Yves Sauve and Frederic Gallard
1. Introduction
“…once development was ended, the founts of growth and regeneration of the axons and dendrites dried up irrevocably. In adult centres the nerve paths are something fixed, ended, immutable. Everything may die, nothing may be regenerated. It is for the science of the future to change, if possible, this harsh decree. Inspired with high ideals, it must work to impede or moderate gradual decay of neurons, to overcome the almost invincible rigidity of their connections, and to re-establish normal nerve paths, when disease has severed centres that were intimately associated.” (Santiago Ramon y Cajal, 1913-14).
Fig.1 Santiago Ramon y Cajal at work (From Cajal Institute; http://www.cajal.csic.es/)
Over 90 years after Ramon y Cajal’s farsighted work, we are still unable to solve the great mystery of regeneration in the adult mammalian central nervous system (CNS). Retinal degeneration leading to loss of photoreceptors and retinal ganglion cells (RGCs) is still largely untreatable, although recent experimental work is beginning to provided possible solutions to these devastating conditions. Other untreatable pathologies leading to loss of sight involve lesions to the CNS visual centers or projection pathways. There are two fundamental experimental approaches presently used to tackle both these problems. One involves reconstructing lost circuitry by replacement with (usually fetal) neuronal tissue and the other is attempting to slow the rate of the degeneration.
2. Reconstruction of Primary Visual Pathways
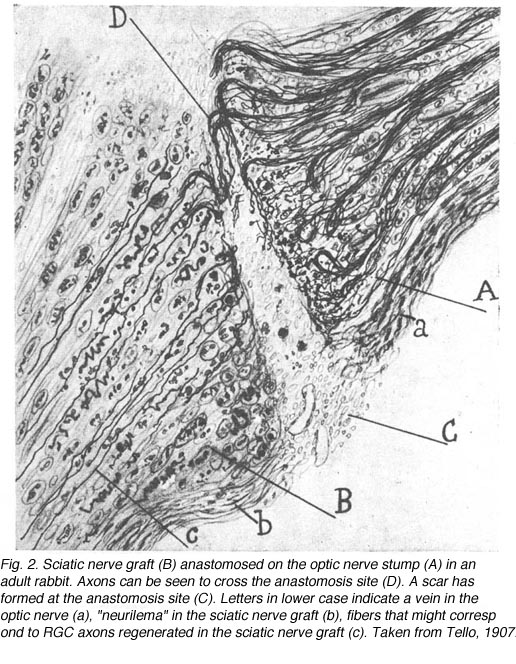
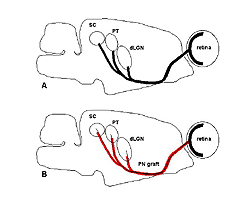
Without any experimental intervention, nerve lesions in the adult CNS of mammals produce only a limited and brief period of abortive sprouting and then to the death of axotomized neurons. In sharp contrast, the peripheral nervous system (PNS) and the immature CNS of mammals, as well as the mature CNS of cold-blooded vertebrates all display varying levels of successful spontaneous regeneration following injury. The development of experimental repair strategies has relied on lessons learned from these systems. Combined with advances in anatomical tracing and immunohistochemical methods, these strategies have revealed the surprising capacity for regeneration and synapse formation in the adult mammalian CNS. For instance, Dr Albert Aguayo and colleagues have used an experimental approach (So and Aguayo, 1985; Vidal-Sanz et al., 1987; Aguayo et al., 1991) based on the concept that regeneration in the PNS is dependent on the permissive environment provided by Schwann cells present in the nerve tube. They suggested that the absence of this permissive environment in the mature CNS was the reason for failure of regeneration. Thus, they postulate that damaged neurons from the mature CNS might be able to regenerate axons if placed in close proximity to Schwann cells. In fact, this hypothesis was suggested by Ramon y Cajal over a century ago and was later tested by Tello in 1907 (Fig. 2) in a series of experiments on rabbits. There was a suggestion that retinal axons could regenerate after axotomy if the cut optic nerve end was anastomosed to a sciatic nerve graft (Tello, 1907). The lack of anatomical tracers at that time did not allow us to see whether such regenerating axons were indeed of CNS origin, namely from retinal ganglion cells (RGCs). The indisputable proof that some CNS neurons do have this regenerative potential had to wait until specific axonal tracing techniques became available in the late 1970s (Richardson et al., 1980; David and Aguayo, 1981). Further work applied to primary visual pathways showed that if directed into the brain (Fig 3), regenerating RGC axons were able to re-establish connections in visual centers of the brainstem (Vidal-Sanz et al., 1987). These connections appeared capable of transmitting visual information (Keirstead et al., 1989; Sauve et al., 1995).
The concept that neural regeneration depends on a permissive environment does not alone explain why most mature mammalian CNS neurons do not regenerate an axon. For instance, in the absence of axonal myelin-associated growth inhibitors such as Nogo, axonal sprouting itself is not improved (Kim et al., 2003). The role of axonal growth inhibitors in axonal regeneration remains a controversial issue (Raisman, 2004). When developing experimental strategies, other factors have to be considered, such as axotomy-induced cell death and secondary degeneration (Schwartz, 2004a), scar formation (Rhodes and Fawcett, 2004), and factors intrinsic to neurons themselves. We know that most CNS neurons loose their capacity for axonal regeneration at a specific point in early development even before myelination (Chen et al., 1995; Shewan et al., 1995; Bouslama-Oueghlani et al., 2003).
The abundant and diverse experiments directed at reconstructing visual circuitry (several will be discussed below) have all uncovered a range of difficulties such as, 1) establishing a permissive interface between sprouting and/or regenerating axons, or between graft and host; 2) providing the optimal conditions for appropriately directed axon outgrowth; 3) achieving sufficient amount of axon outgrowth for proper function; 4) limiting or controlling the local neural responses to initial injury, such as cell death, which may in itself mitigate against effective recovery. A central point to all such work is that visual function should be used as the yardstick for success (see section 8).
3. Requirements for recovery of function following lesions of CNS pathways
The recovery of lost function following axonal interruption in any neuronal system depends on the fulfillment of the following prerequisites:
1) Survival of axotomized neurons and prevention of secondary degeneration
2) Axonal extension of the neurons surviving axotomy
3) Guidance of regenerating axons towards their appropriate target(s)
4) Target innervation and synapse formation onto recipient neurons
5) Supra-threshold activation in target neurons caused by regenerated afferents
6) Restoration of ordered functional connections
7) Preservation of local and downstream circuitry
The retinocollicular pathway is particularly suitable for addressing the above mentioned points for at least three reasons: 1) RGC axons project to the superior colliculus (SC) through the optic nerve, which can be easily lesioned, replaced, and anatomically traced; 2) Because RGCs are excited by light, they can be selectively stimulated in a physiological manner without the use of less selective electrical pulses which would concomitantly excite other pathways; 3) The fact that intact RGC axons arborize preferentially in the superficial layers of the SC onto which they form a point to point representation of the retina (Siminoff et al., 1966; Tiao and Blakemore, 1976; Finlay et al., 1978) allows comparison of the regenerated pathway with the intact, using either morphological or physiological approaches.
We will review the extent to which these requirements can be achieved by using the example of the interrupted retinocollicular pathway and attempts for reconstruction using PN grafts replacing the optic nerve. In brief, the potential for regeneration of optic axons after being damaged was first indicated by studying hamsters in which the optic nerve was cut and a peripheral nerve graft placed in association with the retinal optic fiber layer (So and Aguayo, 1985) (Fig 3). Optic axons grew into this nerve graft and studies in cat, in this instance, showed that the major classes characterized both anatomically and physiologically, contribute to this regenerative growth (Fukuda et al., 1998). Subsequent experiments (Villegas-Perez et al., 1988) refined the approach and were able to show that up to 10% or so of the normal optic projection was able to regenerate into the nerve graft. If the other end of the nerve graft was placed in an appropriate brain region, a proportion of these axons could reach regions normally innervated by them (Vidal-Sanz et al., 1987; Carter et al., 1989). If the central stump was placed in close proximity to a visual center, the axons formed terminal ramifications and synapses within that region (Aviles-Trigueros et al., 2000). If placed at a distance from a visual center, axons were able to grow as much as 6 mm through the brainstem to specific targets of optic input, ignoring in the process other brain regions, including ones that had lost their primary input during the surgical procedure (Carter et al., 1989). While an anatomical study of topological representation of the regenerated axons has not been done, physiological experiments (Sauve et al., 2001a) have indicated that while the precise map encountered in the normal retinotectal projection was not seen, there was indication of a tendency for the naso-temporal representation of the visual field to be appropriately arrayed along the rostro-caudal SC axis. Whether this would be sufficient for an animal to be able to perform a behavior requiring topographically encoded information, such as head tracking, is not clear. The further possibility that some sort of training regimen might improve the tightness of the map has not been explored either, but this preparation does lend itself to such exploration.
4. Promoting the survival of axotomized RGCs
a) RGCs die after optic nerve transection
Following a complete intraorbital lesion of the adult mammalian optic nerve, the majority of RGCs are lost within 2 weeks (Villegas-Perez et al., 1988, 1993; Berkelaar et al., 1994; Peinado-Ramon et al., 1996). Axotomized RGCs start dying after one week post-lesion, via programmed cell death. This process, known as apoptosis, is also responsible for the naturally occurring cell death of RGCs during development. Cells undergoing apoptosis, such as axotomized RGCs, are characterized by the condensation of their nucleus, DNA fragmentation and the formation of apoptotic bodies (Berkelaar et al., 1994; Quigley at al., 1995). The pathways responsible for the apoptotic death of axotomized RGCs involve the activation of caspases type 3, 8, 9, and CPP32-like (Kermer et al., 1998, 1999b, 2000a; Weishaupt et al., 2003; Cheung et al., 2004), and the p38 mitogen activated protein kinase p38MAPK (Kikuchi et al., 2000). The inhibition of either or all of these caspases (Chaudhary et al., 1999; Kermer et al., 1998, 1999a, 2000a) or of p38MAPK (Kikuchi et al., 2000) enhances the survival of axotomized RGCs. Furthermore, optic nerve transection leads to the elevation of proapoptotic protein Bax and the decrease of the antiapoptotic proteins Bcl-2 and Bcl-X (Isenmann et al., 1997). The overexpression of Bcl-2 in transgenic mice has been shown to protect RGCs for axotomy-induced death (Bonfanti et al., 1996; Chierzi et al., 1998). Finally, transfection of RGCs with the X-linked inhibitor of apoptosis or with the caspase inhibitor p35 can both protect RGCs from axotomy-induced cell death (Kugler et al., 2000). The conclusion from these findings is that axotomy-induced death of RGCs involves the activation of not only one but several apoptotic pathways. Therefore, strategies aimed at preventing the death of axotomized RGC would have to target multiple pathways.
b) Strategies to prevent the death of axotomized RGCs
Most strategies developed to prevent RGC death involve the supply of various trophic factors either exogenously, through cell based therapy, or via gene transfection (for reviews see: Yip and So, 1998; Cui et al., 2000; Weishaupt and Bahr, 2001; Koeberle and Bahr, 2004). The rationale for supplying neurotrophins to axotomized RGCs is that their death might be related to the lost of retrogradely supplied trophic factors, such as BDNF, from their targets in the SC (Quigley et al., 2000; Caleo et al., 2000; Spalding et al., 2004). Axotomized RGCs can be rescued by experimentally supplying the retina with BDNF, either via intravitreal injections (Mey and Thanos, 1993; Mansour-Robaey et al., 1994; Peinado-Ramon et al., 1996; Klocker et al., 1998) or via gene transfection of retinal cells (Di Polo et al., 1998). Furthermore, transfection of retinal cells with the high affinity BDNF receptor TrkB also enhances the survival of axotomized RGCs (Cheng et al., 2002). Axotomized RGCs have also been rescued by the experimental addition of several other neurotrophic factors (some of which having additive effects with each other): neurotrophin NT-4/5 (Cohen et al., 1994; Peinado-Ramon et al., 1996); NGF (Carmignoto et al., 1989); GDNF (Klocker et al., 1997; Koeberle and Ball, 1998; Yan et al., 1999); Neurturin (Koeberle and Ball, 2002); and CNTF (Mey and Thanos, 1993; Weise et al., 2000; Watanabe and Fukuda, 2002; Cui and Harvey, 2000). Several growth factors mediate their action by binding to their specific receptors and activating the mitogen activated protein kinase (MAPK) and phosphatidylinositide-3-kinase (PI3K) transduction cascade pathways. For details on secondary messengers and factors interfering with these cascades see reviews by Kaplan and Miller, 2000; Patapoutian and Reichardt, 2001; Koeberle and Bahr, 2004. In brief, neurotrophic factors may prevent axotomy-induced RGC death by interfering with caspase pathways and by promoting the expression of pro-survival genes.
The neurotrophic factors tested to date for their survival effect on axotomized RGCs all have only short term effects, suggesting that trophic withdrawal is not the only trigger for the apoptosis of axotomized RGCs. Blockade of axonal transport (Fagiolini et al., 1997) has minimal effects on the death of RGCs in neonatal rats. Other factors have been identified, among them, the up-regulation of inducible nitric oxide synthase (iNOS) by Muller cells following RGC axotomy (Koeberle and Ball, 1999). Inhibition of NOS has been shown to promote the survival of axotomized RGCs (Koeberle and Ball, 1999), and when done in combination with BDNF, it potentiates the effect of this neurotrophin (Klocker et al., 1998). Modulation of microglial cells and macrophage activity has also been shown to have an effect on the survival of axotomized RGCs (Thanos et al., 1993, Raibon et al., 2002; Koeberle et al., 2004).
Finally, the prevention of axotomy induced RGC death has been explored using a vaccination approach known as “protective autoimmunity” (for a review see Schwartz, 2004b). Dr Michal Schwartz and colleagues recently demonstrated that T cells specific to self-proteins residing in the site of CNS insult can be neuroprotective. With the aim of boosting autoimmunity for neuroprotection without risking the induction of an autoimmune disease, Dr. Schwartz’s group has developed the use of Cop-1 (an FDA-approved drug for the treatment of multiple sclerosis) as an active vaccination for neuroprotection. This approach is currently under investigation as a potential preventive treatment for glaucoma.
5. Promoting the growth of axotomized RGC axons
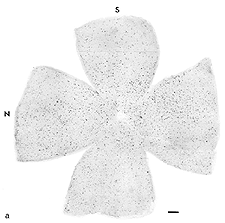
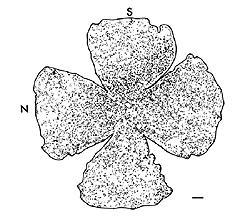
Anastomosis of a PN graft onto the optic nerve stump has been shown to have a survival effect on RGCs (Villegas-Perez et al., 1988; Bahr et al., 1992) as well as acting as an environment permissive to RGC axonal regeneration (So and Aguayo, 1985; Vidal-Sanz et al., 1987). A maximum of 10% of the total RGC population in rodents (10,000 out of 100,000 in rats and hamsters)(Figs. 4a, b and c) can regenerate an axon as far as the distal end of an autologous PN graft, covering a distance of up to 2.5cm. This allows them to reach areas of the primary visual targets such as the contralateral superior colliculus. The RGCs that have regenerated an axon along the PN graft appear to survive longer than the RGCs that have failed to do so (Villegas-Perez et al., 1988). Whether some RGCs have intrinsic survival or axonal regenerative capacities or whether some RGCs simply randomly succeed to regenerate an axon by chance (for instance, proximity to the PN graft stump prior to retrograde degeneration beyond the optic disc) remains to be clarified.
The promotion of RGC axonal regeneration seems to involve different mechanisms than the ones promoting their axonal extension: most trophic factors shown to promote RGC survival fail to promote their axonal regeneration. The discovery of new candidate molecules promoting axonal extension comes in part from the observation that lens scratching induces the production of low molecular weight molecules that can promote both RGC survival and axonal regeneration within the intracranially lesioned optic nerve (Leon et al., 2000). Furthermore, macrophage stimulation, which is associated with the release of similarly acting molecules (Yin et al., 2003) can also promote RGC axonal extension within the lesioned optic nerve. The neutralization of myelin-associated growth inhibitors can promote RGC axonal regeneration within lesioned optic nerves, but only if RGCs are in an “active growth state”, such as stimulated by the small molecules mentioned above (Fischer et al., 2004).
The ability of RGCs to regenerate an axon does not only depend on their environment but also on their intrinsic state. As mentioned in the introduction, most CNS neurons such as RGCs appear to loose their capacity for axonal regeneration during maturation (Chen et al., 1995; Shewan et al., 1995). Some recent experimental manipulations have showed that it might be possible to reverse, at least to some extent, this state. Dr Lisa McKerracher and colleagues have shown that inactivation of the small GTPase Rho can promote RGC axonal regeneration following micro-lesions of the optic nerve in adult rats (for a review see Ellezam et al., 2002). Another approach, investigated by Dr Mary Filbin and colleagues, involves elevating the intracellular level of cyclic AMP in neurons (Cai et al., 2001; for a review see Spencer and Filbin, 2004).
6. Guidance of regenerating RGC axons towards their appropriate target
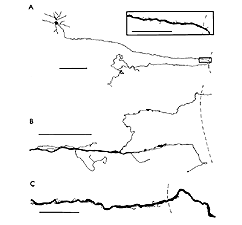
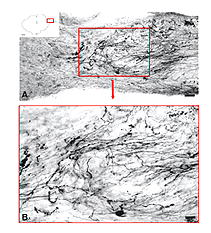
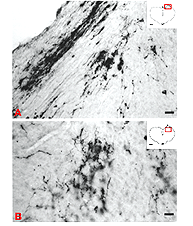
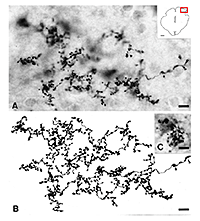
Based on what we know about developmental events, the challenges for regenerating RGC axons to successfully reach their appropriate targets are severe. First, as during development (for a review see Oster et al., 2004), regenerating axons have to navigate within the retina, find their way to the optic disc, exit it, and finally enter into the optic nerve. Following PN grafting (anastomosed on the intraorbitally cut optic nerve stump) combined with intravitreal BDNF (to increase the survival of axotomized RGCs), a minority of RGC axons do successfully regenerate an axon into a peripheral nerve graft. However, many regenerating RGC axons never actually exit the optic disc (Sawai et al., 1996). Several axons from surviving RGCs appear to turn sharply just before the optic disc, growing in the opposite direction, forming numerous collateral branches and loops, with erratic growing patterns (Fig 5).
A second challenge facing the regenerating RGC axon consists of navigating across the optic chiasma by crossing or not crossing the midline, again depending on the RGC type and/or location in the retina (for a review see Williams et al., 2004). After which, regenerating RGC axons, again depending on their types, have to extend all the way into their appropriate target(s); some individual RGCs even have to send collaterals to multiple targets, such as both the superior colliculus (SC) and the dorsolateral geniculate nucleus (dLGN) and even more as shown in Figure 6).
By using PN grafts, it has been possible to guide regenerating RGC axons into their appropriate targets such as the SC (Vidal-Sanz et al., 1987; Carter et al., 1989; Keirstead et al., 1989; Sauve et al., 1995; Thanos and Mey, 1995; Sauve et al., 2001a), the dLGN (Carter and Jhaveri, 1997), the pretectal nuclei (Fig 7) (Aviles-Trigueros et al., 2000), or deliberately into inappropriate targets such as the inferior colliculus or cerebellum (Zwimpfer et al., 1992).
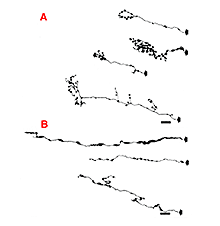
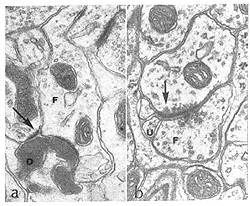
However, in some animals following insertion of the distal end of the PN graft into a chosen target, only a minority or even none of the regenerating RGC axons can enter a target (Aviles-Trigueros et al., 2000). In such cases, several RGC axons (anterogradely labeled with a tracer from the eye) are confined to a neuroma-like formation at the interface between the graft and the CNS target (Fig. 8).
The cause of the formation of the neuroma-like endings is unclear. Three main factors have to be considered: 1) glial reactions that might be associated with the lack of axonal penetration into the CNS target; 2) the presence of inhibitory molecules, which may also be responsible for the curtailed growth observed in CNS targets, and that are known to be expressed in the damaged CNS (Dusart et al., 1999) and at the PNS-CNS interface (Liuzzi and Lasek, 1987; Bandtlow et al., 1990; Smith et al., 1990; Davies et al., 1997, 1999; Fawcett, 1997); and 3) the perturbation, due to graft insertion procedure, of the tri-dimensional structure of the extracellular matrix that might act as “guiding railways” coated with molecules that have growth inhibitory or promoting properties such as laminin, vimentin, and chondroitin sulphate proteoglycans (CSPG).
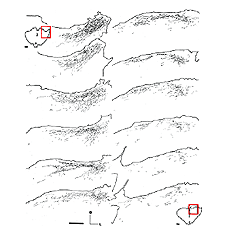
In some instances, PN grafts cannot be directly inserted into appropriate targets due to the small size of the nucleus. In the study of Aviles-Trigueros et al. (2000) successful innervation of the olivary pretectal nucleus (OPN) and the nucleus of the optic tract (NOT) was achieved by inserting the distal end of a PN graft into the superficial aspect of the midbrain, between these two nuclei, by carefully avoiding touching them. Under such circumstances, some axons were seen crossing the midline to innervate the contralateral nuclei. This led the investigators to insert the nerves some distance further away from the nuclei. Axons although coursing for long distances through the brainstem, still showed selectivity for optic target regions (Figs. 9, 10, 11). This occurred even though some nuclei within their trajectory were deafferented by the surgery associated with graft insertion.
The specificity of innervation of visual and non-visual targets would appear to be at odds with the observation of Zwimpfer et al. (1992) showing innervation of the cerebellum by optic axons regenerating through peripheral nerve grafts. There is however a significant difference in experimental design in that in the study of Aviles-Trigueros et al. (2000), the axons have a “choice” of optic or non-optic targets whereas in the cerebellum study, no choice is available. To complicate the issue, there is evidence that when two of the principal targets of retinofugal axons, the SC and dLGN, are ablated in newborn hamsters and the somatosensory (ventrobasal) or auditory (medial geniculate) thalamic nuclei are partially deafferented, the optic axons form permanent, abnormal connections in the latter nuclei (Doug, 1986). The mechanisms responsible for “apparent” preference of appropriate target in studies such as by Aviles-Trigueros et al. (2000) are still unclear.
In addition to PN grafting, several studies have reported successful RGC axonal regeneration within the severed optic nerve (Eitan et al., 1994; Berry et al., 1996; Leon et al., 2000; Fischer et al., 2001; Ellezam et al., 2002; Li et al., 2003). However, it remains unclear as to what is the growth pattern of individual regenerating RGC axons, especially at the level of the optic chiasm, and distally.
7. Arborization and synapse formation by RGC axons regenerating into their CNS targets
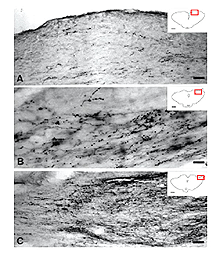
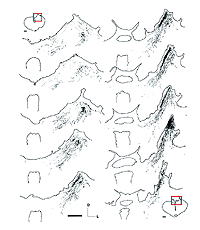
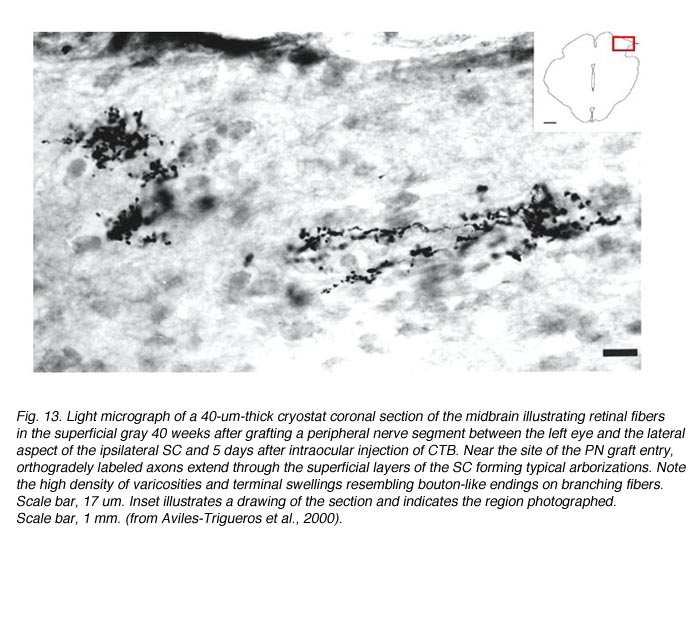
Studies involving PN grafting to bridge the retinofugal pathways have shown that regenerating RGC axons can form distinct arborizations in the target which they reinnervate (Vidal-Sanz et al., 1987; Carter et al., 1991, 1998; Aviles-Trigueros et al., 2000). The study of Aviles-Trigueros et al. (2000) suggests that regenerated retinal axons adopt distinctive patterns of terminal arborizations depending on the target they reinnervate. For instance, while axons entering the OPN show little ramification and swellings reminiscent of the typical retinal innervation of this nucleus, in the NOT axons tend to show more profuse ramifications and arborizations with terminal swellings (Figs. 12 and 13).
These types of terminals are reminiscent of the terminals previously described for such retinorecipient nuclei in another rodent (Ling et al., 1998). In this context, what is also remarkable, is the similarity of the morphology of the elaborate arborizations found in the superficial layers of the superior colliculus (Fig. 14) with that described in normal animals (Ling et al., 1998). This indicates further specificity of terminal arborization within the retinorecipient reinnervated target. Thus, it appears that the morphology of the arborization is dictated by the recipient region, more than by the type of retinal fiber arriving to target (Carter and Jhaveri, 1997), and that regenerating axons modify their arbors to adapt to the local conditions of the target nucleus.
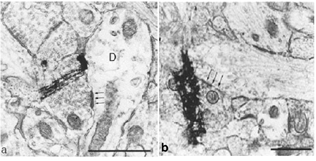
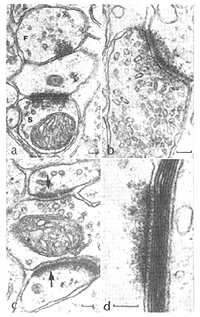
In 1987, Vidal-Sanz et al. provided experimental evidence that regenerating RGC axons were able to re-establish connections in visual centers of the brainstem (Fig. 15) (Vidal-Sanz et al., 1987).
These connections appear to persist for the life span of the rodent, i.e. up to 2 years of age (Vidal-Sanz et al., 1991). The type of synaptic contacts formed, the ratios of contacts to terminal perimeter, and the domains of the postsynaptic neurons contacted are similar to those of intact retinofugal pathways (Carter et al., 1989). Therefore, regenerated RGC axons can establish well-differentiated synapses with neurons in the SC. Based on their results, Carter et al. (1989) concluded that “The synaptic differentiation attained by such reformed retinocollicular projections suggests that regenerating CNS axons and their target neurons in the adult mammalian brain may retain or reexpress certain molecular determinants of normal connectivity”. There are however morphological differences between the regenerated and control synapses: 1) larger size of some regenerated terminals; 2) greater mean length of the regenerated synapses; and 3) higher proportion of contacts with dendrites that contain vesicles in regenerated versus intact synapses.
8. Generation of action potentials in target neurons
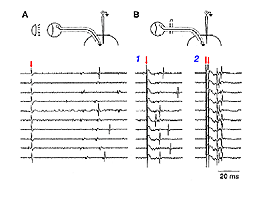
Dr Sue Keirstead and colleagues provided electrophysiological evidence that the synapses such as the ones described at the electron-microscopic level by Vidal-Sanz et al. (1987) could mediate the transynaptic activation of neurons in the superior colliculus of adult mammals (Keirstead et al 1989). Extracellular recordings in the superior colliculus 15 to 18 weeks after PN graft insertion into the SC revealed excitatory and inhibitory postsynaptic responses to visual stimulation of the eye that had received PN anastomosis onto its completely cut optic nerve (Fig 16). Specific stimulation protocols (involving paired electrical stimulation of the PN graft) were used to verify that post-synaptic activity could be elicited in the reinnervated SC.
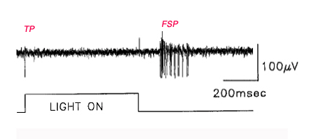
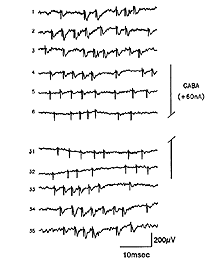
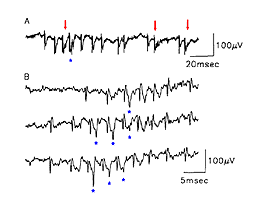
Further studies by Sauve et al. (1995), using the same experimental preparation, indicated that each element of a typical bursting response to light (excitatory type of response, Fig 17) consists of a terminal potential (TP) arising from a regenerated RGC axon terminal arborization, followed by a longer duration focal synaptic potential (FSP) that is selectively blocked by GABA (Fig. 18). FSPs are extracellular changes in potential that reflect the summation of excitatory post-synaptic potentials (EPSPs) in neurons within the terminal field of the regenerated RGC axon (Fig. 18). In some instances, superimposed on these FSPs are spikes (Fig. 19), which arise after three to four consecutive closely spaced impulses from RGCs (as inferred from the TPs).
The results from Sauve et al. (1995) indicate that terminal arborizations of individual regenerated RGC axons can synapse with multiple neurons in the SC and that convergence of inputs from regenerated RGC axons is not required for activation of SC neurons in response to light.
Finally, in vitro studies by Turner et al. (2001) indicate that the deafferentation of the SC, caused by optic nerve cut, and a surgical approach to insert the PN graft into the SC together, lead to ultra-structural changes reflected functionally at the synaptic level in the target structure, even after potential RGC axonal regeneration. Such changes are likely to compromise the ability of the target structure to function normally during information processing. Therefore, although axons regenerating along peripheral nerve grafts can make functional synaptic connections, their efficacy in activating the target structure will probably be compromised by the local changes in synaptic connectivity.
9. Restoration of retinotopy
Is restoration of retinotopy needed for recovery of function?
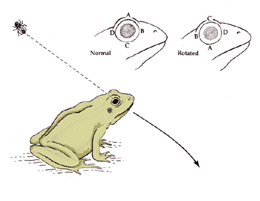
Yes it is, for the appropriate execution of visually guided behaviors. We owe the proof to the 1981 Physiology Nobel Prize co-winner Dr Roger W. Sperry (Fig. 20) who ingeniously took advantage of the spontaneous functional recovery of the retinotectal system in frogs (reviewed in Gaze, 1970).
Fig. 20. Photograph of Nobelist Roger Sperry
Sperry’s impressive demonstration involved sectioning a frog’s optic nerve and rotating the eye by 180Á (Sperry, 1950). After spontaneous regeneration of the retinotectal pathway, the frog’s attempt to catch a prey resulted in an attack directed to the diametrically opposed direction (Fig. 21). The frog’s behavior gave clues as to how the regenerating RGC axons had reconnected in the tectum; note: the structure equivalent to SC is named “tectum” in lower vertebrates. Sperry inferred that the regenerating axons had returned to their original position in the tectum, regardless of the new position they occupied in the rotated eye. This gave experimental support for his chemospecificity theory (Sperry, 1963), which stipulates that “The connections are governed by intrinsic specificity of the advancing fibre tip plus that of the various cellular elements it encounters in its outgrowth” (Sperry, 1965).
We can learn about the extent to which retinotopic projections have to be re-established in order to achieve behavioral recovery, by comparing species that have various levels of regeneration of their retinotectal system, and examining the level of visually guided behavior they can recover. The capacity for RGC axon regeneration in the vertebrate visual pathway is summarized in the study of Dunlop et al. (2004). For instance, there is variability between various species of lizards. Some achieve a stable restoration of retinotectal organization (accompanied by restoration of visually guided behaviors), while others (Ctenophorus ornatus) fail to maintain a retinotopic ordering of the regenerated projection, and lose their capacity to perform visually guided tasks (Dunlop et al., 2003). However, training on a visual task has been shown to improve the outcome of optic nerve regeneration in Ctenophorus ornatus lizards (Beazley et al., 2003).
Guidance cues in the injured retinotectal pathway
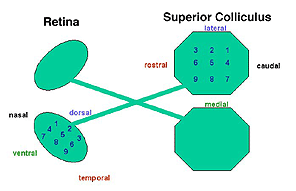
The retinotectal system is the model of choice for studying the mechanisms governing the formation of ordered connections in the CNS. In intact mature rodents, axons from RGCs located in the temporal retina project to the rostral (anterior) part of the contralateral SC while axons from RGCs located in the nasal retina project to the caudal (posterior) contralateral SC (Siminoff et al., 1966; Tiao and Blakemore, 1976; Finlay et al., 1978). Ventral RGCs project axons medially and dorsal RGCs project axons laterally in the contralateral SC (Fig. 22).
Fig. 22. Ventral view schematic of retinotectal projections
In order to elucidate the mechanisms involved in axonal guidance, especially with regard to specificity of polarities in the tectum, Dr Friedrich Bonhoeffer and colleagues developed an in vitro assay, known as the “stripe assay” (Walter et al., 1987a,b). The basis of this assay involves growing retinal explants on stripes made up of tissues alternating from rostral and caudal parts of the tectum. Results using tissues from developing chicks or rodents show that temporal RGC axons avoid stripes made up of caudal tectal tissue while nasal RGC axons grow equally well on either rostral or nasal tectal tissue stripes (Walter et al., 1987a,b; Godement and Bonhoeffer, 1989; Roskies and O’Leary, 1994), or show a preference for nasal stripes providing specific pre-treatments (von Boxberg et al., 1993). This preference appears to be developmentally regulated in a way that is lost in the mature system. However, Dr Mathias Bahr and colleagues showed that this capacity can be partially restored following optic nerve cut in adult rats (Bahr and Eschweiler, 1991, 1993; Wizenmann et al., 1993). Their results indicate that guidance cues might be re-expressed in the deafferented retinotectal system of adult mammals and that these cues might retain some level of function.
Several axonal guidance molecules and their respective receptors have been identified. Among them, the best studied comprise these four classes: netrins, semaphorins, slits and ephrins (for a review see Koeberle and Bahr, 2004). In the goldfish, in which spontaneous regeneration occurs, Eph/ephrins are upregulated as gradients at the time that topography is restored during optic nerve regeneration (King et al, 2003). Furthermore, the Eph/ephrin system is required to restore topography since blocking their interactions in vivo with fusion proteins results in abnormal topography (Rodger et al., 2004). In rodents, in which spontaneous regeneration does not occur, most of the known guidance molecules have been shown to be modulated following deafferentation. However, while some molecules are up-regulated to levels similar to those achieved in development, some (such as the ephrin receptor EphA5 in the retina and the ephrin-B) are actually down-regulated. Therefore it remains improbable that these various changes might actually recapitulate developmental events. How experimental manipulations might achieve such recapitulation of developmental events is for now a mind-boggling puzzle.
Can RGC axon regenerated through a PN graft resume topological specificity when reinnervating the superior colliculus? (Example from the study of Sauve et al., 2001a)
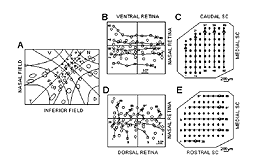
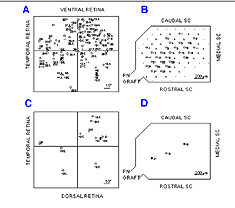
The study of Sauve et al. (2001a) indicates that regenerating mammalian RGC axon terminals do not form a precise retinotopic map (compared to normal intact animals, Fig. 23) when reinnervating the SC (Fig. 24).
However, superimposed on the apparent randomness of distribution of RGC terminals, there appears to be a small but nonetheless statistically significant tendency for these terminals to array themselves appropriately within the rostrocaudal axis of the SC. Because the PN graft tip was placed at different locations in different animals, the assessment of topography was of necessity a comparison of the relative positions of reinnervating axon terminals for each animal rather than an identification of the absolute position of each terminal. It must also must be emphasized that the method used by Sauve et al. (2001a) assessed terminal positions but not trajectories of regenerating RGC axons. Nonetheless, these results suggest that factor(s) may be present in the reinnervated SC, as in the newly innervated SC, that can influence the direction of axonal growth and/or the area within which arborization and synapse formation occurs.
Topographic ordering of projections during normal development of retinofugal pathways is thought to reflect at least two processes, 1) an initial pathfinding to the approximately correct area directed by spatially specific molecular cues as first suggested by Sperry (1963), and, 2) a subsequent phase of refinement of the projection due to activity dependent processes in which near simultaneous firing of neighboring RGCs (for a review see Wong, 1999) serves mutually to stabilize the connections of their shared target neurons in the LGN or tectum (Shatz, 1990, Cline 1991; Penn et al., 1998; Debsky and Cline, 2002; Schmidt, 2004). Initial pathfinding of RGC axons in the tectum may be very precise as in frogs and fish (Holt and Harris, 1983; Stuermer et al., 1992), or more exuberant and diffuse, as in rodents (Simon and O’Leary, 1992a, b). Computer simulations suggest that a combination of positional cues and activity dependent mechanisms gives rise to a very precise retinotectal topology in a variety of experimental situations (Fraser and Perkel, 1990; Honda, 1998), for example even if a molecular gradient is only transiently expressed during the initiation of innervation of the tectum (Hua et al., 1993).
During regeneration of the retinotectal pathway in frogs and fish, the initial topography is only roughly organized. Functional synapses are formed indiscriminately by regenerating goldfish RGC axons as they enter the tectum. These may be unstable if inappropriately located (Matsumoto et al., 1987; Meyer and Kageyama, 1999). Projections become refined into a more precise retinotopic map over a period of several weeks by mechanisms that depend upon ongoing activity in neighboring RGC axons (Udin and Fawcett, 1988; Cline 1991).
In vitro experiments have shown that molecules with topological specificity with respect to the rostral and caudal tectum or SC are transiently expressed in the neonatal mammalian SC (Walter et al., 1987a). These topologically specific markers disappear after the retino-collicular pathway is laid down, but reappear about 2 weeks after denervation of the SC (Wizenmann et al., 1993). Such positionally specific markers may be more strongly expressed in deafferented SC than in embryonic SC (Bahr and Wizenmann, 1996). The experimental results of Sauve et al. (2001) are consistent with the possibility that a gradient of such positionally specific markers could serve as an influence on the exploration of the SC by regenerating RGC axons (Baier and Bonhoeffer, 1992; Wizenmann and Bahr, 1997, 1998).: either by exerting a repulsive or tropic effect on their axonal growth cones (Walter et al., 1987a,b; Boxberg et al., 1993; Nakamoto et al., 1996; Ichijo and Bonhoeffer, 1998, Davenport et al., 1998), or by influencing their branching patterns (Roskies and O’Leary, 1994). Positionally specific markers could also influence the deployment of regenerating RGC axons within the nerve graft as they approach the SC (Goodhill, 1998).
The question arises as to why the effects of these factor(s), if present, are so minimally expressed or so difficult to document in the reinnervated mammalian SC. In the reinnervated SC, the extent of exploration by a regenerated RGC axon is 1 mm or less (Carter et al. 1994). More extensive exploration of the SC is perhaps limited by the presence of factors inhibitory to axonal growth (Caroni and Schwab, 1988; McKerracher et al. 1994; Smith-Thomas et al., 1994; Ghosh and David, 1997) that are present in the adult animal as well as by the developmental down-regulation of growth permissive molecules (McLoon et al., 1988; Rager et al., 1996) and receptors (Cohen et al., 1986; de Curtis and Reichardt, 1993). This is in contrast to the situation during normal development, where RGC axons from all portions of the retina initially innervate the entire SC (Simon and O’Leary, 1992a,b). In the PN graft regeneration paradigm, no more than 10% of the normal total number of RGCs usually regenerate their axons across the PN graft and only a portion of these reinnervate the SC (Vidal-Sanz et al., 1987). Many axons terminate growth immediately after penetrating the CNS (Aviles-Trigueros et al. 2000). One way to increase sprouting of regenerating RGC axons in the SC could be to provide brain-derived neurotrophic factor (BDNF) and chondroitinase ABC (Tropea et al., 2003). However, even in these conditions, with surviving RGCs widely separated in the retina and their axon terminals widely dispersed within the SC, the influence of activity-dependent mechanisms in shaping the topological pattern of innervation would be expected, a priori, to be much more limited than in normal development. Furthermore, with little competition among axons for synaptic sites, it is possible that inappropriately located synapses, once formed, would be much more stable than in the reinnervated frog or goldfish tectum. Such premature formation of synapses could in turn curtail the further exploration of the SC by regenerated RGC axons.
10. Preservation of local and downstream circuitry
In the study of Turner et al. (2001), the local synaptic connectivity in the superficial gray layer of the superior colliculus (SC) was assessed following RGC axonal regeneration through a peripheral nerve graft into the rat SC, using in vitro brain slice techniques. Repair was achieved between the ipsilateral eye and SC, following bilateral lesion of optic nerves and ablation of ipsilateral occipital cortex.
Impact of deafferentation
The normal rat superficial gray layer (SGL) of the SC receives the majority (90%) of its excitatory input from the retina (Lund and Lund 1971, Fig 25) and the remainder (10%) from the visual cortex (Harvey and Worthington, 1980).
Interconnectivity within the SGL appears to be mediated by local GABAergic interneurons (Mize, 1988; Pinard et al., 1991). Both the GABAergic processes of local circuit interneurons (Lund and Lund, 1971; Houser et al., 1983) and the axon terminals of other projections proliferate (Gerrikagoitia et al., 2000; Garcia del Cano et al., 2002) following optic deafferentation. These new neural processes appear to form additional synapses or to take up positions apposed to the postsynaptic densities left vacant by the degenerating retinal afferents in the SC (Lund and Lund, 1971). As a result, the synapse to neuron ratio stays the same in the SC (Smith and Bedi, 1998), although it is not known whether these synaptic contacts are functional. Since the subsequent repair of retinal afferents is also going to alter the balance between excitatory and inhibitory inputs, the connectivity between restored retinal afferents and target neurons is likely to be very different from that in the normal superficial gray layer (SGL).
There is as yet no evidence for local excitatory connections in the SGL. Electrophysiological studies are consistent with the anatomical organization, in that retinal input to the SGL is monosynaptic and there is no evidence for local network related activity even when inhibition is blocked (Isa et al., 1998). In addition, contralateral enucleation 14 days prior to recording is sufficient to result in a complete loss of excitatory inputs to the SGL in vitro following intracollicular stimulation (Miyamoto et al 1990). However, the study of Turner et al. (2001) suggest that this is not always the case: optic tract stimulation 3-9 months after optic nerve transection leads to excitation in the SC and this is likely due to the recruitment of corticocollicular projections, which run close to the optic tract (Bourassa and Deschenes, 1995). Indeed, anatomical studies have shown that 30-45 days after contralateral enucleation there is a reactive synaptogenesis of corticocollicular terminals (Garcia del Cano et al., 2002). The nature of this responsiveness also suggests that this cortical input recruits local recurrent excitatory connections that have been formed as part of the reactive process to retinal deafferentation.
Evidence of new inputs following PN-graft repair
Following combined retinal and cortical deafferentation (as part of the surgery for PN bridging the retinocollicular pathway), all normal excitatory inputs to the SGL are abolished. However, there is evidence from the study of Turner et al., (2001) for the presence of both spontaneous and evoked EPSP-like events in SC slices from the PN graft repair preparation. This suggests that excitatory inputs can form new synaptic contacts on neurons within the SGL. These connections are likely to be due to reactive sprouting of excitatory neurons either in the SGL itself or the deeper layers of the SC, such as the intermediate gray layer (IGL). Since both the SGL and IGL contain neurons that have dendrites that extend across the SO, this could provide a substrate on which such reactive synaptogenesis could form a recurrent excitatory network. Certainly, the delay (20-30ms) in the onset of the network depolarizations following electrical stimulation (reported by Turner et al., 2001) suggests that sprouted excitatory input is poly-synaptic, resulting from the recruitment of a relatively remote population of neurons, as may be located in the IGL. Indeed, the structure of the IGL is conducive to bursting activity since IGL neurons form a recurrent collateral network in normal animals and produce long lasting synaptic responses in the presence of bicuculline (Isa et al., 1998). The network reorganization in the PN graft preparation (recorded in vitro, (Turner et al., 2001) is not that surprising, in view of the impact of deafferentation in other structures, e.g. cerebral cortex (Salin et al., 1995) or the dentate gyrus of the hippocampus (Laurberg and Zimmer 1981). In both cases, this process leads to sprouting of excitatory axons to form new recurrent excitatory connections. One consequence of the reactive events found in most SC slices (from PN graft repair preparation), is that they are likely to obscure efficacy of regenerated RGC axons that had established monosynaptic connections of retinorecipient neurons.
Impact of PN-graft repair surgery on the SGL function: the future for repair strategies
It is clear from Turner et al.’s work (2001) that intrinsic changes in response patterns will impact the way the SGL will function during visual stimulation. Keirstead et al. (1989) demonstrated that, in comparison to normal controls, restoration of functional retinal inputs into the SC, using the PN-graft method, had delays in the onset of post-synaptic action (Fortin et al., 1999). This delay probably reflects the fact that the underlying synaptic responses involve the recruitment of recurrent excitatory connections. It may also reflect the profound impact that GABAergic inhibition has in controlling this network. The impact of visual deafferentation needs to be assessed throughout the SC, in order to identify the locus/loci of the reactive changes that underlie(s) altered network behavior. In addition, for improvements in current strategies of pathway repair, there is the need to limit or reverse these deafferentation-induced changes because firstly, this would allow a better assessment of the efficacy of new connections, and secondly it would probably improve system function.
In conclusion, axonal regeneration to a target alone clearly does not guarantee functional recovery. The study of Turner et al. (2001) highlights the fact that the functional state of the target area is of fundamental importance for whether normal function can be restored, once axonal reinnervation has been achieved.
11. Evidence for some level of recovery of function in the PN-bridged retinofugal pathways
The question of whether visual functions can be mediated by regenerated axons has been explored by looking at 1) the pupillary light reflex, a conditioned response, 2) EEG desynchronization and 3) behavioral arousal. It was found that the regenerated pathway could mediate pupillary constriction to light (Thanos, 1992). Further studies (Whiteley et al., 1998) showed that at best, the response amplitude and latency could be within normal range, but one noticeable difference was that while in normals, repetitive stimulation gave repeated responses of similar amplitude, regenerated pathways showed substantially reduced amplitudes with successive stimulation. The conditioned response studied, i.e. escape from a shuttle box in response to a light flash as a predictor of an electric shock, showed that this task could be performed in rats with regenerated optic input (Sasaki et al., 1993). Finally, animals were tested to demonstrate whether the regenerated pathways could mediate EEG desynchronization behavior to light (Sasaki et al., 1996). This depended on the fact that, under normal conditions, rats show a slow wave sleep pattern. A sensory stimulus, such as a flash of light or auditory signal, desynchronizes this high amplitude slow wave activity, replacing it with low amplitude high frequency activity. When presented with a light flash, blinded rats do not show this response, but animals with nerve grafts connected into the SC do. Associated with this, the rats also show a range of behavioral arousals.
12. Visual Function Assessment
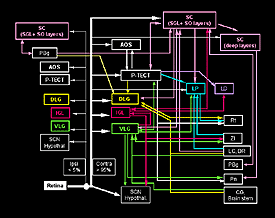
Visual function runs at many levels from unconscious responses to perception. These various functions are generally mediated through specific primary visual centers. Unconscious responses include driving circadian rhythms (relayed through the suprachiasmatic nucleus), photophobic responses (not specifically localized to any specific visual center), pupillary light reflex (mediated by the olivary pretectal nucleus), orienting responses (involving the superior colliculus, SC) and head tracking to moving stripes (involving the SC also, but requiring cortical input for higher spatial frequencies). Perception requires elaborate decoding and integration of a set of visual signals to achieve a representational image at the level of the cortex.
For some visually driven functions, such as circadian rhythms and photophobia, all that is needed is to recognize a gradient, either temporal or spatial of light and dark. For the pupillary light reflex, ability to encode the light intensity is also important, since the amount of pupillary constriction depends on brightness. For orienting responses and head tracking, some level of topographic encoding must be present. For perception, a much more elaborate degree of encoding must be achieved. In any attempt to reconstruct the damaged visual system, or indeed to limit its deterioration, the ideal is to recreate or preserve exactly the normal substrates of the various visually driven behaviors. This is rarely possible and indeed may not always be necessary. The CNS can sometimes adapt to an imperfect sensory input and demonstrate a level of adaptation sufficient to achieve a normal response (Kaas et al., 1983; Merzenich et al., 1984; Gilbert, 1998; Xerri et al., 1998): even in an intact animal the system is able to operate over a wide range of luminance and contrast sensitivity (MacEvoy and Paradiso, 2001). It is also apparent, especially in conditions involving re-establishment of connections, that specific visual responses can be modulated by associated events and may be interdependent.
To reconstruct a particular function therefore, it is necessary for axons to innervate the appropriate brain region, subserving that function. Beyond this it may also be necessary for a topological representation of the retina to be resumed within the region. Despite the likely neural circuit remodeling in a retinorecipient nucleus previously devoid of visual inputs, information processing within this particular nucleus should be relatively normal and this becomes particularly important when cortical functions are involved. Finally the information must have ‘significance’ to the animal so that it can elaborate a suitable response strategy or develop a percept.
13. References
Aguayo AJ, Rasminsky M, Bray GM, Carbonetto S, McKerracher L, Villegas-Perez MP, Vidal-Sanz M, Carter DA. Degenerative and regenerative responses of injured neurons in the central nervous system of adult mammals. Philos Trans R Soc Lond B Biol Sci. 1991;331:337–343. [PubMed]
Aviles-Trigueros M, Sauve Y, Lund RD, Vidal-Sanz M. Selective innervation of retinorecipient brainstem nuclei by retinal ganglion cell axons regenerating through peripheral nerve grafts in adult rats. J Neurosci. 2000;20:361–374. [PubMed]
Bahr M, Eschweiler GW. Regenerating adult rat retinal axons reconnect with target neurons in-vitro. Neuroreport. 1991;2:581–584. [PubMed]
Bahr M, Eschweiler GW, Wolburg H. Precrushed sciatic nerve grafts enhance the survival and axonal regrowth of retinal ganglion cells in adult rats. Exp Neurol.1992;116:13–22. [PubMed]
Bahr M, Eschweiler GW. Formation of functional synapses by regenerating adult rat retinal ganglion cell axons in midbrain target regions in vitro. J Neurobiol.1993;24:456–473. [PubMed]
Bahr M, Wizenmann A. Retinal ganglion cell axons recognize specific guidance cues present in the deafferented adult rat superior colliculus. J Neurosci.1996;16:5106–5116. [PubMed]
Baier H, Bonhoeffer F. Axon guidance by gradients of a target-derived component. Science. 1992;255:472–475. [PubMed]
Bandtlow C, Zachleder T, Schwab ME. Oligodendrocytes arrest neurite growth by contact inhibition. J Neurosci. 1990;10:3837–3848. [PubMed]
Beazley LD, Rodger J, Chen P, Tee LB, Stirling RV, Taylor AL, Dunlop SA. Training on a visual task improves the outcome of optic nerve regeneration. J Neurotrauma. 2003;20:1263–1270. [PubMed]
Berkelaar M, Clarke DB, Wang YC, Bray GM, Aguayo AJ. Axotomy results in delayed death and apoptosis of retinal ganglion cells in adult rats. J Neurosci.1994;14:4368–4374. [PubMed]
Berry M, Carlile J, Hunter A. Peripheral nerve explants grafted into the vitreous body of the eye promote the regeneration of retinal ganglion cell axons severed in the optic nerve. J Neurocytol. 1996;25:147–170. [PubMed]
Bonfanti L, Strettoi E, Chierzi S, Cenni MC, Liu XH, Martinou J-C, Maffei L, Rabacchi SA. Protection of retinal ganglion cells from natural and axotomy-induced cell death in neonatal transgenic mice overexpressing bcl-2. J Neurosci. 1996;16:4186–4194. [PubMed]
Bourassa J, Deschenes M. Corticothalamic projections from the primary visual cortex in rats: a single fiber study using biocytin as an anterograde tracer.Neuroscience. 1995;66:253–263. [PubMed]
Bouslama-Oueghlani L, Wehrle R, Sotelo C, Dusart I. The developmental loss of the ability of Purkinje cells to regenerate their axons occurs in the absence of myelin: an in vitro model to prevent myelination. J Neurosci. 2003;23:8318–8329. [PubMed]
Cai D, Qiu J, Cao Z, McAtee M, Bregman BS, Filbin MT. Neuronal cyclic AMP controls the developmental loss in ability of axons to regenerate. J Neurosci.2001;21:4731–4739. [PubMed]
Cajal SR. (1913–1914). Estudios Sobre la Degeneraci—n y Regeneraci—n del Sistema Nervioso. Madrid: Moya. Translated into English as Degeneration and Regeneration of the Nervous System (R. M. May, tran. and Ed.). London: Oxford University Press, 1928. Reprinted and edited with additional translations by J. DeFelipe and E. G. Jones (), Cajal’s Degeneration and Regeneration of the Nervous System. New York: Oxford University Press;1991.
Caleo M, Menna E, Chierzi S, Cenni MC, Maffei L. Brain-derived neurotrophic factor is an anterograde survival factor in the rat visual system. Curr Biol.2000;10:1155–1161. [PubMed]
Carmignoto G, Maffei L, Candeo P, Canella R, Comelli C. Effect of NGF on the survival of rat retinal ganglion cells following optic nerve section. J Neurosci.1989;9:1263–1272. [PubMed]
Caroni P, Schwab ME. Antibody against myelin-associated inhibitor of neurite growth neutralizes nonpermissive substrate properties of CNS white matter.Neuron. 1988;1:85–96. [PubMed]
Carter DA, Bray GM, Aguayo AJ. Regenerated retinal ganglion cell axons can form well-differentiated synapses in the superior colliculus of adult hamsters. J Neurosci. 1989;9:4042–4050. [PubMed]
Carter DA, Aguayo AJ, Bray GM. Retinal ganglion cell terminals in the hamster superior colliculus: an ultrastructural study. J Comp Neurol. 1991;311:97–107.[PubMed]
Carter DA, Bray GM, Aguayo AJ. Long-term growth and remodeling of regenerated retino-collicular connections in adult hamsters. J Neurosci. 1994;14:590–598.[PubMed]
Carter DA, Jhaveri S. Retino-geniculate axons regenerating in adult hamsters are able to form morphologically distinct terminals. Exp Neurol. 1997;146:315–322.[PubMed]
Carter DA, Bray GM, Aguayo AJ. Regenerated retinal ganglion cell axons form normal numbers of boutons but fail to expand their arbors in the superior colliculus.J Neurocytol. 1998;27:187–196. [PubMed]
Chaudhary P, Ahmed F, Quebada P, Sharma SC. Caspase inhibitors block the retinal ganglion cell death following optic nerve transection. Brain Res Mol Brain Res. 1999;67:36–45. [PubMed]
Chen DF, Jhaveri S, Schneider GE. Intrinsic changes in developing retinal neurons result in regenerative failure of their axons. Proc Natl Acad Sci U S A.1995;92:7287–7291. [PubMed] [Free Full text in PMC]
Cheng L, Sapieha P, Kittlerova P, Hauswirth WW, Di Polo A. TrkB gene transfer protects retinal ganglion cells from axotomy-induced death in vivo. J Neurosci.2002;22:3977–3986. [PubMed]
Cheung ZH, Chan YM, Siu FK, Yip HK, Wu W, Leung MC, So KF. Regulation of caspase activation in axotomized retinal ganglion cells. Mol Cell Neurosci.2004;25:383–393. [PubMed]
Chierzi S, Cenni MC, Maffei L, Pizzorusso T, Porciatti V, Ratto GM, Strettoi E. Protection of retinal ganglion cells and preservation of function after optic nerve lesion in bcl-2 transgenic mice. Vision Res. 1998;38:1537–1543. [PubMed]
Cline HT. Activity-dependent plasticity in the visual systems of frogs and fish. Trends Neurosci. 1991;14:104–111. [PubMed]
Cohen A, Bray GM, Aguayo AJ. Neurotrophin-4/5 (NT-4/5) increases adult rat retinal ganglion cell survival and neurite outgrowth in vitro. J Neurobiol.1994;25:953–959. [PubMed]
Cohen J, Burne JF, Winter J, Bartlett P. Retinal ganglion cells lose response to laminin with maturation. Nature. 1986;322:465–467. [PubMed]
Cui Q, So KF, Yip HK. Major biological effects of neurotrophic factors on retinal ganglion cells in mammals. Biol Signals Recept. 1998;7:220–226. [PubMed]
Cui Q, Harvey AR. CNTF promotes the regrowth of retinal ganglion cell axons into murine peripheral nerve grafts. Neuroreport. 2000;11:3999–4002. [PubMed]
Davenport RW, Thies E, Zhou R, Nelson PG. Cellular localization of ephrin-A2, ephrin-A5, and other functional guidance cues underlies retinotopic development across species. J Neurosci. 1998;18:975–986. [PubMed]
David S, Aguayo AJ. Axonal elongation into peripheral nervous system “bridges” after central nervous system injury in adult rats. Science. 1981;214:931–933.[PubMed]
Davies SJA, Fitch MT, Memberg SP, Hall AK, Raisman G, Silver J. Regeneration of adult axons in white matter tracts of the central nervous system. Nature.1997;390:680–684. [PubMed]
Davies SJA, Goucher DR, Doller C, Silver J. Robust regeneration of adult sensory axons in degenerating white matter of the adult rat spinal cord. J Neurosci.1999;19:5810–5822. [PubMed]
Debski EA, Cline HT. Activity-dependent mapping in the retinotectal projection. Curr Opin Neurobiol. 2002;12:93–99. [PubMed]
de Curtis I, Reichardt LF. Function and spatial distribution in developing chick retina of the laminin receptor alpha 6 beta 1 and its isoforms. Development.1993;118:377–388. [PubMed]
Di Polo A, Aigner LJ, Dunn RJ, Bray GM, Aguayo AJ. Prolonged delivery of brain-derived neurotrophic factor by adenovirus-infected Muller cells temporarily rescues injured retinal ganglion cells. Proc Natl Acad Sci U S A. 1998;95:3978–3983. [PubMed] [Free Full text in PMC]
Dunlop SA, Stirling RV, Rodger J, Symonds AC, Bancroft WJ, Tee LB, Beazley LD. Failure to form a stable topographic map during optic nerve regeneration:abnormal activity-dependent mechanisms. Exp Neurol. 2003;184:805–815. [PubMed]
Dunlop SA, Tee LB, Stirling RV, Taylor AL, Runham PB, Barber AB, Kuchling G, Rodger J, Roberts JD, Harvey AR, Beazley LD. Failure to restore vision after optic nerve regeneration in reptiles: interspecies variation in response to axotomy. J Comp Neurol. 2004;478:292–305. [PubMed]
Dusart I, Morel MP, Wehrle R, Sotelo C. Late axonal sprouting of injured Purkinje cells and its temporal correlation with permissive changes in the glial scar. J Comp Neurol. 1999;408:399–418. [PubMed]
Eitan S, Solomon A, Lavie V, Yoles E, Hirschberg DL, Belkin M, Schwartz M. Recovery of visual response of injured adult rat optic nerves treated with transglutaminase. Science. 1994;264:1764–1768. [PubMed]
Ellezam B, Dubreuil C, Winton M, Loy L, Dergham P, Selles-Navarro I, McKerracher L. Inactivation of intracellular Rho to stimulate axon growth and regeneration.Prog Brain Res. 2002;137:371–380. [PubMed]
Fagiolini M, Caleo M, Strettoi E, Maffei L. Axonal transport blockade in the neonatal rat optic nerve induces limited retinal ganglion cell death. J Neurosci.1997;17:7045–7052. [PubMed]
Fawcett JW. Astrocytic and neuronal factors affecting axon regeneration in the damaged central nervous system. Cell Tissue Res. 1997;290:371–377. [PubMed]
Finlay BL, Schneps SE, Wilson KG, Schneider GE. Topography of visual and somatosensory projections to the superior colliculus of the golden hamster. Brain Res. 1978;142:223–235. [PubMed]
Fischer D, Heiduschka P, Thanos S. Lens-injury-stimulated axonal regeneration throughout the optic pathway of adult rats. Exp Neurol. 2001;172:257–272.[PubMed]
Fischer D, He Z, Benowitz LI. Counteracting the Nogo receptor enhances optic nerve regeneration if retinal ganglion cells are in an active growth state. J Neurosci. 2004;24:1646–1651. [PubMed]
Fortin S, Chabli A, Dumont I, Shumikhina S, Itaya SK, Molotchnikoff S. Maturation of visual receptive field properties in the rat superior colliculus. Brain Res Dev Brain Res. 1999;112:55–64. [PubMed]
Fraser SE, Perkel DH. Competitive and positional cues in the patterning of nerve connections. J Neurobiol. 1990;21:51–72. [PubMed]
Frost DO. Development of anomalous retinal projections to nonvisual thalamic nuclei in Syrian hamsters: a quantitative study. J Comp Neurol. 1986;252:95–105.[PubMed]
Fukuda Y, Watanabe M, Sawai H, Miyoshi T. Functional recovery of vision in regenerated optic nerve fibers. Vision Res. 1998;38:1545–1553. [PubMed]
Garcia del Cano G, Gerrikagoitia I, Martinez-Millan L. Plastic reaction of the rat visual corticocollicular connection after contralateral retinal deafferentiation at the neonatal or adult stage: axonal growth versus reactive synaptogenesis. J Comp Neurol. 2002;446:166–178. [PubMed]
Gaze RM (1970) The formation of nerve connections, Academic Press, New York.
Gerrikagoitia I, Garcia Del Cano G, Martinez-Millan L. Changes of the cholinergic input to the superior colliculus following enucleation in neonatal and adult rats.Brain Res. 2001;898:61–72. [PubMed]
Gilbert CD. Adult cortical dynamics. Physiol Rev. 1998;78:467–485. [PubMed]
Ghosh A, David S. Neurite growth-inhibitory activity in the adult rat cerebral cortical gray matter. J Neurobiol. 1997;32:671–683. [PubMed]
Godement P, Bonhoeffer F. Cross-species recognition of tectal cues by retinal fibers in vitro. Development. 1989;106:313–320. [PubMed]
Goodhill GJ. Mathematical guidance for axons. Trends Neurosci. 1998;21:226–231. [PubMed]
Harvey AR, Worthington DR. The projection from different visual cortical areas to the rat superior colliculus. J Comp Neurol. 1990;298:281–292. [PubMed]
Honda H. Topographic mapping in the retinotectal projection by means of complementary ligand and receptor gradients: a computer simulation study. J Theor Biol. 1998;192:235–246. [PubMed]
Holt CE, Harris WA. Order in the initial retinotectal map in Xenopus: a new technique for labelling growing nerve fibres. Nature. 1983;301:150–152. [PubMed]
Houser CR, Lee M, Vaughn JE. Immunocytochemical localization of glutamic acid decarboxylase in normal and deafferented superior colliculus: evidence for reorganization of gamma-aminobutyric acid synapses. J Neurosci. 1983;3:2030–2042. [PubMed]
Hua SE, Massone LL, Houk JC. Model of topographic map development guided by a transiently expressed repulsion molecule. Neuroreport. 1993;4:1319–1322.[PubMed]
Ichijo H, Bonhoeffer F. Differential withdrawal of retinal axons induced by a secreted factor. J Neurosci. 1998;18:5008–5018. [PubMed]
Isa T, Endo T, Saito Y. The visuo-motor pathway in the local circuit of the rat superior colliculus. J Neurosci. 1998;18:8496–8504. [PubMed]
Isenmann S, Wahl C, Krajewski S, Reed JC, Bahr MB. Up-regulation of Bax protein in degenerating retinal ganglion cells precedes apoptotic cell death after optic nerve lesion in the rat. Eur J Neurosci. 1997;9:1763–1772. [PubMed]
Kaas JH, Merzenich MM, Killackey HP. The reorganization of somatosensory cortex following peripheral nerve damage in adult and developing mammals. Annu Rev Neurosci. 1983;6:325–356. [PubMed]
Kaplan DR, Miller FD. Neurotrophin signal transduction in the nervous system. Curr Opin Neurobiol. 2000;10:381–391. [PubMed]
Kermer P, Klocker N, Labes M, Šhr MB. Inhibition of CPP32-like proteases rescues axotomized retinal ganglion cells from secondary cell death in vivo. J Neurosci.1998;18:4656–4662. [PubMed]
Kermer P, Klocker N, Labes M, Thomsen S, Srinivasan A, Bahr M. Activation of caspase-3 in axotomized rat retinal ganglion cells in vivo. FEBS Lett.1999;453:361–364. [PubMed]
Kermer P, Klocker N, Bahr M. Long-term effect of inhibition of ced 3-like caspases on the survival of axotomized retinal ganglion cells in vivo. Exp Neurol.1999;158:202–205. [PubMed]
Kermer P, Ankerhold R, Klocker N, Krajewski S, Reed JC, Bahr M. Caspase-9: involvement in secondary death of axotomized rat retinal ganglion cells in vivo.Brain Res Mol Brain Res. 2000;85:144–150. [PubMed]
Keirstead SA, Rasminsky M, Fukuda Y, Carter DA, Aguayo AJ, Vidal-Sanz M. Electrophysiologic responses in hamster superior colliculus evoked by regenerating retinal axons. Science. 1989;246:255–257. [PubMed]
Kikuchi M, Tenneti L, Lipton SA. Role of p38 mitogen-activated protein kinase in axotomy-induced apoptosis of rat retinal ganglion cells. J Neurosci.2000;20:5037–5044. [PubMed]
Kim JE, Li S, GrandPre T, Qiu D, Strittmatter SM. Axon regeneration in young adult mice lacking Nogo-A/B. Neuron. 2003;38:187–199. [PubMed]
King CE, Wallace A, Rodger J, Bartlett C, Beazley LD, Dunlop SA. Transient up-regulation of retinal EphA3 and EphA5, but not ephrin-A2, coincides with re-establishment of a topographic map during optic nerve regeneration in goldfish. Exp Neurol. 2003;183:593–599. [PubMed]
Klocker N, Braunling F, Isenmann S, Bahr M. In vivo neurotrophic effects of GDNF on axotomized retinal ganglion cells. Neuroreport. 1997;8:3439–3442.[PubMed]
Klocker N, Cellerino A, Bahr M. Free radical scavenging and inhibition of nitric oxide synthase potentiates the neurotrophic effects of brain-derived neurotrophic factor on axotomized retinal ganglion cells in vivo. J Neurosci. 1998;18:1038–1046. [PubMed]
Koeberle PD, Ball AK. Effects of GDNF on retinal ganglion cell survival following axotomy. Vision Res. 1998;38:1505–1515. [PubMed]
Koeberle PD, Ball AK. Nitric oxide synthase inhibition delays axonal degeneration and promotes the survival of axotomized retinal ganglion cells. Exp Neurol.1999;158:366–381. [PubMed]
Koeberle PD, Ball AK. Neurturin enhances the survival of axotomized retinal ganglion cells in vivo: combined effects with glial cell line-derived neurotrophic factor and brain-derived neurotrophic factor. Neuroscience. 2002;110:555–567. [PubMed]
Koeberle PD, Gauldie J, Ball AK. Effects of adenoviral-mediated gene transfer of interleukin-10, interleukin-4, and transforming growth factor-beta on the survival of axotomized retinal ganglion cells. Neuroscience. 2004;125:903–920. [PubMed]
Koeberle PD, Bahr M. Growth and guidance cues for regenerating axons: where have they gone? J Neurobiol. 2004;59:162–180. [PubMed]
Kugler S, Straten G, Kreppel F, Isenmann S, Liston P, Bahr M. The X-linked inhibitor of apoptosis (XIAP) prevents cell death in axotomized CNS neurons in vivo.Cell Death Differ. 2000;7:815–824. [PubMed]
Laurberg S, Zimmer J. Lesion-induced sprouting of hippocampal mossy fiber collaterals to the fascia dentata in developing and adult rats. J Comp Neurol.1981;200:433–459. [PubMed]
Lemann W, Saper CB, Rye DB, Wainer BH. Stabilization of TMB reaction product for electron microscopic retrograde and anterograde fiber tracing. Brain Res Bull. 1985;14:277–281. [PubMed]
Leon S, Yin Y, Nguyen J, Irwin N, Benowitz LI. Lens injury stimulates axon regeneration in the mature rat optic nerve. J Neurosci. 2000;20:4615–4626. [PubMed]
Li Y, Sauve Y, Li D, Lund RD, Raisman G. Transplanted olfactory ensheathing cells promote regeneration of cut adult rat optic nerve axons. J Neurosci.2003;23:7783–7788. [PubMed]
Ling C, Schneider GE, Jhaveri S. Target-specific morphology of retinal axon arbors in the adult hamster. Vis Neurosci. 1998;15:559–579. [PubMed]
Liuzzi FJ, Lasek RJ. Astrocytes block axonal regeneration in mammals by activating the physiological stop pathway. Science. 1987;237:642–645. [PubMed]
Lund RD, Lund JS. Synaptic adjustment after deafferentation of the superior colliculus of the rat. Science. 1971;171:804–807. [PubMed]
MacEvoy SP, Paradiso MA. Lightness constancy in primary visual cortex. Proc Natl Acad Sci U S A. 2001;98:8827–8831. [PubMed] [Free Full text in PMC]
Mansour-Robaey S, Clarke DB, Wang YC, Bray GM, Aguayo AJ. Effects of ocular injury and administration of brain-derived neurotrophic factor on survival and regrowth of axotomized retinal ganglion cells. Proc Natl Acad Sci U S A. 1994;91:1632–1636. [PubMed] [Free Full text in PMC]
Matsumoto N, Kometami M, Nagano K. Regenerating retinal fibers of the goldfish make temporary and unspecific but functional synapses before forming the final retinotopic projection. Neuroscience. 1987;22:1103–1110. [PubMed]
McKerracher L, David S, Jackson DL, Kottis V, Dunn RJ, Braun PE. Identification of myelin-associated glycoprotein as a major myelin-derived inhibitor of neurite growth. Neuron. 1994;13:805–811. [PubMed]
McLoon SC, McLoon LK, Palm SL, Furcht LT. Transient expression of laminin in the optic nerve of the developing rat. J Neurosci. 1988;8:1981–1990. [PubMed]
Merzenich MM, Nelson RJ, Stryker MP, Cynader MS, Schoppmann A, Zook JM. Somatosensory cortical map changes following digit amputation in adult monkeys. J Comp Neurol. 1984;224:591–605. [PubMed]
Mey J, Thanos S. Intravitreal injections of neurotrophic factors support the survival of axotomized retinal ganglion cells in adult rats in vivo. Brain Res.1993;602:304–317. [PubMed]
Meyer RL, Kageyama GH. Large-scale synaptic errors during map formation by regeneration of optic axons in the goldfish. J Comp Neurol. 1999;409:299–312.[PubMed]
Miyamoto T, Sakurai T, Okada Y. Masking effect of NMDA receptor antagonists on the formation of long-term potentiation (LTP) in superior colliculus slices from the guinea pig. Brain Res. 1990;518:166–172. [PubMed]
Mize RR. Immunocytochemical localization of gamma-aminobutyric acid (GABA) in the cat superior colliculus. J Comp Neurol. 1988;276:169–187. [PubMed]
Nakamoto M, Cheng HJ, Friedman GC, McLaughlin T, Hansen MJ, Yoon CH, O’Leary DD, Flanagan JG. Topographically specific effects of ELF-1 on retinal axon guidance in vitro and retinal axon mapping in vivo. Cell. 1996;86:755–766. [PubMed]
Oster SF, Deiner M, Birgbauer E, Sretavan DW. Ganglion cell axon pathfinding in the retina and optic nerve. Semin Cell Dev Biol. 2004;15:125–136. [PubMed]
Patapoutian A, Reichardt LF. Trk receptors: mediators of neurotrophin action. Curr Opin Neurobiol. 2001;11:272–280. [PubMed]
Peinado-Ramon P, Salvador M, Villegas-Perez MP, Vidal-Sanz M. Effects of axotomy and intraocular administration of NT-4, NT-3, and brain-derived neurotrophic factor on the survival of adult rat retinal ganglion cells. A quantitative in vivo study. Invest Ophthalmol Vis Sci. 1996;37:489–500. [PubMed]
Penn AA, Riquelme PA, Feller MB, Shatz CJ. Competition in retinogeniculate patterning driven by spontaneous activity. Science. 1998;279:2108–2112.[PubMed]
Pinard R, Benfares J, Lanoir J. Electron microscopic study of GABA-immunoreactive neuronal processes in the superficial gray layer of the rat superior colliculus: their relationships with degenerating retinal nerve endings. J Neurocytol. 1991;20:262–276. [PubMed]
Quigley HA, Nickells RW, Kerrigan LA, Pease ME, Thibault DJ, Zack DJ. Retinal ganglion cell death in experimental glaucoma and after axotomy occurs by apoptosis. Invest Ophthalmol Vis Sci. 1995;36:774–786. [PubMed]
Quigley HA, McKinnon SJ, Zack DJ, Pease ME, Kerrigan-Baumrind LA, Kerrigan DF, Mitchell RS. Retrograde axonal transport of BDNF in retinal ganglion cells is blocked by acute IOP elevation in rats. Invest Ophthalmol Vis Sci. 2000;41:3460–3466. [PubMed]
Rager G, Morino P, Schnitzer J, Sonderegger P. Expression of the axonal cell adhesion molecules axonin-1 and Ng-CAM during the development of the chick retinotectal system. J Comp Neurol. 1996;365:594–609. [PubMed]
Raibon E, Sauve Y, Carter DA, Gaillard F. Microglial changes accompanying the promotion of retinal ganglion cell axonal regeneration into peripheral nerve grafts.J Neurocytol. 2002;31:57–71. [PubMed]
Raisman G. Myelin inhibitors: does NO mean GO? Nat Rev Neurosci. 2004;5:157–161. [PubMed]
Rhodes KE, Fawcett JW. Chondroitin sulphate proteoglycans: preventing plasticity or protecting the CNS? J Anat. 2004;204:33–48. [PubMed] [Free Full text in PMC]
Richardson PM, McGuinness UM, Aguayo AJ. Axons from CNS neurons regenerate into PNS grafts. Nature. 1980;284:264–265. [PubMed]
Rodger J, Vitale PN, Tee LB, King CE, Bartlett CA, Fall A, Brennan C, O’Shea JE, Dunlop SA, Beazley LD. EphA/ephrin-A interactions during optic nerve regeneration: restoration of topography and regulation of ephrin-A2 expression. Mol Cell Neurosci. 2004;25:56–68. [PubMed]
Roskies AL, O’Leary DDM. Control of topographic retinal axon branching by inhibitory membrane-bound molecules. Science. 1994;265:799–803. [PubMed]
Salin P, Tseng GF, Hoffman S, Parada I, Prince DA. Axonal sprouting in layer V pyramidal neurons of chronically injured cerebral cortex. J Neurosci.1995;15:8234–8245. [PubMed]
Sasaki H, Inoue T, Iso H, Fukuda Y, Hayashi Y. Light-dark discrimination after sciatic nerve transplantation to the sectioned optic nerve in adult hamsters. Vision Res. 1993;33:877–880. [PubMed]
Sasaki H, Coffey P, Villegas-Perez MP, Vidal-Sanz M, Young MJ, Lund RD, Fukuda Y. Light induced EEG desynchronization and behavioral arousal in rats with restored retinocollicular projection by peripheral nerve graft. Neurosci Lett. 1996;218:45–48. [PubMed]
Sauve Y, Sawai H, Rasminsky M. Functional synaptic connections made by regenerated retinal ganglion cell axons in the superior colliculus of adult hamsters. J Neurosci. 1995;15:665–675. [PubMed]
Sauve Y, Sawai H, Rasminsky M. Topological specificity in reinnervation of the superior colliculus by regenerated retinal ganglion cell axons in adult hamsters. J Neurosci. 2001;21:951–960. [PubMed]
Sauve Y, Girman SV, Wang S, Lawrence JM, Lund RD. Progressive visual sensitivity loss in the Royal College of Surgeons rat: perimetric study in the superior colliculus. Neuroscience. 2001;103:51–63. [PubMed]
Sawai H, Clarke DB, Kittlerova P, Bray GM, Aguayo AJ. Brain-derived neurotrophic factor and neurotrophin-4/5 stimulate growth of axonal branches from regenerating retinal ganglion cells. J Neurosci. 1996;16:3887–3894. [PubMed]
Schmidt JT. Activity-driven sharpening of the retinotectal projection: the search for retrograde synaptic signaling pathways. J Neurobiol. 2004;59:114–133.[PubMed]
Schwartz M. Optic nerve crush: protection and regeneration. Brain Res Bull. 2004;62:467–471. [PubMed]
Schwartz M. Vaccination for glaucoma: dream or reality? Brain Res Bull. 2004;62:481–484. [PubMed]
Shatz CJ. Impulse activity and the patterning of connections during CNS development. Neuron. 1990;5:745–756. [PubMed]
Shewan D, Berry M, Cohen J. Extensive regeneration in vitro by early embryonic neurons on immature and adult CNS tissue. J Neurosci. 1995;15:2057–2062.[PubMed]
Siminoff R, Schwassmann HO, Kruger L. An electrophysiological study of the visual projection to the superior colliculus of the rat. J Comp Neurol. 1966;127:435–444. [PubMed]
Simon DK, O’Leary DDM. Responses of retinal axons in vivo and in vitro to position-encoding molecules in the embryonic superior colliculus. Neuron.1992;9:977–989. [PubMed]
Simon DK, O’Leary DDM. Development of topographic order in the mammalian retino collicular projection. J Neurosci. 1992;12:1212–1232. [PubMed]
Smith GM, Rutishauser U, Silver J, Miller RH. Maturation of astrocytes in vitro alters the extent and molecular basis of neurite outgrowth. Dev Biol. 1990;138:377–390. [PubMed]
Smith SA, Bedi KS. Unilateral eye enucleation in adult rats causes neuronal loss in the contralateral superior colliculus. J Anat. 1997;190:481–490. [PubMed] [Free Full text in PMC]
Smith-Thomas LC, Fok-Seang J, Stevens J, Du J-S, Muir E, Faissner F, Geller HM, Rogers JH, Fawcett JW. An inhibitor of neurite outgrowth produced by astrocytes. J Cell Sci. 1994;107:1687–1695. [PubMed]
So KF, Aguayo AJ. Lengthy regrowth of cut axons from ganglion cells after peripheral nerve transplantation into the retina of adult rats. Brain Res. 1985;328:349–354. [PubMed]
Spalding KL, Rush RA, Harvey AR. Target-derived and locally derived neurotrophins support retinal ganglion cell survival in the neonatal rat retina. J Neurobiol.2004;60:319–327. [PubMed]
Spencer T, Filbin MT. A role for cAMP in regeneration of the adult mammalian CNS. J Anat. 2004;204:49–55. [PubMed] [Free Full text in PMC]
Sperry RW. Neural basis of the spontaneous optokinetic response produced by visual inversion. J Comp Physiol Psychol. 1950;43:482–489. [PubMed]
Sperry RW. Chemoaffinity in the orderly growth of nerve fiber patterns and connections. Proc Natl Acad Sci U S A. 1963;50:703–709. [PubMed]
Sperry RW. (1965) Embryogenesis of behavioral nerve nets. In: DeHann RL, Ursprung H, editors. Organogenesis. New York: Holt, Rinehart and Winston. p.161-186.
Stelzner DJ, Bohn RC, Strauss JA (1986) Regeneration of the frog optic nerve. Comparisons with development. Neurochem Pathol. 5:255-288.
Stuermer CA, Bastmeyer M, Bahr M, Strobel G, Paschke K. Trying to understand axonal regeneration in the CNS of fish. J Neurobiol. 1992;23:537–550.[PubMed]
Tello F. La regeneration dans les voies optiques. Trab Lab Invest Biol Univ Madr. 1907;5:237–248.
Thanos S. Adult retinofugal axons regenerating through peripheral nerve grafts can restore the light-induced pupilloconstriction reflex. Eur J Neurosci.1992;4:691–699. [PubMed]
Thanos S, Mey J, Wild M. Treatment of the adult retina with microglia-suppressing factors retards axotomy-induced neuronal degradation and enhances axonal regeneration in vivo and in vitro. J Neurosci. 1993;13:455–466. [PubMed]
Thanos S, Mey J. Type-specific stabilization and target-dependent survival of regenerating ganglion cells in the retina of adult rats. J Neurosci. 1995;15:1057–1079. [PubMed]
Tiao YC, Blakemore C. Functional organization in the superior colliculus of the golden hamster. J Comp Neurol. 1976;168:483–503. [PubMed]
Tropea D, Caleo M, Maffei L. Synergistic effects of brain-derived neurotrophic factor and chondroitinase ABC on retinal fiber sprouting after denervation of the superior colliculus in adult rats. J Neurosci. 2003;23:7034–7044. [PubMed]
Turner JP, Sauve Y, Lund RD, Salt TA. Retinotectal synaptic transmission in vitro after the repair of cut optic nerve with a peripheral nerve graft. Soc Neurosci Abstr. 2001;2799.Gaze RM. The formation of nerve connections. New York: Academic Press. 1970100.
Udin SB, Fawcett JW. Formation of topographic maps. Annu Rev Neurosci. 1988;11:289–327. [PubMed]
Vidal-Sanz M, Bray GM, Villegas-Perez MP, Thanos S, Aguayo AJ. Axonal regeneration and synapse formation in the superior colliculus by retinal ganglion cells in the adult rat. J Neurosci. 1987;7:2894–2909. [PubMed]
Vidal-Sanz M, Bray GM, Aguayo AJ. Regenerated synapses persist in the superior colliculus after the regrowth of retinal ganglion cell axons . J Neurocytol.1991;20:940–952. [PubMed]
Villegas-Perez MP, Vidal-Sanz M, Bray GM, Aguayo AJ. Influences of peripheral nerve grafts on the survival and regrowth of axotomized retinal ganglion cells in adult rats. J Neurosci. 1988;8:265–280. [PubMed]
Villegas-Perez MP, Vidal-Sanz M, Rasminsky M, Bray GM, Aguayo AJ. Rapid and protracted phases of retinal ganglion cell loss follow axotomy in the optic nerve of adult rats. J Neurobiol. 1993;24:23–36. [PubMed]
von Boxberg Y, Deiss S, Schwarz U. Guidance and topographic stabilization of nasal chick retinal axons on target-derived components in vitro. Neuron.1993;10:345–357. [PubMed]
Walter J, Henke-Fahle S, Bonhoeffer F. Avoidance of posterior tectal membrane by temporal retinal axons. Development. 1987;101:909–913. [PubMed]
Walter J, Kern-Veitis B, Huf J, Stolze B, Bonhoeffer F. Recognition of position specific properties of tectal cell membranes by retinal axons in vitro. Development.1987;101:685–696. [PubMed]
Watanabe M, Fukuda Y. Survival and axonal regeneration of retinal ganglion cells in adult cats. Prog Retin Eye Res. 2002;21:529–553. [PubMed]
Weise J, Isenmann S, Klocker N, Kugler S, Hirsch S, Gravel C, Bahr M. Adenovirus-mediated expression of ciliary neurotrophic factor (CNTF) rescues axotomized rat retinal ganglion cells but does not support axonal regeneration in vivo. Neurobiol Dis. 2000;7:212–223. [PubMed]
Weishaupt JH, Bahr M. Degeneration of axotomized retinal ganglion cells as a model for neuronal apoptosis in the central nervous system – molecular death and survival pathways. Restor Neurol Neurosci. 2001;19:19–27. [PubMed]
Weishaupt JH, Diem R, Kermer P, Krajewski S, Reed JC, Bahr M. Contribution of caspase-8 to apoptosis of axotomized rat retinal ganglion cells in vivo. Neurobiol Dis. 2003;13:124–135. [PubMed]
Whiteley SJ, Sauve Y, Aviles-Trigueros M, Vidal-Sanz M, Lund RD. Extent and duration of recovered pupillary light reflex following retinal ganglion cell axon regeneration through peripheral nerve grafts directed to the pretectum in adult rats. Exp Neurol. 1998;154:560–572. [PubMed]
Williams SE, Mason CA, Herrera E. The optic chiasm as a midline choice point. Curr Opin Neurobiol. 2004;14:51–60. [PubMed]
Wizenmann A, Thies E, Klostermann S, Bonhoeffer F, Bahr M. Appearance of target-specific guidance information for regenerating axons after CNS lesions.Neuron. 1993;11:975–983. [PubMed]
Wizenmann A, Bahr M. Growth characteristics of ganglion cell axons in the developing and regenerating retinotectal projection of the rat. Cell Tissue Res.1997;290:395–403. [PubMed]
Wizenmann A, Bahr M. Growth preferences of adult rat retinal ganglion cell axons in retinotectal cocultures. J Neurobiol. 1998;35:379–387. [PubMed]
Wong RO. Retinal waves and visual system development. Annu Rev Neurosci. 1999;22:29–47. [PubMed]
Xerri C, Merzenich MM, Peterson BE, Jenkins W. Plasticity of primary somatosensory cortex paralleling sensorimotor skill recovery from stroke in adult monkeys. J Neurophysiol. 1998;79:2119–2148. [PubMed]
Yin Y, Cui Q, Li Y, Irwin N, Fischer D, Harvey AR, Benowitz LI. Macrophage-derived factors stimulate optic nerve regeneration. J Neurosci. 2003;23:2284–2293.[PubMed]
Yip HK, So KF. Axonal regeneration of retinal ganglion cells: effect of trophic factors. Prog Retin Eye Res. 2000;19:559–575. [PubMed]
Zwimpfer TJ, Aguayo AJ, Bray GM. Synapse formation and preferential distribution in the granule cell layer by regenerating retinal ganglion cell axons guided to the cerebellum of adult hamsters. J Neurosci. 1992;12:1144–1159. [PubMed]