Mahnoosh Farsaii and Victoria P. Connaughton
1. Introduction.
The AII amacrine cell is characterized by a multifaceted connectivity and physiology. It is unique among amacrine cells in that it participates predominantly in the vertical flow of information though the inner retina, contributing to center mechanisms, rather than in lateral inhibitory pathways. As its multilayer stratification, and synaptic partners, suggest, the AII transmits both rod and cone-driven signals within the ON- and OFF- retinal pathways to the inner retina. This chapter summarizes the morphological and physiological characteristics of AII cells focusing on data obtained from four mammalian species: rat, cat, rabbit, and monkey.
2. Morphology and Distribution.
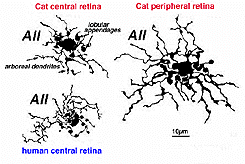
The AII amacrine cell, originally described by Kolb and Famiglietti (1974; Famiglietti & Kolb, 1975) is the most studied amacrine cell of the vertebrate retina. It has been identified in a variety of mammalian retinas. The AII is a narrow-field, bistratified amacrine cell that is involved in synaptic contacts throughout the IPL. Its morphology has been identified using a variety of staining methods, such as Golgi techniques (Fig.1a) (Kolb & Famiglietti, 1974; Famiglietti & Kolb, 1975), Lucifer and HRP labeling of recorded neurons (Nelson, 1982), and fluorescent dyes such as DAPI (4,6-diamidi no-2-phenylindole) or Lucifer Yellow (Vaney, 1985, 1991; Mills & Massey, 1991).
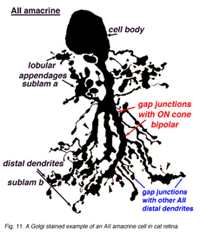 Fig. 1a. Golgi stained image of an AII amacrine cell in cat retina. |
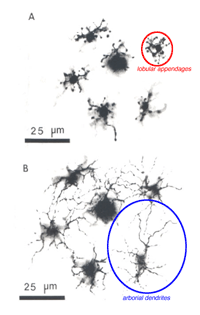
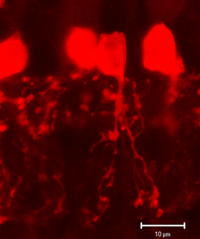 Fig. 1b. AII amacrine cells immunocytochemically stained with anti-parvalbumen in rodent retina (courtesy Nicolas Cuenca). |
More recently, immunocytochemical methods targeting various calcium binding proteins (parvalbumin and calretinin) have been employed to selectively label AII cells in rabbit (Casini et al., 1995; Massey & Mills, 1999) and rodent (Fig. 1b) (Wassle et al., 1993). Primate AII cells are also labeled with calretinin (Wassle et al., 1995; Mills & Massey, 1999 ), though not selectively (Kolb et al., 2002). Regardless of technique used, the AII cell has a consistent morphology (Figure 1a) with a round or oval shaped cell body 8-10um in diameter located in the proximal inner nuclear layer. In peripheral retina, the cell body often extends into the IPL. The distribution of AII cell bodies is non-random, forming a regular mosaic (Vaney, 1985; Mills & Massey, 1991; Kolb et al., 2000). Calculated regularity indices range from 4.0 (inferior retina) to 5.4 (temporal midperipheral retina) in rabbit (Vaney et al., 1991a), cat (Vaney, 1985) and rat (Wassle et al., 1993); a lower value was calculated in monkey (Wassle et al., 1995). AII dendritic trees are reported to be more regularly distributed than their cell bodies, with their processes filling in gaps between adjacent cell bodies to get full coverage of the IPL (Vaney et al., 1991a; Wassle et al., 1995).
The bistratified nature of AII cells arises from two distinct dendritic trees (Figures 1a, b). The dendritic tree in sublamina a (s1 and s2) is composed of short, thin processes ending in lobular appendages that radiate in all directions from typically a single primary dendrite (Famiglietti & Kolb, 1975; Kolb & Famiglietti, 1976; Vaney et al., 1991b; Strettoi et al., 1992). Quantitative analysis of serial sections of one particular rabbit AII cell (Strettoi et al., 1992) identified 21 lobular appendages ranging in diameter from 1.6 – 5.5um. In contrast, the processes that form the dendritic tree in sublamina b are called the distal (or arboreal) dendrites and terminate predominantly in stratum 5. The distal dendrites are thin processes that possess spines along their length and branch more widely than the lobular appendages (Figure 1a). The above characteristics are conserved in rabbit, rat, cat, and macaque monkey retinas (Kolb et al., 1976; Vaney, 1985; Kolb et al., 1992; Wassle et al., 1993; Wassle et al., 1995; Mills & Massey, 1999).
AII distribution and morphology is different in central versus peripheral retina. AII density is greatest in extrafoveal central retina and decreases with increasing eccentricity. Simultaneously, both dendritic fields enlarge with eccentricity (Fig. 2a) (Kolb et al., 1981; Mills & Massey, 1991; Vaney et al., 1991a; Vaney et al., 1991b; Wassle et al., 1993), with a more pronounced change reported for the distal dendrites. For example, AII cell density in central cat retina is maximal at 5300 cells/mm2 and decreases to 500-800 cells/mm2 in peripheral retina. The diameter of the lobular appendage field increases from 16um (central retina) to 30um (8mm eccentricity); whereas, the diameter of the distal arbor expands from 18um to 58um (Vaney, 1985) (Fig.2b). This morphological trend has also been identified in other mammalian retinas.
The distal dendrites in sublamina b of adjacent AII cells overlap. The amount of overlap has been quantified by calculating the coverage (dendritic field area x cell density) of each cell (Casini et al., 1995). In rabbit, a coverage value of 1.8 was calculated for distal dendrites in the central retina (visual streak) and 2.4 in the inferior retina. The greatest overlap is seen in the superior peripheral retina, where a coverage value of ~10 was calculated (Vaney et al., 1991a). In contrast, there is little or no overlap between the lobular dendrites in sublamina a of adjacent rabbit AII cells, as coverage varied from ~1.0 in the inferior retina and ~0.8 in the superior retina (Vaney et al., 1991a) were calculated. These values show that the lobular appendages maintain a constant coverage over the entirety of the retinal area; while the distal processes vary in overlap along both central-to-peripheral and superior-to-inferior axes. Similar differences in AII dendritic processes have been reported in cat, monkey, and rat, suggesting differences in function between AII processes in the distal and proximal IPL (Vaney, 1985; Wassle et al., 1993; Wassle et al., 1995).
3. Synaptic connectivity.
Chemical inputs to distal dendrites in sublamina b
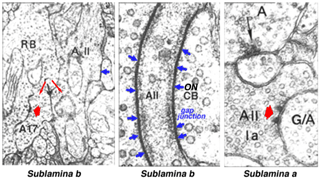
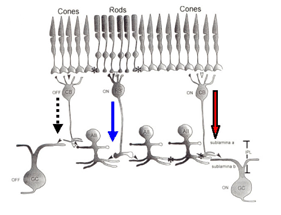
The AII receives direct glutamatergic inputs in sublamina b (s5) from rod bipolar cells (RBC)(Fig.3). At this synapse, the AII forms one member of the postsynaptic dyad (Kolb & Famiglietti, 1974; Famiglietti & Kolb, 1975; Strettoi et al., 1990; Strettoi et al., 1992; Chun et al., 1993; Kim et al., 1998); the other member (discussed below) is a GABA-containing amacrine cell known as A17 in cat and S1/S2 in rabbit (Massey et al., 1992). The latter cell types make reciprocal synapses back onto the RBC terminal at GABAc receptors on the RBC (Sandell et al., 1989; Grunert & Wassle, 1990; Strettoi et al., 1990; Chun et al., 1993; Kim etal., 1998; Zhang et al., 2002). Each AII receives input from several RBCs.
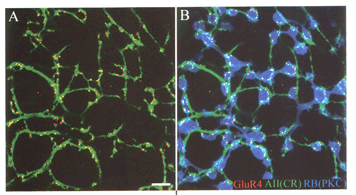
In all mammals, physiological studies show glutamate-elicited currents in AII amacrine cells are mediated by AMPA-preferring receptors (Boos et al., 1993; Cohen & Miller, 1999; Morkve et al., 2002) sensitive to the non-selective antagonist CNQX and the AMPA receptor specific compounds GYKI-52466 and cyclothiazide (Morkve et al., 2002; Singer & Diamond, 2003; Veruki et al., 2003). The receptors are formed from GluR3 (Dacheux & Raviola, 1986; Qin & Pourcho, 1999a, 1999b; Gabriel et al., 2002) and/or GluR4 (Ghosh et al., 2001; Li et al., 2002) subunits (Fig. 4), which confer high calcium permeability to the channel.
NMDA responses have also been identified on rat (Hartveit & Veruki, 1997) and rabbit (Bloomfield & Xin, 2000) AII cells. These findings contradict immunocytochemical studies which note the absence of NMDA receptor subunits on AII dendritic processes (Hartveit et al., 1994; Goebel et al., 1998; Fletcher et al., 2000; Grunert et al., 2002). Recent findings indicate NMDA responses do not mediate transmission at the RBC-to-AII synapse (Singer & Diamond, 2003), suggesting these receptors, when present, may be located extra-synaptically. The NMDA response in AII cells may also depend on an intracellular signaling pathway, as the response runs down quickly in patch clamp studies (Hartveit & Veruki, 1997).
Though one AII amacrine cell is associated with almost all RBC dyads rod bipolar connections comprise only ~30% of the inputs to AII cells (Strettoi et al., 1992). These cells also receive chemical inputs from other amacrine cells within s1 and s5 in rabbit; s1, s3-s5 in rat and cat and in monkey. The amacrine cell(s) presynaptic to AII distal dendrites have not generally been identified, except for dopaminergic amacrine cells in cat (Kolb et al., 1990). Amacrine contacts comprise ~40% of the synaptic inputs in sublamina b according to Strettoi et al., (1992).
Electrical connections of distal dendrites in sublamina b
AIIs are electrically coupled to ON cone bipolar cells (CBC) throughout sublamina b (Fig. 3) (Kolb and Famiglietti, 1974: Famiglietti and Kolb, 1975: Kolb, 1979; Strettoi et al., 1992; Veruki & Hartveit, 2002a) and to other AII cells in s5 (Strettoi et al., 1990; Strettoi et al., 1992; Strettoi et al., 1994). AII/AII junctions are structurally formed from connexin 36 (Feigenspan et al., 2001; Mills et al., 2001). Signal propagation is bidirectional, promoting synchronized responses (i.e., action potentials) among connected cells (Veruki & Hartveit, 2002b).
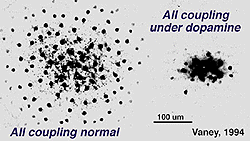
Dopamine application reduces AII/AII coupling (Mills & Massey, 1995) through the activation of D1-type dopamine receptors (Hampson et al., 1992) and the formation of cAMP (Fig. 5a)(Hampson et al., 1992; Cooper et al., 1996). In contrast, gap junctions formed between AII and ON-CBC are heterologous, formed by connexin 36 on AII processes and either connexin 36 or another (possibly 45) connexin on CBC (Feigenspan et al., 2001; Mills et al., 2001; Lin et al., 2005). These junctions are also bidirectional (Cohen & Miller, 1999; Bloomfield & Xin, 2000; Trexler et al., 2001), though signals are more effectively propagated in the direction, AII to ON-CBC (Veruki & Hartveit, 2002a). The permeability of AII/ON-CBC channels appears to be regulated by nitric oxide and cGMP (Mills & Massey, 1995). Nitric oxide activates guanylate cyclase, increasing intracellular cGMP, and decreases coupling between these cell types.
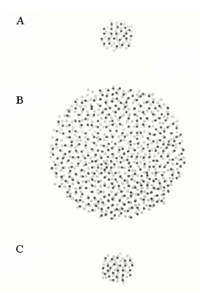
The reported extent of coupling among AII cells varies greatly, from modeling estimates that each AII is coupled to three other AII cells (Vardi & Smith, 1996) to dye-coupling studies showing the network can range from ~20 to >300 coupled cells (Vaney, 1991; Bloomfield et al., 1997). This difference in coupling extent is dependent upon adaptational state (Fig. 5b). Changes in AII/AII coupling have been shown to reflect changes in the diameter of the receptive field center (Figure 5b). For example, in dark-adapted tissue, one AII cell is coupled to a network of ~20 other cells, and the corresponding diameter of the receptive field center averages ~74 um (Bloomfield et al., 1997). When the intensity of light is increased, but still within the operating range of the cell (~2.25 log intensity unit), both the extent of coupling and the diameter of the receptive field center increase (Fig. 5b). Further increases in light intensity actually results in a decrease in coupling to levels comparable to those seen in dark-adapted conditions (Fig.5b). Thus, the cells are extensively coupled over their operating range, but uncouple at the edges of this range.
What is the benefit of varying the extent of coupling? AII/AII coupling is integral in optimizing the signal-to-noise ratio of the AII network (Bloomfield et al., 1997; Bloomfield & Dacheux, 2001) as it allows the smaller correlated rod signals to be increased while uncorrelated noise is decreased (Smith & Vardi, 1995; Vardi & Smith, 1996). This would be important when the retina is operating at dim light intensities (mesopic conditions) as increased coupling maximizes the area from which each AII can merge spatially correlated signals, thereby increasing signal to noise ratio. In dark-adapted conditions, however, a miniscule number of photons are intermittently absorbed by the rods and the correlated signals are few (Bloomfield & Dacheux, 2001). As coupled AII cells all receive common inputs, AII coupling would strengthen the response by summing correlated signals and reducing noise (Smith & Vardi, 1995; Vardi & Smith, 1996). However, given that the number of correlated signals reaching the AII network is few, extensive coupling at these low light levels is of no advantage as it would increase background noise, causing the faint signals to spread laterally and decrease the overall amplitude of the response. Coupling is also reduced in light-adapted conditions, reducing the transmission, and loss, of cone signals through the AII network facilitating the high spatial acuity in photopic conditions (Bloomfield & Dacheux, 2001). Further, the high signal-to-noise levels in the cone system reduce the need for spatial averaging.
Gap junctions between AII/ON-CBC provide the mechanism through which rod signals reach ON-ganglion cells (Kolb, 1979) via the circuit: rod to rod bipolar to AII to ON-cone bipolar cell to ON-ganglion cell (Fig.6, red circle, blue square). As these gap junctions are also bidirectional, they provide an alternate pathway through which AIIs receive cone-driven inputs (DeVries & Baylor, 1995; Cohen & Miller, 1999; Xin & Bloomfield, 1999). The alternative route would be through rod/cone gap junctions thus getting rod signals into cone photoreceptors themselves (Nelson, 1977). Rod signals would be transmitted to cone bipolar cells and then pass to AIIs in the circuit rod -cone – ON-cone bipolar -AII (DeVries & Baylor, 1995). Signals originating in cone photoreceptors would be relayed via this path as well and cone-driven responses have been recorded in AII cells (Nelson,1982; Xin & Bloomfield, 1999). Photopic inputs from ON-CBC depolarize AII cells which, in turn, hyperpolarize OFF-CBC (Fig. 6) suppressing light-adapted OFF-center responses (Xin & Bloomfield, 1999). As noted above, AII/ON-CBC junctions appear to be modulated by NO, but not by light as physiological studies report changes in background light levels do not alter the observed overall extent of AII/ON-CBC coupling (Bloomfieldm et al. 1997) and cone-driven responses can be recorded from AII cells in photopic conditions (Nelson, 1882; Xin & Bloomfield, 1999).
Given the morphological diversity of CBC types, it is useful to identify which specific cell(s) is/are coupled to AIIs to better understand the underlying circuitry. Mills and Massey (1996) identified electrical coupling between AIIs and the calbindin-positive bipolar cell in rabbit. However, as only one-quarter of the bipolar cells (23%) tracer-coupled to AIIs are calbindin-positive, more than one type of ON-CBC probably makes gap junctions with AIIs (Massey & Mills, 1996; Kim et al., 2004). In agreement with this, a recent study by Veruki & Harveit (2002a) examining the electrical properties of AII/ON-CBC gap junctions in rat identified four types of bipolar cells, namely CB5-CB8 electrically coupled to AIIs in rat (Euler et al., 1996). Furthermore the latest evidence from Masland’s group suggest that some of the ON cone bipolars coupled to AII cells express connexin 45 but not connexin 36 (Lin et al., 2005. In cat, the cb5 bipolar cell was found to be coupled to AII processes (Nelson & Kolb, 1983). The cb5 bipolar cell probably corresponds to the calbindin bipolar cell in rabbit, as the latter makes the greatest number of gap junctions with AII cells (Strettoi et al., 1994).
Thus, electrical coupling within the AII network functions to maximize signal transmission to postsynaptic ganglion cells. As AIIs receive both rod- and cone-driven inputs, the caliber of the signal varies depending on stimulus intensity. Consequently, the dynamics of the AII network must also vary continuously. In contrast, synaptic transmission through AII/ON-CBC gap junctions is not affected by adaptational state (Bloomfield et al., 1997), suggesting this pathway may function on a sustained level continually relaying signals independent of light level to ganglion cells.
Synapses of the lobular appendages in sublamina a
The lobular appendages are the major chemical synaptic output sites of AII amacrine cells (Fig. 3)(Famiglietti and Kolb, 1975; Strettoi et al., 1992). These appendages form reciprocal, inhibitory synapses in s1-s2 (Strettoi et al., 1992) with OFF-CBC terminals (Kolb & Nelson, 1983; Strettoi et al., 1992; Chun et al., 1993; Merighi et al., 1996). These synapses account for 90% of the output from AII cells in rabbit (Strettoi et al., 1992). AII amacrine cells in rabbit rarely synapse directly onto OFF-ganglion cells whereas in cat and monkey synapses to OFF ganglion cells are common (Kolb, 1979). Inhibitory signals from AIIs are passed to OFF-CBC, decreasing glutamate release onto OFF-type ganglion cells during light stimulation (Fig. 6, blue circle).
Merighi et al (1996) have shown in rabbit that at least two different OFF-CBC types (DAPI-Ba1 and DAPI-Ba2) are postsynaptic to AII cell lobular appendages in different regions of the IPL. The axon terminal of DAPI-Ba1 cells ramifies in s2, while DAPI-Ba2 cells are multistratified with boutons located within s1-s3. In cat, cb1 and cb2 bipolar cells (Kolb et al., 1981), which correspond to DAPI-Ba2 and DAPI-Ba1 cells, respectively, contact AII cells in sublamina a. In primates, AII lobular appendages participate in reciprocal synapses with calbindin positive DB3 axon terminals in s2 (Jacoby & Marshak, 2000). Another bipolar cell in macaque, DB2, is similar in stratification pattern to the rabbit DAPI-Ba2.
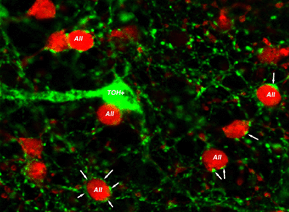
Both AII dendrites and the cell body are postsynaptic to other amacrine cells in S1. Double labeling studies have shown that the dendrites of dopaminergic amacrine cells encircle AII somata, forming characteristic rings as shown in Figure 7 (Pourcho, 1982; Voigt & Wassle, 1987; Kolb et al., 1991). These dopaminergic inputs are believed to mediate changes in AII/AII coupling that occur with adaptational state. However, recent findings show that direct contacts between the dopaminergic processes and AII somata are GABAergic rather than dopaminergic (since dopaminergic amacrines are said to be also GABA positive, Contini & Raviola, 2003). Thus, AII cells may receive direct GABAergic inputs at the cell body which could also regulate AII cell light responses. These GABA-elicited responses, from GABAA receptor activation, would result in a faster modification of AII responses compared to dopamine actions which are mediated through slower second messenger cascades and may be considered paracrine in nature. It has to be mentioned here that the dopaminergic amacrine cell is now considered to contain glutamate (Jones and Marc, 2004) and if this is the case, a faster transmitter action would also be expected.
4. Physiological responses
AII cells themselves are inhibitory glycinergic neurons (Pourcho, 1996; Menger et al., 1998). Their strongest response, and unique role, is during scotopic levels of illumination. In this case, the AII assists in increasing both the speed of transmission and the amplitude of the rod signals seen by the ganglion cells (Nelson, 1982).
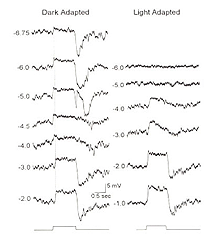
AII amacrine cells are also called the “rod amacrine cell” because they have response characteristics, such as threshold, saturation level, and spectral sensitivities, similar to rods (Nelson, 1982; Bloomfield & Dacheux, 2001). These cells display complex physiological responses that serve both rod and cone retinal pathways and are sensitive to adaptational state (Fig. 8a). In general, AIIs generate TTX-sensitive action potentials and respond to the exogenous application of GABA, glycine, and glutamate (Boos et al., 1993). AIIs are classified as ON-center amacrine cells that display center-surround antagonism (Nelson, 1982; Dacheux & Raviola, 1986; Xin & Bloomfield, 1999) when dark-adapted; surround antagonism is abolished in light-adapted cells (Xin & Bloomfield, 1999).
Generation of the ON-center response
Based on the synaptic connections of AII cells, ON-center responses could be generated via two pathways: (1) direct rod-driven inputs from (ON-type) RBC at chemical synapses or (2) direct cone-driven inputs from (ON-type) CBC via electrical synapses. Physiological recordings showing rod- and cone-driven responses in AII cells and pharmacological studies indicate ON-center responses are blocked by the application of APB (2-amino-4-phosphonobutyric acid), indicating they are generated by ON-type bipolar cells (Xin & Bloomfield, 1999).
How is the ON-center response generated? Interestingly, signals transmitted within both of the above pathways can generate the center response, with the contributions of each pathway varying depending on the intensity of the stimulus (Figure 8b). In dark-adapted conditions, center responses are derived from rod-driven or cone-driven inputs transmitted directly to AIIs from rod BCs or from cone BCs through gap junctions. Exogenous application of either 8-bromo-cGMP or SNAP, which uncouple AII/ON-CBC gap junctions, does not block scotopic ON-center responses but does block the cone signal component. Bright stimuli saturate the rods reducing rod-driven inputs to AIIs. In these conditions, ON-center responses are blocked when AII/ON-CBC junctions are pharmacologically uncoupled (Xin & Bloomfield, 1999). Thus, the ON-center responses in AII cells comes from either rods or cones. Rod signals depolarize AIIs in dark-adapted (scotopic) conditions; cone-driven signals are relayed in light (photopic) conditions (Figure 8a and b). Interestingly, a temporary OFF-center response has also been identified in light-adapted AII cells. This response is blocked by CNQX and is believed to be generated via direct glutamatergic inputs from OFF-cone bipolar cells onto AII lobular appendages in sublamina a (Xin & Bloomfield, 1999).
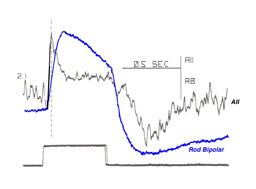
The AII light response is biphasic, with an initial transient depolarization followed by a more sustained component (Figure 9). This allows the AII to respond faster to a light stimulus than the presynaptic rod bipolar cell and to reach its maximum amplitude more rapidly. In fact, at the peak of the AII response, the rod bipolar response is one third of its maximum amplitude (see Figure 9). The AII cell also repolarizes at a faster rate (Nelson, 1982). The transient nature of the AII light response is unexpected as both presynaptic RBC (Dacheux & Raviola, 1986) and rod photoreceptors generate slow sustained responses. This leads to the conclusion that the change from a sustained to a transient signal originates in the AII and serves to quicken rod signals relayed to the inner retina (Nelson, 1982). What underlying mechanism accounts for the change in response kinetics? This question was answered in a recent paper by Singer & Diamond (2003). They examined the RBC/AII synapse using dual patch clamp techniques in rat retinal slices. The transient component of the AII response was not due to feedback inhibition onto RBC or to postsynaptic AMPA receptor desensitization, because it was present when either picrotoxin or cyclothiazide was applied. However, the EPSC waveform did vary with calcium influx into the presynaptic terminal suggesting that activation of calcium channels limits the rate of exocytosis. The authors conclude that the initial transient component of the AII light response is due to the rapid rate of neurotransmitter release from RBC terminals coupled to the fast kinetics of postsynaptic AMPA receptors (Singer & Diamond, 2003).
Generation of the OFF-surround response
In the initial physiological characterization of AIIs, surround responses were only identified in some cells (Nelson, 1982). Subsequently, it was shown that the antagonistic surround is present only when cells are dark-adapted (Xin & Bloomfield, 1999; Bloomfield & Xin, 2000). A reasonable hypothesis, given AII morphology, would be that the surround is generated through connections between AII cells and OFF-CBC, as this synapse would be functioning in dark-adapted conditions. Hoever, the surround response is blocked by CNQX (consistent with a role for OFF-CBC), and is also blocked by APB, suggesting the surround originates at rod bipolar synapse(s) in the IPL (sublamina b), although RBC themselves do not display center/surround organization (Xin & Bloomfield, 1999; Bloomfield & Xin, 2000).
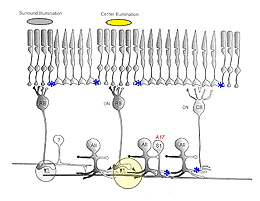
Two pathways have been proposed to account for the circuitry underlying the OFF-surround response (Fig. 10)). First, another amacrine cell may synapse directly with and provide inhibitory inputs to AII distal dendrites, as suggested by anatomical studies. Second, inhibitory feedback synapses onto RBC terminals from A17 amacrine cells may generate surround responses (i.e., (Bloomfield & Xin, 2000; Volgyi et al., 2002). Recent evidence favors this latter pathway, which is summarized below (Figure 10).
The A17 amacrine cell in cat, rat, and monkey is equivalent to the S1/S2 indolamine-accumulating amacrine cell in rabbit (Sandell et al., 1989). This cell, like the AII, is rod-driven (Nelson & Kolb, 1985) and transmission from RBC to A17 cells in rat is blocked by CNQX (Hartveit, 1999). In other species, however, immunocytochemical studies have identified metabotropic glutamate receptors (Cai & Pourcho, 1999) and GluR1/2 receptor subunits (Ghosh et al., 2001; Li et al., 2002) on A17 dendrites. These findings suggest that information flow across the RBC dyad may be mediated by several different glutamatergic mechanisms (Ghosh et al., 2001; Li et al., 2002). Cat A17 cells display a sustained light response and lack an antagonistic surround (Nelson & Kolb, 1985); whereas, center-surround antagonism has been reported for rat A17 cells (Menger & Wassle, 2000).
A17 and S1/S2 amacrine cells make reciprocal contacts onto RBC terminals. A17 cells contain GABA (Porcho and Goebel, 1983; Grunert & Wassle, 1990; Massey et al., 1992; Kim et al., 1998) and inhibit RBC through the activation of GABAc receptors (Zhang et al., 2002; Dong & Hare, 2003). This GABAergic feedback is modulated by light intensity, the activation of GABAA receptors (Dong & Hare, 2003) also present on rod bipolar terminals (Euler & Wassle, 1998; Fletcher & Wassle, 1999; McGillem et al., 2000; Volgyi et al., 2002), and the activation of presynaptic mGluR1/5 receptors (Euler & Wassle, 1998). Physiologically, this feedback changes rod bipolar responses, making the scotopic b-wave more transient (Dong & Hare, 2003), and may be involved in altering calcium currents in RBC terminals (Pan, 2001), ultimately modifying glutamate release contributing to the surround mechanism in both ganglion cells (Shields & Lukasiewicz, 2003) and AII amacrine cells (Volgyi et al., 2002).
In cat, the A17 cell is always the other member of the postsynaptic dyad with AII amacrine distal processes, although other amacrine cell types also receive inputs from rod bipolar cells (Kolb & Nelson, 1983). In contrast, in rabbit, either the S1 or the S2 indolamine-accumulating amacrine cell could partner with the AII at the dyad. Why is this synapse more complex in rabbit? The answer can be found in the differences in morphology and coupling extent between these two cell types. S1 cells are wide-field amacrine cells that display extensive coupling with other S1 cells (i.e., up to ~130 cells coupled), while S2 cells are smaller and not as extensively coupled (maximum of 35 cells coupled) (Li et al., 2002). These differences suggest that S1 cells provide a wide-field component to the AII surround response, while S2 cells provide inputs from a more local circuit (Li et al., 2002; Zhang et al., 2002).
5. Role in duplex vision.
The AII amacrine is the place where rod and cone pathways, as well as ON- and OFF-pathways cross. It is central to scotopic (night) vision. The complex physiology of AII cells is paralleled by their diffuse morphology: their processes are located throughout the IPL and synapses have been observed within all strata (i.e., chemical synapses in s1, s2, and s5; electrical synapses in s3, s4, and s5). Due to these connections, and the parallel retinal pathways, AII amacrine cells always receive inputs, providing the inner retina with a sustained inhibitory pathway (Cohen & Miller, 1999).
Within the rod pathway (or scotopic levels of light), light stimulated decrease in rod photoreceptor glutamate release depolarizes rod bipolar cells, in turn causing the release of glutamate from rod bipolar terminals onto the postsynaptic dyad elements: the indolamine-accumulating or the A17 amacrine cell and the AII amacrine cell. Glutamate excites the AII, resulting in three simultaneous synaptic events: (1) the release of glycine onto OFF-CBC, ultimately inhibiting OFF-ganglion cells, (2) the depolarization of ON-CBC (via AII/ON-CBC gap junctions) and the subsequent depolarization of ON-ganglion cells, and (3) and increase in signal to noise ratio due to the spread of the signal among the coupled AII network. In addition, cat, monkey and rat AII cells synapse directly onto OFF-ganglion cells, resulting in direct glycinergic inhibition of these cells (i.e Muller et al. 1988). AII responses are modified by inhibitory inputs from depolarized A17 (S1/S2) cells which feedback onto rod bipolar terminals. The overall effect is inhibition of the OFF-pathway and excitation in the ON-pathway both by rod-driven signals.
In bright light (photopic) conditions, ON- and OFF-center responses have been recorded from AII cells (Xin and Bloomfield, 1999). Both responses are generated via inputs from CBC, with the transient OFF-center response arising from glutamatergic inputs from OFF-CBC and the ON-response arising transmission through ON-CBC/AII gap junctions. While both ON- and OFF-responses may be found in the same AII cell, the ON-center response predominates. These inputs from ON-CBC depolarize the AII cells, causing glycinergic inhibition of OFF-CBC and OFF-ganglion cells (Muller et al., 1988). In addition, under bright light conditions, coupling within the AII network is reduced, preventing the dissipation of cone signals prior to reaching the ganglion cells. The overall result, again, is that the OFF-pathway is inhibited by the ON-pathway while high acuity cone signals are maintained. In this way the AII mixes not only rod and cone signals, but ON- and OFF-pathways.
Thus, the inclusion of the AII in the rod circuit (scotopic conditions) is required for the vertical flow of information within the IPL, as it functions to amplify the signal, increase transience, and reduce noise prior to reaching the ganglion cells. In cone-driven circuits, inputs from ON-CBCs directly stimulate AIIs and ON-ganglion cells. The AII response is a depolarization, with robust ON-center response, resulting in glycine release and the inhibition of OFF-type responses. These glycinergic inputs to postsynaptic CBC and/or ganglion cells allow ON-signals to predominate in the inner retina under these conditions. Thus, AII is always receiving inputs, though the type of input varies (depending on stimulus intensity) causing the AII cell to “shift its focus” from one input pathway to another and from one function to another.
6. References.
Bloomfield SA, Xin D, Osborne T. Light-induced modulation of coupling between AII amacrine cells in the rabbit retina. Vis Neurosci. 1997;14:565–576.[PubMed]
Bloomfield SA, Xin D. Surround inhibition of mammalian AII amacrine cells is generated in the proximal retina. J Physiol. 2000;523:771–783. [PubMed]
Bloomfield SA, Dacheux RF. Rod vision: pathways and processing in the mammalian retina. Prog Retin Eye Res. 2001;20:351–384. [PubMed]
Boos R, Schneider H, Wassle H. Voltage- and transmitter-gated currents of AII-amacrine cells in a slice preparation of the rat retina. J Neurosci.1993;13:2874–2888. [PubMed]
Boycott, B. B. & Dowling, J. E. (1966). Organization of the primate retina: electron microscopy. Philosophical Transactions of the Royal Society, London. B 166, 80-111.
Boycott, B. B. & Kolb, H. (1973). The connections between bipolar cells and photoreceptors in the retina of the domestic cat. Journal of Comparative Neurology 148, 91-114. [PubMed]
Cai W, Pourcho RG. Localization of metabotropic glutamate receptors mGluR1alpha and mGluR2/3 in the cat retina. J Comp Neurol. 1999;407:427–437.[PubMed]
Casini G, Rickman DW, Brecha NC. AII amacrine cell population in the rabbit retina: identification by parvalbumin immunoreactivity. J Comp Neurol.1995;356:132–142. [PubMed]
Chun MH, Han S-H, Chung J-W, Wassle H. Electron microscopic analysis of the rod pathway of the rat retina. J Comp Neurol. 1993;332:421–432.[PubMed]
Cohen ED, Miller RF. The network-selective actions of quinoxalines on the neurocircuitry operations of the rabbit retina. Brain Res. 1999;831:206–228.[PubMed]
Contini M, Raviola E. GABAergic synapses made by a retinal dopaminergic neuron. Proc Natl Acad Sci U S A. 2003;100:1358–1363. [PubMed] [Free Full text in PMC]
Cooper JR, Bloom FE, Roth RH. The biochemical basis of neuropharmacology. New York: Oxford University Press. 1996
Dacey DM. Primate retina: cell types, circuits, and color opponency. Prog Retin Eye Res. 1999;18:737–763. [PubMed]
Dacheux RF, Raviola E. The rod pathway in the rabbit retina: a depolarizing bipolar and amacrine cell. J Neurosci. 1986;6:331–345. [PubMed]
DeVries SH, Baylor DA. An alternative pathway for signal flow from rod photoreceptors to ganglion cells in mammalian retina. Proc Natl Acad Sci U S A.1995;92:10658–10662. [PubMed] [Free Full text in PMC]
Dong C-J, Hare WA. Temporal modulation of scotopic visual signals by A17 amacrne cells in mammalian retina in vivo. J Neurophysiol. 2003;89:2159–2166. [PubMed]
Dowling, J. E. (1987). The retina: an approachable part of the brain. Belknap Press of Harvard University Press, Cambridge, MA
Euler T, Schneider H, Wassle H. Glutamate responses of bipolar cells in a slice preparation of the rat retina. J Neurosci. 1996;16:2934–2944. [PubMed]
Euler T, Wassle H. Different contributions of GABAA and GABAC receptors to rod and cone bipolar cells in a rat retinal slice preparation. J Neurophysiol.1998;79:1384–1395. [PubMed]
Famiglietti EV, Kolb H. A bistratified amacrine cell and synaptic circuitry in the inner plexiform layer of the retina. Brain Res. 1975;84:293–300. [PubMed]
Feigenspan A, Teubner B, Willecke K, Weiler R. Expression of neuronal connexin 36 in AII amacrine cells of the mammalian retina. J Neurosci.2001;21:230–239. [PubMed]
Fletcher EL, Koulen P, Wassle H. GABAA and GABAC receptors on mammalian rod bipolar cells. J Comp Neurol. 1998;396:351–365. [PubMed]
Fletcher EL, Wassle H. Indoleamine-accumulating amacrine cells are presynaptic to rod bipolar cells through GABAC receptors. J Comp Neurol.1999;413:155–167. [PubMed]
Fletcher EL, Hack I, Brandstatter JH, Wassle H. Synaptic localization of NMDA receptor subunits in the rat retina. J Comp Neurol. 2000;420:98–112.[PubMed]
Gabriel R, De Souza S, Ziff EB, Witkovsky P. Association of the AMPA receptor-related postsynaptic density proteins GRIP and ABP with subsets of glutamate-sensitive neurons in the rat retina. J Comp Neurol. 2002;449:129–140. [PubMed]
Ghosh KK, Haverkamp S, Wassle H. Glutamate receptors in the rod pathway of the mammalian retina. J Neurosci. 2001;21:8636–8647. [PubMed]
Goebel DJ, Aurelia JL, Tai Q, Jojich L, Poosch MS. Immunocytochemical localization of the NMDA-R2A receptor subunit in the cat retina. Brain Res.1998;808:141–154. [PubMed]
Grunert U, Wassle H. GABA-like immunoreactivity in the Macaque monkey retina: a light and electron microscopic study. J Comp Neurol. 1990;297:509–524. [PubMed]
Grunert U, Martin PR. Rod bipolar cells in the macaque monkey retina: immunoreactivity and connectivity. J Neurosci. 1991;11:2742–2758. [PubMed]
Grunert U, Wassle H. Glycine receptors in the rod pathway of the macaque monkey retina. Vis Neurosci. 1996;13:101–115. [PubMed]
Grunert U, Haverkamp S, Fletcher EL, Wassle H. Synaptic distribution of ionotropic glutamate receptors in the inner plexiform layer of the primate retina. J Comp Neurol. 2002;447:138–151. [PubMed]
Hampson EC, Vaney DI, Weiler R. Dopaminergic modulation of gap junction permeability between amacrine cells in mammalian retina. J Neurosci.1992;12:4911–4922. [PubMed]
Hartveit E, Brandstatter JH, Sassoe-Pognetto M, Laurie DJ, Seeburg PH, Wassle H. Localization and developmental expression of the NMDA receptor subunit NR2A in the mammalian retina. J Comp Neurol. 1994;348:570–582. [PubMed]
Hartveit E, Veruki ML. AII amacrine cells express functional NMDA receptors. Neuroreport. 1997;8:1219–1223. [PubMed]
Hartveit E. Reciprocal synaptic interactions between rod bipolar cells and amacrine cells in the rat retina. J Neurophysiol. 1999;81:2923–2936. [PubMed]
Jacoby RA, Marshak DW. Synaptic connections of DB3 diffuse bipolar cell axons in macaque retina. J Comp Neurol. 2000;416:19–29. [PubMed]
Jacoby, R. A., Wiechmann, A. F., Amara, S. G., Leighton, B. H. & Marshak, D. W. Diffuse bipolar cells provide input to off parasol ganglion cells in the macaque retina. Journal of Comparative Neurology (2000) 416, 6-18. [PubMed]
Jones BW, Watt CB, Marc RE. Dopamine amacrine and interplexiform cells exhibit glutamatergic signatures. Rockville (MD): Association for Research in Vision and Ophthalmology. 2004
Kim I-B, Lee M-Y, Oh S-J, Kim K-Y, Chun M-H. Double-labeling techniques demonstrate that rod bipolar cells are under GABAergic control in the inner plexiform layer of the rat retina. Cell Tissue Res. 1998;292:17–25. [PubMed]
Kim I.-B., Park M.T.-H.K., Lee E.-J., Kang M.-S., Chun M.-H. Synaptic circuitries of two types of ON-cone bipolar cells in the rabbit retina. Investigative Ophthalmology and Visual Science. 2004;45:E-abstract 5357.
Kolb H, Famiglietti EV. Rod and cone pathways in the inner plexiform layer of the cat retina. Science. 1974;186:47–49. [PubMed]
Kolb H, Famiglietti EV. Rod and cone pathways in the retina of the cat. Invest Ophthalmol. 1976;15:935–945.
Kolb H, Famiglietti EV Jr, Nelson R. Neural connections in the inner plexiform layer of the cat’s retina. In: The structure of the eye. Vol. III. 1976.
Kolb H. The inner plexiform layer in the retina of the cat: electron microscope observation. J Neurocytol. 1979;8:295–329. [PubMed]
Kolb H, Nelson R, Mariani A. Amacrine cells, bipolar cells, and ganglion cells of the cat retina: a Golgi study. Vision Res. 1981;21:1081–1114. [PubMed]
Kolb H, Nelson R. Rod pathways in the retina of the cat. Vision Res. 1983;23:301–312. [PubMed]
Kolb H, Cuenca N, Wang H-H, Dekorver L. The synaptic organization of the dopaminergic amacrine cell in the cat retina. J Neurocytol. 1990;19:343–366.[PubMed]
Kolb H, Cuenca N, Dekorver L. Postembedding immunocytochemistry for GABA and glycine reveals the synaptic relationships fo the dopaminergic amacrine cell of the cat retina. J Comp Neurol. 1991;310:267–284. [PubMed]
Kolb H, Linberg KA, Fisher SK. Neurons of the human retina: a Golgi study. J Comp Neurol. 1992;318:147–187. [PubMed]
Kolb H, E. Fernandez, R. Nelson, R. (2000). Webvision: the organization of the vertebrate retina [online]. Available from: http://webvision.med.utah.edu/.
Kolb H, Zhang L, Dekorver L, Cuenca N. A new look at calretinin-immunoreactive amacrine cell types in the monkey retina. J Comp Neurol. 2002;453:168–184. [PubMed]
Li W, Trexler EB, Massey SM. Glutamate receptors at rod bipolar ribbon synapses in the rabbit retina. J Comp Neurol. 2002;448:230–248. [PubMed]
Lin B, Jakobs TC, Masland RH. Different functional types of bipolar cells use different gap-junctional proteins. J Neurosci. 2005;25:6696–6701. [PubMed]
MacNeil MA, Masland RH. Extreme diversity among amacrine cells: implications for function. Neuron. 1998;20:971–982. [PubMed]
MacNeil MA, Heussy JK, Dacheux RF, Raviola E, Masland RH. The shapes and numbers of amacrine cells: matching photofilled with Golgi-stained cells in the rabbit retina and comparison with other mammalian species. J Comp Neurol. 1999;413:305–326. [PubMed]
Mariani AP. Amacrine cells of the rhesus monkey retina. J Comp Neurol. 1990;301:382–400. [PubMed]
Massey SC, Mills SL, Marc RE. All indolamine-accumulating cells in the rabbit retina contain GABA. J Comp Neurol. 1992;322:275–291. [PubMed]
Massey SC, Mills SL. A calbindin-immunoreactive cone bipolar cell type in the rabbit retina. J Comp Neurol. 1996;366:15–33. [PubMed]
Massey SC, Mills SL. Antibody to calretinin stains AII amacrine cells in the rabbit retina: double-label and confocal analyses. J Comp Neurol. 1999;411:3–18. [PubMed]
McGillem GS, Rotolo TC, Dacheux RF. GABA responses of rod bipolar cells in rabbit retinal slices. Vis Neurosci. 2000;17:381–389. [PubMed]
Menger N, Pow DV, Wassle H. Glycinergic amacrine cells of the rat retina. J Comp Neurol. 1998;401:34–46. [PubMed]
Menger N, Wassle H. Morphological and physiological properties of the A17 amacrine cell of the rat retina. Vis Neurosci. 2000;17:769–780. [PubMed]
Merighi A, Raviola E, Dacheux R. Connections of two types of flat cone bipolars in the rabbit retina. J Comp Neurol. 1996;371:164–178. [PubMed]
Mills SL, Massey SC. Labeling and distribution of AII amacrine cells in the rabbit retina. J Comp Neurol. 1991;304:491–501. [PubMed]
Mills SL, Massey SC. Differential properties of two gap junctional pathways made by AII amacrine cells. Nature. 1995;377:734–737. [PubMed]
Mills SL, Massey SC. AII amacrine cells limit scotopic acuity in central macaque retina: a confocal analysis of calretinin labeling. J Comp Neurol.1999;411:19–34. [PubMed]
Mills SL, O’Brien JJ, Li W, O’Brien J, Massey SC. Rod pathways in the mammalian retina use connexin 36. J Comp Neurol. 2001;436:336–350. [PubMed] [Free Full text in PMC]
Morkve SH, Veruki ML, Hartveit E. Functional characteristics of non-NMDA-type ionotropic glutamate receptor channels in AII amacrine cells in rat retina. J Physiol. 2002;542:147–165. [PubMed]
Muller F, Wassle H, Voight T. Pharmacological modulation of rod pathway in the cat retina. J Neurophysiol. 1988;59:1657–1672. [PubMed]
Nelson R. Cat cones have rod input: a comparison of the response properties of cones and horizontal cell bodies in the retina of the cat. J Comp Neurol.1977;172:109–136. [PubMed]
Nelson R. AII amacrine cells quicken the time course of rod signals in the cat retina. J Neurophysiol. 1982;47:928–947. [PubMed]
Nelson R, Kolb H. Synaptic patterns and response properties of bipolar and ganglion cells in the cat retina. Vision Res. 1983;23:1183–1195. [PubMed]
Nelson R, Kolb H. A17: a broad-field amacrine cell in the rod system of the cat retina. J Neurophysiol. 1985;54:592–614. [PubMed]
Pan Z-H. Voltage-activated Ca2+ channels and ionotropic GABA receptors localized at axon terminals of mammalian retinal bipolar cells. Vis Neurosci.2001;18:279–288. [PubMed]
Pourcho RG. Dopaminergic amacrine cells in the cat retina. Brain Res. 1982;252:101–109. [PubMed]
Pourcho RG, Goebel DJ. Neuronal subpopulations in cat retina which accumulate the GABA agonist (3H)muscimol: a combined Golgi and autoradiographic study. J Comp Neurol. 1983;219:25–35. [PubMed]
Pourcho RG. Neurotransmitters in the retina. Curr Eye Res. 1996;15:797–803. [PubMed]
Qin P, Pourcho RG. Localization of AMPA-selective glutamate receptor subunits in the cat retina: a light- and electron-microscopic study. Vis Neurosci.1999;16:169–177. [PubMed]
Qin P, Pourcho RG. AMPA-selective glutamate receptor subunits GluR2 and GluR4 in the cat retina: an immunocytochemical study. Vis Neurosci.1999;16:1105–1114. [PubMed]
Sandell JH, Masland RH, Raviola E, Dacheux RF. Connections of the indoleamine-accumulating cells in the rabbit retina. J Comp Neurol. 1989;283:303–313. [PubMed]
Shields CR, Lukasiewicz PD. Spike-dependent GABA inputs to bipolar cell axon terminals contribute to lateral inhibition of retinal ganglion cells. J Neurophysiol. 2003;89:2449–2458. [PubMed]
Singer JH, Diamond JS. Sustained Ca2+ entry elicits transient postsynaptic currents at a retinal ribbon synapse. J Neurosci. 2003;23:10923–10933.[PubMed]
Slaughter MM, Miller RF. 2-Amino-4-phosphonobutyric acid: a new pharmacological tool for retina research. Science. 1981;211:182–185. [PubMed]
Slaughter MM, Miller RF. Characterization of an extended glutamate receptor on the ON bipolar neuron in the vertebrate retina. J Neurosci. 1985;5:224–233. [PubMed]
Smith RG, Vardi N. Simulation of the AII amacrine cell of mammalian retina: functional consequences of electrical coupling and regenerative membrane properties. Vis Neurosci. 1995;12:851–860. [PubMed]
Strettoi E, Dacheux RF, Raviola E. Synaptic connections of rod bipolar cells in the inner plexiform layer of the rabbit retina. J Comp Neurol. 1990;295:449–466. [PubMed]
Strettoi E, Raviola E, Dacheux RF. Synaptic connections of the narrow-field, bistratified rod amacrine cell (AII) in the rabbit retina. J Comp Neurol.1992;325:152–168. [PubMed]
Strettoi E, Dacheux RF, Raviola E. Cone bipolar cells as interneurons in the rod pathway of the rabbit retina. J Comp Neurol. 1994;347:139–149.[PubMed]
Trexler EB, Li W, Mills SL, Massey SC. Coupling from AII amacrine cells to ON cone bipolar cells is bidirectional. J Comp Neurol. 2001;437:408–422.[PubMed]
Vaney DI. The morphology and topographic distribution of AII amacrine cells in the cat retina. Proc R Soc Lond B Biol Sci. 1985;224:475–488. [PubMed]
Vaney DI, Gynther IC, Young HM. Rod-signal interneurons in the rabbit retina: 2. AII amacrine cells. J Comp Neurol. 1991;310:154–169. [PubMed]
Vaney DI, Young HM, Gynther IC. The rod circuit in the rabbit retina. Vis Neurosci. 1991;7:141–154. [PubMed]
Vaney DI. Many diverse types of retinal neurons show tracer coupling when injected with biocytin or neurobiotin. Neurosci Lett. 1991;125:187–190.[PubMed]
Vardi N, Smith RG. The AII amacrine network: coupling can increase correlated activity. Vision Res. 1996;36:3743–3757. [PubMed]
Veruki ML, Hartveit E. Electrical synapses mediate signal transmission in the rod pathway of the mammalian retina. J Neurosci. 2002;22:10558–10566.[PubMed]
Veruki ML, Hartveit E. AII (rod) amacrine cells for a network of electrically coupled interneurons in the mammalian retina. Neuron. 2002;33:935–946.[PubMed]
Veruki ML, Morkve SH, Hartveit E. Functional properties of spontaneous EPSCs and non-NMDA receptors in rod amacrine (AII) cells in the rat retina. J Physiol. 2003;549:759–774. [PubMed]
Voigt T, Wassle H. Dopaminergic innervation of A II amacrine cells in the mammalia retina. J Neurosci. 1987;7:4115–4128. [PubMed]
Volgyi B, Xin D, Bloomfield SA. Feedback inhibition in the inner plexiform layer underlies the surround-mediated responses of AII amacrine cells in the mammalian retina. J Physiol. 2002;539:603–614. [PubMed]
Wassle H, Grunert U, Rohrenbeck J. Immunocytochemical staining of AII-amacrine cells in the rat retina with antibodies to parvalbumin. J Comp Neurol.1993;332:407–420. [PubMed]
Wassle H, Grunert U, Chun M-H, Boycott BB. The rod pathway of the macaque monkey retina: identification of AII-amacrine cells with antibodies against calretinin. J Comp Neurol. 1995;361:537–551. [PubMed]
Xin D, Bloomfield SA. Comparison of the responses of AII amacrine cells in the dark- and light-adapted rabbit retina. Vis Neurosci. 1999;16:653–665.[PubMed]
Zhang J, Li W, Trexler EB, Massey SC. Confocal analysis of reciprocal feedback at rod bipolar terminals in the rabbit retina. J Neurosci. 2002;22:10871–10882. [PubMed]
Mahnoosh Farsaii and Victoria P. Connaughton
Last Updated: July, 2011.