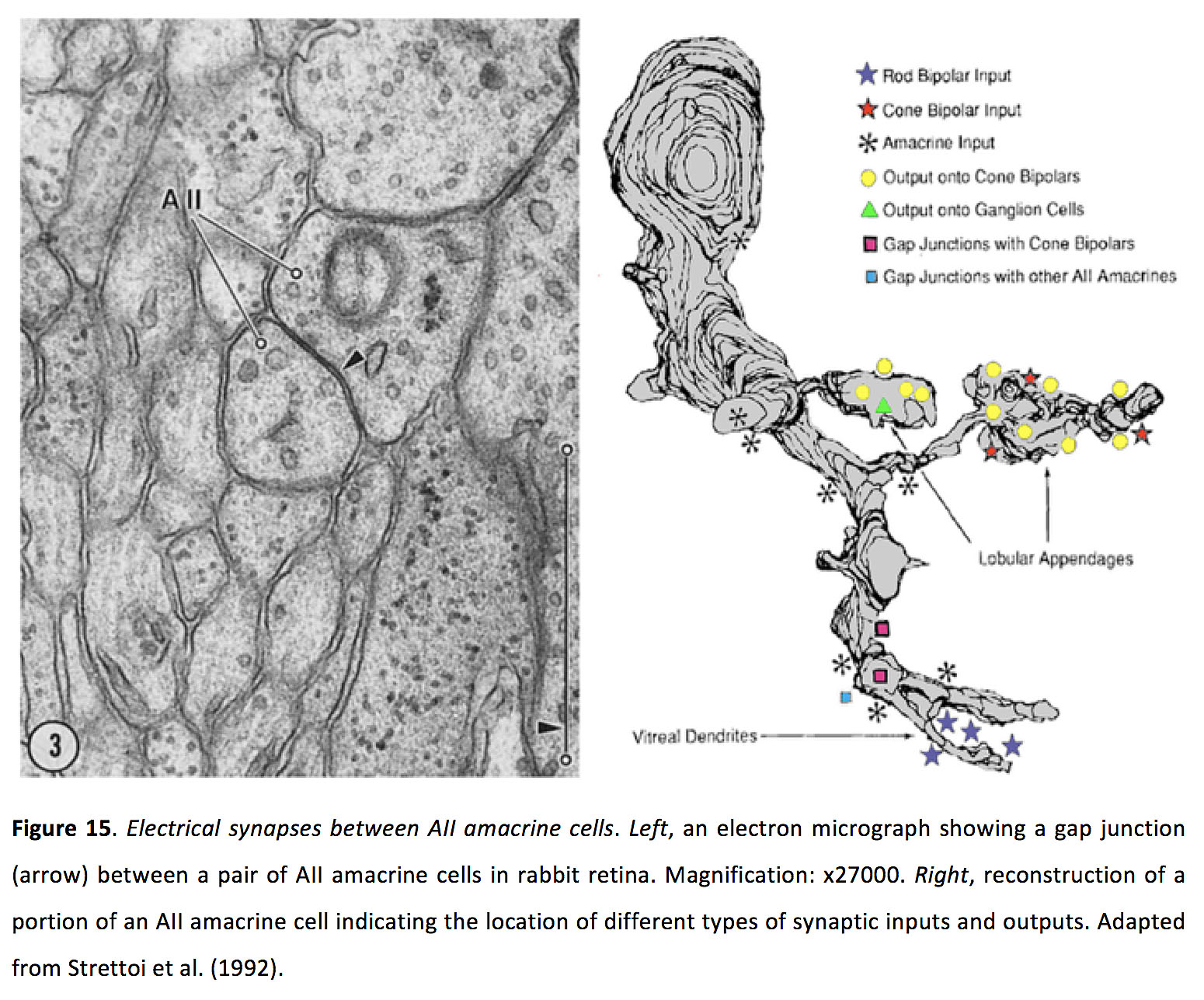 Figure 15. Electrical synapses between AII amacrine cells. Left, an electron micrograph showing a gap junction (arrow) between a pair of AII amacrine cells in rabbit retina. Magnification: x27000. Right, reconstruction of a portion of an AII amacrine cell indicating the location of different types of synaptic inputs and outputs. Adapted from Strettoi et al. (1992) (69).
Figure 15. Electrical synapses between AII amacrine cells. Left, an electron micrograph showing a gap junction (arrow) between a pair of AII amacrine cells in rabbit retina. Magnification: x27000. Right, reconstruction of a portion of an AII amacrine cell indicating the location of different types of synaptic inputs and outputs. Adapted from Strettoi et al. (1992) (69).
AII amacrine cells appear to form gap junctions with ON cone bipolar cells with Cx36. Coupling between AIIamacrine cells is bidirectional and symmetric, with a junctional conductance of ~700 pS and a coupling coefficient of ~ 0.3 (Figure 16) (78).
 Figure 16. Properties of electrical synapses between AII amacrine cells. A, A paired recording from AIIamacrine cells in a slice from rat retina (top view, DIC; bottom view, fluorescence from Lucifer yellow delivered via the patch pipettes). B,C, Paired voltage clamp recordings, with cells held at -60 mV, and voltage steps (-30 mV and +10 mV) injected into one of the cells while current is monitored in the other cell. D,E, Paired current clamp recordings when current steps (-50, -25, 0 and +25 pA) are injected into one of the cells while voltage is monitored in the other cell. Taken from Veruki and Hartveit (2002b) (78).
Figure 16. Properties of electrical synapses between AII amacrine cells. A, A paired recording from AIIamacrine cells in a slice from rat retina (top view, DIC; bottom view, fluorescence from Lucifer yellow delivered via the patch pipettes). B,C, Paired voltage clamp recordings, with cells held at -60 mV, and voltage steps (-30 mV and +10 mV) injected into one of the cells while current is monitored in the other cell. D,E, Paired current clamp recordings when current steps (-50, -25, 0 and +25 pA) are injected into one of the cells while voltage is monitored in the other cell. Taken from Veruki and Hartveit (2002b) (78).
Other amacrine cell types form gap junctions with amacrine or ganglion cells with electrical synapses comprising either Cx36 or Cx45 (96, 97). nNOS-2 amacrine cells, which are the main source of nitric oxide in the mouse retina, have been shown be extensively interconnected with what appear to be Cx45 containing gap junctions, and exhibit a bidirectional and symmetrical conductance, with a junctional conductance that was modeled to be very large (4.4 nS, Figure 17) (98). The measured coupling coefficient for nNOS-2 amacrine cell gap junctions was ~0.08 (98).
 Figure 17. Tracer coupling between nNOS-2 amacrine cells in mouse retina. A, Tracer coupling when a single amacrine cell in the center of the field of view was injected with Neurobiotin (green). B (top), Injecting an nNOS-2 amacrine cell with Neurobiotin in the presence of the gap junction blocker meclofenamic acid results in no neighbouring cells being tracer coupled. Red and pink cells indicate the positions of nearby nNOS-2 amacrine cells in both the ganglion cell layer (GCL, pink) and the inner nuclear layer (INL, red). B (bottom), Side projection of a Neurobiotin (green) filled nNOS-2 amacrine cell co-stained with choline acetyltransferace (ChAT) which labels starburst amacrine and helps to delineate the inner plexiform layer. C, A 2-photon image from an acute retinal preparation showing that Alexa Fluor 488 (green), delivered to a single nNOS-2 amacrine via a patch pipette, can spread throughout the coupled network. Blue represents tdTomato transgenically labelled nNOS-2 amacrine cells. Taken from Jacoby et al. (2018) (98).
Figure 17. Tracer coupling between nNOS-2 amacrine cells in mouse retina. A, Tracer coupling when a single amacrine cell in the center of the field of view was injected with Neurobiotin (green). B (top), Injecting an nNOS-2 amacrine cell with Neurobiotin in the presence of the gap junction blocker meclofenamic acid results in no neighbouring cells being tracer coupled. Red and pink cells indicate the positions of nearby nNOS-2 amacrine cells in both the ganglion cell layer (GCL, pink) and the inner nuclear layer (INL, red). B (bottom), Side projection of a Neurobiotin (green) filled nNOS-2 amacrine cell co-stained with choline acetyltransferace (ChAT) which labels starburst amacrine and helps to delineate the inner plexiform layer. C, A 2-photon image from an acute retinal preparation showing that Alexa Fluor 488 (green), delivered to a single nNOS-2 amacrine via a patch pipette, can spread throughout the coupled network. Blue represents tdTomato transgenically labelled nNOS-2 amacrine cells. Taken from Jacoby et al. (2018) (98).
Light adaptation state appears to strongly control coupling strength in amacrine cells (94). Similar to horizontal cells, tracer coupling experiments indicate that AII amacrine cells exhibit minimal coupling during both very dim and bright conditions, and strong coupling during mid light conditions (Figure 18) (94).
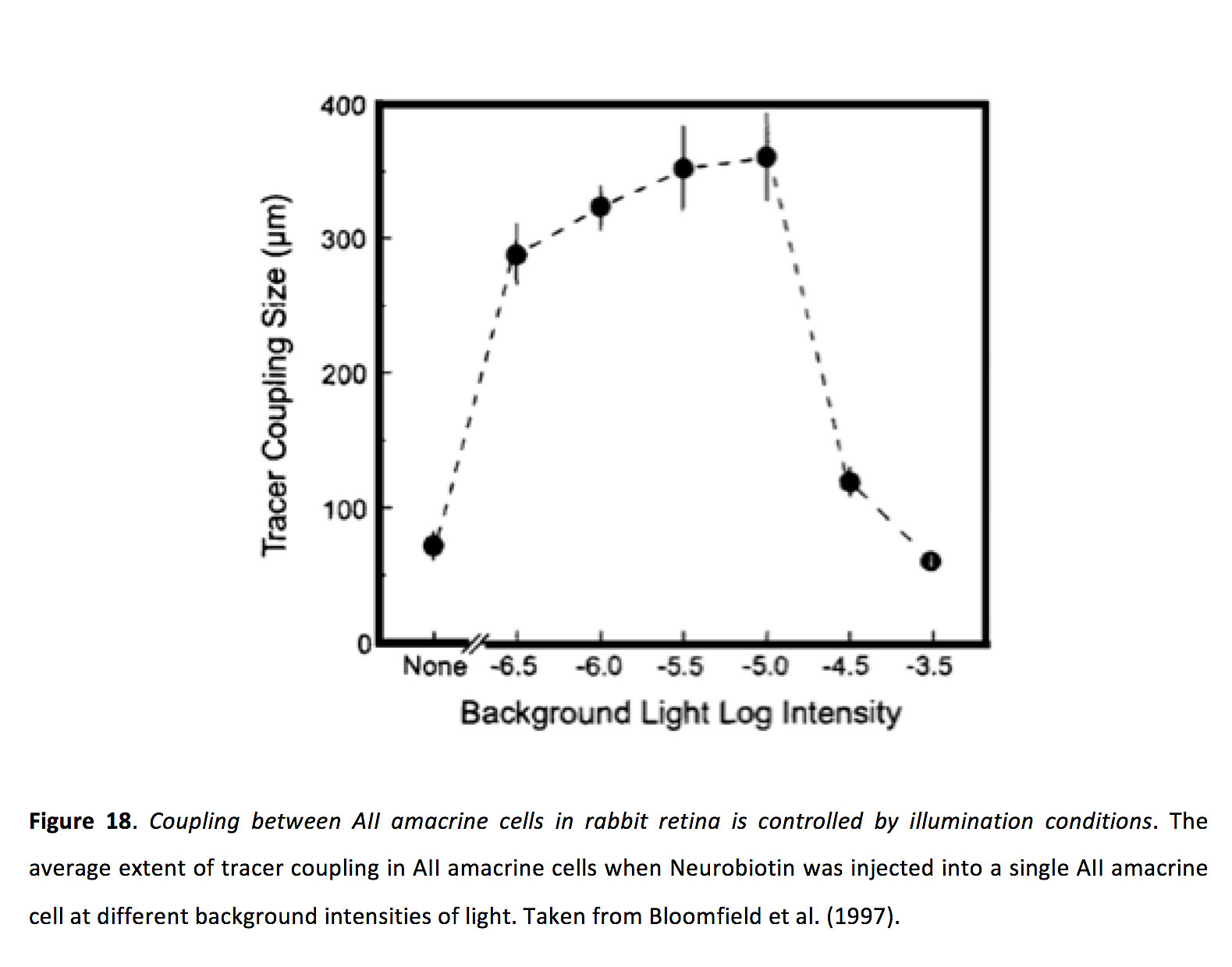 Figure 18. Coupling between AII amacrine cells in rabbit retina is controlled by illumination conditions. The average extent of tracer coupling in AII amacrine cells when Neurobiotin was injected into a single AIIamacrine cell at different background intensities of light. Taken from Bloomfield et al. (1997) (200).
Figure 18. Coupling between AII amacrine cells in rabbit retina is controlled by illumination conditions. The average extent of tracer coupling in AII amacrine cells when Neurobiotin was injected into a single AIIamacrine cell at different background intensities of light. Taken from Bloomfield et al. (1997) (200).
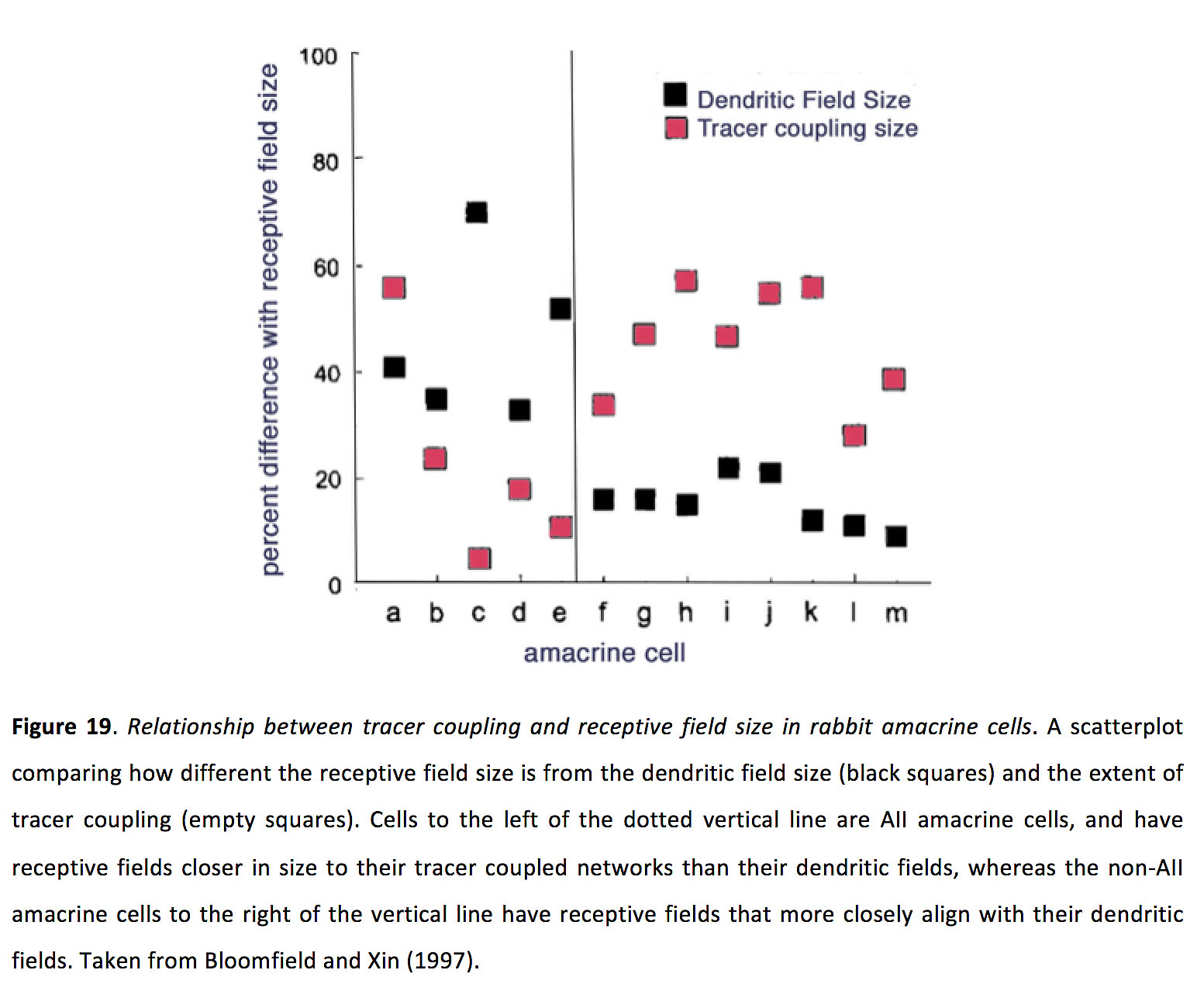
Such changes in coupling strength appear to strongly influence receptive field size, as AII amacrine cell receptive fields can be 2-3 times larger than their dendritic fields in the light-sensitized state (94). However, a direct relationship between coupling strength and receptive field size does not appear to hold across all amacrine cell types. For instance, unlike AII amacrine cells, work with some other types of amacrine cells did not indicate a strong effect of light adaptation on receptive field size, despite the fact that tracer coupling experiments showed light-adaptation dependent changes in tracer coupling patterns (Figure 19) (94). Why the effect of gap junction coupling on receptive field size does not appear consistent across all amacrine cell types remains unclear, though it could have to do with the fact that the dendrites of some amacrine cell types appear to perform local computations (99), which might therefore not require global changes in receptive field size for changes in junctional conductance to have an effect on circuit function. Another possibility for the difference of light adaptation on amacrine cell coupling could be differential regulation by dopamine. Indeed, gap junction coupling of A8 amacrine cells, which are coupled to both ON and OFF bipolar cells with Cx36 gap junctions, does not appear to be modulated by dopamine receptor D1 activation, in contrast to AII amacrine cells (100). Finally, in mouse nNOS-2 amacrine cells, there appears to be a remarkable self-regulation between light activation and coupling state. nNOS-2 cells are extensively electrically coupled in the dark-adapted retina, whereas upon light activation they produce nitric oxide which in turn decouples the network (98). Whether this light-activated decoupling affects receptive field size remained to be tested.
Figure 19. Relationship between tracer coupling and receptive field size in rabbit amacrine cells. A scatterplot comparing how different the receptive field size is from the dendritic field size (black squares) and the extent of tracer coupling (pink squares). Cells to the left of the dotted vertical line are AII amacrine cells and have receptive fields closer in size to their tracer coupled networks than their dendritic fields, whereas the non- AIIamacrine cells to the right of the vertical line have receptive fields that more closely align with their dendritic fields. Taken from Bloomfield and Xin (1997) (200).
Ganglion cells
Ganglion cells, of which there are upwards of 30 types (101-103), receive excitatory glutamatergic input from bipolar cells and inhibitory GABAergic and glycinergic input from amacrine cells, and send their signals, via the optic nerve, to multiple higher visual areas (6, 7) (See Webvision chapter Visual Responses of Ganglion Cells). Ganglion cells also form dendrodendritic gap junctions with amacrine cells and other ganglion cells (Figure 20) (91, 95, 104-108).
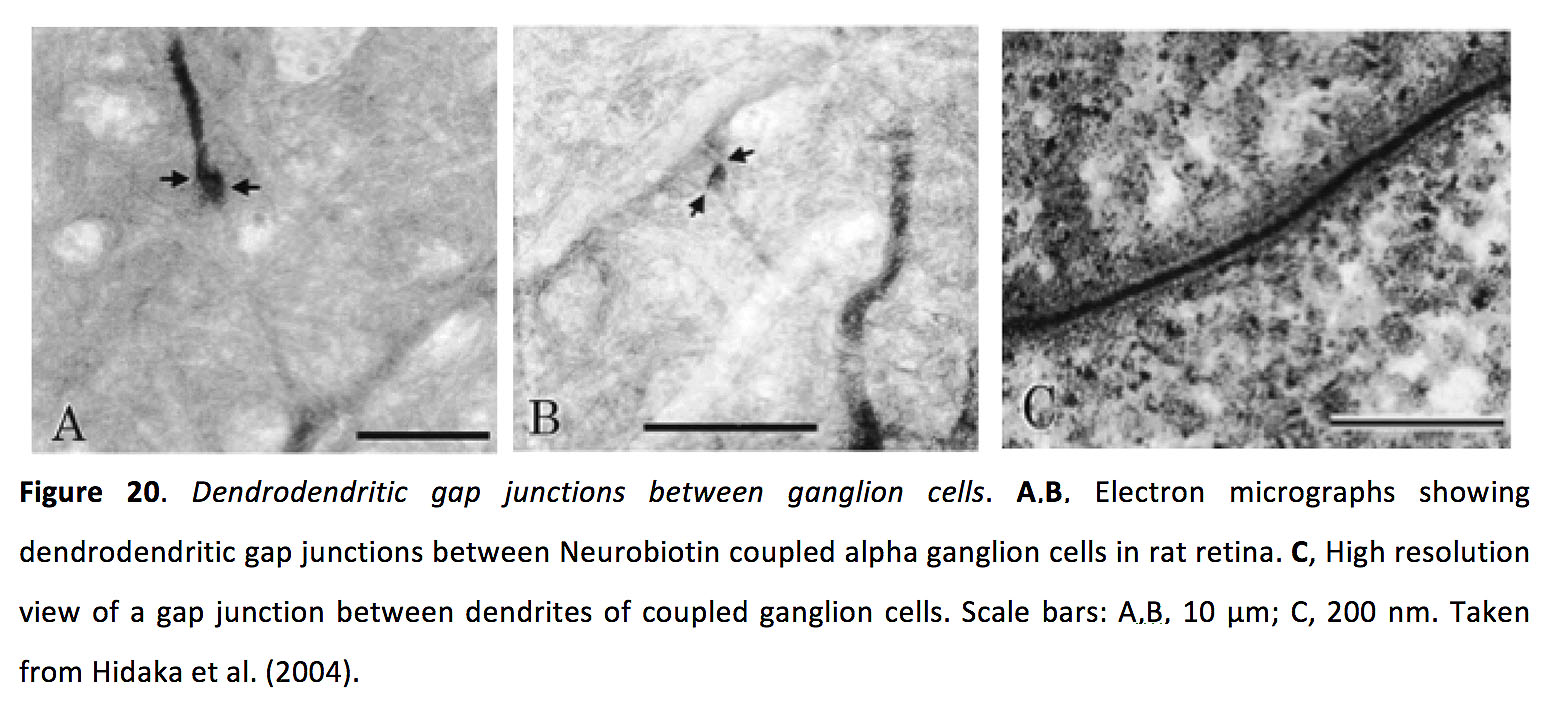 Figure 20. Dendrodendritic gap junctions between ganglion cells. A,B, Electron micrographs showing dendrodendritic gap junctions between Neurobiotin coupled alpha ganglion cells in rat retina. C, High resolution view of a gap junction between dendrites of coupled ganglion cells. Scale bars: A,B, 10 µm; C, 200 nm. Taken from Hidaka et al. (2004) (106).
Figure 20. Dendrodendritic gap junctions between ganglion cells. A,B, Electron micrographs showing dendrodendritic gap junctions between Neurobiotin coupled alpha ganglion cells in rat retina. C, High resolution view of a gap junction between dendrites of coupled ganglion cells. Scale bars: A,B, 10 µm; C, 200 nm. Taken from Hidaka et al. (2004) (106).
Ganglion cell gap junctions are frequently comprised of connexin 36 (Figure 21) (96, 109-111), though some contain connexin 45 (109) and possibly also connexin 30.2 (109).
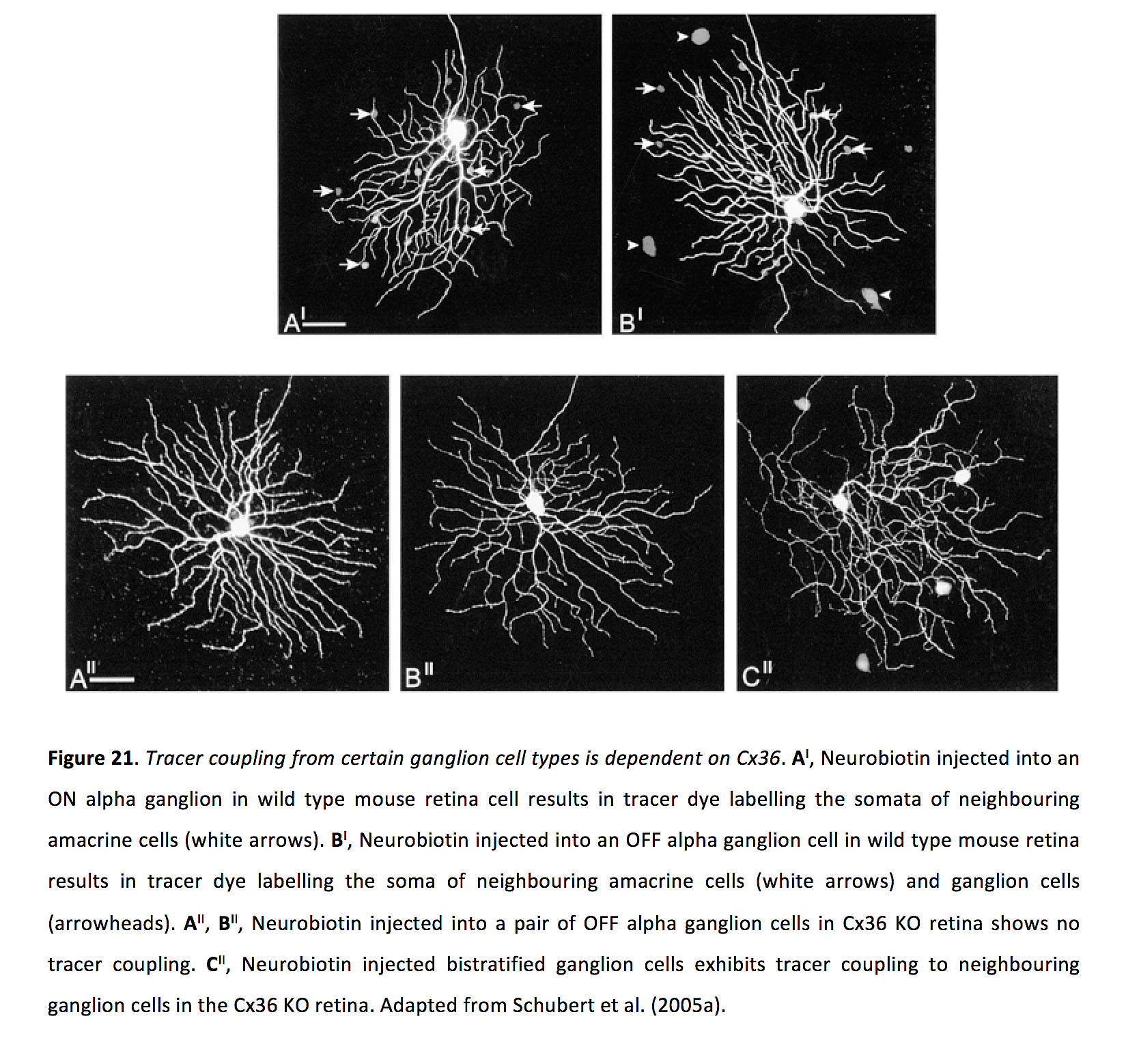 Figure 21. Tracer coupling from certain ganglion cell types is dependent on Cx36. AI, Neurobiotin injected into an ON alpha ganglion in wild type mouse retina cell results in tracer dye labelling the somata of neighbouring amacrine cells (white arrows). BI, Neurobiotin injected into an OFF alpha ganglion cell in wild type mouse retina results in tracer dye labelling the soma of neighbouring amacrine cells (white arrows) and ganglion cells (arrowheads). AII, BII, Neurobiotin injected into a pair of OFF alpha ganglion cells in Cx36 KO retina shows no tracer coupling. CII, Neurobiotin injected into a bistratified ganglion cell shows tracer coupling to neighbouring ganglion cells in the Cx36 KO retina. Adapted from Schubert et al. (2005a) (201).
Figure 21. Tracer coupling from certain ganglion cell types is dependent on Cx36. AI, Neurobiotin injected into an ON alpha ganglion in wild type mouse retina cell results in tracer dye labelling the somata of neighbouring amacrine cells (white arrows). BI, Neurobiotin injected into an OFF alpha ganglion cell in wild type mouse retina results in tracer dye labelling the soma of neighbouring amacrine cells (white arrows) and ganglion cells (arrowheads). AII, BII, Neurobiotin injected into a pair of OFF alpha ganglion cells in Cx36 KO retina shows no tracer coupling. CII, Neurobiotin injected into a bistratified ganglion cell shows tracer coupling to neighbouring ganglion cells in the Cx36 KO retina. Adapted from Schubert et al. (2005a) (201).
Ganglion cell to ganglion cell coupling appears to be exclusively homologous, with no coupling between different ganglion cell types (95, 105, 112, 113), though possibly there are exceptions (114). Furthermore, distinct ganglion cell subtypes have unique coupling patterns (Figure 22) (95). Less is known about ganglion cell-amacrine cell coupling, though tracer coupling work suggests that specific ganglion cells form gap junctions with specific types of amacrine cells (Figure 22) (95).
 Figure 22. Diverse coupling patterns of different retinal ganglion cell types. Schematic outlining differential coupling patterns (as determined via tracer coupling experiments) of 22 types of ganglion cells in the mouse retina. Dendritic stratification patterns in the inner plexiform layer (IPL) for each ganglion cell (black) and its coupled amacrine cells (grey) are shown. Large unfilled circles in the ganglion cell layer indicate tracer coupled ganglion cells. Filled grey circles represent coupled amacrine cells (the size of the circle varies depending the soma size, and if also labelled, amacrine cell dendrites/axons are shown). Taken from Völgyi et al. (2009) (95).
Figure 22. Diverse coupling patterns of different retinal ganglion cell types. Schematic outlining differential coupling patterns (as determined via tracer coupling experiments) of 22 types of ganglion cells in the mouse retina. Dendritic stratification patterns in the inner plexiform layer (IPL) for each ganglion cell (black) and its coupled amacrine cells (grey) are shown. Large unfilled circles in the ganglion cell layer indicate tracer coupled ganglion cells. Filled grey circles represent coupled amacrine cells (the size of the circle varies depending the soma size, and if also labelled, amacrine cell dendrites/axons are shown). Taken from Völgyi et al. (2009) (95).
Gap junctions between ganglion cells are bidirectional and have a symmetrical junctional conductance of around 1 nS and a coupling coefficient of around 0.15 (Figure 23) (106, 113).
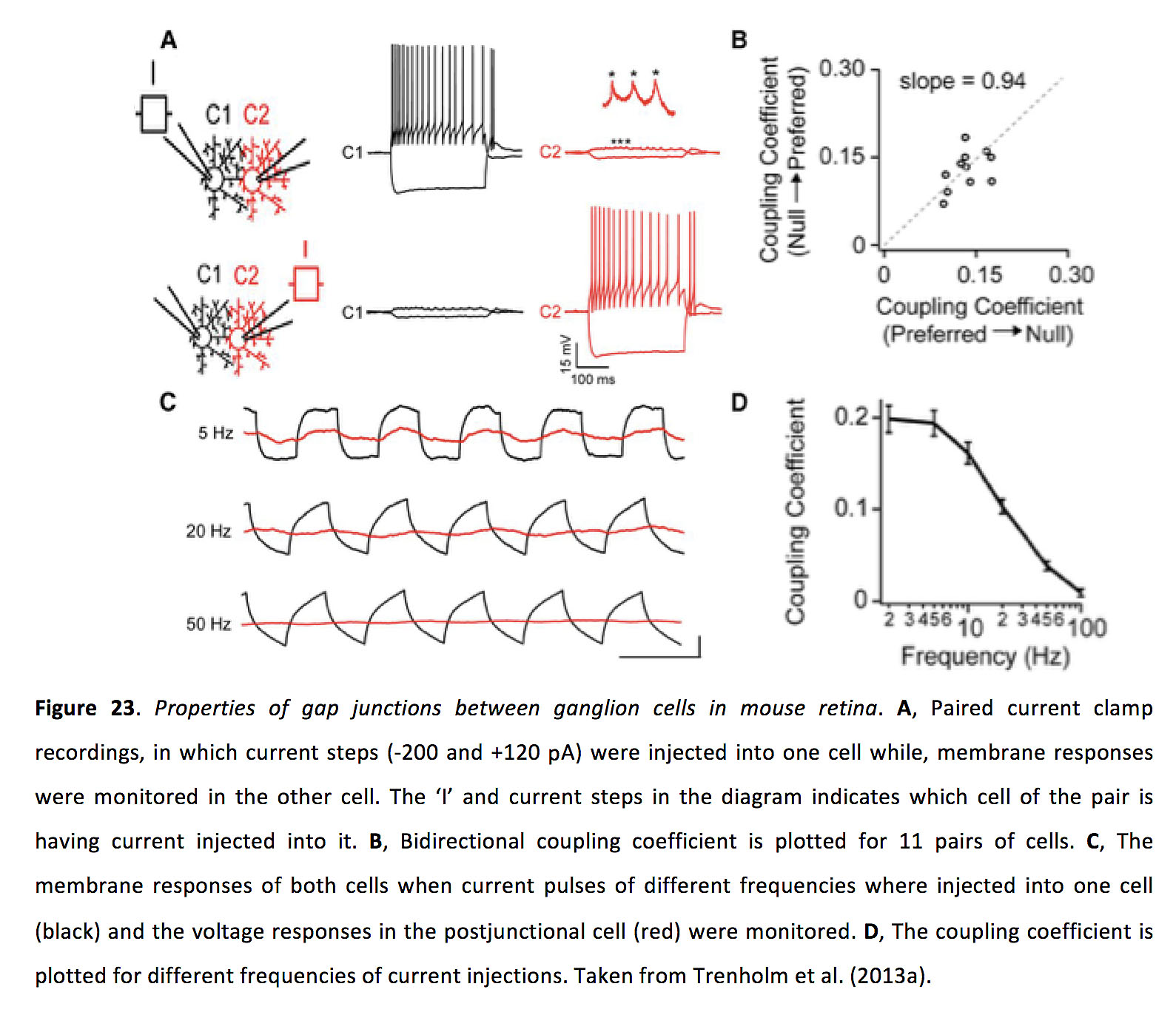 Figure 23. Properties of gap junctions between ganglion cells in mouse retina. A, Paired current clamp recordings, in which current steps (-200 and +120 pA) were injected into one cell while, membrane responses were monitored in the other cell. The ‘I’ and current steps in the diagram indicates which cell of the pair is having current injected into it. B, Bidirectional coupling coefficient is plotted for 11 pairs of cells. C, The membrane responses of both cells when current pulses of different frequencies where injected into one cell (black) and the voltage responses in the postjunctional cell (red) were monitored. D, The coupling coefficient is plotted for different frequencies of current injections. Taken from Trenholm et al. (2013a) (113).
Figure 23. Properties of gap junctions between ganglion cells in mouse retina. A, Paired current clamp recordings, in which current steps (-200 and +120 pA) were injected into one cell while, membrane responses were monitored in the other cell. The ‘I’ and current steps in the diagram indicates which cell of the pair is having current injected into it. B, Bidirectional coupling coefficient is plotted for 11 pairs of cells. C, The membrane responses of both cells when current pulses of different frequencies where injected into one cell (black) and the voltage responses in the postjunctional cell (red) were monitored. D, The coupling coefficient is plotted for different frequencies of current injections. Taken from Trenholm et al. (2013a) (113).
In ganglion cells, light adaptation appears to control coupling strength, with coupling being reduced in the dark adapted state and increased in the light adapted state (at least as has been shown for OFF α cells in the rabbit retina (115); though this may not be the case for all ganglion cell types (111)). Interestingly, while increased coupling strength does not seem to alter the size of the classical (spiking) receptive field (115, 116), gap junction inputs from neighboring cells endow electrically coupled ganglion cells with extensive subthreshold excitatory surround receptive fields (113). Furthermore, for many ganglion cells, receptive field size actually decreases in light adapted conditions (117-119), likely due to a strengthening of inhibitory surround responses arising from either horizontal and/or amacrine cells. For instance, it was found that for mouse ON and OFF α-like ganglion cells (59) and directionally selective ganglion cells (60), surround inhibition in ganglion cells is provided by wide-field amacrine cells, and this surround inhibition appears to be activated only at light intensities that activate cones. In this bright light, amacrine-cell-mediated surround inhibition is thought to rely on electrical coupling between a wide-field amacrine and ON cone bipolar cells. Knocking out Cx36 eliminates the bright light induced reduction in receptive field size of α-like ganglion cells (59). As such, classical receptive field size in ganglion cells appears to be most strongly controlled by amacrine cell mediated surround inhibition: one recent study suggests that horizontal cells may only contribute as little as ~15% to the surround inhibition of ganglion cells (58).
Much less is known about the properties of ganglion cell-amacrine cell coupling. Nonetheless, the gap junction strength appears to be strong enough such that current injected into one ganglion cell can pass via gap junctions into an amacrine cell, and then via additional gap junctions into a nearby ganglion cell, driving measurable responses in the indirectly coupled ganglion cell (Figure 24) (120).
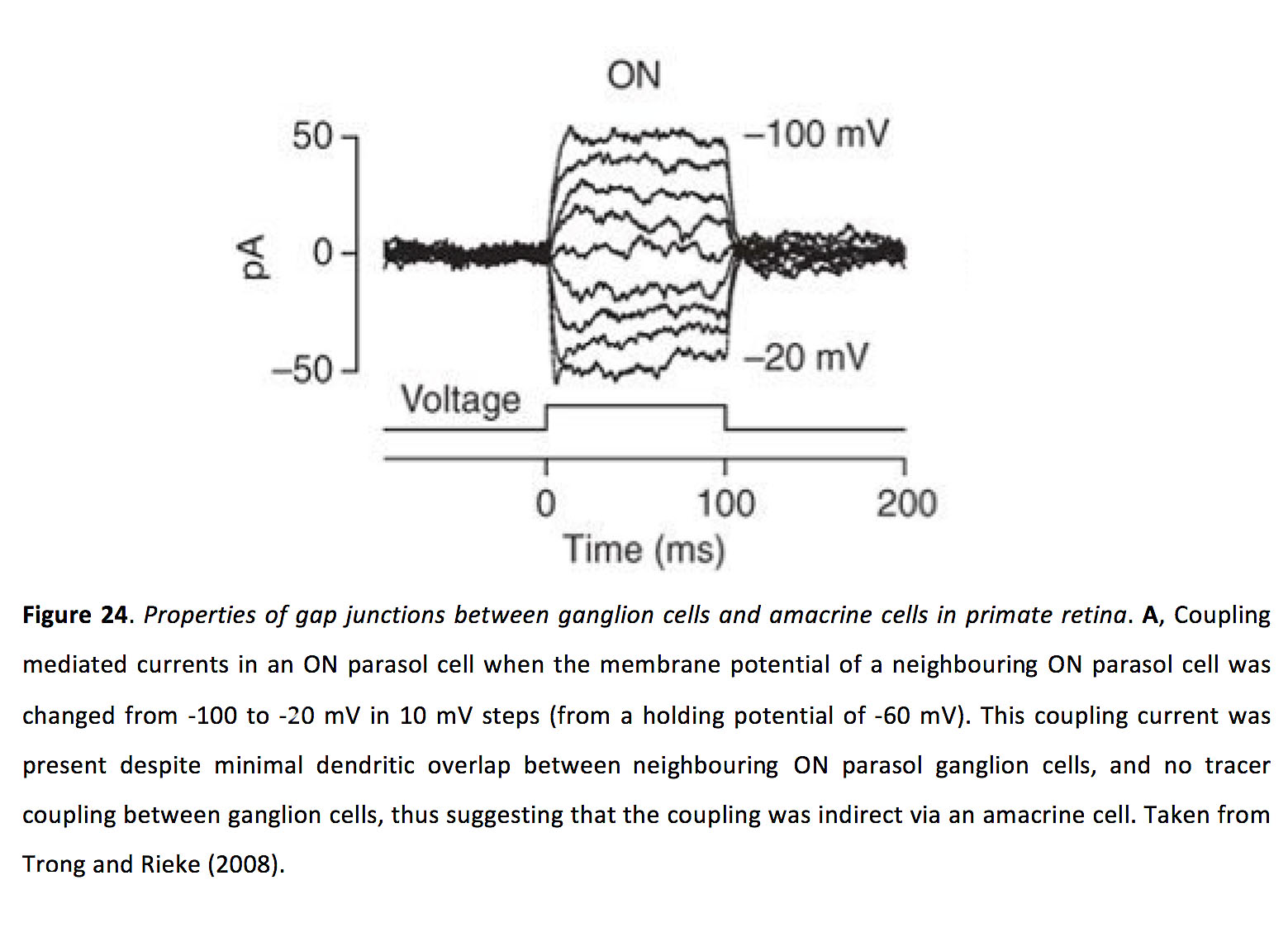 Figure 24. Properties of gap junctions between ganglion cells and amacrine cells in primate retina. A, Coupling mediated currents in an ON parasol cell when the membrane potential of a neighbouring ON parasol cell was changed from -100 to -20 mV in 10 mV steps (from a holding potential of -60 mV). This coupling current was present despite minimal dendritic overlap between neighbouring ON parasol ganglion cells, and no tracer coupling between ganglion cells, thus suggesting that the coupling was indirect via an amacrine cell. Taken from Trong and Rieke (2008) (120).
Figure 24. Properties of gap junctions between ganglion cells and amacrine cells in primate retina. A, Coupling mediated currents in an ON parasol cell when the membrane potential of a neighbouring ON parasol cell was changed from -100 to -20 mV in 10 mV steps (from a holding potential of -60 mV). This coupling current was present despite minimal dendritic overlap between neighbouring ON parasol ganglion cells, and no tracer coupling between ganglion cells, thus suggesting that the coupling was indirect via an amacrine cell. Taken from Trong and Rieke (2008) (120).
Gap junctions and scotopic vision
In dark (scotopic) light conditions, when rods are the active photoreceptor, gap junctions are critical for passing rod-mediated signals throughout the retina. In the classical rod pathway, rods form glutamatergic synapses with rod bipolar cells, which in turn form glutamatergic synapses with AII amacrine. These AII amacrine cells then transfer rod-generated signals to the ON cone bipolar cells, via gap junctions with ON cone bipolar cells. In addition, AII amacrine cells also make glycinergic synapses with OFF bipolar cells (Figure 25 A) (64, 79, 80, 121-124). In the second route, rod signals pass directly into cones, via rod-cone electrical synapses, and are thus passed onto the inner retina (Figure 25 B) (36, 122, 125, 126). In the third route, some rods appear to form glutamatergic synapses with OFF bipolar cells, and pooling of signals between electrically coupled rods has been hypothesized to increase sensitivity of this pathway (Figure 25 C) (17, 127, 128).
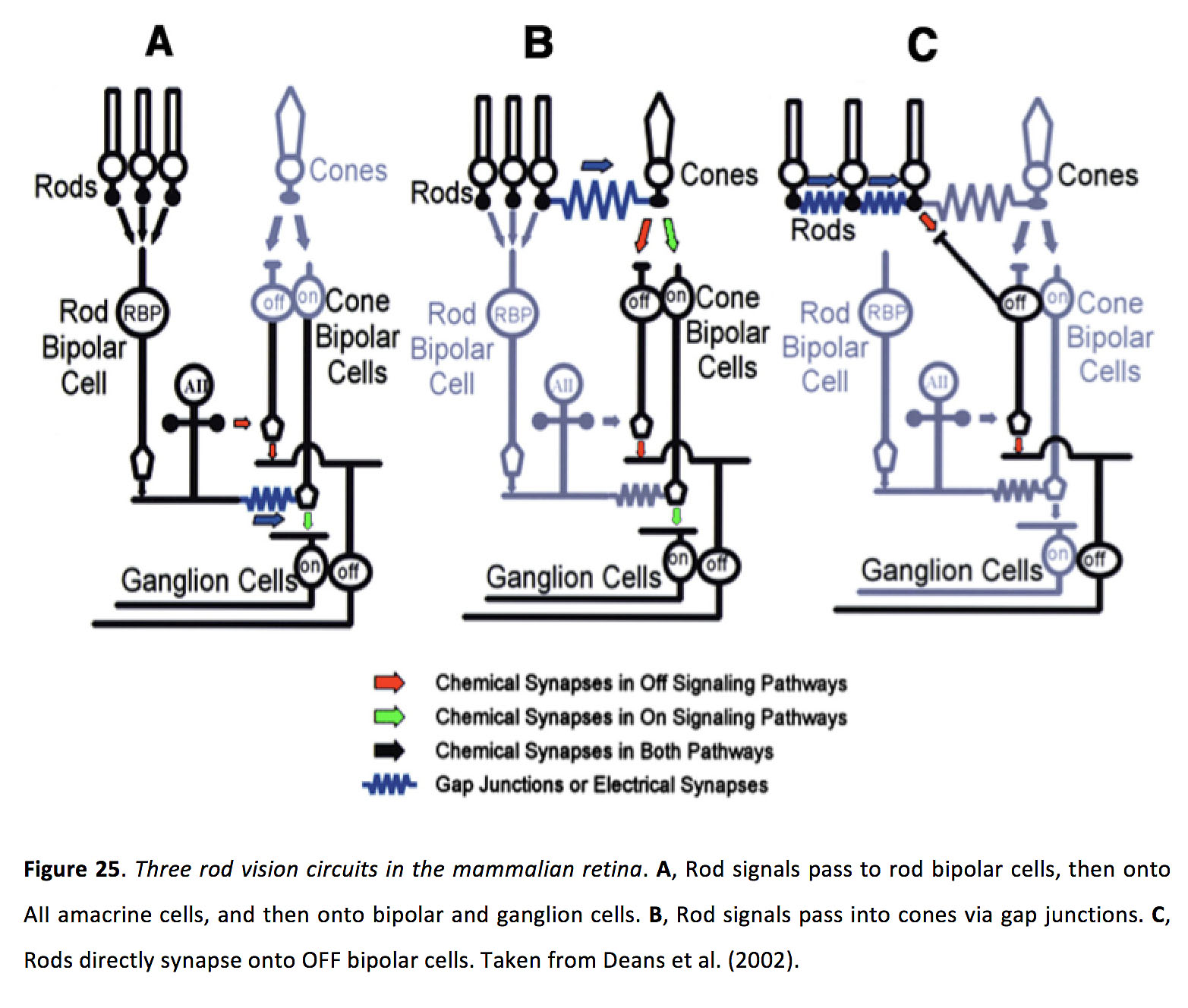 Figure 25. Three rod vision circuits in the mammalian retina. A, Rod signals pass to rod bipolar cells, then onto AII amacrine cells, and then onto bipolar and ganglion cells. B, Rod signals pass into cones via gap junctions. C, Rods directly synapse onto OFF bipolar cells. Taken from Deans et al. (2002) (70).
Figure 25. Three rod vision circuits in the mammalian retina. A, Rod signals pass to rod bipolar cells, then onto AII amacrine cells, and then onto bipolar and ganglion cells. B, Rod signals pass into cones via gap junctions. C, Rods directly synapse onto OFF bipolar cells. Taken from Deans et al. (2002) (70).
Consistent with an important role for gap junction signaling during rod-mediated scotopic vision, knocking out Cx36 greatly impairs rod-mediated responses in the inner retina (Figure 26) (70, 122, 126). Additionally, gap junctions between certain types of amacrine and ganglion cells could also aid in high-sensitivity detection of dim light (129).
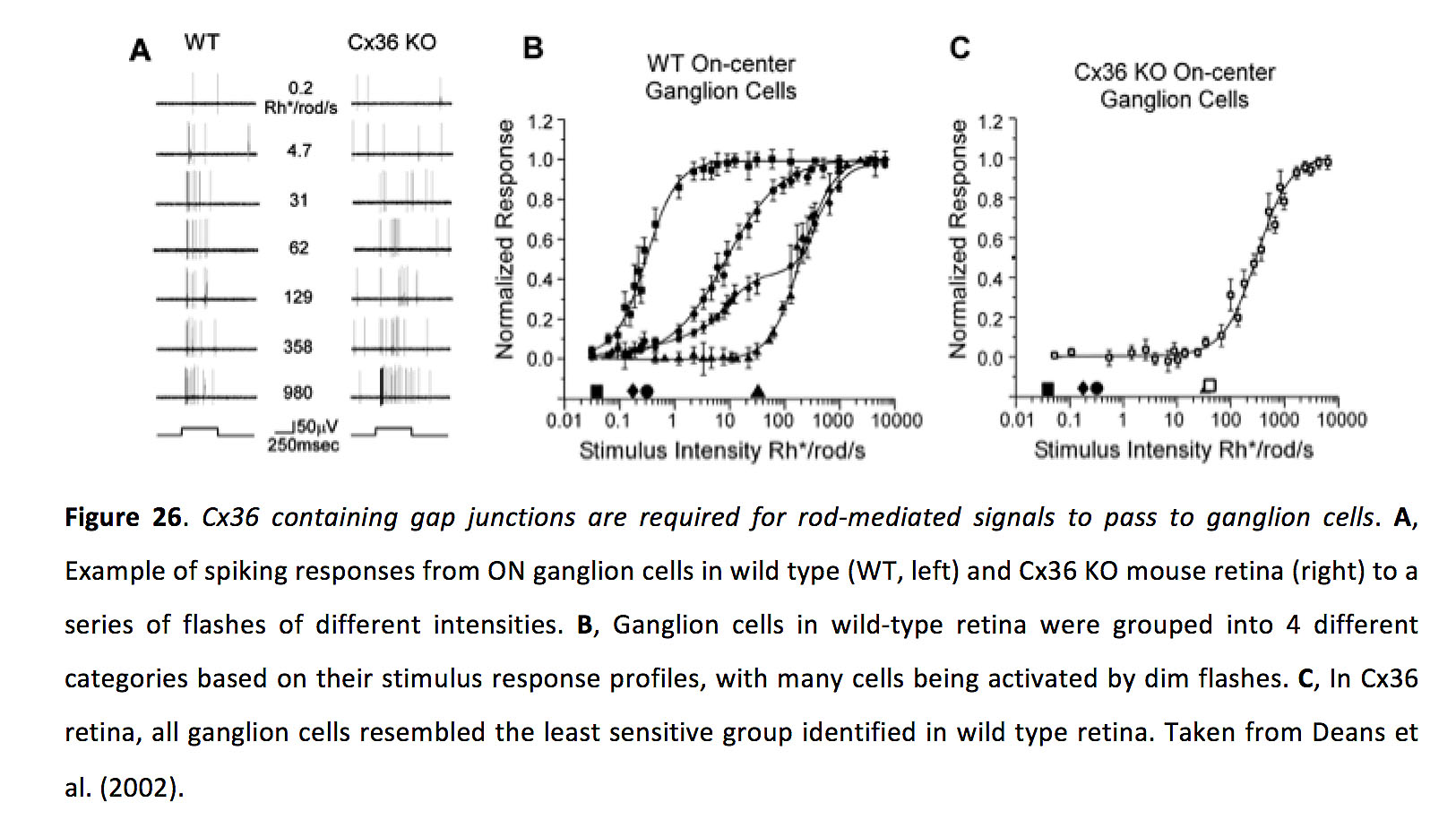 Figure 26. Cx36 containing gap junctions are required for rod-mediated signals to pass to ganglion cells. A, Example of spiking responses from ON ganglion cells in wild type (WT, left) and Cx36 KO mouse retina (right) to a series of flashes of different intensities. B, Ganglion cells in wild-type retina were grouped into 4 different categories based on their stimulus response profiles, with many cells being activated by dim flashes. C, In Cx36 retina, all ganglion cells resembled the least sensitive group identified in wild type retina. Taken from Deans et al. (2002) (70).
Figure 26. Cx36 containing gap junctions are required for rod-mediated signals to pass to ganglion cells. A, Example of spiking responses from ON ganglion cells in wild type (WT, left) and Cx36 KO mouse retina (right) to a series of flashes of different intensities. B, Ganglion cells in wild-type retina were grouped into 4 different categories based on their stimulus response profiles, with many cells being activated by dim flashes. C, In Cx36 retina, all ganglion cells resembled the least sensitive group identified in wild type retina. Taken from Deans et al. (2002) (70).
Gap junctions are thought to play another important role in rod signaling by allowing rods to increase their signal-to-noise ratio. Intrinsic noise in photoreceptors is unique to individual cells, whereas gap junctional coupling allows light-evoked signals to be shared, resulting in more effective detection of relatively weak signals and a decrease in response variability. Note however that these gains may result in a loss of absolute sensitivity and resolution in dim conditions (12, 13, 15, 32, 130-132). A final possible role for gap junctions in rod signaling relates to the nature of synaptic transmission between rods and rod bipolar cells. There appears to be significant signal clipping (i.e. saturation of synaptic transmission) between rods and rod bipolar cells, and it has been posited that electrical coupling between rods could allow spatially-restricted visual stimuli to activate several rods, via junctional spread, and that this activated group of rods would then more effectively activate bipolar cells (133).
Gap junctions and synchrony
One of the best described roles for gap junctions in the CNS is that they can synchronize activity between coupled cells (134, 135). Classical work in the retina revealed that neighboring retinal ganglion cells exhibit significant spike synchrony (136-139). Subsequent studies verified that electrical synapses are responsible for driving diverse types of fine-scale synchrony between ganglion cells, in many species (112, 116, 140-143).
Correlations between neighbouring pairs of ganglion cells are generally classified, based on temporal characteristics, as either narrow, medium or broad (Figure 27) (140, 142, 144). Narrow synchrony occurs within ± 2 ms, with a trough in the cross-correlogram at 0 ms delay. Medium correlations have a cross-correlogram peak at 0 ms delay, and occur somewhere between 2-10 ms (144), ~ 25-50 ms (140), and less than 100 ms (142). Broad correlations generally exhibit a 0 ms delay peak in the cross-correlogram, and occur on a timescale of around 50 ms (144), greater than 50 ms (140) and greater than 100 ms (142), though between pairs of ON and OFF ganglion cells, broad correlations can exhibit a trough at 0 ms, indicative of a slow anti-correlation (137, 138, 144).
 Figure 27. Correlated spike activity in pairs of retinal ganglion cells. A, Schematic cross-correlogram outlining broad (purple), medium (red) and narrow (blue) spike correlations between pairs of retinal ganglion cells. The inset shows a zoomed in view of the narrow correlations, with a trough in the cross-correlogram at 0 ms delay. B, Schematic of the retinal synaptic locations where broad (purple), medium (blue) and narrow (red) correlations are thought to arise. Taken from Trenholm and Awatramani (2017) (197).
Figure 27. Correlated spike activity in pairs of retinal ganglion cells. A, Schematic cross-correlogram outlining broad (purple), medium (red) and narrow (blue) spike correlations between pairs of retinal ganglion cells. The inset shows a zoomed in view of the narrow correlations, with a trough in the cross-correlogram at 0 ms delay. B, Schematic of the retinal synaptic locations where broad (purple), medium (blue) and narrow (red) correlations are thought to arise. Taken from Trenholm and Awatramani (2017) (197).
Pharmacological and genetic knockout experiments have been performed to tease apart the role of electrical and chemical synaptic transmission in these different types of ganglion cell correlations. Pharmacological studies indicate that narrow and medium correlations are unaffected by a cocktail of chemical synaptic blockers, but are inhibited by application of gap junction blockers (140). Cx36 knockout experiments have indicated that broad correlations may also rely upon gap junction signaling (142). Based on these studies, it is thought that broad correlations arise initially in photoreceptors. In contrast, medium correlations are thought to be generated when pairs of ganglion cells are indirectly coupled via an amacrine cell (116, 140, 142, 144, 145), and narrow correlations form between reciprocally coupled ganglion cells (106, 111, 112, 116, 139, 140, 142, 143). In the rest of this section, we mostly focus on homologous coupling between ganglion cells and narrow correlations.
It is thought that narrow spike synchrony arises when one neuron fires an action potential and thus biases spike firing in its neighbor within a short time window. However, as gap junctions act as low-pass filters which significantly reduce the amplitude of action potential mediated signals as they pass across the junction (Figure 28) (134, 135, 146), it was not immediately evident how such small signals can generate such robust synchrony (120).
 Figure 28. Gap junctions act as low-pass filters. Left, An equivalent circuit of a gap junction. Right, Electrical signals pass through gap junctions with a short delay, and high frequency presynaptic signal components are more highly attenuated upon passing to the postsynaptic cell. Taken from Bennett and Zukin (2004) (146).
Figure 28. Gap junctions act as low-pass filters. Left, An equivalent circuit of a gap junction. Right, Electrical signals pass through gap junctions with a short delay, and high frequency presynaptic signal components are more highly attenuated upon passing to the postsynaptic cell. Taken from Bennett and Zukin (2004) (146).
While it has been suggested that an action potential in one ganglion cell can directly drive a spike in a coupled neighbour (112), more direct experimental evidence indicates that action potentials in one ganglion cell practically never drive suprathreshold responses in coupled ganglion cells (143). Consistent with this finding, early work in the field suggested that a spike in one ganglion cell will directly drive a spike in a coupled neighbor with a success rate of less than 5% (139). Furthermore, the classical receptive field of ganglion cells is not significantly increased by the presence of gap junctions (but see also112, 113, 116). Why is a spike in one ganglion cell ineffective in directly driving spikes in a neighour? It turns out that in ganglion cells, coupled spikelets – at least when measured with a patch-pipette at the post-junctional soma – are small in amplitude (Figure 23; ~ 1 mV), though they do arrive with little delay (< 1 ms) (113). As such, coupling between ganglion cells is similar to coupling between most other neurons in the CNS, in which spiking in a pre-junctional neuron only drives subthreshold activity in post-junctional neighbors (134). How then do subthreshold coupled spikelets drive synchronous activity in coupled ganglion cells?
ON-OFF directionally selective (DS) ganglion cells have been an ideal model for studying how coupled spikelets can drive synchrony via non-linear dendritic signaling. DS ganglion cells generate Na+-channel dependent, TTX-sensitive dendritic spikes that trigger action potentials with high reliability (147, 148). There are at least four types of ON-OFF DS ganglion cells, coding the cardinal directions (149). For reasons that are not entirely clear, only ON-OFF DS cells that prefer superior motion in the visual field (ventral motion on the retina) exhibit homologous electrical coupling in the adult mammalian retina, at least in rabbit and mouse retinas (Figure 29) (105, 113, 150).
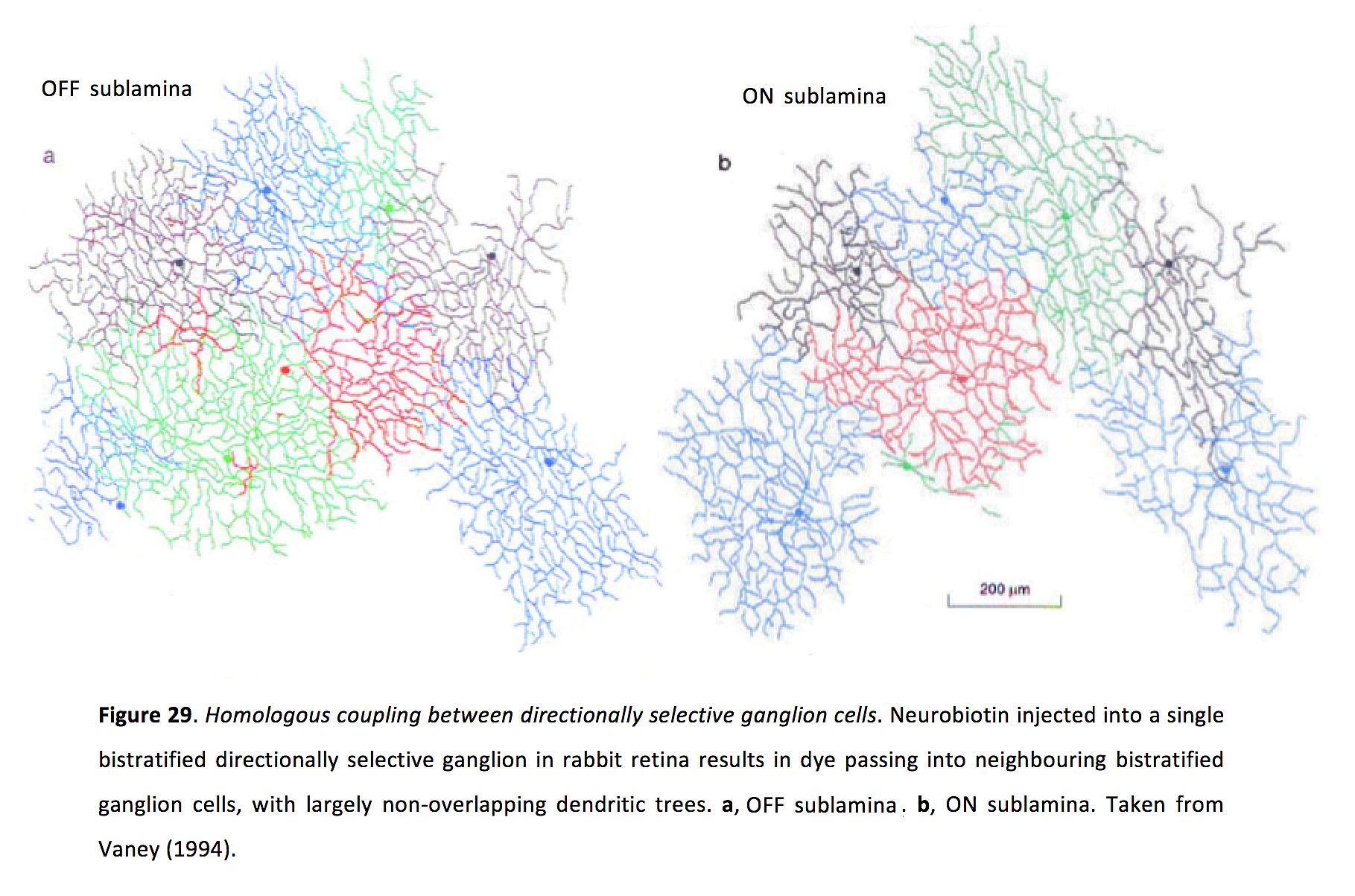 Figure 29. Homologous coupling between directionally selective ganglion cells. Neurobiotin injected into a single bistratified directionally selective ganglion in rabbit retina results in dye passing into neighbouring bistratified ganglion cells, with largely non-overlapping dendritic trees. a, OFF sublamina. b, ON sublamina. Taken from Vaney (1994) (105).
Figure 29. Homologous coupling between directionally selective ganglion cells. Neurobiotin injected into a single bistratified directionally selective ganglion in rabbit retina results in dye passing into neighbouring bistratified ganglion cells, with largely non-overlapping dendritic trees. a, OFF sublamina. b, ON sublamina. Taken from Vaney (1994) (105).
Interestingly, when coupled spikelets were simulated via current injection at the soma (with a patch pipette) in coupled DS ganglion cells, they did not significantly modulate action potential timing, raising the possibility that their site of action was in the dendrites (143). Furthermore, coupled spikelets do not appear large enough to directly initiate dendritic spikes themselves. It turns out that to drive spike synchrony in neighbouring cells, coupled spikelets must interact non-linearly with chemical synaptic inputs in the overlapping dendrites of neighbouring ganglion cells (143). Flashing light at a shared location of receptive overlap between a pair of neighboring coupled ganglion cells drives significant spike correlations, whereas flashing two spots of light at two different spatial locations to activate the same pair of ganglions at the same time but with non-overlapping chemical synaptic inputs, does not drive significant correlations (Figure 30) (143).
 Figure 30. Non-linear interactions between coupled spikelets and chemical synaptic inputs drive correlated spiking. A flash of light drives an action potential (AP) in the central ganglion cell. The action potential back-propagates into the dendritic tree (red arrows), crosses gap junctions (GJs), which become coupled spikelets in the dendrites of coupled neighbors. Coupled spikelets integrate with light-evoked chemical synaptic inputs from bipolar cells to trigger dendritic spikes that in turn propagate to the soma (black arrows), where they generate a synchronized action potential. Taken from Lanore and Silver (2014) (202).
Figure 30. Non-linear interactions between coupled spikelets and chemical synaptic inputs drive correlated spiking. A flash of light drives an action potential (AP) in the central ganglion cell. The action potential back-propagates into the dendritic tree (red arrows), crosses gap junctions (GJs), which become coupled spikelets in the dendrites of coupled neighbors. Coupled spikelets integrate with light-evoked chemical synaptic inputs from bipolar cells to trigger dendritic spikes that in turn propagate to the soma (black arrows), where they generate a synchronized action potential. Taken from Lanore and Silver (2014) (202).
Despite its prevalence, it remains unclear what role correlated ganglion cell activity plays in higher visual processing. One hypothesized role is that it could enhance synaptic transmission in retinorecipient cells. In the lateral geniculate nucleus (LGN), retinal inputs with short inter-spike intervals greatly increase synaptic efficacy (151, 152). Considering that multiple neighbouring ganglion cells converge onto individual LGN relay neurons (153-156), ganglion cell synchrony appears to be a highly plausible strategy for effectively driving LGN neurons. Another hypothesized role for ganglion cell synchrony is that it enhances bandwidth of the optic nerve (145, 157). Since correlated ganglion cell spikes tend to occur when light activates overlapping areas between neighbouring ganglion cell receptive fields (Figure 31) (145, 157), correlated activity contains unique spatial information which could subsequently be decoded separately from uncorrelated activity.
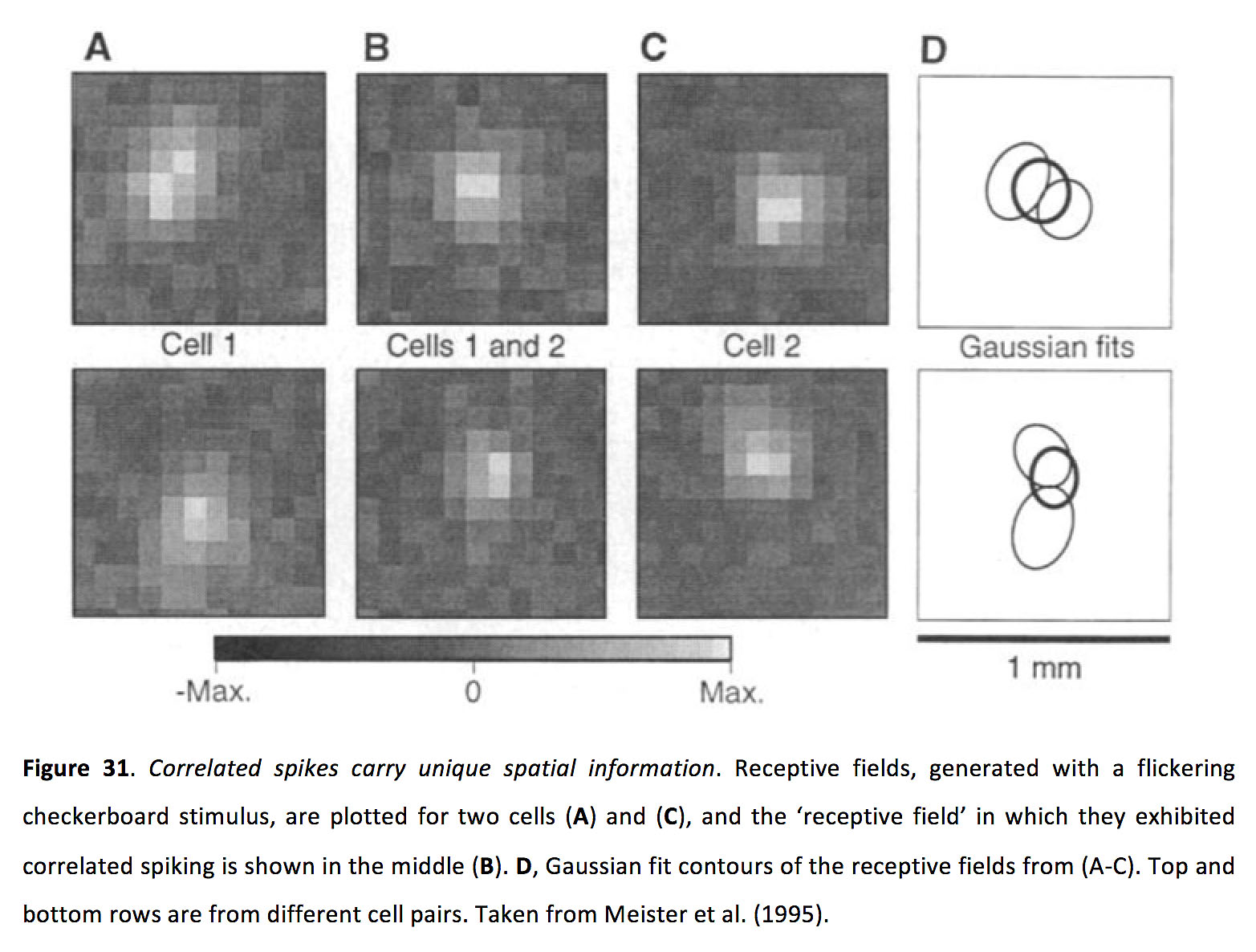 Figure 31. Correlated spikes carry unique spatial information. Receptive fields, generated with a flickering checkerboard stimulus, are plotted for two cells (A) and (C), and the ‘receptive field’ in which they exhibited correlated spiking is shown in the middle (B). D, Gaussian fit contours of the receptive fields from (A-C). Top and bottom rows are from different cell pairs. Taken from Meister et al (1995) (157).
Figure 31. Correlated spikes carry unique spatial information. Receptive fields, generated with a flickering checkerboard stimulus, are plotted for two cells (A) and (C), and the ‘receptive field’ in which they exhibited correlated spiking is shown in the middle (B). D, Gaussian fit contours of the receptive fields from (A-C). Top and bottom rows are from different cell pairs. Taken from Meister et al (1995) (157).
Furthermore, since the strength of narrow gap junction mediated ganglion cell correlations appears to decrease with increasing spike rates (143, 158), though an inhibitory mechanism may sometimes concomitantly lower spike rates and reduce medium-scale gap-junction mediated synchrony (159), correlated ganglion cell activity could relay information to downstream targets independent from spike rate (Figure 32). Whether these types of parallel decoding strategies are actually implemented in higher visual areas remains to be examined.
 Figure 32. Correlated spikes contain information that is independent from the spike rate. a (top), Spikes are recorded from a pair of coupled ganglion cells when (left) a bright bar of light is flashed that covers both cells’ receptive fields, (middle) the same bar of light is shown, but at low contrast, or (right) the same contrast of stimuli is shown as on the left, but is only presented to each cell in a portion of their receptive fields that is not shared between to the two cells, but that drives a similar amount of spiking as the middle stimulus. a (bottom), Cross-correlograms for the stimuli described above. b, Decreasing the contrast results in lower spike rates but higher spike correlations. c, Not stimulating the region of receptive field overlap between the pair of cells reduces spike rate but decreases correlation strength. Taken from Trenholm et al. (2014) (143).
Figure 32. Correlated spikes contain information that is independent from the spike rate. a (top), Spikes are recorded from a pair of coupled ganglion cells when (left) a bright bar of light is flashed that covers both cells’ receptive fields, (middle) the same bar of light is shown, but at low contrast, or (right) the same contrast of stimuli is shown as on the left, but is only presented to each cell in a portion of their receptive fields that is not shared between to the two cells, but that drives a similar amount of spiking as the middle stimulus. a (bottom), Cross-correlograms for the stimuli described above. b, Decreasing the contrast results in lower spike rates but higher spike correlations. c, Not stimulating the region of receptive field overlap between the pair of cells reduces spike rate but decreases correlation strength. Taken from Trenholm et al. (2014) (143).
Aside from the above described forms of synchrony, which tend to be restricted to neighbouring retinal neurons, gap junctions also appear to play an important role in synchronizing activity across wide regions of the retina. Classic work with paired recordings revealed the presence of long-range correlated activity in the retina across distances larger than 20 degrees of visual space (160). Such synchrony appeared to arise when pairs of distally located ganglion cells were responding to a visual image that fell upon their receptive fields as well as the space in between them (160). More recent work has shown that this long-range synchrony arises from gap junction connectivity between ganglion cells, likely via an intermediary widefield amacrine cell (161). Remarkably, as was originally hypothesized (160), this long-range synchrony appears to play an important behavioral role in an animal’s ability to bind together distal portions of the same visual image (161).
Gap junctions and moving visual stimuli
The classical view of the excitatory retinal signaling pathway is of a vertical circuit from photoreceptors to bipolar cells to ganglion cells. Placing gap junctions between neighbouring cells adds a lateral excitatory connection to this vertical pathway. Unsurprisingly, adding such a lateral excitatory pathway endows retinal circuits with distinct advantages when it comes to processing moving visual stimuli.
Motion sensitivity
Lateral gap junction connectivity, in both bipolar cells and ganglion cells, has been shown to selectively modify and enhance responses to moving stimuli. In mouse retina, it has been shown that gap junction inputs to bipolar cells (which can represent > 30% of their excitatory synaptic input), can endow bipolar cells with supralinear spatial summation, which selectively enhances processing of spatio-temporally correlated inputs (i.e. stimuli moving in a coherent direction). This results in enhanced responses to motion in retinal ganglion cells (Figure 33) (84). Related work has been done in primate retina, in which bipolar cell gap junction coupling appears to contribute to motion sensitivity of parasol ganglion cells (162). The general principles underlying motion facilitation in gap junction networks in bipolar cells appears to be similar to that described in more detail in a later section on ganglion cells (163).
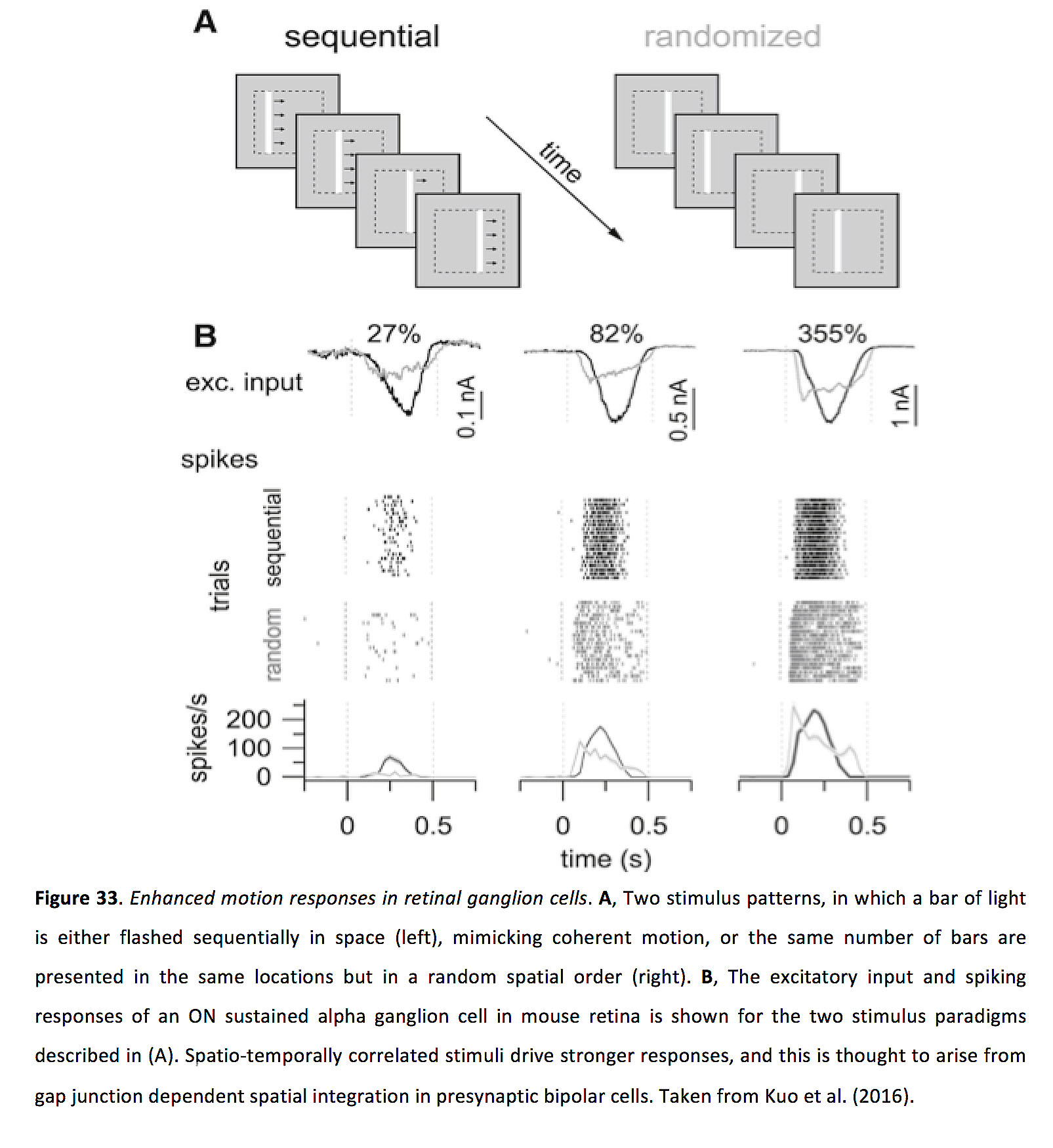 Figure 33. Enhanced motion responses in retinal ganglion cells. A, Two stimulus patterns, in which a bar of light is either flashed sequentially in space (left), mimicking coherent motion, or the same number of bars are presented in the same locations but in a random spatial order (right). B, The excitatory input and spiking responses of an ON sustained alpha ganglion cell in mouse retina is shown for the two stimulus paradigms described in (A). Spatio-temporally correlated stimuli drive stronger responses, and this is thought to arise from gap junction dependent spatial integration in presynaptic bipolar cells. Taken from Kuo et al. (2016) (84).
Figure 33. Enhanced motion responses in retinal ganglion cells. A, Two stimulus patterns, in which a bar of light is either flashed sequentially in space (left), mimicking coherent motion, or the same number of bars are presented in the same locations but in a random spatial order (right). B, The excitatory input and spiking responses of an ON sustained alpha ganglion cell in mouse retina is shown for the two stimulus paradigms described in (A). Spatio-temporally correlated stimuli drive stronger responses, and this is thought to arise from gap junction dependent spatial integration in presynaptic bipolar cells. Taken from Kuo et al. (2016) (84).
Finally, in mouse retina, at low light levels, homologously coupled direction selective ganglion cells have broader tuning curves than their uncoupled counterparts. As such, gap junction connectivity appears to allow these cells to strike a balance, in low light levels, between detecting dim moving stimuli and accurately reporting the direction of movement (Figure 34) (111).
Figure 34. The direction tuning of electrically coupled directionally selective ganglion cells changes at different light levels. I, J, The direction selective index (DSI) and tuning width (high tuning width values mean a cell responds strongly to a wider angle of directions of motion) are plotted for direction selective ganglion cells coding the four cardinal directions (note that only superior coding direction selective cells are electrically coupled). K, Normalized tuning curves, to 8 directions of moving stimuli, are plotted for the four directions of ganglion cells, at dim (top) and bright (bottom) light levels. Taken from Yao et al. (2018) (111).
Motion anticipation
When light is flashed on the retina, it takes roughly 50 ms or more for a ganglion cell to fire a light-evoked action potential. Most of this delay is due to phototransduction in rods and cones, the relatively slow process whereby incoming photons are converted into an electro-chemical signal. This delay poses a problem, given that the same retinal circuity can process both static and moving stimuli. For static images, this delay does not pose a significant problem, as when a ganglion cell spikes the stimulus it is encoding is still in the appropriate position. In contrast, when a moving image activates photoreceptors in one location, the image will have moved to a different spatial location by the time the ganglion cells vertically connected to the photoreceptors at the earlier position begin to respond. The lag between where a ganglion cell reports an image is, compared to where the image actually is in space, increases as movement gets faster. One method that has been hypothesized for the retina to address this problem is for ganglion cells to dynamically shift the peak of their receptive field toward the leading edge of a moving stimulus (164, 165), which could theoretically be read out by downstream targets as anticipation if they decode position based on peak spike rate. Another method that has been proposed to compensate for the lag that arises when coding moving stimuli involves using gap junctions to allow upstream retinal ganglion cells to alert their downstream neighbors to the presence of an incoming image. This phenomenon, termed lag normalization, arises in homologously coupled retinal ganglion cells which exhibit a coupling-mediated subthreshold excitatory receptive field that surrounds their receptive field center (Figure 35) (113).
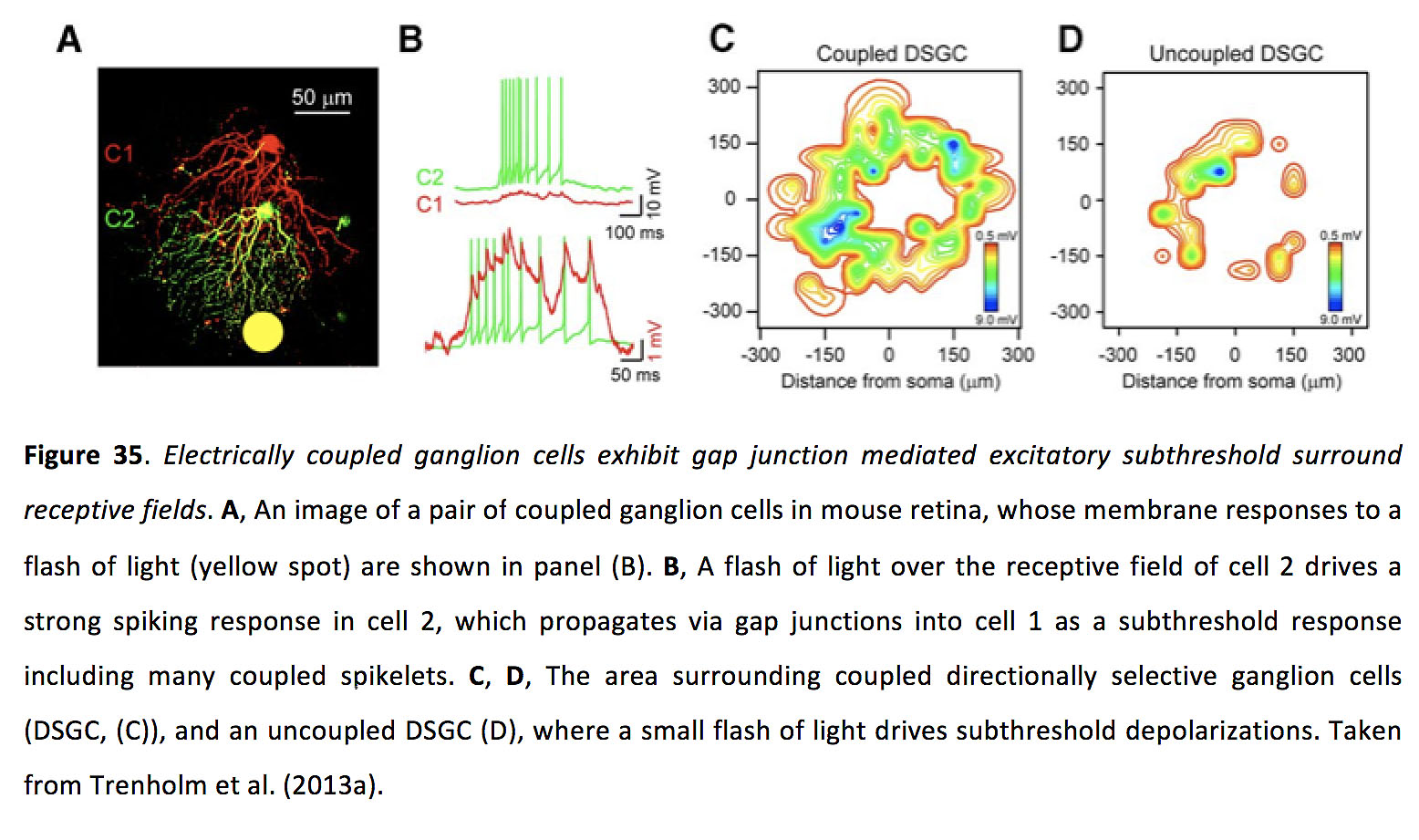 Figure 35. Electrically coupled ganglion cells exhibit gap junction mediated excitatory subthreshold surround receptive fields. A, An image of a pair of coupled ganglion cells in mouse retina, whose membrane responses to a flash of light (yellow spot) are shown in panel (B). B, A flash of light over the receptive field of cell 2 drives a strong spiking response in cell 2, which propagates via gap junctions into cell 1 as a subthreshold response including many coupled spikelets. C, D, The area surrounding coupled directionally selective ganglion cells (DSGC, (C)), and an uncoupled DSGC (D), where a small flash of light drives subthreshold depolarizations. Taken from Trenholm et al. (2013a) (113).
Figure 35. Electrically coupled ganglion cells exhibit gap junction mediated excitatory subthreshold surround receptive fields. A, An image of a pair of coupled ganglion cells in mouse retina, whose membrane responses to a flash of light (yellow spot) are shown in panel (B). B, A flash of light over the receptive field of cell 2 drives a strong spiking response in cell 2, which propagates via gap junctions into cell 1 as a subthreshold response including many coupled spikelets. C, D, The area surrounding coupled directionally selective ganglion cells (DSGC, (C)), and an uncoupled DSGC (D), where a small flash of light drives subthreshold depolarizations. Taken from Trenholm et al. (2013a) (113).
For moving images, the gap junction inputs spread laterally from one ganglion cell to another, and are able to combine with weak bipolar cell mediated chemical synaptic inputs to allow the ganglion cell to begin responding outside of the receptive area that drove spiking activity to static stimuli (166). Moreover, when these coupled ganglion cells are stimulated with images moving at various speeds, they can initiate their responses at nearly the same spatial location (i.e. they ‘lag normalize’), in contrast to uncoupled ganglion cells which initiate their responses at positions further and further in space when presented with faster and faster speeds of moving images (Figure 36) (166).
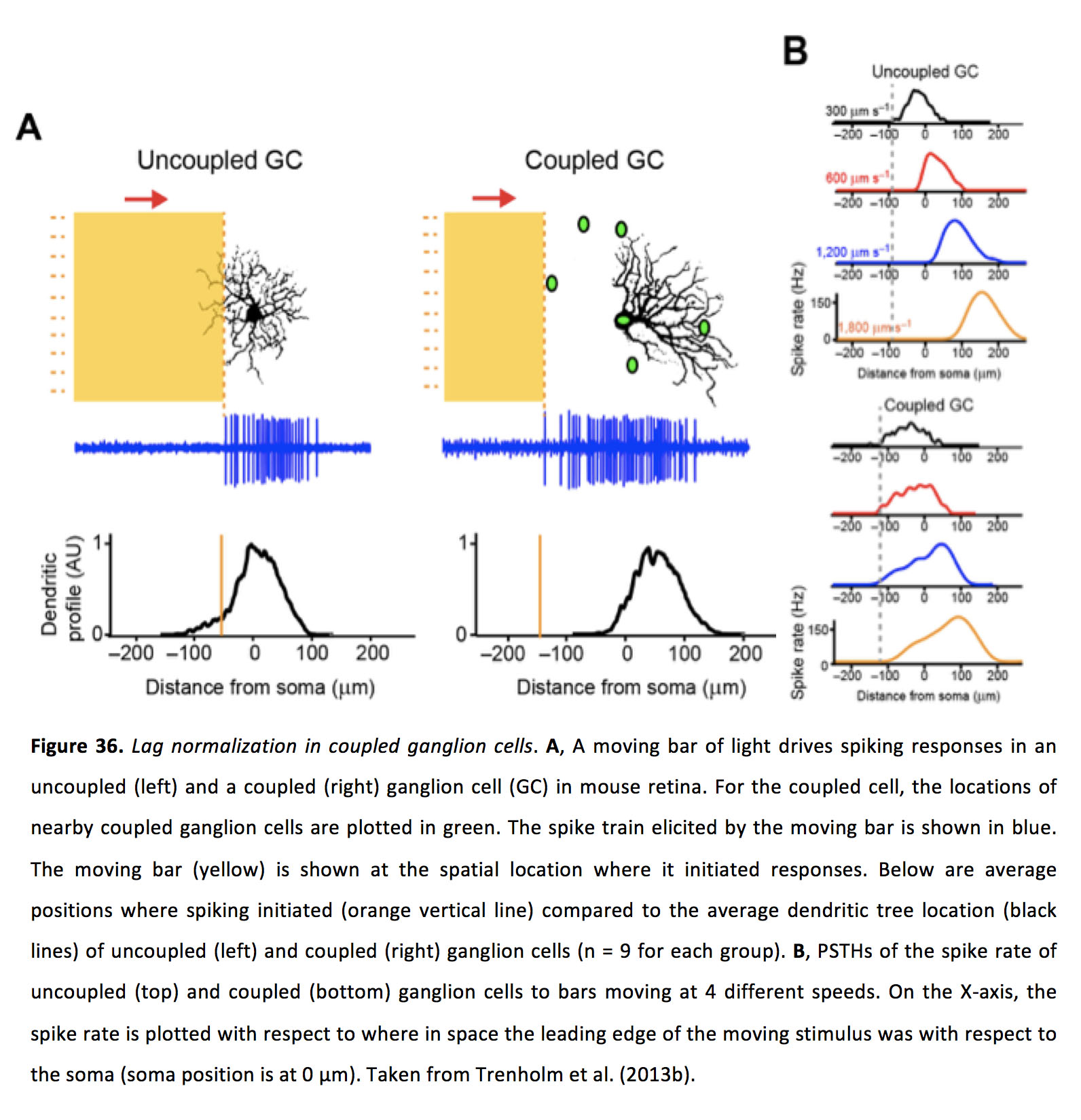 Figure 36. Lag normalization in coupled ganglion cells. A, A moving bar of light drives spiking responses in an uncoupled (left) and a coupled (right) ganglion cell (GC) in mouse retina. For the coupled cell, the locations of nearby coupled ganglion cells are plotted in green. The spike train elicited by the moving bar is shown in blue. The moving bar (yellow) is shown at the spatial location where it initiated responses. Below are average positions where spiking initiated (orange vertical line) compared to the average dendritic tree location (black lines) of uncoupled (left) and coupled (right) ganglion cells (n = 9 for each group). B, PSTHs of the spike rate of uncoupled (top) and coupled (bottom) ganglion cells to bars moving at 4 different speeds. On the X-axis, the spike rate is plotted with respect to where in space the leading edge of the moving stimulus was with respect to the soma (soma position is at 0 µm). Taken from Trenholm et al. (2013b) (166).
Figure 36. Lag normalization in coupled ganglion cells. A, A moving bar of light drives spiking responses in an uncoupled (left) and a coupled (right) ganglion cell (GC) in mouse retina. For the coupled cell, the locations of nearby coupled ganglion cells are plotted in green. The spike train elicited by the moving bar is shown in blue. The moving bar (yellow) is shown at the spatial location where it initiated responses. Below are average positions where spiking initiated (orange vertical line) compared to the average dendritic tree location (black lines) of uncoupled (left) and coupled (right) ganglion cells (n = 9 for each group). B, PSTHs of the spike rate of uncoupled (top) and coupled (bottom) ganglion cells to bars moving at 4 different speeds. On the X-axis, the spike rate is plotted with respect to where in space the leading edge of the moving stimulus was with respect to the soma (soma position is at 0 µm). Taken from Trenholm et al. (2013b) (166).
Looming
Effective detection of looming visual stimuli, like an approaching predator, can be a matter of life or death. Accordingly, looming (or approaching) visual stimuli drive behavioral reflexes that are found across many species, including humans (167-171). In the retina, it appears that lateral gap junction connectivity – between AII amacrine cells and ON cone bipolar cells – plays a role in allowing a specific retinal ganglion cell to preferentially detect looming visual images. In mouse retina, the looming sensitive cell is an OFF ganglion cell, termed the PV-5 cell (124). When static decrements of light are presented over the receptive field of this cell, they evoke transient OFF excitation, followed by transient ON inhibition when the light level returns to baseline (124). As a large dark stimulus gets smaller over the receptive field of this cell, it exclusively drives ON inhibition and no excitatory OFF response, and thus does not result in spiking. On the other hand, if the same stimulus is now played backwards and the black area expands over the receptive field of the cell, it exclusively drives OFF excitation that drives robust spiking responses. Based on the described response properties, this cell should also respond reasonably well to moving stimuli that pass across the receptive field, which would drive a mix of OFF excitation and ON inhibition. However, the ON inhibitory input to the PV-5 cell is very fast and appears to arise via gap junction signals passed from ON cone bipolar cells to AII amacrine cells, which weaken the effect of concomitant excitatory OFF inputs. Indeed, when Cx36 was knocked out, PV-5 cells no longer responded preferentially to looming stimuli over stimuli that simply moved across the receptive field (124). In mice, this retinal cell type is thought to underlie innate freezing/fleeing behavioral responses when dark looming stimuli are presented above the head of a mouse (172). Whether similar retinal circuitry underlies behavioral responses to looming visual stimuli in primates remains to be tested.
Gap junctions, retinal degeneration and spontaneous activity
While, as outlined above, gap junctions serve many functions in the healthy retina, they also promote aberrant neuronal activity following retinal degeneration (rd). In rodent rd models, following photoreceptor degeneration, instead of becoming silent many ganglion cells exhibit spontaneous oscillations with a frequency of around 10 Hz (Figure 37) (173-179). Understanding the source of this aberrant activity is important since it could underlie phosphenes experienced by patients suffering from vision loss (180, 181), and could impact the success of therapeutic strategies including electrical implants (182) and optogenetic vision therapy (183).
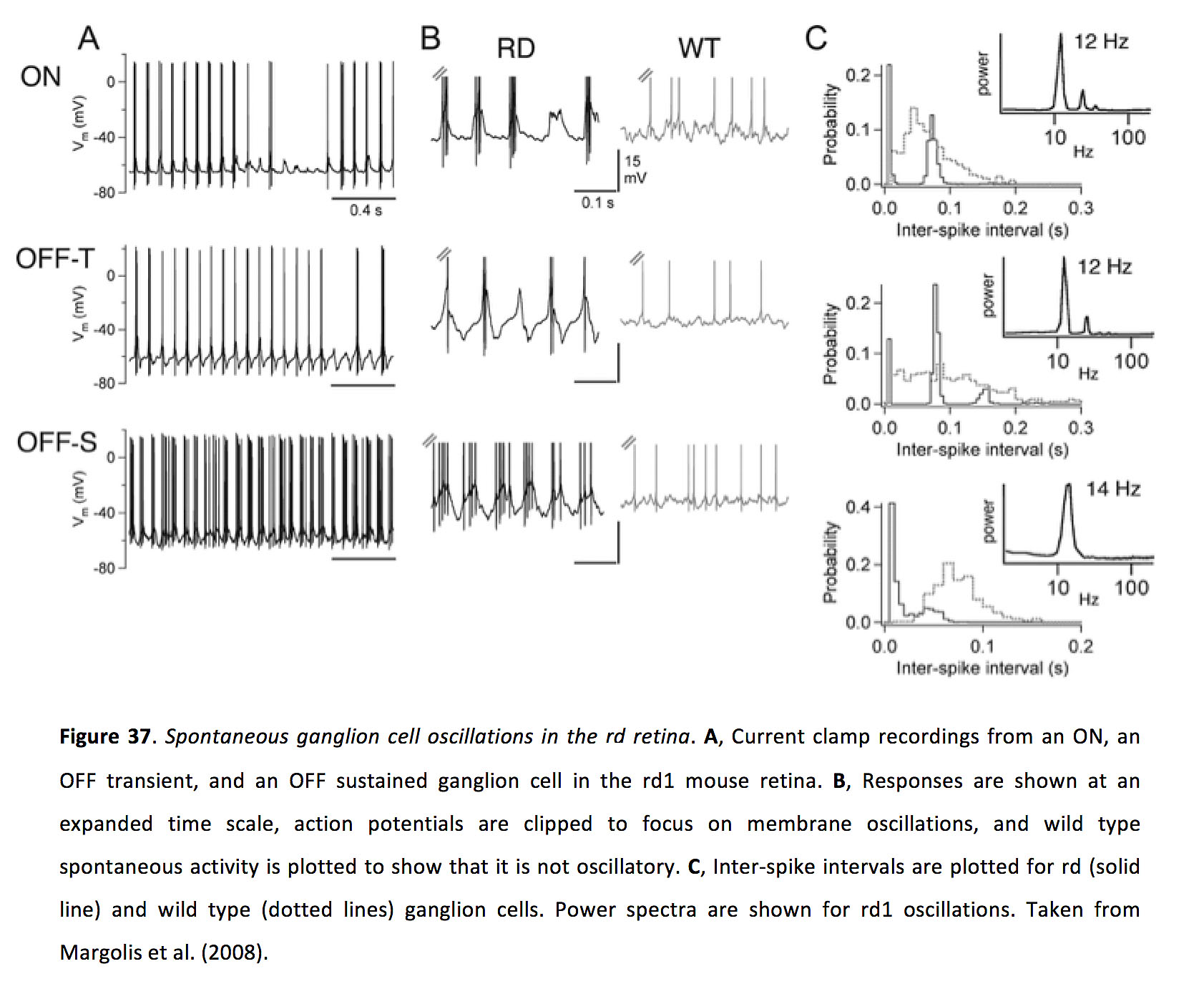 Figure 37. Spontaneous ganglion cell oscillations in the rd retina. A, Current clamp recordings from an ON, an OFF transient, and an OFF sustained ganglion cell in the rd1 mouse retina. B, Responses are shown at an expanded time scale, action potentials are clipped to focus on membrane oscillations, and wild type spontaneous activity is plotted to show that it is not oscillatory. C, Inter-spike intervals are plotted for rd (solid line) and wild type (dotted lines) ganglion cells. Power spectra are shown for rd1 oscillations. Taken from Margolis et al. (2008) (175).
Figure 37. Spontaneous ganglion cell oscillations in the rd retina. A, Current clamp recordings from an ON, an OFF transient, and an OFF sustained ganglion cell in the rd1 mouse retina. B, Responses are shown at an expanded time scale, action potentials are clipped to focus on membrane oscillations, and wild type spontaneous activity is plotted to show that it is not oscillatory. C, Inter-spike intervals are plotted for rd (solid line) and wild type (dotted lines) ganglion cells. Power spectra are shown for rd1 oscillations. Taken from Margolis et al. (2008) (175).
In rd retina, ganglion cells oscillate as the result of receiving excitatory and inhibitory oscillatory inputs (174, 175, 179, 184). Application of chemical synaptic blockers inhibits ganglion cell oscillations, indicating that spontaneous oscillations do not arise in ganglion cells themselves (174, 176), but see also (185). In contrast, membrane oscillations in ON cone bipolar cells and AII amacrine cells, which also occur with a frequency of around 10 Hz, persist upon application of chemical synaptic blockers, indicating that spontaneous oscillations arise intrinsically within the coupled network of ON cone bipolar cells and AII amacrine cells (174). As evidence that gap junctions between bipolar cells and AII amacrine cells are important in this pathophysiology, pharmacologically blocking gap junctions inhibits oscillations in ganglion cells (176, 186), AII amacrine cells and ON cone bipolar cells (Figure 38) (186). Furthermore, spontaneous ganglion cell oscillations were greatly reduced in rd mice in which connexin 36 was knocked out (187).
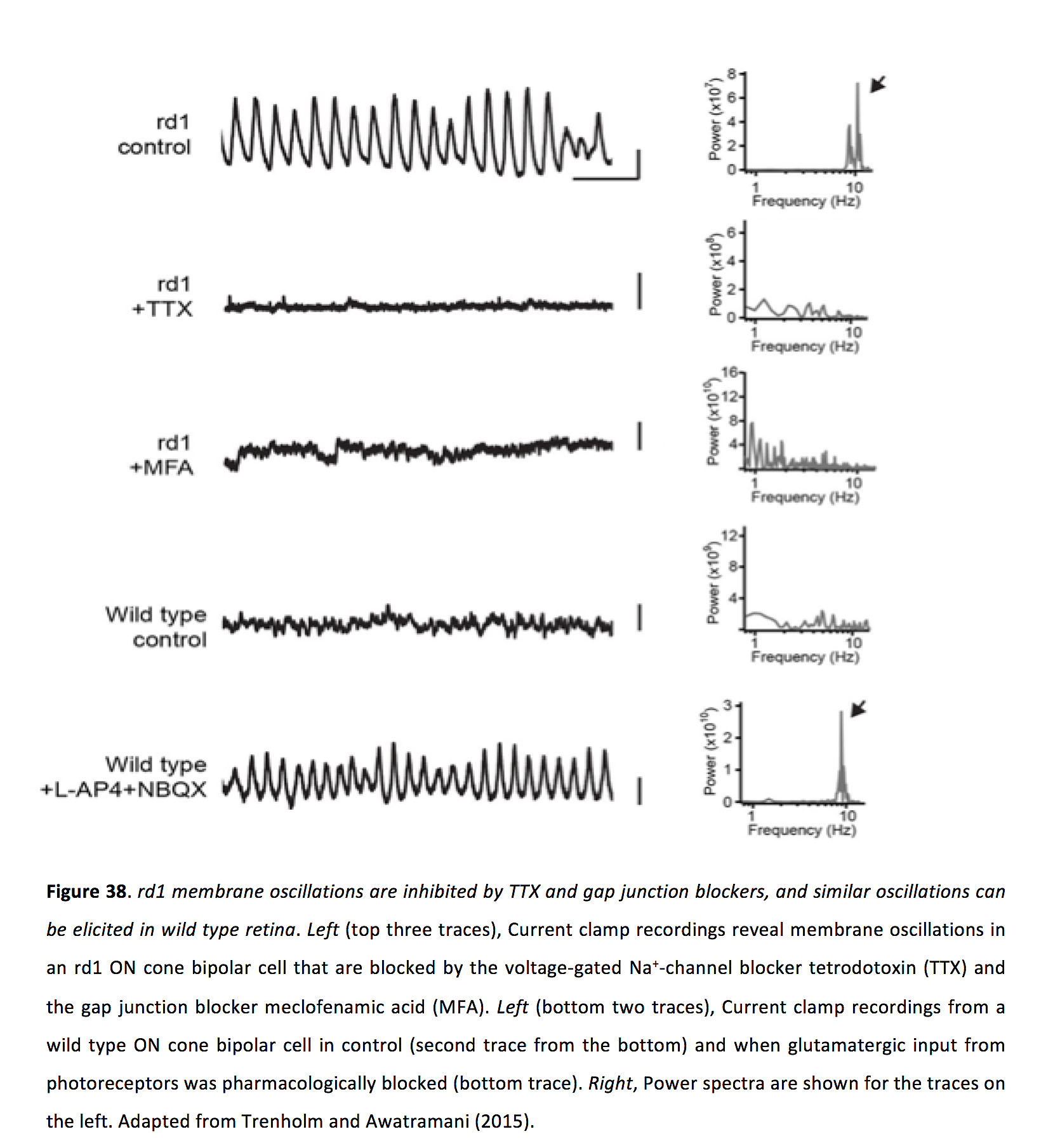 Figure 38. rd1 membrane oscillations are inhibited by TTX and gap junction blockers, and similar oscillations can be elicited in wild type retina. Left (top three traces), Current clamp recordings reveal membrane oscillations in an rd1 ON cone bipolar cell that are blocked by the voltage-gated Na+-channel blocker tetrodotoxin (TTX) and the gap junction blocker meclofenamic acid (MFA). Left (bottom two traces), Current clamp recordings from a wild type ON cone bipolar cell in control (second trace from the bottom) and when glutamatergic input from photoreceptors was pharmacologically blocked (bottom trace). Right, Power spectra are shown for the traces on the left. Adapted from Trenholm and Awatramani (2015) (203).
Figure 38. rd1 membrane oscillations are inhibited by TTX and gap junction blockers, and similar oscillations can be elicited in wild type retina. Left (top three traces), Current clamp recordings reveal membrane oscillations in an rd1 ON cone bipolar cell that are blocked by the voltage-gated Na+-channel blocker tetrodotoxin (TTX) and the gap junction blocker meclofenamic acid (MFA). Left (bottom two traces), Current clamp recordings from a wild type ON cone bipolar cell in control (second trace from the bottom) and when glutamatergic input from photoreceptors was pharmacologically blocked (bottom trace). Right, Power spectra are shown for the traces on the left. Adapted from Trenholm and Awatramani (2015) (203).
Considering that there is extensive coupling between ON cone bipolar cells and AII amacrine cells in wild type retina, what occurs during retinal degeneration that promotes aberrant network activity? Experiments in wild type retina suggest that the main trigger for spontaneous activity relates to altered glutamate release from photoreceptors, as either pharmacologically blocking photoreceptor output in wild type retina or photo-bleaching the wild type retina with bright light (both of which would hyperpolarize the AII amacrine/ON cone bipolar cell network) can drive spontaneous activity similar to that found in the rd retina (186), though recent work also implicates retinoic acid in this hyperactivity (188). Consistent with the former idea, the resting membrane potential of AII amacrine cells in the degenerating retina appears to be hyperpolarized compared to those in the wild type retina (189). Such hyperpolarization could activate rhythmogenic conductances, such as Ih, which is present in some ON cone bipolar cells and has been shown to modulate oscillations in the rd retina (186). These oscillations have also been shown to be reliant on TTX-sensitive Na+ channels in AII amacrine cells (186). Finally, it has been shown that gap junctional coupling of AII amacrine cells in the rd retina appears to be increased (as measured via an increase in phosphorylated Cx36), which could exacerbate aberrant activity following photoreceptor degeneration (190). As such, membrane conductances and gap junctions in the AII amacrine and ON bipolar cell network act in tandem following photoreceptor degeneration to elicit spontaneous membrane oscillations (Figure 39).
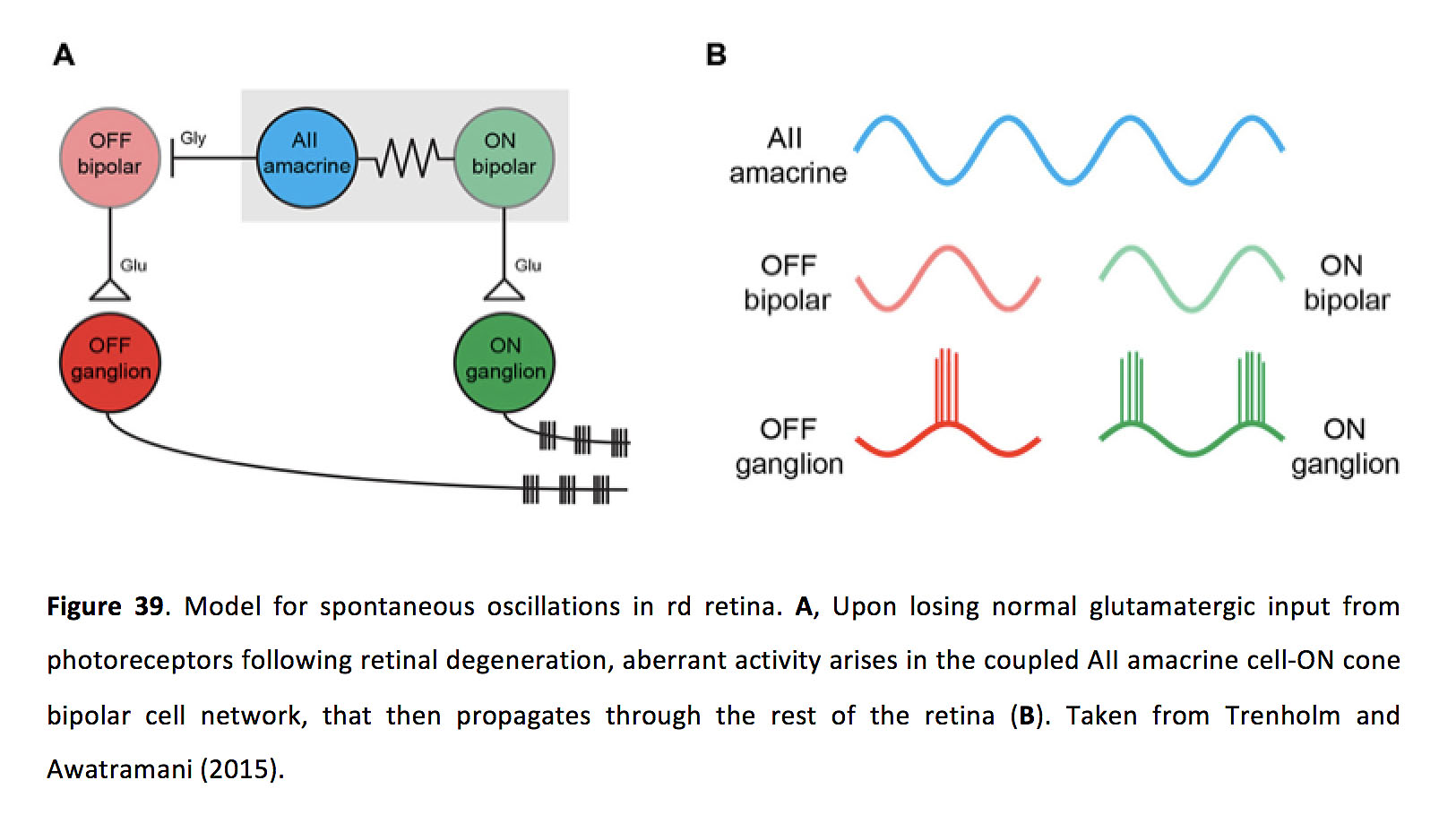 Figure 39. Model for spontaneous oscillations in rd retina. A, Upon losing normal glutamatergic input from photoreceptors following retinal degeneration, aberrant activity arises in the coupled AII amacrine cell-ON cone bipolar cell network, that then propagates through the rest of the retina (B). Taken from Trenholm and Awatramani (2015) (203).
Figure 39. Model for spontaneous oscillations in rd retina. A, Upon losing normal glutamatergic input from photoreceptors following retinal degeneration, aberrant activity arises in the coupled AII amacrine cell-ON cone bipolar cell network, that then propagates through the rest of the retina (B). Taken from Trenholm and Awatramani (2015) (203).
Conclusions
It is well established that the retina is extensively gap junction coupled, and that these connections endow retinal circuits with a plethora of functional properties. In the retina, gap junctions are associated with night vision, controlling receptive field size, signal correlation, spatial integration, and motion detection. Despite these findings, many unknowns remain. First, many critical experiments into the roles of gap junctions in the retina have employed either pharmacological agents or Cx36 KO to examine the roles of gap junctions in retinal circuits. However, simultaneously blocking gap junctions at all levels of the retina makes it difficult to assign roles for specific electrical synapses between given cell types. As such, future research will need to endeavor to modify gap junctions in specific cell types (111). Second, related to the first point, while it is clear that the retina splits incoming visual signals into over 30 distinct retinal channels (made up of distinct cell types, connected in distinct ways), unique roles for gap junctions in each of these channels is only beginning to be studied. Third, given that gap junctions are known to play important roles in retinal development (191), and may affect susceptibility to disease pathologies (192-195), it will be important to examine whether gap junctions play precise cell-type-specific roles in development (196) and disease. Finally, in addition to increasing our knowledge into the fine details of roles for gap junctions in microcircuits, it will be important to characterize different manners in which gap junction connections are plastic, and thus allow circadian rhythm, light adaptation, or neuronal activity to gate their activity and modify circuit function.
Acknowledgements
This work was supported by Canada Research Chairs to ST and GBA.
About the authors
Dr. Stuart Trenholm received his PhD in neuroscience from Dalhousie University, in Halifax Canada, in the lab of Dr. Gautam Awatramani. Subsequently he was a postdoctoral fellow in neuroscience in the lab of Dr. Botond Roska, at the Friedrich Miescher Institute for Biomedical Research in Basel, Switzerland. He is now an Assistant Professor at the Montreal Neurological Institute at McGill University, in Montreal, Canada. His lab examines the neuronal circuitry underlying visual perception and studies vision rehabilitation strategies.
Dr. Gautam Awatramani received his B.S. from the University of Rochester (1995) and then did his Ph.D. in Physiology and Biophysics under the supervision of Dr. Malcolm Slaughter at SUNY Buffalo. He was a postdoctoral fellow at the Vollum Institute (Portland, OR, 2000-2004) with Dr. Laurence Trussell, at the University of British Columbia (Vancouver, B.C, 2005-2007) with Dr. Tim Murphy and at the Friedrich Miescher Institute (Basel, CH, 2008) with Dr. Botond Roska. He then started his own lab at Dalhousie University (Halifax 2008-2011) and subsequently moved to the University of Victoria (Victoria, B.C.) where he is currently an Associate Professor/CRC chair in Physiology. His work currently focusses on understanding the synaptic mechanisms underlying the generation of direction selectivity in the retina.
References
- Masland, R.H., The fundamental plan of the retina. Nature Neuroscience. 2001; 4(9):877-886. [PubMed]
- Gollisch, T. and M. Meister, Eye smarter than scientists believed: neural computations in circuits of the retina. Neuron. 2010; 65(2):150-164. [PubMed]
- Azeredo da Silveira, R. and B. Roska, Cell types, circuits, computation. Current Opinion in Neurobiology. 2011; 21(5):664-671. [PubMed]
- Masland, R.H., The neuronal organization of the retina. Neuron. 2012; 76(2):266-280. [PubMed]
- Diamond, J.S., Inhibitory Interneurons in the Retina: Types, Circuitry, and Function. Annual Review of Vision Science. 2017; 3:1-24. [PubMed]
- Martersteck, E.M., K.E. Hirokawa, M. Evarts, A. Bernard, X. Duan, Y. Li, L. Ng, S.W. Oh, B. Ouellette, J.J. Royall, M. Stoecklin, Q. Wang, H. Zeng, J.R. Sanes, and J.A. Harris, Diverse Central Projection Patterns of Retinal Ganglion Cells. Cell Reports. 2017; 18(8):2058-2072. [PubMed]
- Robles, E., E. Laurell, and H. Baier, The retinal projectome reveals brain-area-specific visual representations generated by ganglion cell diversity. Current biology: CB. 2014; 24(18):2085-2096. [PubMed]
- Ribelayga, C.P. and J. O’Brien, Chapter 10 – Circadian and Light-Adaptive Control of Electrical Synaptic Plasticity in the Vertebrate Retina, in Network Functions and Plasticity, J. Jing, Editor. 2017, Academic Press. p. 209-241. [PubMed]
- Baylor, D.A., M.G. Fuortes, and P.M. O’Bryan, Receptive fields of cones in the retina of the turtle. The Journal of Physiology. 1971; 214(2):265-294. [PubMed]
- Copenhagen, D.R. and W.G. Owen, Coupling between rod photoreceptors in a vertebrate retina. Nature. 1976; 260(5546):57-59. [PubMed]
- Custer, N.V., Structurally specialized contacts between the photoreceptors of the retina of the axolotl. The Journal of Comparative Neurology. 1973; 151(1):35-56. [PubMed]
- DeVries, S.H., X. Qi, R. Smith, W. Makous, and P. Sterling, Electrical coupling between mammalian cones. Current biology: CB. 2002; 12(22):1900-1907. [PubMed]
- Hornstein, E.P., J. Verweij, P.H. Li, and J.L. Schnapf, Gap-junctional coupling and absolute sensitivity of photoreceptors in macaque retina. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2005; 25(48):11201-11209. [PubMed]
- Hornstein, E.P., J. Verweij, and J.L. Schnapf, Electrical coupling between red and green cones in primate retina. Nature Neuroscience. 2004; 7(7):745-750. [PubMed]
- Li, P.H., J. Verweij, J.H. Long, and J.L. Schnapf, Gap-junctional coupling of mammalian rod photoreceptors and its effect on visual detection. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2012; 32(10):3552-3562. [PubMed]
- Raviola, E. and N.B. Gilula, Gap junctions between photoreceptor cells in the vertebrate retina. Proceedings of the National Academy of Sciences of the United States of America. 1973; 70(6):1677-1681. [PubMed]
- Tsukamoto, Y., K. Morigiwa, M. Ueda, and P. Sterling, Microcircuits for night vision in mouse retina. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2001; 21(21):8616-8623. [PubMed]
- Feigenspan, A., U. Janssen-Bienhold, S. Hormuzdi, H. Monyer, J. Degen, G. Söhl, K. Willecke, J. Ammermüller, and R. Weiler, Expression of connexin36 in cone pedicles and OFF-cone bipolar cells of the mouse retina. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2004; 24(13):3325-3334. [PubMed]
- Kántor, O., Z. Benkő, A. Énzsöly, C. Dávid, A. Naumann, R. Nitschke, A. Szabó, E. Pálfi, J. Orbán, M. Nyitrai, J. Németh, Á. Szél, Á. Lukáts, and B. Völgyi, Characterization of connexin36 gap junctions in the human outer retina. Brain Structure & Function. 2016; 221(6):2963-2984. [PubMed]
- Lee, E.-J., J.-W. Han, H.-J. Kim, I.-B. Kim, M.-Y. Lee, S.-J. Oh, J.-W. Chung, and M.-H. Chun, The immunocytochemical localization of connexin 36 at rod and cone gap junctions in the guinea pig retina. The European Journal of Neuroscience. 2003; 18(11):2925-2934. [PubMed]
- O’Brien, J.J., X. Chen, P.R. Macleish, J. O’Brien, and S.C. Massey, Photoreceptor coupling mediated by connexin36 in the primate retina. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2012; 32(13):4675-4687. [PubMed]
- Li, H., A.Z. Chuang, and J. O’Brien, Photoreceptor coupling is controlled by connexin 35 phosphorylation in zebrafish retina. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2009; 29(48):15178-15186. [PubMed]
- Li, H., Z. Zhang, M.R. Blackburn, S.W. Wang, C.P. Ribelayga, and J. O’Brien, Adenosine and dopamine receptors coregulate photoreceptor coupling via gap junction phosphorylation in mouse retina. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2013; 33(7):3135-3150. [PubMed]
- Zhang, J. and S.M. Wu, Connexin35/36 gap junction proteins are expressed in photoreceptors of the tiger salamander retina.The Journal of Comparative Neurology. 2004; 470(1):1-12. [PubMed]
- Asteriti, S., C. Gargini, and L. Cangiano, Connexin 36 expression is required for electrical coupling between mouse rods and cones. Visual Neuroscience. 2017; 34:E006. [PubMed]
- Kolb, H., The organization of the outer plexiform layer in the retina of the cat: electron microscopic observations. Journal of Neurocytology. 1977; 6(2):131-153. [PubMed]
- Li, W. and S.H. DeVries, Separate blue and green cone networks in the mammalian retina. Nature Neuroscience. 2004; 7(7):751-756. [PubMed]
- Tsukamoto, Y., P. Masarachia, S.J. Schein, and P. Sterling, Gap junctions between the pedicles of macaque foveal cones. Vision Research. 1992; 32(10):1809-1815. [PubMed]
- Zhang, J. and S.M. Wu, Physiological properties of rod photoreceptor electrical coupling in the tiger salamander retina. The Journal of Physiology. 2005; 564(Pt 3):849-862. [PubMed]
- Werblin, F.S., Transmission along and between rods in the tiger salamander retina. The Journal of Physiology. 1978; 280:449-470. [PubMed]
- Gao, F., J.-J. Pang, and S.M. Wu, Sign-preserving and sign-inverting synaptic interactions between rod and cone photoreceptors in the dark-adapted retina. The Journal of Physiology. 2013; 591(22):5711-5726. [PubMed]
- Attwell, D., M. Wilson, and S.M. Wu, A quantitative analysis of interactions between photoreceptors in the salamander (Ambystoma) retina. The Journal of Physiology. 1984; 352:703-737. [PubMed]
- Ribelayga, C., Y. Cao, and S.C. Mangel, The circadian clock in the retina controls rod-cone coupling. Neuron. 2008; 59(5):790-801. [PubMed]
- Wu, S.M. and X.L. Yang, Electrical coupling between rods and cones in the tiger salamander retina. Proceedings of the National Academy of Sciences of the United States of America. 1988; 85(1):275-278. [PubMed]
- Asteriti, S., C. Gargini, and L. Cangiano, Mouse rods signal through gap junctions with cones. eLife. 2014; 3:e01386. [PubMed]
- Nelson, R., Cat cones have rod input: a comparison of the response properties of cones and horizontal cell bodies in the retina of the cat. The Journal of Comparative Neurology. 1977; 172(1):109-135. [PubMed]
- Schneeweis, D.M. and J.L. Schnapf, Photovoltage of rods and cones in the macaque retina. Science (New York, N.Y.). 1995; 268(5213):1053-1056. [PubMed]
- Thoreson, W.B. and S.C. Mangel, Lateral interactions in the outer retina. Progress in Retinal and Eye Research. 2012; 31(5):407-441. [PubMed]
- Raviola, E. and N.B. Gilula, Intramembrane organization of specialized contacts in the outer plexiform layer of the retina. A freeze-fracture study in monkeys and rabbits. The Journal of Cell Biology. 1975; 65(1):192-222. [PubMed]
- Hombach, S., U. Janssen-Bienhold, G. Söhl, T. Schubert, H. Büssow, T. Ott, R. Weiler, and K. Willecke, Functional expression of connexin57 in horizontal cells of the mouse retina. The European Journal of Neuroscience. 2004; 19(10):2633-2640. [PubMed]
- Janssen-Bienhold, U., J. Trümpler, G. Hilgen, K. Schultz, L.P.D.S. Müller, S. Sonntag, K. Dedek, P. Dirks, K. Willecke, and R. Weiler, Connexin57 is expressed in dendro-dendritic and axo-axonal gap junctions of mouse horizontal cells and its distribution is modulated by light. The Journal of Comparative Neurology. 2009; 513(4):363-374. [PubMed]
- Dorgau, B., R. Herrling, K. Schultz, H. Greb, J. Segelken, S. Ströh, P. Bolte, R. Weiler, K. Dedek, and U. Janssen-Bienhold, Connexin50 couples axon terminals of mouse horizontal cells by homotypic gap junctions. The Journal of Comparative Neurology. 2015; 523(14):2062-2081. [PubMed]
- O’Brien, J.J., W. Li, F. Pan, J. Keung, J. O’Brien, and S.C. Massey, Coupling between A-type horizontal cells is mediated by connexin 50 gap junctions in the rabbit retina. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2006; 26(45):11624-11636. [PubMed]
- Pan, F., J. Keung, I.-B. Kim, M.B. Snuggs, S.L. Mills, J. O’Brien, and S.C. Massey, Connexin 57 is expressed by the axon terminal network of B-type horizontal cells in the rabbit retina. The Journal of Comparative Neurology. 2012; 520(10):2256-2274. [PubMed]
- Klaassen, L.J., Z. Sun, M.N. Steijaert, P. Bolte, I. Fahrenfort, T. Sjoerdsma, J. Klooster, Y. Claassen, C.R. Shields, H.M.M. Ten Eikelder, U. Janssen-Bienhold, G. Zoidl, D.G. McMahon, and M. Kamermans, Synaptic transmission from horizontal cells to cones is impaired by loss of connexin hemichannels. PLoS biology. 2011; 9(7):e1001107. [PubMed]
- Shields, C.R., J. Klooster, Y. Claassen, M. Ul-Hussain, G. Zoidl, R. Dermietzel, and M. Kamermans, Retinal horizontal cell-specific promoter activity and protein expression of zebrafish connexin 52.6 and connexin 55.5. The Journal of Comparative Neurology. 2007; 501(5):765-779. [PubMed]
- DeVries, S.H. and E.A. Schwartz, Modulation of an electrical synapse between solitary pairs of catfish horizontal cells by dopamine and second messengers. The Journal of Physiology. 1989; 414:351-375. [PubMed]
- Lasater, E.M. and J.E. Dowling, Dopamine decreases conductance of the electrical junctions between cultured retinal horizontal cells. Proceedings of the National Academy of Sciences of the United States of America. 1985; 82(9):3025-3029. [PubMed]
- McMahon, D.G., Modulation of electrical synaptic transmission in zebrafish retinal horizontal cells. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 1994; 14(3 Pt 2):1722-1734. [PubMed]
- McMahon, D.G. and M.P. Mattson, Horizontal cell electrical coupling in the giant danio: synaptic modulation by dopamine and synaptic maintenance by calcium. Brain Research. 1996; 718(1-2):89-96. [PubMed]
- Xin, D. and S.A. Bloomfield, Dark- and light-induced changes in coupling between horizontal cells in mammalian retina. The Journal of Comparative Neurology. 1999; 405(1):75-87. [PubMed]
- Ribelayga, C. and S.C. Mangel, Absence of circadian clock regulation of horizontal cell gap junctional coupling reveals two dopamine systems in the goldfish retina. The Journal of Comparative Neurology. 2003; 467(2):243-253. [PubMed]
- Ribelayga, C. and S.C. Mangel, Tracer coupling between fish rod horizontal cells: modulation by light and dopamine but not the retinal circadian clock. Visual Neuroscience. 2007; 24(3):333-344. [PubMed]
- Mangel, S.C. and J.E. Dowling, Responsiveness and receptive field size of carp horizontal cells are reduced by prolonged darkness and dopamine. Science (New York, N.Y.). 1985; 229(4718):1107-1109. [PubMed]
- Shelley, J., K. Dedek, T. Schubert, A. Feigenspan, K. Schultz, S. Hombach, K. Willecke, and R. Weiler, Horizontal cell receptive fields are reduced in connexin57-deficient mice. The European Journal of Neuroscience. 2006; 23(12):3176-3186. [PubMed]
- Mangel, S.C., Analysis of the horizontal cell contribution to the receptive field surround of ganglion cells in the rabbit retina. The Journal of physiology. 1991; 442(1):211-234. [PubMed]
- Werblin, F.S. and J.E. Dowling, Organization of the retina of the mudpuppy, Necturus maculosus. II. Intracellular recording.Journal of Neurophysiology. 1969; 32(3):339-355. [PubMed]
- Drinnenberg, A., F. Franke, R.K. Morikawa, J. Jüttner, D. Hillier, P. Hantz, A. Hierlemann, R. Azeredo da Silveira, and B. Roska, How Diverse Retinal Functions Arise from Feedback at the First Visual Synapse. Neuron. 2018; 99(1):117-134.e11. [PubMed]
- Farrow, K., M. Teixeira, T. Szikra, T.J. Viney, K. Balint, K. Yonehara, and B. Roska, Ambient illumination toggles a neuronal circuit switch in the retina and visual perception at cone threshold. Neuron. 2013; 78(2):325-338. [PubMed]
- Hoggarth, A., A.J. McLaughlin, K. Ronellenfitch, S. Trenholm, R. Vasandani, S. Sethuramanujam, D. Schwab, K.L. Briggman, and G.B. Awatramani, Specific wiring of distinct amacrine cells in the directionally selective retinal circuit permits independent coding of direction and size. Neuron. 2015; 86(1):276-291. [PubMed]
- Dedek, K., C. Pandarinath, N.M. Alam, K. Wellershaus, T. Schubert, K. Willecke, G.T. Prusky, R. Weiler, and S. Nirenberg, Ganglion cell adaptability: does the coupling of horizontal cells play a role? PloS One. 2008; 3(3):e1714. [PubMed]
- Pandarinath, C., I. Bomash, J.D. Victor, G.T. Prusky, W.W. Tschetter, and S. Nirenberg, A novel mechanism for switching a neural system from one state to another. Frontiers in Computational Neuroscience. 2010; 4:2. [PubMed]
- Wässle, H., C. Puller, F. Müller, and S. Haverkamp, Cone contacts, mosaics, and territories of bipolar cells in the mouse retina.The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2009; 29(1):106-117. [PubMed]
- Kolb, H. and E.V. Famiglietti, Rod and cone pathways in the inner plexiform layer of cat retina. Science (New York, N.Y.). 1974; 186(4158):47-49. [PubMed]
- Lee, S.C.S., A. Meyer, T. Schubert, L. Hüser, K. Dedek, and S. Haverkamp, Morphology and connectivity of the small bistratified A8 amacrine cell in the mouse retina. The Journal of Comparative Neurology. 2015; 523(10):1529-1547. [PubMed]
- Arai, I., M. Tanaka, and M. Tachibana, Active roles of electrically coupled bipolar cell network in the adult retina. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2010; 30(27):9260-9270. [PubMed]
- Jacoby, R.A. and D.W. Marshak, Synaptic connections of DB3 diffuse bipolar cell axons in macaque retina. The Journal of Comparative Neurology. 2000; 416(1):19-29. [PubMed]
- Marc, R.E., W.L. Liu, and J.F. Muller, Gap junctions in the inner plexiform layer of the goldfish retina. Vision Research. 1988; 28(1):9-24. [PubMed]
- Strettoi, E., E. Raviola, and R.F. Dacheux, Synaptic connections of the narrow-field, bistratified rod amacrine cell (AII) in the rabbit retina. The Journal of Comparative Neurology. 1992; 325(2):152-168. [PubMed]
- Deans, M.R., B. Volgyi, D.A. Goodenough, S.A. Bloomfield, and D.L. Paul, Connexin36 is essential for transmission of rod-mediated visual signals in the mammalian retina. Neuron. 2002; 36(4):703-712. [PubMed]
- Han, Y. and S.C. Massey, Electrical synapses in retinal ON cone bipolar cells: subtype-specific expression of connexins.Proceedings of the National Academy of Sciences of the United States of America. 2005; 102(37):13313-13318. [PubMed]
- Hilgen, G., J. von Maltzahn, K. Willecke, R. Weiler, and K. Dedek, Subcellular distribution of connexin45 in OFF bipolar cells of the mouse retina. The Journal of Comparative Neurology. 2011; 519(3):433-450. [PubMed]
- Maxeiner, S., K. Dedek, U. Janssen-Bienhold, J. Ammermüller, H. Brune, T. Kirsch, M. Pieper, J. Degen, O. Krüger, K. Willecke, and R. Weiler, Deletion of connexin45 in mouse retinal neurons disrupts the rod/cone signaling pathway between AII amacrine and ON cone bipolar cells and leads to impaired visual transmission. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2005; 25(3):566-576. [PubMed]
- Dacey, D., O.S. Packer, L. Diller, D. Brainard, B. Peterson, and B. Lee, Center surround receptive field structure of cone bipolar cells in primate retina. Vision Research. 2000; 40(14):1801-1811. [PubMed]
- Kujiraoka, T. and T. Saito, Electrical coupling between bipolar cells in carp retina. Proceedings of the National Academy of Sciences of the United States of America. 1986; 83(11):4063-4066. [PubMed]
- Mills, S.L., Unusual coupling patterns of a cone bipolar cell in the rabbit retina. Visual Neuroscience. 1999; 16(6):1029-1035. [PubMed]
- Umino, O., M. Maehara, S. Hidaka, S. Kita, and Y. Hashimoto, The network properties of bipolar-bipolar cell coupling in the retina of teleost fishes. Visual Neuroscience. 1994; 11(3):533-548. [PubMed]
- Veruki, M.L. and E. Hartveit, AII (Rod) amacrine cells form a network of electrically coupled interneurons in the mammalian retina. Neuron. 2002; 33(6):935-946. [PubMed]
- Marc, R.E., J.R. Anderson, B.W. Jones, C.L. Sigulinsky, and J.S. Lauritzen, The AII amacrine cell connectome: a dense network hub. Frontiers in Neural Circuits. 2014; 8:104. [PubMed]
- Graydon, C.W., E.E. Lieberman, N. Rho, K.L. Briggman, J.H. Singer, and J.S. Diamond, Synaptic Transfer between Rod and Cone Pathways Mediated by AII Amacrine Cells in the Mouse Retina. Current biology: CB. 2018; 28(17):2739-2751.e3. [PubMed]
- Saito, T. and T. Kujiraoka, Characteristics of bipolar-bipolar coupling in the carp retina. The Journal of General Physiology. 1988; 91(2):275-287. [PubMed]
- Berntson, A. and W.R. Taylor, Response characteristics and receptive field widths of on-bipolar cells in the mouse retina. The Journal of Physiology. 2000; 524 Pt 3:879-889. [PubMed]
- Werblin, F.S., Response of retinal cells to moving spots: intracellular recording in Necturus maculosus. Journal of Neurophysiology. 1970; 33(3):342-350. [PubMed]
- Kuo, S.P., G.W. Schwartz, and F. Rieke, Nonlinear Spatiotemporal Integration by Electrical and Chemical Synapses in the Retina. Neuron. 2016; 90(2):320-332. [PubMed]
- Masland, R.H., The tasks of amacrine cells. Visual Neuroscience. 2012; 29(1):3-9. [PubMed]
- Masland, R.H. and J.W. Mills, Autoradiographic identification of acetylcholine in the rabbit retina. The Journal of Cell Biology. 1979; 83(1):159-178. [PubMed]
- Lee, S., K. Kim, and Z.J. Zhou, Role of ACh-GABA cotransmission in detecting image motion and motion direction. Neuron. 2010; 68(6):1159-1172. [PubMed]
- Sethuramanujam, S., A.J. McLaughlin, G. deRosenroll, A. Hoggarth, D.J. Schwab, and G.B. Awatramani, A Central Role for Mixed Acetylcholine/GABA Transmission in Direction Coding in the Retina. Neuron. 2016; 90(6):1243-1256. [PubMed]
- Zhang, D.-Q., T.-R. Zhou, and D.G. McMahon, Functional heterogeneity of retinal dopaminergic neurons underlying their multiple roles in vision. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2007; 27(3):692-699. [PubMed]
- Baden, T. and T. Euler, Retinal Physiology: Non-Bipolar-Cell Excitatory Drive in the Inner Retina. Current biology: CB. 2016; 26(15):R706-R708. [PubMed]
- Xin, D. and S.A. Bloomfield, Tracer coupling pattern of amacrine and ganglion cells in the rabbit retina. The Journal of Comparative Neurology. 1997; 383(4):512-528. [PubMed]
- Li, W., J. Zhang, and S.C. Massey, Coupling pattern of S1 and S2 amacrine cells in the rabbit retina. Visual Neuroscience. 2002; 19(2):119-131. [PubMed]
- Abdel-Majid, R.M., M.L. Archibald, F. Tremblay, and W.H. Baldridge, Tracer coupling of neurons in the rat retina inner nuclear layer labeled by Fluorogold. Brain Research. 2005; 1063(2):114-120. [PubMed]
- Bloomfield, S.A. and D. Xin, A comparison of receptive-field and tracer-coupling size of amacrine and ganglion cells in the rabbit retina. Visual Neuroscience. 1997; 14(6):1153-1165. [PubMed]
- Völgyi, B., S. Chheda, and S.A. Bloomfield, Tracer coupling patterns of the ganglion cell subtypes in the mouse retina. The Journal of Comparative Neurology. 2009; 512(5):664-687. [PubMed]
- Pan, F., D.L. Paul, S.A. Bloomfield, and B. Völgyi, Connexin36 is required for gap junctional coupling of most ganglion cell subtypes in the mouse retina. The Journal of Comparative Neurology. 2010; 518(6):911-927. [PubMed]
- Pang, J.-J., D.L. Paul, and S.M. Wu, Survey on amacrine cells coupling to retrograde-identified ganglion cells in the mouse retina. Investigative Ophthalmology & Visual Science. 2013; 54(8):5151-5162. [PubMed]
- Jacoby, J., A. Nath, Z.F. Jessen, and G.W. Schwartz, A Self-Regulating Gap Junction Network of Amacrine Cells Controls Nitric Oxide Release in the Retina. Neuron. 2018; 100(5):1149-1162.e5. [PubMed]
- Grimes, W.N., J. Zhang, C.W. Graydon, B. Kachar, and J.S. Diamond, Retinal parallel processors: more than 100 independent microcircuits operate within a single interneuron. Neuron. 2010; 65(6):873-885. [PubMed]
- Yadav, S.C., S. Tetenborg, and K. Dedek, Gap Junctions in A8 Amacrine Cells Are Made of Connexin36 but Are Differently Regulated Than Gap Junctions in AII Amacrine Cells. Frontiers in Molecular Neuroscience. 2019; 12:99. [PubMed]
- Dacey, D., Origins of perception: retinal ganglion cell diversity and the creation of parallel visual pathways, in The Cognitive Neurosciences. 2004, MIT Press. [PubMed]
- Sanes, J.R. and R.H. Masland, The types of retinal ganglion cells: current status and implications for neuronal classification.Annual Review of Neuroscience. 2015; 38:221-246. [PubMed]
- Baden, T., P. Berens, K. Franke, M. Román Rosón, M. Bethge, and T. Euler, The functional diversity of retinal ganglion cells in the mouse. Nature. 2016; 529(7586):345-350. [PubMed]
- Vaney, D.I., Many diverse types of retinal neurons show tracer coupling when injected with biocytin or Neurobiotin. Neuroscience Letters. 1991; 125(2):187-190. [PubMed]
- Vaney, D.I., Territorial organization of direction-selective ganglion cells in rabbit retina. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 1994; 14(11 Pt 1):6301-6316. [PubMed]
- Hidaka, S., Y. Akahori, and Y. Kurosawa, Dendrodendritic electrical synapses between mammalian retinal ganglion cells. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2004; 24(46):10553-10567. [PubMed]
- Müller, L.P.d.S., M.T.H. Do, K.-W. Yau, S. He, and W.H. Baldridge, Tracer coupling of intrinsically photosensitive retinal ganglion cells to amacrine cells in the mouse retina. The Journal of Comparative Neurology. 2010; 518(23):4813-4824. [PubMed]
- Reifler, A.N., A.P. Chervenak, M.E. Dolikian, B.A. Benenati, B.Y. Li, R.D. Wachter, A.M. Lynch, Z.D. Demertzis, B.S. Meyers, F.S. Abufarha, E.R. Jaeckel, M.P. Flannery, and K.Y. Wong, All Spiking, Sustained ON Displaced Amacrine Cells Receive Gap-Junction Input from Melanopsin Ganglion Cells. Current biology: CB. 2015; 25(21):2878. [PubMed]
- Schubert, T., S. Maxeiner, O. Krüger, K. Willecke, and R. Weiler, Connexin45 mediates gap junctional coupling of bistratified ganglion cells in the mouse retina. The Journal of Comparative Neurology. 2005; 490(1):29-39. [PubMed]
- Kántor, O., G. Szarka, Z. Benkő, Z. Somogyvári, E. Pálfi, G. Baksa, G. Rácz, R. Nitschke, G. Debertin, and B. Völgyi, Strategic Positioning of Connexin36 Gap Junctions Across Human Retinal Ganglion Cell Dendritic Arbors. Frontiers in Cellular Neuroscience. 2018; 12:409. [PubMed]
- Yao, X., J. Cafaro, A.J. McLaughlin, F.R. Postma, D.L. Paul, G. Awatramani, and G.D. Field, Gap Junctions Contribute to Differential Light Adaptation across Direction-Selective Retinal Ganglion Cells. Neuron. 2018; 100(1):216-228.e6. [PubMed]
- Hu, E.H. and S.A. Bloomfield, Gap junctional coupling underlies the short-latency spike synchrony of retinal alpha ganglion cells.The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2003; 23(17):6768-6777. [PubMed]
- Trenholm, S., A.J. McLaughlin, D.J. Schwab, and G.B. Awatramani, Dynamic tuning of electrical and chemical synaptic transmission in a network of motion coding retinal neurons. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2013; 33(37):14927-14938. [PubMed]
- Cooler, S. and G.W. Schwartz, An on-primary retinal ganglion cell receives off input via a heterotypic RGC gap junction, in Annual Meeting of the Society for Neuroscience. 2019, Society for Neuroscience: Chicago. p. 225.08. [PubMed]
- Hu, E.H., F. Pan, B. Völgyi, and S.A. Bloomfield, Light increases the gap junctional coupling of retinal ganglion cells. The Journal of Physiology. 2010; 588(Pt 21):4145-4163. [PubMed]
- DeVries, S.H., Correlated firing in rabbit retinal ganglion cells. Journal of Neurophysiology. 1999; 81(2):908-920. [PubMed]
- Barlow, H.B., R. Fitzhugh, and S.W. Kuffler, Change of organization in the receptive fields of the cat’s retina during dark adaptation. The Journal of Physiology. 1957; 137(3):338-354. [PubMed]
- Peichl, L. and H. Wässle, The structural correlate of the receptive field centre of alpha ganglion cells in the cat retina. The Journal of Physiology. 1983; 341:309-324. [PubMed]
- Muller, J.F. and R.F. Dacheux, Alpha ganglion cells of the rabbit retina lose antagonistic surround responses under dark adaptation. Visual Neuroscience. 1997; 14(2):395-401. [PubMed]
- Trong, P.K. and F. Rieke, Origin of correlated activity between parasol retinal ganglion cells. Nature Neuroscience. 2008; 11(11):1343-1351. [PubMed]
- Nelson, R., AII amacrine cells quicken time course of rod signals in the cat retina. Journal of Neurophysiology. 1982; 47(5):928-947. [PubMed]
- Völgyi, B., M.R. Deans, D.L. Paul, and S.A. Bloomfield, Convergence and segregation of the multiple rod pathways in mammalian retina. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2004; 24(49):11182-11192. [PubMed]
- Ivanova, E., U. Müller, and H. Wässle, Characterization of the glycinergic input to bipolar cells of the mouse retina. The European Journal of Neuroscience. 2006; 23(2):350-364. [PubMed]
- Münch, T.A., R.A. da Silveira, S. Siegert, T.J. Viney, G.B. Awatramani, and B. Roska, Approach sensitivity in the retina processed by a multifunctional neural circuit. Nature Neuroscience. 2009; 12(10):1308-1316. [PubMed]
- Trexler, E.B., W. Li, and S.C. Massey, Simultaneous contribution of two rod pathways to AII amacrine and cone bipolar cell light responses. Journal of Neurophysiology. 2005; 93(3):1476-1485. [PubMed]
- Abd-El-Barr, M.M., M.E. Pennesi, S.M. Saszik, A.J. Barrow, J. Lem, D.E. Bramblett, D.L. Paul, L.J. Frishman, and S.M. Wu, Genetic dissection of rod and cone pathways in the dark-adapted mouse retina. Journal of Neurophysiology. 2009; 102(3):1945-1955. [PubMed]
- Soucy, E., Y. Wang, S. Nirenberg, J. Nathans, and M. Meister, A novel signaling pathway from rod photoreceptors to ganglion cells in mammalian retina. Neuron. 1998; 21(3):481-493. [PubMed]
- Li, W., J.W. Keung, and S.C. Massey, Direct synaptic connections between rods and OFF cone bipolar cells in the rabbit retina.The Journal of Comparative Neurology. 2004; 474(1):1-12. [PubMed]
- Murphy, G.J. and F. Rieke, Electrical synaptic input to ganglion cells underlies differences in the output and absolute sensitivity of parallel retinal circuits. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2011; 31(34):12218-12228. [PubMed]
- Fain, G.L., Quantum sensitivity of rods in the toad retina. Science (New York, N.Y.). 1975; 187(4179):838-841. [PubMed]
- Lamb, T.D. and E.J. Simon, The relation between intercellular coupling and electrical noise in turtle photoreceptors. The Journal of Physiology. 1976; 263(2):257-286. [PubMed]
- Tessier-Lavigne, M. and D. Attwell, The effect of photoreceptor coupling and synapse nonlinearity on signal:noise ratio in early visual processing. Proceedings of the Royal Society of London. Series B, Biological Sciences. 1988; 234(1275):171-197. [PubMed]
- Attwell, D., S. Borges, S.M. Wu, and M. Wilson, Signal clipping by the rod output synapse. Nature. 1987; 328(6130):522-524. [PubMed]
- Connors, B.W. and M.A. Long, Electrical synapses in the mammalian brain. Annual Review of Neuroscience. 2004; 27:393-418. [PubMed]
- Pereda, A.E., S. Curti, G. Hoge, R. Cachope, C.E. Flores, and J.E. Rash, Gap junction-mediated electrical transmission: regulatory mechanisms and plasticity. Biochimica Et Biophysica Acta. 2013; 1828(1):134-146. [PubMed]
- Arnett, D. and T.E. Spraker, Cross-correlation analysis of the maintained discharge of rabbit retinal ganglion cells. The Journal of Physiology. 1981; 317:29-47. [PubMed]
- Mastronarde, D.N., Correlated firing of cat retinal ganglion cells. I. Spontaneously active inputs to X- and Y-cells. Journal of Neurophysiology. 1983; 49(2):303-324. [PubMed]
- Mastronarde, D.N., Correlated firing of cat retinal ganglion cells. II. Responses of X- and Y-cells to single quantal events. Journal of Neurophysiology. 1983; 49(2):325-349. [PubMed]
- Mastronarde, D.N., Interactions between ganglion cells in cat retina. Journal of Neurophysiology. 1983; 49(2):350-365. [PubMed]
- Brivanlou, I.H., D.K. Warland, and M. Meister, Mechanisms of concerted firing among retinal ganglion cells. Neuron. 1998; 20(3):527-539. [PubMed]
- Greschner, M., J. Shlens, C. Bakolitsa, G.D. Field, J.L. Gauthier, L.H. Jepson, A. Sher, A.M. Litke, and E.J. Chichilnisky, Correlated firing among major ganglion cell types in primate retina. The Journal of Physiology. 2011; 589(Pt 1):75-86. [PubMed]
- Völgyi, B., F. Pan, D.L. Paul, J.T. Wang, A.D. Huberman, and S.A. Bloomfield, Gap junctions are essential for generating the correlated spike activity of neighboring retinal ganglion cells. PloS One. 2013; 8(7):e69426. [PubMed]
- Trenholm, S., A.J. McLaughlin, D.J. Schwab, M.H. Turner, R.G. Smith, F. Rieke, and G.B. Awatramani, Nonlinear dendritic integration of electrical and chemical synaptic inputs drives fine-scale correlations. Nature Neuroscience. 2014; 17(12):1759-1766. [PubMed]
- Mastronarde, D.N., Correlated firing of retinal ganglion cells. Trends in Neurosciences. 1989; 12(2):75-80. [PubMed]
- Schnitzer, M.J. and M. Meister, Multineuronal firing patterns in the signal from eye to brain. Neuron. 2003; 37(3):499-511. [PubMed]
- Bennett, M.V.L. and R.S. Zukin, Electrical Coupling and Neuronal Synchronization in the Mammalian Brain. Neuron. 2004; 41(4):495-511. [PubMed]
- Oesch, N., T. Euler, and W.R. Taylor, Direction-selective dendritic action potentials in rabbit retina. Neuron. 2005; 47(5):739-750. [PubMed]
- Sivyer, B. and S.R. Williams, Direction selectivity is computed by active dendritic integration in retinal ganglion cells. Nature Neuroscience. 2013; 16(12):1848-1856. [PubMed]
- Oyster, C.W. and H.B. Barlow, Direction-selective units in rabbit retina: distribution of preferred directions. Science (New York, N.Y.). 1967; 155(3764):841-842. [PubMed]
- Xu, Z., Q. Zeng, X. Shi, and S. He, Changing coupling pattern of The ON-OFF direction-selective ganglion cells in early postnatal mouse retina. Neuroscience. 2013; 250:798-808. [PubMed]
- Usrey, W.M., J.B. Reppas, and R.C. Reid, Paired-spike interactions and synaptic efficacy of retinal inputs to the thalamus.Nature. 1998; 395(6700):384-387. [PubMed]
- Carandini, M., J.C. Horton, and L.C. Sincich, Thalamic filtering of retinal spike trains by postsynaptic summation. Journal of Vision. 2007; 7(14):20.1-11. [PubMed]
- Cleland, B.G., M.W. Dubin, and W.R. Levick, Simultaneous recording of input and output of lateral geniculate neurones. Nature: New Biology. 1971; 231(23):191-192. [PubMed]
- Chen, C. and W.G. Regehr, Developmental remodeling of the retinogeniculate synapse. Neuron. 2000; 28(3):955-966. [PubMed]
- Morgan, J.L., D.R. Berger, A.W. Wetzel, and J.W. Lichtman, The Fuzzy Logic of Network Connectivity in Mouse Visual Thalamus. Cell. 2016; 165(1):192-206. [PubMed]
- Rompani, S.B., F.E. Müllner, A. Wanner, C. Zhang, C.N. Roth, K. Yonehara, and B. Roska, Different Modes of Visual Integration in the Lateral Geniculate Nucleus Revealed by Single-Cell-Initiated Transsynaptic Tracing. Neuron. 2017; 93(4):767-776.e6. [PubMed]
- Meister, M., L. Lagnado, and D.A. Baylor, Concerted signaling by retinal ganglion cells. Science (New York, N.Y.). 1995; 270(5239):1207-1210. [PubMed]
- Xiao, L., M. Zhang, D. Xing, P.-J. Liang, and S. Wu, Shifted encoding strategy in retinal luminance adaptation: from firing rate to neural correlation. Journal of Neurophysiology. 2013; 110(8):1793-1803. [PubMed]
- Ackert, J.M., S.H. Wu, J.C. Lee, J. Abrams, E.H. Hu, I. Perlman, and S.A. Bloomfield, Light-induced changes in spike synchronization between coupled ON direction selective ganglion cells in the mammalian retina. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2006; 26(16):4206-4215. [PubMed]
- Neuenschwander, S. and W. Singer, Long-range synchronization of oscillatory light responses in the cat retina and lateral geniculate nucleus. Nature. 1996; 379(6567):728-732. [PubMed]
- Roy, K., S. Kumar, and S.A. Bloomfield, Gap junctional coupling between retinal amacrine and ganglion cells underlies coherent activity integral to global object perception. Proceedings of the National Academy of Sciences of the United States of America. 2017; 114(48):E10484-E10493. [PubMed]
- Manookin, M.B., S.S. Patterson, and C.M. Linehan, Neural Mechanisms Mediating Motion Sensitivity in Parasol Ganglion Cells of the Primate Retina. Neuron. 2018; 97(6):1327-1340.e4. [PubMed]
- Murphy-Baum, B.L. and G.B. Awatramani, An Old Neuron Learns New Tricks: Redefining Motion Processing in the Primate Retina. Neuron. 2018; 97(6):1205-1207. [PubMed]
- Berry, M.J., I.H. Brivanlou, T.A. Jordan, and M. Meister, Anticipation of moving stimuli by the retina. Nature. 1999; 398(6725):334-338. [PubMed]
- Johnston, J. and L. Lagnado, General features of the retinal connectome determine the computation of motion anticipation.eLife. 2015; 4. [PubMed]
- Trenholm, S., D.J. Schwab, V. Balasubramanian, and G.B. Awatramani, Lag normalization in an electrically coupled neural network. Nature Neuroscience. 2013; 16(2):154-156. [PubMed]
- Ball, W. and E. Tronick, Infant responses to impending collision: optical and real. Science (New York, N.Y.). 1971; 171(3973):818-820. [PubMed]
- Ishikane, H., M. Gangi, S. Honda, and M. Tachibana, Synchronized retinal oscillations encode essential information for escape behavior in frogs. Nature Neuroscience. 2005; 8(8):1087-1095. [PubMed]
- King, S.M., C. Dykeman, P. Redgrave, and P. Dean, Use of a distracting task to obtain defensive head movements to looming visual stimuli by human adults in a laboratory setting. Perception. 1992; 21(2):245-259. [PubMed]
- Schiff, W., J.A. Caviness, and J.J. Gibson, Persistent fear responses in rhesus monkeys to the optical stimulus of “looming”.Science (New York, N.Y.). 1962; 136(3520):982-983. [PubMed]
- Temizer, I., J.C. Donovan, H. Baier, and J.L. Semmelhack, A Visual Pathway for Looming-Evoked Escape in Larval Zebrafish.Current biology: CB. 2015; 25(14):1823-1834. [PubMed]
- Yilmaz, M. and M. Meister, Rapid innate defensive responses of mice to looming visual stimuli. Current biology: CB. 2013; 23(20):2011-2015. [PubMed]
- Biswas, S., C. Haselier, A. Mataruga, G. Thumann, P. Walter, and F. Müller, Pharmacological analysis of intrinsic neuronal oscillations in rd10 retina. PloS One. 2014; 9(6):e99075. [PubMed]
- Borowska, J., S. Trenholm, and G.B. Awatramani, An intrinsic neural oscillator in the degenerating mouse retina. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2011; 31(13):5000-5012. [PubMed]
- Margolis, D.J., G. Newkirk, T. Euler, and P.B. Detwiler, Functional stability of retinal ganglion cells after degeneration-induced changes in synaptic input. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2008; 28(25):6526-6536. [PubMed]
- Menzler, J. and G. Zeck, Network oscillations in rod-degenerated mouse retinas. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2011; 31(6):2280-2291. [PubMed]
- Stasheff, S.F., Emergence of sustained spontaneous hyperactivity and temporary preservation of OFF responses in ganglion cells of the retinal degeneration (rd1) mouse. Journal of Neurophysiology. 2008; 99(3):1408-1421. [PubMed]
- Stasheff, S.F., M. Shankar, and M.P. Andrews, Developmental time course distinguishes changes in spontaneous and light-evoked retinal ganglion cell activity in rd1 and rd10 mice. Journal of Neurophysiology. 2011; 105(6):3002-3009. [PubMed]
- Yee, C.W., A.H. Toychiev, and B.T. Sagdullaev, Network deficiency exacerbates impairment in a mouse model of retinal degeneration. Frontiers in Systems Neuroscience. 2012; 6:8. [PubMed]
- Lepore, F.E., Spontaneous visual phenomena with visual loss: 104 patients with lesions of retinal and neural afferent pathways.Neurology. 1990; 40(3 Pt 1):444-447. [PubMed]
- Murtha, T. and S.F. Stasheff, Visual dysfunction in retinal and optic nerve disease. Neurologic Clinics. 2003; 21(2):445-481. [PubMed]
- Weiland, J.D. and M.S. Humayun, Retinal prosthesis. IEEE transactions on bio-medical engineering. 2014; 61(5):1412-1424. [PubMed]
- Busskamp, V., S. Picaud, J.A. Sahel, and B. Roska, Optogenetic therapy for retinitis pigmentosa. Gene Therapy. 2012; 19(2):169-175. [PubMed]
- Yee, C.W., A.H. Toychiev, E. Ivanova, and B.T. Sagdullaev, Aberrant synaptic input to retinal ganglion cells varies with morphology in a mouse model of retinal degeneration. The Journal of Comparative Neurology. 2014; 522(18):4085-4099. [PubMed]
- Sekirnjak, C., L.H. Jepson, P. Hottowy, A. Sher, W. Dabrowski, A.M. Litke, and E.J. Chichilnisky, Changes in physiological properties of rat ganglion cells during retinal degeneration. Journal of Neurophysiology. 2011; 105(5):2560-2571. [PubMed]
- Trenholm, S., J. Borowska, J. Zhang, A. Hoggarth, K. Johnson, S. Barnes, T.J. Lewis, and G.B. Awatramani, Intrinsic oscillatory activity arising within the electrically coupled AII amacrine-ON cone bipolar cell network is driven by voltage-gated Na+ channels. The Journal of Physiology. 2012; 590(10):2501-2517. [PubMed]
- Ivanova, E., C.W. Yee, R. Baldoni, and B.T. Sagdullaev, Aberrant activity in retinal degeneration impairs central visual processing and relies on Cx36-containing gap junctions. Experimental Eye Research. 2016; 150:81-89. [PubMed]
- Telias, M., B. Denlinger, Z. Helft, C. Thornton, B. Beckwith-Cohen, and R.H. Kramer, Retinoic Acid Induces Hyperactivity, and Blocking Its Receptor Unmasks Light Responses and Augments Vision in Retinal Degeneration. Neuron. 2019; 102(3):574-586. e5. [PubMed]
- Choi, H., L. Zhang, M.S. Cembrowski, C.F. Sabottke, A.L. Markowitz, D.A. Butts, W.L. Kath, J.H. Singer, and H. Riecke, Intrinsic bursting of AII amacrine cells underlies oscillations in the rd1 mouse retina. Journal of Neurophysiology. 2014; 112(6):1491-1504. [PubMed]
- Ivanova, E., C.W. Yee, and B.T. Sagdullaev, Increased phosphorylation of Cx36 gap junctions in the AII amacrine cells of RD retina. Frontiers in Cellular Neuroscience. 2015; 9:390. [PubMed]
- Cook, J.E. and D.L. Becker, Gap-junction proteins in retinal development: new roles for the “nexus”. Physiology (Bethesda, Md.). 2009; 24:219-230. [PubMed]
- Akopian, A., T. Atlasz, F. Pan, S. Wong, Y. Zhang, B. Völgyi, D.L. Paul, and S.A. Bloomfield, Gap junction-mediated death of retinal neurons is connexin and insult specific: a potential target for neuroprotection. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2014; 34(32):10582-10591. [PubMed]
- Kranz, K., F. Paquet-Durand, R. Weiler, U. Janssen-Bienhold, and K. Dedek, Testing for a gap junction-mediated bystander effect in retinitis pigmentosa: secondary cone death is not altered by deletion of connexin36 from cones. PloS One. 2013; 8(2):e57163. [PubMed]
- Paschon, V., G.S.V. Higa, R.R. Resende, L.R.G. Britto, and A.H. Kihara, Blocking of connexin-mediated communication promotes neuroprotection during acute degeneration induced by mechanical trauma. PloS One. 2012; 7(9):e45449. [PubMed]
- Striedinger, K., E. Petrasch-Parwez, G. Zoidl, M. Napirei, C. Meier, U.T. Eysel, and R. Dermietzel, Loss of connexin36 increases retinal cell vulnerability to secondary cell loss. The European Journal of Neuroscience. 2005; 22(3):605-616. [PubMed]
- Arroyo, D.A., L.A. Kirkby, and M.B. Feller, Retinal Waves Modulate an Intraretinal Circuit of Intrinsically Photosensitive Retinal Ganglion Cells. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2016; 36(26):6892-6905. [PubMed]
- Trenholm, S. and G.B. Awatramani, Chapter 9 – Dynamic Properties of Electrically Coupled Retinal Networks, in Network Functions and Plasticity, J. Jing, Editor. 2017, Academic Press. p. 183-208. [PubMed]
- Baldridge, W.H., Triphasic adaptation of teleost horizontal cells. Progress in Brain Research. 2001; 131:437-449. [PubMed]
- Veruki, M.L. and E. Hartveit, Electrical synapses mediate signal transmission in the rod pathway of the mammalian retina. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2002; 22(24):10558-10566. [PubMed]
- Bloomfield, S.A., D. Xin, and T. Osborne, Light-induced modulation of coupling between AII amacrine cells in the rabbit retina.Visual Neuroscience. 1997; 14(3):565-576. [PubMed]
- Schubert, T., J. Degen, K. Willecke, S.G. Hormuzdi, H. Monyer, and R. Weiler, Connexin36 mediates gap junctional coupling of alpha-ganglion cells in mouse retina. The Journal of Comparative Neurology. 2005; 485(3):191-201. [PubMed]
- Lanore, F. and R.A. Silver, Reading dendritic activity with gap junctions. Nature Neuroscience. 2014; 17(12):1625-1627. [PubMed]
- Trenholm, S. and G.B. Awatramani, Origins of spontaneous activity in the degenerating retina. Frontiers in Cellular Neuroscience. 2015; 9:277. [PubMed]