Helga Kolb
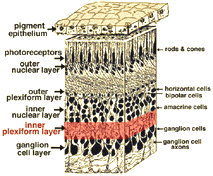
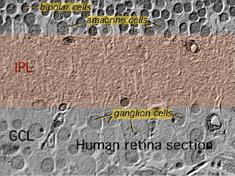
1. Bipolar, amacrine and ganglion cells interact in the inner plexiform layer.
The axonal endings of bipolar cells bring information from the outer plexiform layer (OPL) to the neuropil of the inner plexiform layer (IPL). Here bipolar cells talk to different varieties of functionally specialized amacrine cells and to dendrites of the various ganglion cells (Figs 1 and 2). The neuropil is a confusing network of interconnecting profiles that, to be understood, has to be investigated at the higher magnification afforded by the electron microscope over, the light microscope, and with knowledge gained from Golgi-staining for morphology, and intracellular electrophysiology for function of individual cells in the network.
|
|
2. Ultrastructure of the neuropil of the inner plexiform layer.
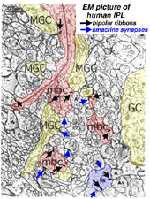
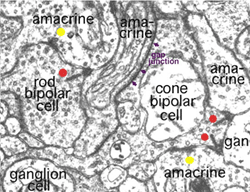
A view of a small part of the neuropil of the inner plexiform layer is shown in Figure 3. Bipolar cell axon terminals (red profiles) are vesicle-filled profiles, containing irregular, long mitochondria and neurotubules. Their synapses are typified by a small synaptic ribbon pointing into a wedge with two post synaptic profiles (known as a dyad) at the apex (arrows in Fig. 3 and red dots in bipolar cell profiles, Fig. 4a) (Dowling and Boycott, 1966). Amacrine cell dendrites are also vesicle-filled and have round mitochondria and sometimes neurofilaments as well as neurotubules (Fig. 3). Amacrine profiles vary between being very small cross sections through thin straight tubes, and larger varicosities budding off the dendrites.
Fig. 3. Electron micrograph of the neuropil of the IPL
Typically amacrine cells synapse upon other profiles (bipolar axons, amacrine cells or ganglion cell dendrites) in the enlarged varicosities at, what is known as a conventional synapse: the synapse consists of synaptic vesicles clustered at a pre- and post membrane density (blue arrows in Fig. 3, yellow dots in amacrine profile, Fig 4a) (Dowling and Boycott, 1966). Ganglion cell dendrites (yellow profiles, Figs. 3) are recognizable as profiles lacking synaptic vesicles but containing ribosomes, neurotubules and filaments. Ganglion cell dendrites are seen to be postsynaptic to bipolar ribbon synapses and amacrine conventional synapses (Figs. 4a and b). Bipolar cells are also postsynaptic to many amacrine synapses. Often amacrine cells make, what are called reciprocal synapses, to the bipolar cell axons from which they receive ribbon synapses (yellow dots, Fig. 4a).
Fig. 4. Electron micrograph of reciprocal synapses in the IPL
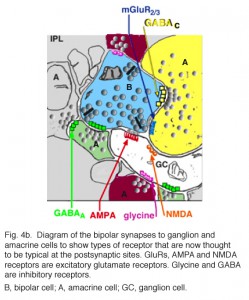
In the last ten years many studies have shown that different glutamate receptors (glutamate being the excitatory amino acid that is the neurotransmitter of the bipolar cells, See chapter on glutamate) is present on the postsynaptic dendrites of amacrine and ganglion cells. These include AMPA, NMDA and GluRs of different types (Fig. 4b) (Grunert et al., 2002). The postsynaptic receptors of IPL processes for the inhibitory neurotransmitter glycine, of amacrine cells, are the various glycine receptors (Fig. 4b). For the inhibitory transmitter GABA, of other amacrine cells, the receptors are typically GABAA subtypes (Grunert, 2000; Kneussel and Betz, 2000), but in some cases, are GABAc subtypes (Fig. 4b). The GABAc receptors are typical of the reciprocal synapses from the wide field amacrine cell (A17) at the ribbon synapse in the rod bipolar axon terminal (Chavez at al., 2010).
This reciprocal synapse is in essence a feed-back synapse to the bipolar cell axon in the inner plexiform layer, with the same strategic importance as the horizontal cell dendrite making a feed-back synapse at the photoreceptor ribbon synapse, in the outer plexiform layer. It will be remembered that such a local circuit in the outer plexiform layer was thought to provide the bipolar cell response with a center-surround organization. It has been suggested that another surround mechanism coming from the amacrine cells could be added at this reciprocal (feed-back) synapse in the inner plexiform layer.
3. Different morphological types of amacrine and ganglion cells.
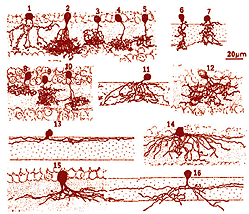
It is clear that there are many different kinds of amacrine cell and ganglion cell branching in the IPL of the human retina. From Golgi staining studies we know that there are at least 25 different amacrine cell types in the monkey and human retina (Mariani, 1990; Kolb et al. 1992). Some drawings of different amacrine cells as seen after Golgi staining in wholemounts of human retina, are shown below (Fig. 5).
Fig. 5. Amacrine cells in human retina
The amacrine cells are classified into different types on morphological characteristics of dendritic tree size such as small, medium and large, branching characteristics (i.e. tufted, varicose, linear, beaded and radiate) and, most importantly, on the stratification of their dendrites in the IPL (Mariani, 1990; Kolb et al., 1992). The neuropil of the IPL was arbitrarily divided into 5 strata by Cajal (1892), because he appreciated the fact that cells branching in disparate strata could not make synaptic interactions while those that costratified could. This 5 strata descriptive classification scheme has been used by morphologists since to classify retinal cells on their dendritic branching level. See, for example in Fgigure 6 below, some amacrine cells have dendrites in strata 1 and 2 (called broadly stratified types, cell 8 below), or strata 1 and 5 (called bistratified types, cell 1, below), stratum 1 or any of the 5 strata (called monostratified, cell 13, below) or dendrite through all strata 1 to 5 (called diffuse types, cells 12 and 14, Fig. 6).
Fig. 6. Stratification of amacrine cells in human retina
The above amacrine cells have been stained by the Golgi procedure which, as we have seen, is very good for showing the isolated complete shape and size of a nerve cell. Newer techniques are also providing valuable information on cell shapes and sizes: techniques such as using intracellular staining through a microelectrode and immunocytochemistry to stain cells with antibodies to their neurotransmitter or synthesising enzymes.
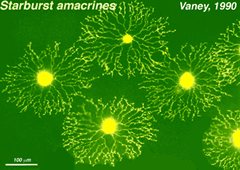
These are some different amacrine cell types stained by injection of dyes through a microelectrode inserted into the cell body. The cells that look like exploding fireworks, are called “starburst” cells (Fig. 7) and are known to use acetylcholine as their neurotransmitter (Famiglietti, 1983; Masland 1988; Vaney, 1990).
Fig.7. Starbust amacrine cells
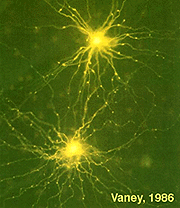
Another type of amacrine cell, thought to contain GABA as a neurotransmitter, and can be well stained with serotonin antibodies has been stained by the same technique (Fig. 8) (Vaney, 1986). This is the A17 cell of the rod system that makes reciprocal synapses at the rod bipolar ribbon (Fig. 4b).
Using similar morphological techniques of Golgi staining, immunocytochemisry and intracellular staining, the ganglion cells of the retina have been classified into various different types.
Fig.8. Serotonin-containing amacrine cells
4. Different morphological types of ganglion cells.
Using similar morphological techniques of Golgi staining, immunocytochemisry and intracellular staining, the ganglion cells of the retina have been classified into various different types.
|
|
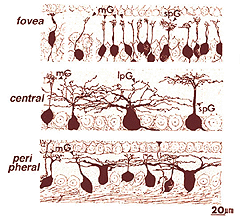
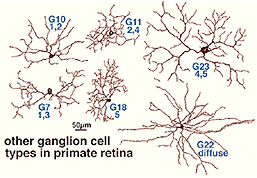
As many as 25 different types exist in mammalian and human retinas, a few of which are shown in Figures 9 and 10, in both wholemount view and sectioned view. As with the amacrine cells, ganglion cells are classified onto the different types by cell body size, dendritic tree spread, branching patterns (e.g. radiate or tufted) and branching level in the 5 strata of the IPL. In both cat and human retinas ganglion cells can be from small to large, diffuse, bistratified and various monostratified large-field types with different stratification levels in the inner plexiform layer (Figs. 9 and 10).
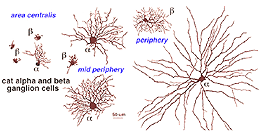
Both amacrine and ganglion cells increase in dendritic tree span with eccentricity from the fovea. The very smallest dendritic fields for all cells types being possessed by cells of the fovea (Fig. 9) or area centralis (Fig. 11) for the common ganglion cells of the cat retina (alpha and beta types). The same ganglion cell types are ten times the size in dendritic tree spread in peripheral retina (Boycott and Wassle, 1974; Kolb et al., 1981). For this reason classification of amacrines and ganglion cells has to always take eccentricity from the fovea into account and compare cells in similar areas of retina to be sure that they are indeed different cell types (Fig. 11).
Fig. 11. Cat ganglion cells
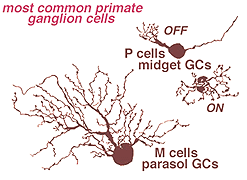
In the human retina, the commonest ganglion cell types are the parasol and the midget ganglion cells (Fig. 12) (Polyak, 1941; Rodieck et al., 1985; Kolb et al., 1992; Dacey, 1993). They are also known as P cells (midget ganglion cells) because of their projection to the parvocellular layers, and M cells (parasol ganglion cells) because of their projection to the magnocellular layers of the lateral geniculate nucleus (Shapley and Perry, 1982). We shall return to these ganglion cell types in a later chapter.
Fig. 12. Primate ganglion cells
5. Stratification of amacrine and ganglion cells in relationship to bipolar cell axons.
One may ask why amacrine cells and ganglion cells should be classified on stratification level of their dendrites, particularly if they look the same in other respects such as field size, cell body size and dendritic morphology. It was clear more than 100 years ago to Cajal (1894) that dendrites of amacrine and ganglion cells and axons of bipolar cells were arranged to costratify in some meaningful manner. The meaning of costratifications and orderly arrangements of branching levels, other than the obvious of making sure certain cell types made synapses with certain other cell types, escaped him though.
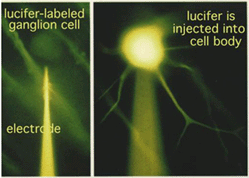
We had to wait for 70 years before the answer was found in the physiological responses of the different nerve cells of the retina. In the 1970s it became possible to make intracellular recordings from the neurons of the retina with dye filled glass microelectrodes. Physiologists were able to record both the response of the impaled cell to light and to fill the cell with a fluorescent dye such as lucifer to see what type of cell it was (see Fig. 13, left) (Kaneko, 1970; Nelson et al., 1978).
Fig.13 . Experimental procedure for impaling ganglion cell in the retina
CLICK HERE to see an animation of the iontophoresis of Lucifer dye into a ganglion cell via a microlectrode (mp4 movie)
CLICK HERE to see an animation of the physiological recording procedure
(mp4 movie)
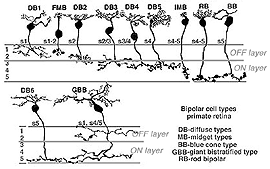
In the fifties and sixties it became apparant from ganglion cell recordings in the mammalian retina that the visual message leaving the retina was in the form of ganglion cell axon spike discharges that occurred either when a spot of light stimulated the retina (ON discharge to light), or when the spot of light was turned off (OFF discharge). Ganglion cells were responding to either one or the other of the change of state of light. The one group was stimulated by the light brighter than background and the other by light darker than background (Kuffler, 1953; Enroth-Cugell and Robson, 1966). Next came intracellular recordings from the five basic cell types in a mudpuppy retina, which revealed that cells preceding ganglion cells also fell into the two physiological types i.e. bipolar and amacrine cells could give ON or OFF discharges (Werblin and Dowling, 1969; Kaneko, 1970). Knowing now that bipolar cells could be ON- or OFF-center to a spot of light (they could have the opposite response in a surround concentrically around their central receptive field; mentioned in the chapter on the outer plexiform layer and dealt with in detail in later chapters on circuitry for rod and cone signals), it was reasonable to suggest that OFF-center bipolar chains excited OFF ganglion cells and that ON-center bipolar cell chains excited ON ganglion cells. The typical bipolar cell types that might form the bipolar chains driving ganglion cells of the primate retina are shown below.
Fig. 14. Bipolar cell types in primate retina
These bipolar cells, as described in the previous section on the outer plexiform layer, make different types of synapses with cone pedicles and rod spherules. Some types make basal contacts on the surface of the cone pedicles and others make invaginating contact to end close to the synaptic ribbons in both rod spherules and cone pedicles.
It is now thought that bipolar cells respond to light just like the photoreceptor with a slow hyperpolarization when the bipolar dendrite is in the basal junction position. In contrast, the other type of contact, invaginating to the ribbon synapse, causes the bipolar to respond to light with an inverted sign compared with the photoreceptor. It gives a slow depolarizing response. Thus the nature of the postsynaptic membrane channels on the cone bipolar cell dendrite is the important designator of the response sign the bipolar will have. The hyperpolarizing bipolar types are the start of OFF-center channels and the depolarizing types are the start of ON-center channels through the retina.
The bipolar cells shown above (Fig. 14), will be noticed to have different axonal ending levels in the inner plexiform layer e.g. DB1 has an axon ending in stratum 1 neuropil close to the amacrine cell layer of the inner nucelar layer, while imb has an axon ending at the opposite side of the inner plexiform layer in straum 5 against the ganglion cell bodies. It seems obvious now, but it took a lot of painstaking serial section recontruction to show (Kolb, 1979; Kolb and DeKorver, 1991) that DB1 and imb made contacts only with ganglion cells that had dendritic stratification in the same neuropil as the respective bipolar axonal terminals. At the level of information transfer between bipolar cells and ganglion cells in the IPL, only excitatory channels are present (Raviola and Raviola, 1982) so the type of signal transmitted to the ganglion cell, as either ON- or OFF-center, is essentially determined by the bipolar cells contacting it.
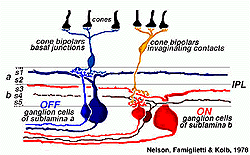
Fig. 15. Organization of ON- and OFF-center ganglion cells
In order to keep the ON and OFF channels separate through the ganglion cells to the brain, the inner plexiform layer is divided into two functionally discrete sublaminae, called a (the 2 strata below the amacrine cell bodies) andb (the other 3 strata stretching to the ganglion cell bodies) (Famigletti and Kolb, 1976). Interactions are only allowed between basal-contacting cone bipolar types and one set of ganglion cells in sublamina a, while invaginating-contacting cone bipolar cells can only interact with another set of ganglion cells branching in sublamina b (see above). Gouras (1971) was the first to suggest that this specificity of bipolar to ganglion cell contacts underlay ON-center and OFF-center midget ganglion cell responses in monkey.
Later Nelson (Nelson, et al., 1978) conclusively proved this hypothesis by means of intracellular recording and marking experiments in ganglion cells of cat (Figs 15 annd 16).
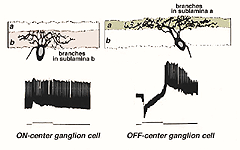
Fig. 16. Intracellular recordings of ON-center and OFF-center ganglion cells
CLICK HERE to see an animation of the physiological recording of ON and OFF beta ganglion cells
(Quicktime movie)
Figure 16 shows the intracellular recordings made from two different ganglion cells of Nelson and coauthors’ study (1978). One cell proves to be ON-center, giving a burst of spikes riding on a depolarization of the membrane as soon as the light flash goes on (Fig. 16, left). In contrast, the other cell is OFF-center, giving a hyperpolarization to the membrane when the light flash is on, but a burst of spikes riding on the depolarization when the light flash is over (Fig. 16 right). The ON-center ganglion cell has dendrites restricted to branching in sublamina b and has synaptic input from invaginating types of bipolar cell. The OFF-center cell has dendrites reaching higher to branch only in sublamina a and gets synapses from basal junction contacting types of bipolar cell.
Thus it is, that there is a definite functional architecture to the inner plexiform layer of the mammalian retina and, in fact, to all vertebrate retinas. Stratification of the neuropil is formed by specific levels of branching of bipolar, amacrine and ganglion cells so that specialized circuits of interactions are set up. In addition there has, during the course of evolution, been an imposition of a broad division of the two halves of the inner plexiform layer into the top half allowing only interactions for the OFF-center ganglion cell pathways and the bottom half, only for the ON-center ganglion cell pathways. Occasionally ON center bipolars pass by ON ganglion cells (melanopsin or ipRGC cells) and amacrine cells (transient dopaminergic amacrine cells) and direct synapses to them thereby “breaking the rule” (Hoshi et al., 2009). However on the whole, bipolar and ganglion cell are responsible for the bisublaminar ON and OFF channel organization in the IPL. Many amacrine cells are diffuse or bistratified and serve to connect up the ON- and OFF-center neuropils, while still others stratify in such a manner at the border between the two functional neuropils, to receive both ON and OFF inputs to drive them. Such amacrine cells may be the majority in the vertebrate retina and are thought to be involved more in temporal facets of retinal performance. They may be important for fast transfer of information, i.e. for speeding up signals. The roles of some of these amacrine cells will become clearer in later chapters.
Last updated March 25, 2012.
5. References.
Boycott, B.B. and Wassle, H. (1974) The morphological types of ganglion cells of the domestic cat’s retina. J. Physiol. (Lond.), 240, 397-419.
Cajal, S.R. (1892) The Structure of the Retina. (Transl. Thorpe, S.A. and Glickstein, M.), Thomas, Springfield, Il., 1972.
Dacey, D.M. (1993) The mosaic of midget ganglion cells in the human retina. J. Neurosci. 13, 5334-5355.
Dowling, J.E. and Boycott, B.B. (1966) Organization of the primate retina: electron microscopy. Proc. R. Soc., B, (Lond.) 166, 80-111.
Enroth-Cugell, C. and Robson, J.G. (1966) The contrast sensitivity of retinal ganglion cells of the cat. J. Physiol.( Lond.), 187, 517-552.
Famiglietti, E.V. (1983) ‘Starburst’ amacrine cells and cholinergic neurons: mirror-symmetric ON and OFF amacrine cells of rabbit retina. Brain Res. 261, 138-144.
Famiglietti, E.V. and Kolb, H. (1976) Structural basis for ON- and OFF-center responses in retinal ganglion cells. Science, 194, 193-195.
Gouras, P. (1971) The function of the midget system in primate color vision. Vision Res. (Suppl) 3, 397-410.
Kaneko, A. (1970) Physiological and morphological identification of horizontal, bipolar and amacrine cells in goldfish retina. J. Physiol. (Lond), 207, 623-633.
Kolb, H. (1979) The inner plexiform layer in the retina of the cat: electron microscopic observations. J. Neurocytol. 8, 295-329.
Kolb, H. and DeKorver, L. (1991) Midget ganglion cells of the parafovea of the human retina: A study by electron microscopy and serial section reconstructions. J. Comp. Neurol., 303, 617-636.
Kolb, H., Linberg, K.A. and Fisher, S.K. (1992) The neurons of the human retina: a Golgi study. J. Comp. Neurol. 318, 147-187.
Kolb, H., Nelson, R. and Mariani, A. (1981) Amacrine cells, bipolar cells and ganglion cells of the cat retina: A Golgi study. Vision Res. 21, 1081-1114.
Kuffler, S.W. (1953) Discharge patterns and functional organization of mammalian retina. J. Neurophysiol. 16, 37-68.
Mariani, A.P. (1990) Amacrine cells of the rhesus monkey retina. J. Comp. Neurol. 301, 382-400.
Masland, R.H. (1988) Amacrine Cells. Trends in Neuroscience 11, 405-410.
Nelson, R., Famiglietti, E.V. and Kolb, H.(1978) Intracellular staining reveals different levels of stratification for on-center and off-center ganglion cells in the cat retina. J. Neurophysiol. 4, 427-483.
Polyak, S.L. (1941) The Retina. The University of Chicago Press, Chicago, Ill.
Raviola, E. and Raviola, G. (1982) Structure of the synaptic membranes in the inner plexiform layer of the retina: A freeze-fracture study in monkeys and rabbits. J. Comp. Neurol. 209, 233-248.
Rodieck, R.W., Binmoeller, K.F. and Dineen, J. (1985) Parasol and midget ganglion cells of the human retina. J. Comp. Neurol. 233, 115-132.
Shapley, R. and Perry, V.H. (1986) Cat and monkey retinal ganglion cells and their visual functional roles. Trends in Neuroscience 9, 229-235.
Vaney, D.I. (1986) Morphological identification of serotonin-accumulating neurons in the living retina. Science 233, 444-446.
Vaney, D.I. (1990) The mosaic of amacrine cells in the mammalian retina. Prog. Ret. Res. 9, 49-100.
Werblin, F.S. and Dowling, J.E. (1969) Organization of the retina of the mudpuppy, Necturus maculosus. II. Intracellular recording. J. Neurophysiol. 32, 339-355.