Donnell J. Creel
Introduction
One of the evolutionary characteristics of the mammalian visual system is the increase of binocular overlap of vision as eyes progress from being located on side of the head such as the guinea pig to the frontal position in Haplorrhine primates. Concomitantly as the proportion of temporal retina increases the number of uncrossed optic neurons at the optic chiasm expands from as little as 1% in guinea pigs to approximately 45% in primates including human beings (Polyak, 1957; Neveu & Jeffery, 2007). Figure 1 illustrates the approximate distribution of crossed and uncrossed retinal ganglion cells (RGCs) in the visual pathways of normally pigmented human beings.
C. L. Sheridan (1965) compared the interocular visual pathways in “split brain” ocularly pigmented (hooded) rats and albino rats. Sheridan concluded “Perhaps the paucity of uncrossed fibers that characterized rodents in general is even further reduced in the albino”. That year Lund (1965) verified Sheridan’s hypothesis anatomically. Lund stated albino rats display no organized uncrossed optic fibers. Lund agreed with previous estimates that pigmented rats possess up to 10% uncrossed optic fibers. Figure 2 is a diagram of crossed and uncrossed optic projections in wild-type rodents.
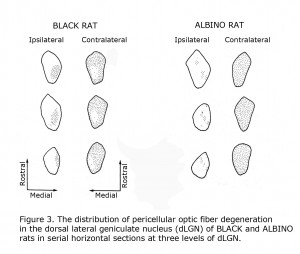
Mammals with few uncrossed retinal ganglion cell fibers (RGCs) such as rodents and lagomorphs do not exhibit the laminated dorsal lateral geniculate nucleus (dLGN) seen in carnivores and primates including human beings. Carnivores and Haplorrhine primate dLGN contain up to six monocular layers with point-to-point representation of visual space organized in columns through these layers. The 90%+ crossed RGCs at the optic chiasm in rodents and lagomorphs fill the contralateral dLGN, with only a pocket of the ipsilateral dLGN devoted to the fewer uncrossed fibers. Figure 3 illustrates RGC fiber terminations in horizontal sections through the dLGN comparing black and albino rats. The fewer uncrossed fibers in albino rodents are also projected to the dLGN in a fragmented fashion as opposed to a close organization in pigmented rodents.
2. Visual learning studies.
A retrospective review of visual learning experiments, which isolated uncrossed optic pathways in rats, published 1924 to 1966 indicated that ocularly pigmented rats can learn visual discriminations using uncrossed optic fibers, whereas albino rats cannot (Lashley, 1924; Chang, 1936; Muntz & Sutherland, 1964; Sheridan, 1965; Creel & Sheridan, 1966). This correlation was not noticed until 1965. These studies were all designed similar to depicted in Figure 4 of rat visual pathways. A combination of limiting visual input to one eye with ablation of visual cortex restricted visual information in rats to either crossed 90-95% of RGCs or 5-10% of uncrossed RGCs. Studies reported pigmented rats can learn visual pattern discriminations using uncrossed pathways. The uncrossed terminations in albino rats are minimal. Albino rats do not learn visual pattern discriminations when limited to uncrossed visual pathways.
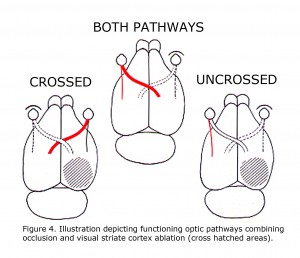 Figure 4. Illustration depicting functioning optic pathways combining occlusion and visual striate cortex ablation (cross hatched areas).
Figure 4. Illustration depicting functioning optic pathways combining occlusion and visual striate cortex ablation (cross hatched areas).
3. Siamese cats.
For several years the anatomical anomaly of few uncrossed RGCs at the optic chiasm in albinos appeared to be limited to rats and rabbits (Giolli & Guthrie, 1969). Guillery (1969) described aberrant retinogeniculate organization in Siamese cats, but the connection to albinism was not recognized.
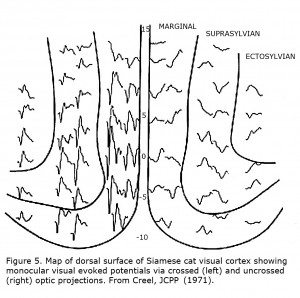
The first link between Siamese cats and albinism, and suggestion that reduced uncrossed optic fibers likely is a “highly general transspecies phenomenon in albinic mammals” was in 1971 (Creel, 1971a, b). Siamese cats possess a tyrosinase-locus mutation that is a temperature sensitive pigmentation defect, i.e. pigment forms only on the cold parts of the body similar to Himalayan mice, rats and rabbits. This mutation is sometimes referred to as the Siamese – Himalayan temperature effect. Cortical mapping of visual projection areas in normally pigmented and Siamese cats demonstrated significant differences in their uncrossed optic pathways. Figure 5 depicts monocular visual evoked potentials recorded from a Siamese cat’s cortical visual area showing preponderance of contralateral innervation.
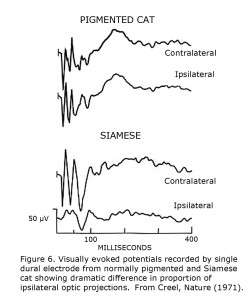
These cat studies discovered that a single recording site over each visual cortical projection area could reflect differences between pigmented and albino cats (Figure 6). This observation was the basis for testing human albinos using scalp-recorded visually evoked potentials (Creel et al., 1974).
4. Albino animal studies.
Many animal studies in the 1970s reported that all albino mammals with oculocutaneous, or only ocular albinism, demonstrate reduced uncrossed optic projections (Sanderson et al., 1975; Creel & Giolli, 1976; Guillery et al., 1979). A review with figures characterizing types of visual organization in the Siamese cat is R. W. Guillery’s 1974 publication in Scientific American. One human albino brain (Guillery et al., 1975, Hickey & Guillery, 1979) and an albino monkey brain were studied verifying similar anomalies in albino primates (Gross & Hickey, 1980; Guillery et al., 1984). Defects identified in albino mammals encompass a decreased number of photoreceptors, foveal/area centralis hypoplasia, misrouting of the temporal retinal ganglion cell axons across the chiasm, variation of central RGC terminations, vascular intrusion into area centralis, anomalous cortical organization, and fewer cones and RGCs in macular area (Elschnig, 1913; Fulton et al., 1978; Wilson et al., 1988; Bridge et al., 2014; McKetton et al., 2014).
The organization of the visual system varies considerably among mammals. Haplorrhine primates display well-defined foveae, foveal avascular zone, and large numbers of uncrossed optic fibers, whereas rodents and even carnivores exhibit only a central fixation area in the retina. In the domestic cat there is a trend towards an avascular zone with only capillary vessels found in the area centralis. Mammals vary not only in numbers of optic nerve fibers that decussate but further in embryonic development and proportion of optic projections terminating in various levels of the optic system from suprachiasmatic nuclei, to midbrain, to geniculate nuclei, to cortex.
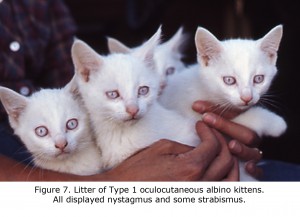
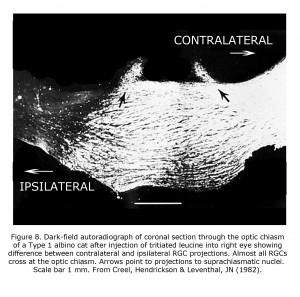
An animal model manifesting dramatic effects of misrouting is the albino cat. Figure 7 is a photograph of a litter of albino cats homologous to Type 1 tyrosinase-locus oculocutaneous human albinism (OCA1) with no ability to make pigment. All these cats had observable nystagmus and many strabismus. Figure 8 pictures the optic chiasm of an OCA1 albino cat showing almost all RGCs cross at the chiasm.
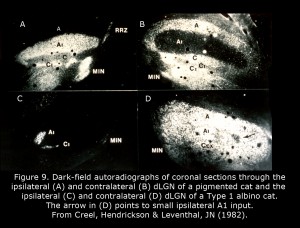
Figure 9 depicts the geniculate nuclei of a Type 1 albino cat compared to a normally pigmented cat. The dLGN of albino cats, which include only one small pocket of ipsilateral RGC terminations, is almost as primitive as a rodent dLGN. Four patterns of geniculocortical organization are described in albino mammals (Hubel & Wiesel, 1973; Guillery, 1974, 1984; Leventhal & Creel, 1985; Hoffmann et al., 2003). All forms likely exist in human albinos. Abnormal proportions of ipsilateral and contralateral RGCs continue to mid-brain nuclei probably providing the basis for atypical optokinetic nystagmus in albino mammals.
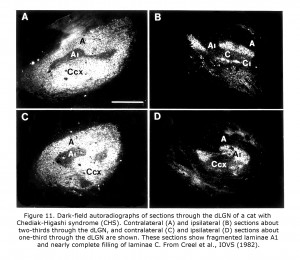
Few of the rare forms albinism has been investigated anatomically in carnivores or primates. One rare form that has is the Chediak-Higashi syndrome (CHS), which occurs in mammals including human beings and cats. Decreased oculocutaneous pigmentation, enlarged cytoplasmic granules, increased susceptibility to infections, and hemorrhagic tendencies characterize CHS. Ocular anomalies include pale irides and albinotic fundi. Cats with CHS exhibit photophobia and prolonged postrotatory nystagmus. Figure 10 depicts littermate cats of a Blue Smoke Persian strain. The hypopigmented cat on the left is affected with CHS. Visual projections of CHS cats misroute at the optic chiasm fragmenting layer A1 of the dLGN into several islands, similar to the disruption of A1 outlined in the Siamese cat (Creel et al., 1982). Visual and auditory anomalies resembling those in albinos were described in human beings with CHS using evoked potentials (Creel et al., 1983).
Phylogenetically older connections are less abnormal in albino mammals. Retinohypothalamic projections predate vertebrates, existing in early chordates that possess retinal pigment matching vertebrates (Vopalensky et al, 2012). Melanopsin-sensitive retinal ganglion cells are completely crossed or bilaterally projecting to suprachiasmatic nuclei, and not affected (Lucas et al., 2003; Hattar et al., 2006). The phylogenetically older bilateral projections to suprachiasmatic nuclei in albino cat (Figure 8) appear to be unaffected (Creel et al., 1982). The crossed/uncrossed ration of approximately 1.4:1 of retinal ganglion fibers to the suprachiasmatic nuclei in albino cats corresponds to the ration reported for normally pigmented cats (Hendrickson et al., 1972). The later-evolving vertebrate rod-cone-RPE-ganglion cell system is vulnerable to alteration in albino mammals whereas the phylogenetically older melanopsin pathway is generally not.
Most research regarding visual and auditory anomalies associated with albinism has used tyrosinase, c-locus, models. The majority of mammalian albino mutations are not associated with tyrosinase loci. Non-c locus albino mutations associated with similar visual and auditory anomalies are located on half of the human chromosomes ranging from Chediak-Higashi Syndrome (CHS) on 1q42 to X-linked ocular albinism locus Xp22.3, most of which phenotypically and chemically vary considerably from classic c-locus albinism. Tyrosinase-based, c-locus, albinism are readily available models, but do not provide the answer for the widespread association of visual and auditory anomalies with all forms of retinal hypopigmentation.
5. Genetic mechanisms.
Sprinkled through publications since the 1970s are suggestions that genetic affects are paramount. Sanderson et al. (1974) and Sanderson (1975) in the mink and rabbit, and Creel & Giolli (1972, 1976) in guinea pigs and rats early addressed genetic factors modifying expression of anomalies in ocularly hypopigmented models. In the cat model there are indications of genetic control including reports of heterozygote effects in pigmented cats (Ault et al., 1995; Leventhal et al., 1985). Carriers of the genes for human albinism do not show detectable visual anomalies (Hoffmann et al., 2006). Up to 90% of obligate carriers of X-linked ocular albinism exhibit pigmentary mosaicism in their ocular fundus, a lyonization effect, but with no further visual anomalies (Summers, 2009).
Many candidate agents, including signaling and transcription factors have been suggested that plausibly control targeting of retinal ganglion cells coursing through the chiasm and other visual features associated with albinism. Likely many of these conclusions are correct within the model studied, but not a general solution within mammals. Each species appear to vary. For example, significant differences between mouse and human embryogenesis specific to chiasm formation are described (Neveu et al., 2006). These differences include that the nodal point of neurogenesis in the human/primate retina is the fovea, while in rodents it is the optic nerve head. In rodents uncrossed cells are born early for their retinal location. This is not the case in primates. Consequently temporal patterning of axon arrival at the optic chiasm and their course through the chiasm are different between man and mouse. In addition there are no absolutes. The expression of these genetically determined traits has a statistical probability of occurrence (Creel & Giolli, 1976).
Variation can also be seen in the drift of retinal evolution even in Haplorrhine primates. Old World and New World monkeys evolved different retinal color vision receptors (Dulai et al., 1999) and different cortical visual organization (Hendrickson et al., 1978). Another example of variability concerning pigment chemistry is that human tyrosinase-related sequence locus on chromosome 11pll.2 is described in gorilla, but not chimpanzee (Oetting et al., 1993). Even minor differences in pigment metabolism alter metabolic proteins, which possibly could initiate a cascade of effects on visual embryogenesis.
Due to millions of years of divergent evolution the micro mechanisms of axon guidance underlying optic neuron decussation and target fate are likely idiosyncratic for each species. Pax2, Pax6, SOX2 appear to be necessary for ocular development. Only the most fundamental, ancient, conserved loci such as SOX, ROBO 3 and 4, homeobox (VSX2), Sonic hedgehog (Shh) and PAX2/PAX 6 interaction are likely perpetual and contributing to guidance and angiogenesis decisions in most mammals (Azuma, 2000; Arendt, 2003; Kozmik, 2005, 2008; Herbert & Stainier, 2011,). The conservation of some genes spans hundreds of millions of years. SOX21, active in developing retina and inner ear, occupies the same human loci (13q31-32) as DCT (L-dopachrome tautomerase), active in melanogenesis and tyrosine metabolism, has maintained 90-100% identity across species for hundreds of millions of years of divergent evolution (Woolfe et al., 2004, 2005). Worth noting is mutations of ROBO 3 and 4 are associated with non-decussation of the pyramidal motor pathways (Jen et al., 2004). ROBO 3 and 4 may affect visual pathway decussation by interacting with tyrosinase loci on same arm of chromosome 11q. Tyrosinase loci 11q13-22 are millions of base pairs from loci 11q23-24.2, but possibly communicate by interacting with noncoding RNA target sites close in three-dimensional space such as by chromosomal looping rather than linear sequence such as suggested for the X chromosome (Dimond and Fraser, 2013; Engreitz et al., 2013). Chromosome 11q possibly contains a domain coordinating tyrosine metabolism and coding for decussation (Dixon et al., 2012).
More intra-genome communication is probably taking place than currently credited. In addition to control of visual embryogenesis by protein coding, embryogenesis is plausibly affected by contributions from noncoding DNA or conserved noncoding portions of the genome. Noncoding DNA sequences are located near or within developmental gene loci that likely contribute to modulation or expression, and provide communication to bridge the gap between loci (Woolfe et al., 2004, 2005; Woolfe & Elgar 2008). Species-specific differences appear to originate more in noncoding space (Lee, 2012). Species likely evolve unique noncoding sequences contributing to variation in embryogenesis between species, and presumably are responsible for singular features in human CNS development (Prabhakar et al., 2006).
The chromosomal proximity of tyrosinase loci and ROBO 3 and 4 is not replicated in most mammalian genomes, but primate vision is the most complicated of mammals, and interactive gene control likely is more centralized in human beings. In other mammals loci on different chromosomes plausibly communicate to coordinate their expression (Kioussis, 2005). The “ROBO code” is likely a code of gene expression not an environmental interaction during embryogenesis (Spitzweck et al., 2010).
6. Melanin pigment.
Melanin pigment is a phylogenetically and genetically consistent metabolic vertebrate pathway dating back to at least the evolution of early chordates. The presence of melanin pigment is as fundamental as any characteristic of vertebrates. During mammalian embryonic development melanin pigment is pervasive as is its interaction with neural crest cell migration, along with ocular and auditory system development. Additionally the chemical pathways of melanin pigment, brain catecholamines, liver, thyroid and adrenal gland metabolism maintain a delicate balance anchored by tyrosine metabolism (Creel, 1980). Even in phenotypically pigment-less cavefish that mutated to not express pigment the ability to produce pigment is preserved in neural crest derived melanoblasts (McCauley et al, 2004).
Melanin’s role in visual function can be traced back to the hydra and jellyfish 600 million years ago (Plachetzki et al., 2012). Melanin pigment’s roles in the eye are different than other parts of the body. Melanin pigment is conspicuously present and melanocytes are most active early in vertebrate embryogenesis. Ocular melanocytes are relatively dormant after embryogenesis and unlike extra-ocular melanocytes do not secrete melanin. The genetic basis of pigment cell development and differentiation is conserved from teleosts to mammals. The melanin pigment chemical pathway initiated with tyrosine is expressed similarly from drosophila to primates. The common ancestor of drosophila and primate eye was likely an organism similar to a rag worm 500 million years ago (Arendt et al., 2004). The molecular sequence leading to eye formation in the fly is recapitulated in the developing human eye (Hanson, 2001; Kumar & Moses, 2001). In general, phylogeny is recapitulated in the embryonic development of the visual system.
7. Human albinos.
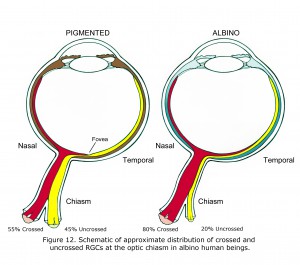
The features associated with hypopigmented retinae vary between species and individuals. Readily observable in most human albinos is anomalous foveal development and lack of vascular sparing of central foveal area of retina (Fulton et al., 1978; McAllister et al., 2010). Less obvious is the predominance of crossed optic fibers at the optic chiasm quantified by visually evoked potential, functional magnetic resonance imaging (fMRI) (McKetton et al., 2014), cortical thickening (Bridge et al., 2014) and aforementioned anatomical studies. Retinal ganglion cells (RGCs) in pigmented retinae that originate from the nasal side of fovea cross at the optic chiasm. RGCs originating temporal side of fovea in pigmented human beings do not cross at the optic chiasm. Figure 12 is a schematic of approximate differences between the optic chiasm of ocularly pigmented and albino human beings.
In mammalian hypopigmented retinae optic nerve ganglion cells that originate from retinae nasal side of fovea, and nearly all RGC neurons originating from up to 15 degrees in temporal retina also cross at the optic chiasm (Figure 12). The proportion is not known in human beings and likely varies considerably. In many albino animals essentially all optic neurons from nasal and temporal retina cross at the optic chiasm. In Figure 12 the 80% crossed and 15 degree shift of vertical meridian in human albinos was estimated based on animal anatomical studies and multifocal visually evoked potential studies (Leventhal et al., 1985, Hoffmann et al., 2006).
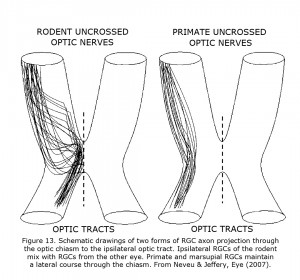
The routing of RGCs through the chiasm of human and other Haplorrhine primates is organized similar to schematic Figure 13 in that uncrossed RGCs originate and remain lateral at the chiasm (Hoyt & Luis, 1963), whereas in rodents and carnivores the nasal and temporal RGCs mix within the chiasm before routing ipsilateral or contralateral (Neveu & Jeffery, 2007; Jeffery et al., 2008). In marsupials, insectivores and primates the RGCs projecting to either hemisphere remain largely segregated with uncrossed fibers located laterally in the chiasm. In rodents and carnivores RGCs mix in the caudal nerve.
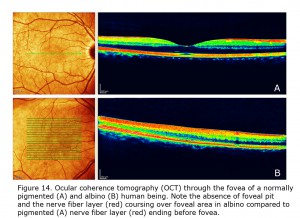
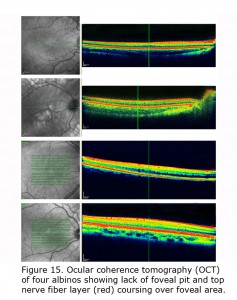
Figure 14 depicts comparison of optical coherence tomography (OCT) through the foveal area of an ocularly pigmented human being (A) and a human albino’s (B) foveal areas. In (A) note the foveal depression and that the nerve fiber layer, i.e. red layer on top, is not present across the foveal area. In most human albino retinae the nerve fiber layer (B) continues over the potential foveal area. Figure 15 pictures OCTs of four human albinos examined at the Moran Eye Center. To view more human albino OCTs see Gargiulo et al. (2011) and McAllister et al. (2010). Foveal under development and considerable variability among albino foveae is likely due to amount of ocular pigment and genetic background (Dorey et al., 2003). Albino human beings manifest a reduction in cone density in the central retina (Wilson et al., 1988). Multifocal ERG studies provide evidence of arrested postnatal development of foveae in albino infants (Kelly & Weiss, 2006). Primates are the only mammals with foveae, but in almost all mammals with albinism the central retina is underdeveloped (Hendrickson et al., 2006; Jeffery, 1997).
An exception is the albino squirrel (Esteve & Jeffery, 1998). Squirrel retinae contain a very large cone population. Rods are disrupted in albino development probably because they develop slightly later in retinal development and that cell cycle events get out of control when rod production is taking place. Cell cycle control is relatively normal early in retinal development when cones are being generated.
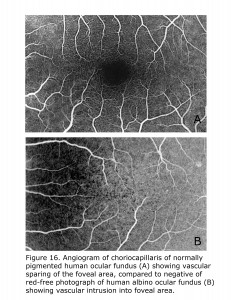
Retinal vascularization in human pigmented retinae spares the foveal area, whereas in albino human retinae small vessels and capillaries intrude into foveal area. Figure 16 compares the central vascular organization in ocularly pigmented and albino human retinae. Retinae of ocularly pigmented human beings exhibit an avascular zone surrounding the fovea, albinos do not. There are likely many modifying factors such as extent of hypopigmentation specific to the loci tempering phenotypic expression.
The neural retina and retinal pigment epithelium (RPE) demonstrate an intimate embryonic relationship to the extent that each can differentiate into the other (Fuhrmann, 2010). An interaction exists between melanin pigment in the RPE and the development of the fovea and choriocapillaris, the blood supply to the retina, and RGC axon guidance (Le et al., 2010). The RPE-derived vascular endothelial growth factor (VEGF) is essential for choriocapillaris development (Marneros et al., 2005; Zhu et al., 2012). Additionally, pigment epithelium derived factor (PEDF) was isolated in the developing fovea suggesting axon guidance genes presumably accompany early mechanisms determining vascular patterning in macular area (Kozulin et al., 2009). The PEDF has a major function in development of the foveal avascular area (Kozulin et al., 2010).
8. Scalp-recorded VEPs in human beings.
In 1974 using scalp-recorded visually evoked potentials (VEPs) four genetic forms of human oculocutaneous albinism exhibited electrophysiological evidence of reduced uncrossed optic fibers (Creel et al, 1974). Similar results were published for human ocular albinos (Creel et al, 1978) and replicated in early studies by Taylor (1978) and Coleman et al. (1979). Reviews of research in this area: Guillery (1996), Hoffmann et al. (2006), Neveu & Jeffery (2007), Bridge et al. (2014).
The best visual stimulus to use recording VEPs from most albinos is a pattern onset/offset with individual checks subtending about 1 degree of arc. For individuals with very poor vision and infants flash stimuli are preferred. In some albinos comparing evoked potentials using the International 10-20 System locations O1 and O2 (Jasper, 1958) demonstrate the misrouting. In most albinos more lateral locations along the same plane about halfway to T5 and T6 (H3 and H4), marked in Figure 17 as blue area, better demonstrate misrouting. An early finding was that with monocular stimulation the “difference” potential comparing 10-20 International System scalp locations O1-O2, or H3-H4 across the midline is an efficient way to exhibit chiasmic misrouting in albinos (Creel et al., 1981). The worst stimulus is pattern reversal, which exacerbates nystagmus in albinos blurring the image.
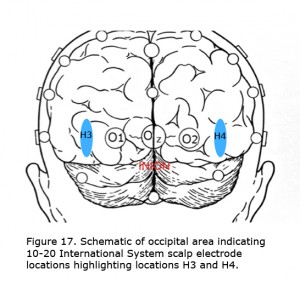 Figure 17. Schematic of occipital area indicating 10-20 International System scalp electrode locations highlighting locations H3 and H4.
Figure 17. Schematic of occipital area indicating 10-20 International System scalp electrode locations highlighting locations H3 and H4.
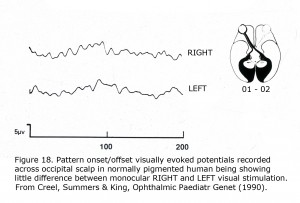
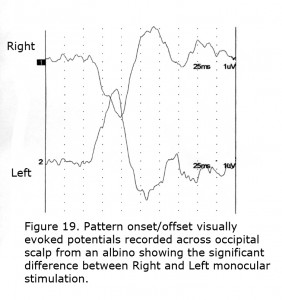
To detect misrouting at the chiasm a minimum of two recording sites is required, one positive electrode and one negative location in each hemisphere about 3 cm above the inion in an adult and 4 cm or more off the midline. This bipolar montage records a “difference” potential, requiring no additional reference electrode. The ground electrode can be attached anywhere on the person. In ocularly pigmented human beings innervation of visual cortex from nasal and temporal retinae is comparable so the “difference” potential recorded between hemispheres would be similar in form whether stimulating the left or right eye (Figure 18). In contrast, most central visual field retinal ganglion fibers (RGCs) cross to the contralateral hemisphere in albinos so the “difference” potential recorded between occipital hemispheres will change polarity across the occipital area when VEPs following monocular stimulation are compared (Figure 19).
An important addition to VEP methodology was the development of multifocal visually evoked potential (mfVEP) technology by Erich Sutter (2010). The first publication of mfVEPs in human albinos was by Michael Hoffmann and colleagues (2006). This group has continued to publish advances in dissecting the human albino visual system using mfVEPs and fMRI studies (von dem Hagen et al., 2008; Hoffmann et al., 2012; Klemen et al., 2012; Bridge et al., 2014; Kaule et al., 2014).
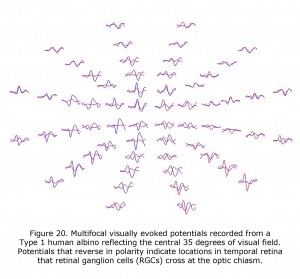
VEPs are of profound value in detecting the misrouting of the optic nerves in human albinism and revealed that an on average a central ±8 degree wide vertical strip in the retina is affected (Hoffmann et al., 2005). The extent of the misrouting varies, depending on pigmentation deficit, between 2 and 15 degrees. This indicates that RGCs originating up to 15 degrees into temporal retina erroneously cross at the optic chiasm. The most important addition of mfVEPs is the spatial characterization of VEP responses. This approach can be combined with the misrouting VEP paradigm (Creel et al., 1981; Apkarian et al., 1983) to yield visual field topography of the abnormal visual field representations typical for albinism as pioneered by Michael Hoffmann et al. (2006). Figure 20 displays mfVEPs recorded from a Type 1 oculocutaneous albino (OCA1). This person has nystagmus and exotropia, but exceptional acuity for a Type 1 oculocutaneous albino. His acuity meets requirements of visual acuity required for a driver’s license. The central visual field stimulated for these mfVEPs extended 35 degrees horizontally and 30 degrees vertically, similar to the perceptual field of a Humphrey 24-2 visual field perimeter test. Potentials that reverse in polarity indicate temporal retinal field areas projecting RGCs that cross the optic chiasm. MfVEPs require reasonably stable fixation (Hoffmann et al., 2005, 2006; Zhang et al., 2008), but they are a useful extension of the classical misrouting VEP paradigm as they permit the detection of locally confined, i.e. only small misrepresentations. Their prime use is to explore the specificity of misrouting for albinism. MfVEP studies confirmed the absence of evidence of misrouting in carriers of OA (Hoffmann et al., 2006).
While fMRI was used to validate the misrouting VEP (von dem Hagen et al., 2008; McKetton et al., 2014), it further assists in uncovering the organization of the human visual cortex in albinism, and higher cortical function (Morland et al., 2001; Hoffmann et al., 2003). These studies indicate that the unrepresentative visual input to each hemisphere is propagated to higher processing stages (Kaule et al., 2014) and is made available for perceptual (Hoffmann et al., 2007; Klemen et al., 2012) and visuo-motor integration (Wolynski et al., 2010). Further, structural MRI investigations underscore that the anatomy of the visual cortex in human albinism appears to be affected more strongly by the foveal hypoplasia than by the misrouting of the optic nerves (von dem Hagen et al., 2005; Bridge et al., 2014).
Maturation and aging of the visual system produce measurable changes reflected in visually evoked potentials. Neveu et al. (2003) described differences in the population of albino human beings ranging from 8 months to 60 years. Visual acuity usually improves until adolescence due to physiological maturation, dampening of nystagmus, finding a favorable head position, and learning how to manage poor acuity including perceptual cues (Kinnear et al., 1985; Weiss & Kelly, 2007; Dijkstal et al., 2012).
Acuity is significantly diminished in most human albinos averaging from 20/80 to 20/200 with a wide range of 20/40 to 20/400. Nystagmus, usually horizontal, pendular or jerky in character is almost always present. A high incidence of strabismus is also seen in albinism including both horizontal and vertical misalignment (Taylor, 1978; Summers, 1996).
Contributing to differences in optic tract development are possible differences in axon sizes and myelination in the albino mammal’s optic tract (Guibal & Baker, 2009). When RGC axons go to the wrong side of the brain they may not myelinate properly. Myelination changes with age add weight to Neveu et al.’s (2003) finding of age-related changes in VEP development.
9. Visual perception.
Studies demonstrate that albino human beings do not fuse binocular stimuli (St John & Timney, 1981; Witkop et al., 1982; Kinnear et al., 1985). Few binocular cells are located in areas 17, 18 and 19 in Siamese cats and cats with complete albinism, disturbing the underlying anatomical connections for stereovision (Di Stefano et al., 1984; Leventhal & Creel, 1985; Kalberlah et al., 2009). Some individuals with albinism manifest coarse binocular fusion with some stereovision (Lee et al., 2001). In addition, while areas 17/18 of the Siamese cat do not contain binocular cells, the postero-medial lateral suprasylvian area (PMLS) includes binocular cells (Bacon et al., 2001). The superior colliculus (SC) also possesses binocular cells (Antonini et al., 1981). The corpus callosum possibly provides an anatomical connection enabling some binocular vision (Berlucchi et al., 1967; Shatz, 1977; Marzi et al., 1980; Zeki & Fries, 1980). Stereovision may be accomplished through these cortico-cortical connections. Although stereovision is dramatically affected in albinos, spatial perception remains (Klemen et al., 2012).
Adjustments improving perception appear to be made at each level as the anomalous visual information in albino visual pathways progress towards the cortex. In the albino cat the disaster at the level of the dLGN where only 46% of cells responded normally improves to 84% in cortical area 17, and to 70% of cells in cortical area 18 (Schmolesky et al., 2000). The competence of the albino visual system improves greatly at the cortical level of cats and human beings (Hoffmann et al., 2007) resulting in near-normal visual perceptual abilities within the limits of an individual’s acuity.
In general the level of pigmentation of an albino correlates negatively with severity of expression of visual anomalies (Dorey et al., 2003; Schmitz et al., 2003). It is likely that the more pigment present in the retina, the more normally routed RGC fibers.
10. Genetic classification of forms of human albinism.
Figure 21. Type 1 oculocutaneous albino (OCA1).
Current classification of forms of albinism is based on genetic locus. Figure 21 is a photograph of an individual with Type 1 oculocutaneous albinism. Chromosomal location has been identified for twenty forms of human albinism on twelve different chromosomes: oculocutaneous albinism (OCA1-7), ocular albinism (OA1), nine forms of Hermansky Pudlak syndrome (HPS1-9), Chediak Higashi syndrome (CHS) and Griscelli syndrome (GS) (see: Westbroek et al., 2011; Montoliu et al., 2014]. An additional syndromic form of albinism to be added is the rare Vici syndrome (VICIS) (Vici et al., 1988; Cullup et al., 2013; Filloux et al., 2014). Phenotypic expression varies considerably within genetic forms of albinism, and even between siblings (Summers et al., 1991; Cheong et al., 1992). Many genes modify expression of albinism (Oetting et al., 2003). For tyrosinase enzymes alone more than 100 mutations are involved in coding (see Albinism Database (http://www.ifpcs.org/albinism/index.html).
Visual abnormalities reported in albinism does not include disorders of hypopigmentation due to embryonic migration defects of neurons and pigment cells from the neural crest such as in Waardenburg syndrome and deaf white cat (W gene), vitiligo or piebald spotting, nor other forms of hypopigmentation such as phenylketonuria. These conditions do not exhibit the visual anomalies.
11. Exceptions to direct association with albinism.
Recently a novel gene mutation on human chromosome 16 was described that is associated with foveal hypoplasia, optic-nerve-decussation defects, and anterior segment dysgenesis (FHONDA) in the absence of pigment deficits (Poulter et al., 2013; Al-Araimi et al., 2013). Current research addresses whether in this condition optic nerve misrouting is initiated by a mechanism that is independent of the one that is associated with the pigment deficit in albinism. While enhanced crossing of the optic nerves at the optic chiasm was previously viewed to be specific for albinism or related only to ocular pigment deficits there may be exceptions. FHONDA may be an important exception. A possible site of common determination of visual abnormalities in FHONDA and albinism could be the similar influences affecting optic anomalies in both such as genetic control by Sonic hedgehog (Shh), VSX2, PAX 2 and PAX 6.
There are reports of optic misrouting in human non-albinos such as some with Kartagener syndrome (Van Genderen et al., 2006), and selected hypopigmented patients with Prader-Willi syndrome (Creel et al., 1986), with microdeletion at tyrosinase locus 15q11-13 superimposed on the most prominent albino sequence. The Kartagener syndrome loci, 15q24-25, are on same arm of chromosome 15q as the most prominent human albino sequence. Few individuals with the Kartagener syndrome show optic misrouting (Hoffmann et al., 2011). Other exceptions are some individuals with congenital stationary night blindness (CSNB) reported to express optic misrouting (Tremblay et al., 1996; Ung et al., 2005). Genetic loci for forms of CSNB range from chromosome Xp11 to Xp22 (Gal et al., 1989). These mutations are located on the same arm of X chromosome located Xp22. Some of these exceptions are possibly due to interaction with albino loci or a common feature to both of dysregulation within these sequences. Communication may be enhanced by noncoding DNA bridging the gap between nearby loci.
12. Underlying mechanisms.
Many studies were published pursuing a causal relationship between retinal pigmentation and determination of neuronal routing at the optic chiasm. No mechanism has been described that can be generalized across species and genetic loci. Prominent theories include the role of tyrosinase or its precursor DOPA as critical in the synthesis of melanin such as reporting addition of DOPA to albino eyes in vitro normalizes patterns of cell production (Ilia & Jeffery, 1999). Ocular albinism (OA1) associated with a mutation of the GPR143 (G-protein-coupled receptor) gene on the X chromosome (Xp22.3) provides evidence of another possible function of DOPA. The GPR143 gene regulates melanin biosynthesis (Giodano et al., 2011). The OA1 GPCR protein encoded by GPR143 gene was found to be a selective DOPA receptor, where both Dopamine and DOPA compete to bind at its site (Roffler-Tarlov et al., 2013). DOPA function in the melanin pathway may also play a role in the downstream effects that affect the developing retina (Lopez et al., 2008).
Other factors influencing ganglion cell targeting at the optic chiasm include, in different species, growth cone action, molecules, and regulatory genes including laminin, NCAM, L1 and cadherins (Jeffery & Erskine, 2005). Evidence that Netrin-1 (Deiner et al., 1997), Slit ligand molecules that are expressed at the optic pathway and their associated axon guidance receptors from the Roundabout (Robo) family (Plump et al., 2002), Sema5A (Oster et al., 2003), Sonic Hedgehog (Shh) (Trousse et al., 2001), transcription factor 2 (Zic-2) regulated by Islet 2 in mice (Petros, 2008; Rebsam et al., 2012), and EphrinB2 (EphB2) (Williams et al., 2003) are also suggested as impacting cell fate. The transcription factor visual system homeobox 2 (VSX2) probably also affects the retinal anomalies (Zou & Levine, 2012; Phillips et al., 2014). Many signals likely vary between species.
13. Vision & Hearing.
Vision and hearing have an ancient history of evolving in parallel traced back to the PaxB gene, which functions as a single gene controlling eye and hearing (mechanoreceptors) genesis in box jellyfish predating the later-appearing separate Pax 2 and Pax 6 genes (Piatigorsky & Kozmik, 2004). The continuity of SOX 21 was mentioned earlier (Woolfe et al., 2005). There are evolutionary connections between eyes and mechanoreceptors of the inner ear to the extent that during evolution, “sensory cells can shift their sensory modalities” (Fritzsch et al., 2005).
There is a history of both functional integration and anomalous syndromes involving the visual and auditory systems such as Usher’s Syndrome, which includes retinitis pigmentosa and congenital deafness. In spite of their differences in embryogenesis further similarities were recently described in retinal and inner ear hair cell physiology. The same Usher protein functions in retinal rod physiology and inner ear hair cell physiology (Yang, 2012; Cosgrove & Zallocchi, 2014) and ribbon synapses are similar in structure and function in both photoreceptors and hair cells (Nouvian et al., 2006). Further metabolic roles of melanin in the inner ear, and the similarities of retinal and inner ear physiology are yet to be elucidated.
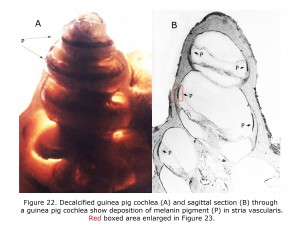
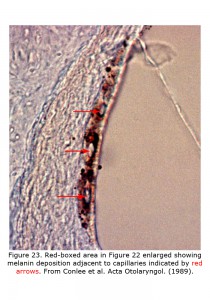
Melanin pigment is located in the stria vascularis of the inner ear (Conlee et al., 1989, 1994). Figure 22 depicts a decalcified cochlea of pigmented guinea pig (A) displaying a band of melanin pigment (P) in the stria vascularis, and a section through a pigmented guinea pig cochlea (B) showing melanin pigment. Figure 23 is an enlargement of an area in a different guinea pig similar to that boxed in Figure 22B illustrating detail of pigment deposition. Melanocytes are adjacent to and wrap around capillaries in the stria vascularis.
In most mammals auditory cues mainly tell vision where to look (Heffner & Heffner, 1992; Feng et al., 2014). Within each species spatial vision and auditory localization evolve in a coordinated manner. Analogous evolutionary and functional relationships exist in vision and audition. The second level termination of visual neurons is cortical including synapses with binocular cells, the substrate for binocular, stereo, and spatial vision. The second level neuron terminations in the auditory system are in the superior olivary complex where binaural cells receive input from each ear giving similar spatial information. The auditory space map in the brainstem is strongly related to the cortical visual space map (Grothe et al., 2010). Visual and auditory space are connected to the extent that in human beings fMRIs depict more recruitment of visual cortex than auditory cortex when blind individuals perform auditory echo localization (Thaler et al., 2011).
Albino mammals are not hearing impaired, per se. The auditory and visual deficits in albino mammals are connected in that similar anomalies are present in processing the highest-level integration of spatial localization.
Auditory anomalies are reported in albino mammals. The absence of melanin pigment in the inner ear of albinos is associated with susceptibility to noise damage (Gottesberge, 1988; Murillo-Cuesta et al., 2010). In human beings and animal models prolonged sensitivity to noise measured as a longer temporary threshold shift (TTS) following exposure to loud sounds is documented (Garber et al., 1982; Conlee et al., 1986; Barrenas & Lindgren, 1990). Cell size and dendritic length in the medial superior olivary nucleus is reduced in albinos (Conlee et al., 1984, 1986). Functionally the reduced cell size in brainstem olivary nuclei was associated with reduced binaural cell responses in medial superior olive of albino cats (Yin et al., 1990). One study demonstrated reduced ipsilateral projections from the cochlear nuclei in hypopigmented ferrets (Moore & Kowalchuk, 1988).
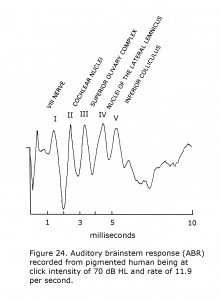
The auditory brainstem response (ABR) is an auditory version of a visually evoked potential except the generators measured end at the level of the superior colliculus. Auditory brainstem responses are recorded by placing a positive electrode on top of the head at 10-20 International System position Cz. A negative pole electrode is placed on ipsilateral earlobe or mastoid. Often the contralateral earlobe or mastoid is used for a ground location. Click stimuli at one or more intensity approximately 70 dB above hearing level threshold (HL) are presented to one ear at click rates of 10 or more per second. Potentials appearing in the first 10 milliseconds evoked by about 2,000 clicks are averaged. Audiologists record a more complicated sequence using several click intensities, tones bursts, and bone conduction testing to evaluate hearing.
A feature of the ABR is that in addition to anomalies in the auditory brainstem pathways, alterations in adjacent brainstem structures such as cranial nerve nuclei IV to VII are often detected. The ABR is of use locating etiology of ocular and facial movement disorders such as Duane’s retraction syndrome, Marcus-Gunn ptosis, blepharospasm, facial cranial nerve palsies, and is a common site of demyelination in patients with multiple sclerosis. Another feature of the ABR is the first five peaks, I – V, are associated with the level of the brainstem generating each component.
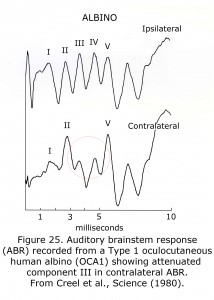
A sample human ABR is depicted with generators labeled in Figure 24. ABRs of most mammals contain 5 or more components in the first 6 milliseconds following click stimuli. ABRs of some rodents include a double component III due to separate medial and lateral olivary nuclei generators. A variant in some human ABRs is coalesced components IV and V into one wave. In albino human beings and animal models component III of ABRs are attenuated, which are generated in region of the medial superior olivary nuclei reflecting the reduced neuronal size and dendritic length in albino mammals (Conlee et al., 1984, 1986; Creel et al., 1980, 1983). Figure 25 illustrates sample abnormal ABRs in a Type 1 oculocutaneous human albino with components III – IV attenuated in amplitude.
14. Eye Movement: Albinism, Dissociated Vertical Deviation, Latent Nystagmus.
Adjacent to the brainstem auditory centers are the nuclei integrating sensory input including the superior colliculus and other nuclei involved in eye alignment and fixation. Abnormal proportions of ipsilateral RGCs in albino mammals include reduced RGCs to mid-brain nuclei that contribute to strabismus, nystagmus, and atypical optokinetic nystagmus in albino mammals. In albino guinea pigs, rabbits and ferrets there are no ipsilateral optic projections to the ventral lateral geniculate nucleus (LGNv), superior colliculus (SC), pretectum, nucleus of the optic tract (NOT) or accessory optic system (AOS) (Giolli & Creel, 1973; Gayer et al., 1989; Zhang & Hoffmann, 1993).
In the normally pigmented cat about 50% of RGCs project via the LGN to the ipsilateral cortex. 24% of RGCs originating in temporal retina project to the ipsilateral superior colliculus (Wässle & Illing, 1980), and about 15% of RGCs project to the ipsilateral pretectum (Koontz et al., 1985). The albino cat has very few ipsilateral projections to the pretectum and almost no ipsilateral projections to superior colliculus (Creel et al., 1982). All albino cats have nystagmus and many have strabismus.
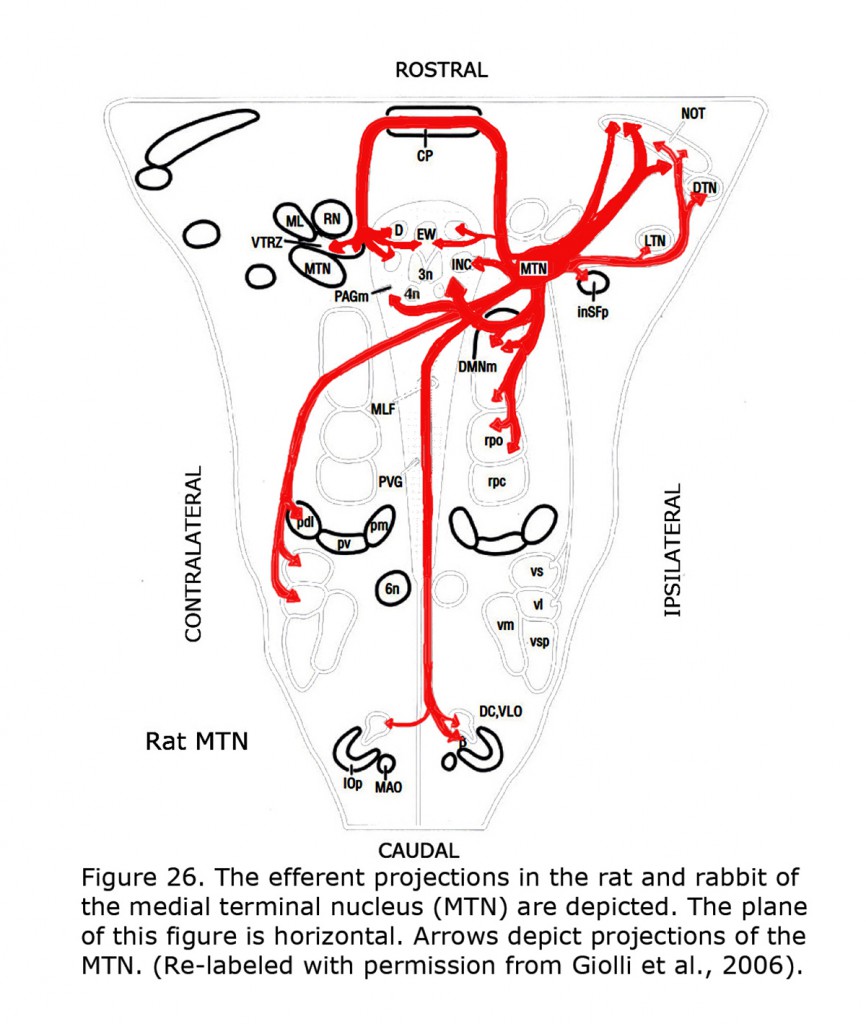
Several types of aberrant optokinetic nystagmus (OKN) are described in human albinos (Collewijn et al., 1978, 1983; Huber-Reggi et al., 2012). The nuclei responsible for the OKN are the terminal nuclei of the accessory optic system: the dorsal terminal nucleus (DTN), lateral terminal nucleus (LTN), medial terminal nucleus (MTN) and the Nucleus of the Optic Tract (NOT).
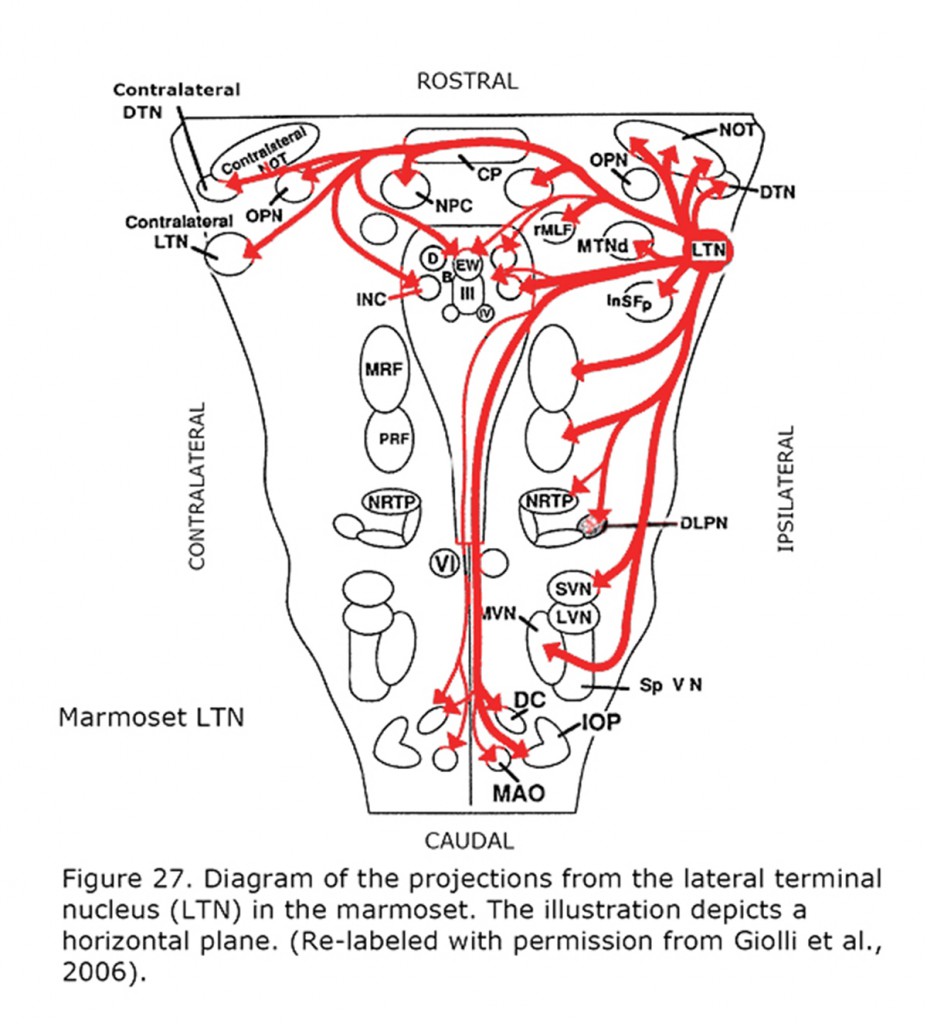
Figure 26 depicts the rat and rabbit accessory optic system showing the connections of the medial terminal nucleus (MTN) (Giolli et al., 2006). Figure 27 illustrates the accessory optic system (AOS) projections from the lateral terminal nucleus (LTN) of a primate, the Marmoset.
Albinism in mammals is associated with most RGCs crossing in the optic chiasm. Few RGCs project to the ipsilateral side ultimately projecting to the cortex and brainstem. The optic chiasm plays a pivotal role in human binocularity. Hemidecussation in the optic chiasm dictates the nasal retinal ganglion cells to cross to the other side of the brain, and temporal retinal ganglion cells to stay on the same side of the brain. Retinotopy directs ganglion cells, one nasal and one temporal from each eye, to pair up at the cortex. In this way each pair finally drives one binocular cell in the visual cortex. Besides hemidecussation in the optic chiasm, the corpus callosum and commissural connections in the brainstem are essential building blocks of mammalian binocularity. Identical binocular retinotopic loci on both sides of the cortex are connected through the corpus callosum. These connections facilitate fusion of the eyes, and the right and left visual hemifield (Pietrasanta, 2012; ten Tusscher, 2014) In albinism, characterized by a substantial reduction in ipsilateral projections of RGCs in the optic chiasm, and poor binocularity, results in less coordination between and loss of association between the eyes, and infantile strabismus occurs. Dissociated Vertical Deviation (DVD) and Latent Nystagmus (LN), both occurring in infantile strabismus, are characterized by binocular motor dissociation. The co-occurrence of both DVD and LN in albinos suggests a common dissociative origin (ten Tusscher, 2014).
There are several theories rooted in the argument that older evolutionary connections dominate during the first several months of human infancy before cortical pathways have matured. Michael C. Brodsky and colleagues propose atavistic reflexes of the accessory optic system generate the dissociated eye movements associated with infantile strabismus (Brodsky & Dell’Osso, 2014; Christoff et al., 2014; Brodsky 2012a,b). They argue that when foveal vision develops slowly in early infancy, the phylogenetically older optokinetic system prominent in lateral-eyed vertebrates that is expressed in first 2 months of infancy, continues to be expressed. Monocular, subcortical, nasotemporal optokinetic asymmetry normal in lateral-eyed vertebrates is suppressed in human beings with cortical binocular vision. They suggest cases of possible infantile nystagmus may be due to genetically determined maturational delay in development of cortical binocular vision allowing phylogenetically older pathways to dominate; “[hypothesizing] that the oculomotor disturbances of infantile strabismus correspond to subcortical binocular visual reflexes that are operative in lateral-eyed animals.”; and that the vestibulocerebellum actively contributes to generating infantile nystagmus (Brodsky & Dell’Osso, 2014).
Marcel ten Tusscher also proposes a fall back to older evolutionary connections including the corpus callosum, lateral geniculate nucleus, lack of normal binocularity at the cortex, and subsequent loss of binocular input to the subcortical centers including the superior colliculus (ten Tusscher, 2010, 2011, 2012, 2014).
The evolution of the eye and the brain provides a possible explanation for both the anomalous eye movements in albino mammals, and the dissociation of infantile esotropia and its motor characteristics. In the course of evolution, the eyes moved from a lateral to a frontal position. In 300 million years, modern mammals, birds and reptiles evolved from a common ancestor, the captorhinidea (Carroll, 1988; Husband, 2001). Consequently, the monocular visual fields progressed forward on the head to overlap resulting in a binocular visual field. In lateral-eyed animals, the retinae project to the contralateral visual cortices only. These projections are also found in binocular mammals and birds with binocular visual fields. In binocular mammals there are also uncrossed projections from the temporal retinae to the visual cortex. Partial chiasmal decussation and the corpus callosum provide the necessary connections for binocular vision to develop. (Aboitiz et al., 1992). Disruption of normal binocular development causes a loss of binocularity in the primary visual cortex and adjacent areas. Outside the primary visual cortex, the contralateral eye dominates while the temporal retinal signal appears to lose influence.
Complete motor dissociation between the eyes often results from sensory dissociation between the eyes. In human achiasma, where all retinal nerve fibers project to the ipsilateral cortex, saccades are executed monocularly. Dissociation with monocular saccades also occurs in animals with lateral eyes, e.g. reptiles. Retinal ganglion cells in these species show 100% crossing in the optic chiasm.
Eye movements in animals with laterally-located eyes are intended to stabilize the visual environment. The vestibular ocular reflex compensates for body movements and the optokinetic reflex compensates for optic flow. Motion sensitive neurons in the retina project to the contralateral brainstem, i.e. the terminal nuclei of the accessory optic system (Figure 27) that comprise the afferents of the optokinetic reflex. The nucleus of the optic tract (NOT) is one of these nuclei. The right NOT senses horizontal movement to the right, and the left NOT senses movement to the left. The major target of the optic tract in lateral-eyed animals, however, is the superior colliculus that is responsible for directing gaze movements and sensory integration. Similar neuroanatomical pathways and eye movements also exist in human beings. Human beings, in addition, demonstrate saccades, pursuit and vergence, all of which are initiated by the visual cortex, not the brainstem. The retinal ganglion cells partially decussating in the optic chiasm is one of the evolutionary solutions for combining information from frontal eyes, enabling a correlated binocular signal in the visual cortex. The binocular human cortex ties the temporal retina of one eye together with the nasal retina of the fellow eye. Binocular visual experience overrules the brainstem through an ipsilateral signal directly from the retina, but even more so, via the visual cortex. The optokinetic reflex interacts with the pursuit system, while the superior colliculus (SC) interacts with the saccade and vergence systems (ten Tusscher, 2014).
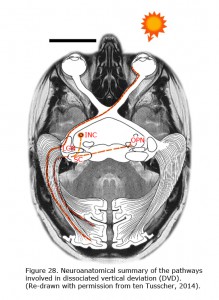
Newborn children have smooth nasally directed monocular pursuit and impaired temporal-directed pursuit (Atkinson, 1979). At birth, the eye is controlled by the contralateral nucleus of the optic tract (NOT) (Hoffmann et al, 1992). The right nucleus of the optic tract senses movement to the right, the left nucleus to the left. The newer pathways (ipsilateral from retina to nucleus of the optic tract (NOT), and the more abundant cortical projection to the NOT are activated through binocular visual experience. When the primary visual cortex does not receive realistic binocular input from both eyes the crossed older pathway dominates. The corticotectal pathway is dominated by the contralateral pathways (Hoffmann, 2003; Mustari et al., 2008). Van Rijn et al. (1997) showed that the same mechanism responsible for LN might be true for DVD (Figure 28). DVD combines vertical vergence with binocular torsional version. The elevating eye shows excyclotorsion while the fixating eye shows incyclotorsion. Van Rijn and ten Tusscher (1997) measured eye movements in DVD and showed that these movements are similar to the eye movements in disparity-induced vertical vergence. They suggested that, like LN that likely results from dissociation in the optokinetic /pursuit pathways, DVD might result from dissociation in the vergence pathways (ten Tusscher 1997, 2010). In LN the optokinetic pathway is driven by the signal that originates in the crossed retinal ganglion cells due to sensory dissociation, and less so the uncrossed cortical influence on the NOT in the brainstem. DVD likely is a supranuclear cortical problem of the pathways responsible for vergence originating in the crossed retinal ganglion cells due to sensory dissociation and less binocular cortical influence on the brainstem level (ten Tusscher, 2010).
DVD appears as a result of inhibition of the uncrossed temporal retinal signal. Beyond the primary visual cortex and the reciprocal connection of the callosum, a biased cortical activation leads to corticotectal stimulation dominated by the nasal retina. Figure 28 depicts the neuroanatomical summary of the pathways involved in DVD projection from nasal retina of the contralateral eye that dominate the superior colliculi in infantile strabismus. With occlusion of the left eye, the nasal bias in ocular dominance in the visual cortex in a patient with infantile strabismus gives rise to dominance of the left visual cortex and the left corticotectal pathway. The left superior colliculus (SC) projects to the left interstitial nucleus of Cajal (INC) and the right omnipause (OPN) neurons that results in elevation of the left eye and counterclockwise cycloversion. The pathway from the superior colliculi (SC) to the interstitial nuclei of Cajal (INC) links the two dimensional cortex and superior colliculus to the three dimensional brainstem. A monocular vertical signal leads to torsional vergence, DVD.
Partial decussation in the optic chiasm appears to be only one of the components important for binocularity. In rodents, e.g. the rat, with 95% crossing in the optic chiasm binocularity of the visual cortex depends on the combination of a signal from the contralateral eye through the geniculate pathway, and a signal from the ipsilateral eye to the contralateral geniculate and back through the corpus callosum. But even in cats that have substantial hemidecussation at the optic chiasm, transection of all crossing retinal ganglion cell axons in the optic chiasm appears not to affect most kinds of disparity (Pietrasanta et al., 2012). In mammals without partial decussation in the optic chiasm and without a corpus callosum, like marsupials, e.g., the wallaby, binocularity is still demonstrated in brainstem neurons (Ibbotson et al., 2002). Binocularity appears to depend on commissural connections between both superior colliculi and between the nuclei of the optic tract. So, mammalian binocularity includes information provided by 1) partial decussation in the optic chiasm, 2) the corpus callosum and 3) commissural connections in the brainstem (ten Tusscher, 2014).
A high percentage of both infantile exotropia (67%) and esotropia (49%) patients were found to have a coexisting ocular or systemic abnormality (Berman & Murphy, 1982). Agenesis of the corpus callosum underlines the importance of the corpus callosum for normal binocular fusion. Strabismus is found in 46% of patients with partial agenesis of the corpus callosum (Goyal et al., 2010). Moreover, hemispherectomy early in life often causes strabismus (Koenraads et al., 2014). Hemidecussation in the optic chiasm allows retinotopic matching between the nasal and the temporal retina. In albinism, many RGCs originating in temporal retina project to the contralateral LGN then to contralateral visual cortex. The border between crossed and uncrossed ganglion cell fibers in most human albinos appears to vary from point of fixation to 6–14 degrees into the temporal retina. Besides prominent misrouting in the chiasm, as in albinism, or agenesis of the corpus callosum, more subtle misrouting may cause a mismatch between temporal and nasal signals or between the direct thalamic and the indirect corpus callosum signal. Also the vertical meridian of one retina may not coincide with the overlapping zone of the other eye. In a primate study, the retinal vertical meridian in one of three monkeys was found not to coincide with the fovea (Fukuda et al., 1989).
Both esotropia induced by prisms or surgery in the sensitive postnatal period, and infantile esotropia change the binocular response of neurons in the primary visual cortex dramatically. After early surgically induced strabismus, neurons in the primary visual cortex, area 17, of kittens were found to be responsive to one eye only. An approximately equal representation of both eyes was found in both hemispheres. These results have often been confirmed, although a small bias of the non-operated eye in the ocular dominance columns sometimes occurred. Also most cortical neurons distal to area 17, the extrastriate cortex, could exclusively be triggered by one eye. An equal representation of both eyes is not found in the extrastriate cortex. The large majority of neurons in, for example, area 18 respond to the contralateral eye exclusively. In contrast to this huge contralateral dominance, only a slight dominance of the non-operated eye was found. In electrophysiological studies cortical neurons representing the temporal retina were found to be unresponsive to a stimulus from the squinting eye. In the early postnatal period, crossing nasal retinal afferents were found to be far less vulnerable than temporal uncrossed retinal afferents (Bisti & Carmignoto, 1986).
Crossed retinal dominance beyond the primary visual cortex appears to explain Dissociated Vertical Deviation and Latent Nystagmus, however, the cause of DVD is often explained differently. In a 2014 overview of a symposium on dissociated vertical divergence (DVD), Christoff et al. (2014) discussed two popular theories on the origin of DVD: a hypothesis that suggests that DVD results from damping of latent nystagmus (LN), and another theory that postulates an atavistic origin: a righting reflex in fish.
Ten Tusscher argues the co-occurrence of both DVD and LN in infantile strabismus may well suggest a common dissociative origin, or perhaps one of these phenomena may be the result of the other. It is postulated to be the latter because damping of latent nystagmus occurs after the start of vertical divergence in DVD. However, DVD is more frequent than latent nystagmus. Moreover, DVD is a unique binocular cyclovertical eye movement. It is suggested to occur in order to suppress a horizontal eye movement and only if this movement is latent. Why does it not occur in manifest latent nystagmus and why not in congenital nystagmus? Ten Tusscher argues the righting reflex observed in fish as the origin of DVD is less likely (ten Tusscher & van Rijn, 2010, 2011). They argue the righting reflex is a postural reflex, not an ocular one and is orchestrated by the torus longitudinalis and the valvula cerebelli, and that both of these have no analogue in the human brain. Moreover, during a righting reflex the eye that is illuminated most, will move downward and the other upward. In DVD, however, blinding an eye by light will cause an upward movement of the blinded eye (the Bielschowsky phenomenon). Also, in contrast with the righting reflex, DVD is influenced by blur and by fixation. DVD combines vertical vergence with binocular torsional version. The elevating eye shows excyclotorsion while the fixating eye shows incyclotorsion. Early research measured eye movements in DVD. DVD characteristics appeared similar to the eye movements in disparity-induced vertical vergence (van Rijn & ten Tusscher, 1997).
Albinism is an example that sensory dissociation may be the result of a change in chiasmal decussation. Dissociated Vertical Deviation (DVD) and Latent Nystagmus (LN), are characterized by binocular motor dissociation with a common dissociative origin. Brain pathology may cause sensory dissociation, which may subsequently lead to motor dissociation. The binocular link between the cortex and the brainstem nuclei like the NOT and the superior colliculus, is a key element in the origin of motor dissociation in infantile strabismus. In primates with infantile strabismus, the visual cortex beyond V1 is dominated by the crossed retinal signal. The equilibrium between the crossed and the uncrossed signal is not established. The brainstem is deprived of normal binocular input. This causes the NOT and superior colliculus to be driven mostly by the contralateral eye (Figure 28). If an eye is connected mostly to the contralateral NOT it will be more sensitive to motion directed towards the nose. This asymmetry causes latent nystagmus during monocular viewing. Fixation, saccades and vergence rely on the information from visual cortex to the superior colliculus. Loss of uncrossed retinal signal to the superior colliculus causes the upward movement of the eye. Cortical deactivation of this system causes Bell’s phenomenon and the upward movement of the eyes during the early stages of general anesthesia that is related to the loss of cortical influence. The two dimensional cortex and superior colliculus are connected to a three dimensional brainstem. Vertical eye movements are associated with torsional movements by way of a pathway from the superior colliculus to the contralateral interstitial nucleus of Cajal (Klier et al., 2007; ten Tusscher, 2011). If the contralateral eye dominates the cortical information to the superior colliculus, vertical vergence and torsional version (DVD) result.
If all features of infantile strabismus can be explained with a general principle based on proven changes in primate neuroanatomy, ten Tusscher suggests Occam’s razor applies; and there is no reason to explain one of its features with a body reflex with characteristics different from DVD originating from the fish brain that phylogenetically bears little relationship to the primate brain, or assume it arises to facilitate viewing during occlusion of one eye. It follows that sensory dissociation beyond V1, most likely due to active suppression by the older evolutionary crossed pathway (crossed retinal dominance) leads to the eye movement dissociation that is typically seen in the infantile strabismus syndrome.
15. Creel theory.
The Creel theory proposes that the lack of melanin pigment initiates atavistic expression of visual and auditory embryogenesis. In albino mammals’ embryogenesis vision and hearing take an analogous step back. Visual anomalies in albino mammals, including human beings, represent a developmental field defect that occurs as a normal developmental state in closely related species (Opitz, 2012). As pointed out by Meckel (1812), primary malformations, in this case complete optic decussation, are not aberrant. Complete decussation of retinal ganglion cells at the optic chiasm is a conventional ancestral state in non-mammalian vertebrates, and reduced non-decussated optic fibers are the norm in ancestral mammals.
In an embryonic environment, neural stem cells lacking positive signals to progress transition into more primitive neural precursor cells (Smuckler et al., 2006). Melanin pigment in the nascent retina, or likely genetic coding for melanin pigment, is the positive inductive signal launching normal retinal development and retinal ganglion cell targeting. When retinal melanin is reduced, retinal embryogenesis defaults to the conserved genetic package existing when the species first evolved with a cascade of consequences.
The vertebrate genome includes coding of genetic past. Charles Darwin (1868) popularized atavism as the term for reappearance of ancestral characteristics in future generations. Most atavistic expression is probably not random, but due to an error in early embryonic environment triggering expression of genes conserved across evolution. Actuating atavistic optic misrouting likely includes several possible events.
Mammals retain the evolutionary base instruction package for complete crossing of the retinal ganglion cells in ancestral vertebrates, and little genetic information re area centralis development, foveal, or vascular sparing of the foveal area. As each mammalian species or subspecies branched off and went their own way the genetic control of visual and auditory system development evolved to fit the environment of the species, revising embryogenesis that existed at time the species became independent.
The essence of this theory is that retinal melanin pigment is so fundamental to retinal, vascular, and early retinal ganglion cell targeting that insufficient retinal melanin triggers a switch resulting in the conventional genetic instructions not to be completed. Mammals with nondecussating retinal ganglion cells are genetically unstable. In albinos genetic instruction defaults to the simpler, more entrenched, platform. An atavistic expression of the conserved, more stable, genetic platform skewed toward complete crossing of retinal ganglion cells at the chiasm, and commensurate visual and auditory anomalies, develops consistent with genetics of each species’ evolutionary origin.
A genome is not comprised of independently functioning loci on chromosomes, but an interactive compilation of coded instructions that reacts to alterations in the genome. The inductive signals for entirely crossed optic fibers are conserved in mammals. The atavistic fallback to this morphology is an easy misstep because of the phylogenetically recent development of the temporal retina, vascular sparing in macula, and foveal development in primates. As suggested by Gehring (2005) regarding early evolution of eyes, morphogenetic pathways are progressively being modified by intercalation of novel genes. Phylogenetically late genetic addendums to conserved instructions are vulnerable to not being expressed if genetic cues, such as sufficient retinal melanin pigmentation, are not present to support variation from highly conserved coding. Coding for reduced pigment in the retina also possibly disrupts silencing of ancestral genes, which dictate complete decussation of optic neurons and more primitive vascular and retinal development.
References
Aboitiz F, Montiel J. One hundred million years of interhemispheric communication: the history of the corpus callosum. Braz J Med Biol Res 2003;36:409–420.
Aboitiz F, Scheibel AB, Fisher RS, Zaidel E. Fiber composition of the human corpus callosum. Brain Res 1992;598:143–153.
Al-Araimi M, Pal B, Poulter JA, van Genderen MM, Carr I, Cudrnak T, Brown L, Sheridan E, Mohamed MD, Bradbury J, Ali M, Inglehearn CF, Toomes C. A new recessively inherited disorder composed of foveal hypoplasia, optic nerve decussation defects and anterior segment dysgenesis maps to chromosome 16q23.3-24.1. Mol Vis. 2013;19:2165-2172.
Antonini A, Berlucchi G, Di Stefano M, Marzi CA. Differences in binocular interactions between cortical areas 17 and 18 and superior colliculus of Siamese cats. J Comp Neurol. 1981;200;597-611.
Apkarian P, Reits D, Spekreijse H, Van Dorp D. A decisive electrophysiological test for human albinism. Electroencephalogr Clin Neurophysiol. 1983;55:513-531.
Arendt D. Evolution of eyes and photoreceptor cell types. Int J Dev Biol. 2003;47:563-571.
Arendt D, Tessmar-Raible K, Snyman H, Dorresteijn AW, Wittbrodt J. Ciliary photoreceptors with a vertebrate-type opsin in an invertebrate brain. Science. 2004;306(5697):869-871. PubMed PMID: 15514158.
Atkinson J. Development of optokinetic nystagmus in the human infant and monkey infant. In: Freeman RD (ed.). Developmental Neurobiology of Vision. New York: Plenum. 1979;277–287.
Ault SJ, Leventhal AG, Vitek DJ, Creel DJ. Abnormal ipsilateral visual field representation in areas 17 and 18 of hypopigmented cats. J Comp Neurol. 1995;354:181-192.
Azuma N. Molecular cell biology on morphogenesis of the fovea and evolution of central vision. Nihon Ganka Gakkai Zasshi. 2000;104:960-985.
Bacon BA, Mimeault D, Lepore F, Guillemot JP. Spatial disparity sensitivity in area PMLS of the Siamese cat. Brain Res. 2001;906:149-156.
Barrenäs ML, Lindgren F. The influence of inner ear melanin on susceptibility to TTS in humans. Scand Audiol. 1990;19(2):97-102. PubMed PMID: 2371541.
Berlucchi G, Gazzaniga MS, Rizzolatti G. Microelectrode analysis of transfer of visual information by the corpus callosum. Arch Italiennes de Biologie. 1967;105:583-596.
Berman EH, Murphy N. The critical period for alteration in cortical binocularity resulting from divergent and convergent strabismus. Dev Brain Res. 1982;2:181–202.
Bisti S, Carmignoto G. Monocular deprivation in kittens differently affects crossed and uncrossed visual pathways. Vision Res 1986;26:875– 884.
Bridge H, von dem Hagen EAH, Davies G, Chambers C, Gouws A, Hoffmann MB, Morland AB. Changes in brain morphology in albinism reflect reduced visual acuity. Cortex. 2014;56:64-72.
Brodsky MC. The accessory optic system: the fugitive visual control system in infantile strabismus. Arch Ophthalmol. 2012a;130(8):1055-1058.
Brodsky MC. An expanded view of infantile esotropia: bottoms up! Arch Ophthalmol. 2012b;130(9):1199-1202.
Brodsky MC, Dell’Osso LF. A unifying neurologic mechanism for infantile nystagmus. JAMA Ophthalmol. 2014;132(6):761-768. doi: 10.1001/jamaophthalmol.2013.5833. Review. PubMed PMID: 24525626.
Carroll RL. Vertebrate paleontology and evolution. 1988. New York: WH Freeman and Company.
Chang MC. Neural mechanisms of monocular vision: I. Disturbance of monocular pattern discrimination in albino rat after destruction of cerebral visual area. Chinese J Psychol. 1936;1:10-20.
Cheong PY, King RA, Bateman JB. Oculocutaneous albinism: variable expressivity of nystagmus in a sibship. J Pediatr Ophthalmol Strabismus. 1992;29:185-188.
Christoff A, Raab EL, Guyton DL, Brodsky MC, Fray KJ, Merrill K, Hennessey CC,Bothun ED, Morrison DG.. DVD—a conceptual, clinical, and surgical overview. JAAPOS 2014;18:378–384.
Coleman J, Sydnor CF, Wolbarsht ML, Bessler M. Abnormal visual pathways in human albinos studied with visually evoked potentials. Exper Neurol. 1979;65:667-679.
Collewijn H, Apkarian P, Spekreijse, H. The oculomotor behaviour of human albinos. Brain. 1985;108:1-28.
Collewijn H, Winterson BJ, Dubois, MFW. Optokinetic eye movements in albino rabbits: Inversion in anterior visual field. Science. 1978;199:1351-1353.
Conlee JW, Abdul-Baqi KJ, McCandless GA, Creel DJ. Differential susceptibility to noise-induced permanent threshold shift between albino and pigmented guinea pigs. Hear Res. 1986;23:81-91.
Conlee JW, Gerity LC, Westenberg IS, Creel DJ. Pigment-dependent differences in the stria vascularis of albino and pigmented guinea pigs and rats. Hear Res. 1994;72:108-24.
Conlee JW, Parks TN, Creel DJ. Reduced neuronal size and dendritic length in the medial superior olivary nucleus of albino rabbits. Brain Res. 1986;363:28-37.
Conlee JW, Parks TN, Schwartz IR, Creel DJ. Comparative anatomy of melanin pigment in the stria vascularis. Evidence for a distinction between melanocytes and intermediate cells in the cat. Acta Otolaryngol. 1989;107:48-58.
Conlee JW, Parks TN, Romero C, Creel DJ. Auditory brainstem anomalies in albino cats: II. Neuronal atrophy in the superior olive. J Comp Neurol. 1984;225:141-8.
Cosgrove D, Zallocchi M. Usher protein functions in hair cells and photoreceptors. Int J Biochem Cell Biol. 2014;46:80-9.
Creel DJ. Visual system anomaly associated with albinism in the cat. Nature. 1971a;231:465-466.
Creel DJ. Differences of ipsilateral and contralateral visually evoked responses in cats: strains compared. J Comp Physiol Psychol. 1971b;77:161-165.
Creel D. Inappropriate use of albino animals as models in research. Pharmacol Biochem Behav. 1980;12:969-977.
Creel D, Boxer LA, Fauci AS. Visual and auditory anomalies in Chediak-Higashi syndrome. Electroencephalogr Clin Neurophysiol. 1983;55:252-257.
Creel DJ, Bendel C, Wiesner GL, Wirtschafter JD, Arthur DC, King RA. Abnormalities of the central visual pathways in Prader-Willi syndrome associated with hypopigmentation. New Eng J Med. 1986;314:1606-1609.
Creel D, Collier LL, Leventhal AG, Conlee JW, Prieur DJ. Abnormal retinal projections in cats with the Chediak-Higashi syndrome. Invest Ophthalmol Vis Sci. 1982;23:798-801.
Creel D, Conlee JW, Parks TN. Auditory brainstem anomalies in albino cats. I. Evoked potential studies. Brain Res. 1983;260:1-9.
Creel DJ, Garber SR, King RA, Witkop CJ, Jr. Auditory brainstem anomalies in human albinos. Science. 1980;209:1253-1255.
Creel DJ and Giolli RA. Retino-geniculostriate projections in guinea pigs: albino and pigmented strains compared. Exper Neurol. 1972;36:411-425.
Creel DJ and Giolli RA. Retino-geniculostriate projections in guinea pigs: albino and pigmented strains compared. Exper Neurol. 1972;36:411-425.
Creel DJ, Giolli RA. Retinogeniculate projections in albino and ocularly hypopigmented rats. J Comp Neurol. 1976;166:445-456.
Creel D, Hendrickson AE, Leventhal AG. Retinal projections in tyrosinase-negative albino cats. J Neurosci. 1982;2:907-911.
Creel DJ, Witkop CJ, Jr., King RA. Asymmetric visually evoked potentials in human albinos: evidence for visual system anomalies. Invest Ophthal Vis Sci. 1974;13:430-440.
Creel DJ, O’Donnell FE, Jr., Witkop CJ, Jr. Visual system anomalies in human ocular albinos. Science. 1978;201:931-933.
Creel DJ, Sheridan CL. Monocular acquisition and interocular transfer in albino rats with unilateral striate ablations. Psychonomic Sci, 1966;6:89-90.
Creel D, Spekreijse H, Reits D. Evoked potentials in albinos: efficacy of pattern stimuli in detecting misrouted optic fibers. Electroencephalogr Clin Neurophysiol. 1981;52:595-603.
Creel DJ, Summers CG, King RA. Visual anomalies associated with albinism. Ophthalmic Paediatr Genet. 1990;11:193-200.
Cullup T, Kho AL, Dionisi-Vici C, Brandmeier B, Smith F, Urry Z, Simpson MA, Yau S, Bertini E, McClelland V, Al-Owain M, Koelker S, Koerner C, Hoffmann GF, Wijburg FA, ten Hoedt AE, Rogers RC, Manchester D, Miyata R, Hayashi M, Said E, Soler D, Kroisel PM, Windpassinger C, Filloux FM, Al-Kaabi S, Hertecant J, Del Campo M, Buk S, Bodi I, Goebel HH, Sewry CA, Abbs S, Mohammed S, Josifova D, Gautel M, Jungbluth H. Recessive mutations in EPG5 cause Vici syndrome, a multisystem disorder with defective autophagy. Nat Genet. 2013;45:83-87.
Darwin C. The variation of animals and plants under domestication, Volume 2, John Murray, 1868.
Deiner, MS, Kennedy, TE, Fazeli, A, Serafini, T, Tessier-Lavigne, M, Sretavan, DW. Netrin-1 and DCC mediate axon guidance locally at the optic disc: loss of function leads to optic nerve hypoplasia. Neuron. 1997;19:575–589.
Di Stefano M, Bedard S, Marzi CA, Lepore F. Lack of binocular activation of cells in area 19 of the Siamese cat. Brain Research 1984;303:391-395.
Dijksta, JM, Cooley SS, Holleschau AM, King RA, Summers CG. Change in visual acuity in albinism in the early school years. J Ped Ophthal Strabis. 2012;49:81-87.
Dimond A, Fraser P. Long Noncoding RNAs Xist in Three Dimensions. Science, 2013;341(6147):720-721.
Dixon JR, Selvaraj S, Yue F, Kim A, Li Y, Shen Y, Hu M, Liu JS, Ren B. Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature. 2012;485(7398):376-380. doi: 10.1038/nature11082. PubMed PMID: 22495300; PubMed Central PMCID: PMC3356448.
Dorey SE, Neveu MM, Burton LC, Sloper JJ, Holder GE. The clinical features of albinism and their correlation with visual evoked potentials. British J Ophthal. 2003;87:767-772.
Dulai KS, von Dornum M, Mollon JD, Hunt DM. The evolution of trichromatic color vision by opsin gene duplication in New World and Old World primates. Genome Res. 1999;9:629-638.
Elschnig A. Zur anatomie des menschlichen albinoauges (Translated: On the anatomy of the human albino eye). Graefes Archivfur Ophthalmologie. 1913;84:401-419.
Engle EC. Human genetic disorders of axon guidance. Cold Spring Harb. Perspect Biol. 2010;2:1-13.
Engreitz JM, Pandya-Jones A, McDonel P, Shishkin A, Sirokman K, Surka C, Kadri S, Xing J, Goren A, Lander ES, Plath K, Guttman M. The Xist lncRNA exploits three-dimensional genome architecture to spread across the X chromosome. Science, 2013;341(6147):1237973.
Esteve JV, Jeffery G. Reduced retinal deficits in an albino mammal with a cone rich retina: a study of the ganglion cell layer at the area centralis of pigmented and albino grey squirrels. Vision Res. 1998;38:937-40.
Feng W, Störmer VS, Martinez A, McDonald JJ, Hillyard SA. Sounds activate visual cortex and improve visual discrimination. J Neurosci. 2014;34(29):9817-9824. doi: 10.1523/JNEUROSCI.4869-13.2014. PubMed PMID: 25031419; PubMed Central PMCID: PMC4099554.
Filloux FM, Hoffman RO, Viskochil DH, Jungbluth H, Creel DJ. Ophthalmologic features of Vici syndrome. J Pediatr Ophthalmol Strabismus. 2014; . doi: 10.3928/01913913-20140423-02 [online advanced release]
Fritzsch B, Piatigorsky J, Tessmar-Raible K, Jékely G, Guy K, Raible F, Wittbrodt J, Arendt D. Ancestry of photic and mechanic sensation? Science. 2005;308:1113-1114
Fukuda Y, Sawai H, Watanabe M, Wakakuwa K & Morigiwa K. Nasotemporal overlap of crossed and uncrossed retinal ganglion cell projections in the Japanese monkey. J Neurosci 1989;9:2353–2373.
Fulton AB, Albert DM, Craft JL. Human albinism light microscopy and electron microscopy study. Arch. Ophthal. 1978;96:305-310.
Fuhrmann S. Eye morphogenesis and patterning of the optic vesicle. Curr Top Dev Biol. 2010;93:61-84.
Gal A, Schinzel A, Orth U, Fraser NA, Mollica F, Craig IW, Kruse T, Mächler M, Neugebauer M, Bleeker-Wagemakers LM. Gene of X-chromosomal congenital stationary night blindness is closely linked to DXS7 on Xp. Hum Genet. 1989;81:315-318.
Garber SR, Turner CW, Creel D, Witkop CJ Jr. Auditory system abnormalities in human albinos. Ear Hear. 1982;3(4):207-210. PubMed PMID: 7117717.
Gargiulo A, Testa F, Rossi S, Di Iorio V, Fecarotta S, de Berardinis T, Iovine A, Magli A, Signorini S, Fazzi E, Galantuomo MS, Fossarello M, Montefusco S, Ciccodicola A, Neri A, Macaluso C, Simonelli F, Surace EM. Molecular and clinical characterization of albinism in a large cohort of Italian patients. Invest Ophthalmol Vis Sci. 2011;14:1281-1289.
Gayer NS, Horsburgh GM, Dreher B. Developmental changes in the pattern of retinal projections in pigmented and albino rabbits. Brain Res Dev Brain Res. 1989;50:33-54.
Gehring WJ. THE WILHEMINE E. KEY 2004 INVITATIONAL LECTURE: New Perspectives on Eye Development and the Evolution of Eyes and Photoreceptors. J Hered. 2005;96:171-184.
Giodano F, Simoes S, Rapaso G. The ocular albinism type 1 (OA1) GPCR is ubiquitinated and its traffic requires endosomal sorting complex responsible for transport (ESCRT) function. Proc Natl Acad Sci U S A, 2011;108:11906-11911.
Giolli RA, Blanks RH, Lui F. The accessory optic system: basic organization with an update on connectivity, neurochemistry, and function. Prog Brain Res. 2006;151:407-440.
Giolli RA, Creel DJ. The primary optic projections in pigmented and albino guinea pigs: an experimental degeneration study. Brain Res. 1973;30;55(1):25-39.
Giolli RA, Guthrie MD The primary optic projections in the rabbit: An experimental degeneration study. J Comp Neurol. 1969;136:99-126.
Goyal R, Watts P & Hourihan M. Ocular findings in pediatric patients with partial agenesis of corpus callosum. J Pediatr Ophthalmol Strabismus. 2010;47:236–241.
Guibal C, Baker GE. Abnormal axons in the albino optic tract. Invest Ophthalmol Vis Sci. 2009;50:5516-5521.
Grothe B, Pecka M, McAlpine D. Mechanisms of sound localization in mammals. Physiol Rev. 2010;90:983-1012.
Guillery RW. An abnormal retinogeniculate projection in Siamese cats. Brain Res. 1969;14:739-741.
Guillery RW. Visual pathways in albinos. Scientific Am. 1974;230:44-54.
Guillery RW, Hickey TL, Kaas JH, Felleman DJ, Debruyn EJ, Sparks DL. Abnormal central visual pathways in the brain of an albino green monkey (Cercopithecus aethiops). J Comp Neurol. 1984;226:165-183.
Guillery RW. Why do albinos and other hypopigmented mutants lack normal binocular vision, and what else is abnormal in their central visual pathways? Eye. 1996;10:217–221.
Guillery RW, Oberdorfer MD, Murphy EH. Abnormal retino-geniculate and geniculo-cortical pathways in several genetically distinct color phases of the mink (Mustela vison). J Comp Neurol. 1979;185:623-55.
Guillery RW, Okoro AN, Witkop CJ, Jr. Abnormal visual pathways in the brain of a human albino. Brain Res. 1975;96:373–377.
Hanson IM. Mammalian homologues of the Drosophila eye specification genes. Semin Cell Dev Biol. 2001 Dec;12(6):475-84.
Hattar S, Kumar M, Park A, Tong P, Tung J, Yau K-W, Berson DM. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J Comp Neurol. 2006;497:326-349.
Heffner RS, Heffner HE. Visual factors in sound localization in mammals. J Comp Neurol. 1992;317:219–232.
Hendrickson AE, Tigges M. Enucleation demonstrates ocular dominance columns in Old World macaque but not in New World squirrel monkey visual cortex. Brain Res. 1985;333:340-344.
Hendrickson A, Troilo D, Possin D, Springer A. Development of the neural retina and its vasculature in the marmoset Callithrix jacchus. J Comp Neurol.2006;497:270-86.
Herbert SP, Stainier DYR. Molecular control of endothelial cell behavior during blood vessel morphogenesis. Nature Rev. Molecular Cell Biology. 2011;12:551-564.
Hoffmann KP, Stone J. Retinal input to the nucleus of the optic tract of the cat assessed by antidromic activation of ganglion cells. Exp Brain Res. 1985;59:395–403.
Hoffmann KP, Distler C, Ilg U (1992): Callosal and superior temporal sulcus contributions to receptive field properties in the macaques monkey’s NOT and DTN. J Comp Neurol. 1992;321:150–162.
Hoffmann MB, Kaule FR, Levin N, Masuda Y, Kumar A, Gottlob I, Horiguchi H, Dougherty RF, Stadler J, Wolynski B, Speck O, Kanowski M, Liao YJ, Wandell BA, Dumoulin SO. Plasticity and stability of the visual system in human achiasma. Neuron. 2012;75:393-401
Hoffmann MB, Lorenz B, Morland AB and Schmidtborn LC. Misrouting of the optic nerves in albinism: Estimation of the extent with visual evoked potentials. Invest Ophthalmol Vis Sci. 2005;46:3892-3898.
Hoffmann MB, Lorenz B, Priesing M and Seufert PS. Assessment of cortical visual field representations with multifocal VEPs in control subjects, patients with albinism and female carriers of ocular albinism. Invest Ophthalmol Vis Sci. 2006;47:3195-3201.
Hoffmann MB, Seufert PS. Simulated nystagmus reduces pattern-reversal more strongly than pattern-onset multifocal visual evoked potentials. Clin Neurophysiol. 2005;116:1723-1732.
Hoffmann MB, Seufert PS, Schmidtborn LC. Perceptual relevance of abnormal visual field representations: static visual field perimetry in human albinism. Br J Ophthalmol. 2007;91:509-13.
Hoffmann MB, Tolhurst DJ, Moore AT, Morland AB. Organisation of the visual cortex in human albinism. J Neurosci. 2003;23:8921-8930.
Hoffmann MB, Wolynski B, Bach M, Meltendorf S, Behrens-Baumann W, Golla F. Optic nerve projections in patients with primary ciliary dyskinesia. Invest Ophthalmol Vis Sci. 2011;52: 4617–4625.
Hoyt WF, Luis O. The primate chiasm. Details of visual fiber organization studied by silver impregnation techniques. Arch Ophthalmol. 1963;70:69-85.
Hubel DH, Wiesel TN. Aberrant visual projections in the Siamese cat. J Physiol. 1973;218:33-62.
Huber-Reggi SP, Chen CC, Grimm L, Straumann D, Neuhauss SCF, Huang MYY. Severity of infantile nystagmus syndrome-like ocular motor phenotype is linked to the extent of the underlying optic nerve projection defect in zebrafish belladonna mutant. J Neurosci. 2012;32:18079-18086.
Husband S, Shimizu T. Evolution of the avian visual system. In: Cook RG (ed.). Avian visual cognition. Comparative cognition press. 2001. Access: http://www.pigeon.psy.tufts.edu/avc/husband/default.htm.
Ibbotson MR, Marotte LR, Mark RF. Investigations into the source of binocular input to the nucleus of the optic tract in an Australian marsupial, the wallaby Macropus eugenii. Exp Brain Res. 2002;147(1):80-88. Epub 2002 Sep 11. PubMed PMID: 12373372.
Irving R, Harrison JM. The superior olivary complex and audition: a comparative study. J Comp Neurol. 1967;130:77-86.
Jasper HH. The ten-twenty electrode system of the International Federation. Electroenceph Clin Neurophysiol. 1958;10:371-375.
Jeffery G. The albino retina: an abnormality that provides insight into normal retinal development. Trends Neurosci. 1997;20:165-169.
Jeffery G, Erskine L. Variations in the architecture and development of the vertebrate optic chiasm. Prog Retin Eye Res. 2005;24(6):721-753. Review. PubMed PMID: 16027026.
Jeffery G, Levitt JB, Cooper HM. Segregated hemispheric pathways through the optic chiasm distinguish primates from rodents. Neuroscience. 2008; 157:637-643.
Jen JC, Chan W-M, Bosley TM, Wan J, Carr JR, Rüb U, Shattuck D, Salamon G, Kudo LC, Ou J, Lin DM, Salih MAM, Kansu T, al Dhalaan H, al Zayed Z,
MacDonald DB, Stigsby B, Plaitakis A, Dretakis EK, Gottlob I, Pieh C, Traboulsi EI, Wang Q, Wang L, Andrews C, Yamada K, Demer JL, Karim S, Alger JR, Geschwind DH, Deller T, Sicotte NL, Nelson SF, Baloh RW, Engle EC. Mutations in a human ROBO gene disrupt hindbrain axon pathway crossing and morphogenesis. Science. 2004;304:1509-1513.
Kalberlah C, Distler C, Hoffmann KP. Sensitivity to relative disparity in early visual cortex of pigmented and albino ferrets. Exp Brain Res. 2009;192:379-389.
Kaule FR, Wolynski B, Gottlob I, Stadler J, Speck O, Kanowski M, Meltendorf S, Behrens-Baumann W, Hoffmann MB. Impact of chiasma opticum malformations on the organization of the human ventral visual cortex. Hum Brain Mapp. 2014 Apr 25. doi: 10.1002/hbm.22534. [Epub ahead of print] PubMed PMID: 24771411.
Kelly JP, Weiss AH. Topographical retinal function in oculocutaneous albinism. Am J Ophthalmol. 2006;141(6):1156-8.
Kinnear PE, Jay B, Witkop CJ, Jr. Albinism. Survey Ophthal. 1985;30:75-101.
Kioussis D. Gene regulation: Kissing chromosomes Nature. 2005;435:579-580.
Klemen J, Hoffmann MB, Chambers CD. Cortical plasticity in the face of congenitally altered input into V1. Cortex. 2012;48:1362-1365.
Klier EM, Wang H, Crawford JD. The interstitial nucleus of Cajal encodes three-dimensional head orientations in fick-like coordinates. J Neurophysiol. 2007;97:604–619.
Koenraads Y, van der Linden DC, van Schooneveld MM, Imhof SM, Gosselaar PH, Porro GL, Braun KP. Visual function and compensatory mechanisms for hemianopia after hemispherectomy in children. Epilepsia. 2014;5:909-917.
Koontz MA, Rodieck RW, Farmer SG. The retinal projection to the cat pretectum. J Comp Neurol. 1985;236:42-59.
Klemen J, Hoffmann MB, Chambers CD. Cortical plasticity in the face of congenitally altered input into V1. Cortex. 2012;48:1362-1365.
Kozmik Z. Pax genes in eye development and evolution. Curr Opin Genet Dev. 2005;15:430-438.
Kosmik Z. The role of Pax genes in eye evolution. Brain Res Bull. 2008;75:335-339.
Kozulin P, Natoli R, O’Brien KM, Madigan MC, Provis JM. Differential expression of anti-angiogenic factors and guidance genes in the developing macula. Mol Vis. 2009;15:45-59.
Kozulin P, Natoli R, Bumsted O’Brien KM, Madigan MC, Provis JM. The cellular expression of antiangiogenic factors in fetal primate macula. Invest Ophthalmol Vis Sci. 2010;51:4298-4306.
Lashley KS. Studies of cerebral function in learning: XII. The theory that synaptic resistance is reduced by the passage of a nerve impulse. Psychol Rev. 1924;31:369-375.
Le YZ, Bai Y, Zhu M, Zheng L. Temporal requirement of RPE-derived VEGF in the development of choroidal vasculature. J Neurochem. 2010;112:1584-1592.
Lee JT. Epigenetic regulation by long noncoding RNAs. Science. 2012; 14;338(6113):1435-1439. doi: 10.1126/science.1231776. Review. PubMed PMID: 23239728.
Lee KA, King RA, Summers CG. Stereopsis in patients with albinism: Clinical correlates. J AAPOS. 2001;5:98-104.
Leventhal AG, Creel DJ. Retinal projections and functional architecture of cortical areas 17 and 18 in the tyrosinase-negative albino cat. J Neurosci. 1985;5:795-807.
Leventhal AG, Vitek DJ, Creel DJ. Abnormal visual pathways in normally pigmented cats that are heterozygous for albinism. Science. 1985;229:1395-1397.
Lopez VM, Decatur CL, Stamer WD, Lynch RM, McKay BS. L-DOPA is an endogenous ligand for OA1. PLoS Biol. 2008;6:e236. doi: 10.1371/journal.pbio.0060236. PubMed PMID: 18828673; PubMed Central PMCID:PMC2553842.
Lucas RJ, Hattar S, Takao M, Berson DM, Foster RG, Yau K-W. Diminished pupillary light reflex at high irradiances in melanopsin-knockout mice. Science. 2003;299:245-247.
Lund RC. Uncrossed visual pathways of hooded and albino rats. Science. 1965;149:1505–1507.
McCauley DW, Hixon E, Jeffery WR. Evolution of pigment cell regression in the cavefish Astyanax: a late step in melanogenesis. Evol Dev. 2004;6(4):209-218. PubMed PMID: 15230961.
Marneros AG, Fan J, Yokoyama Y, Gerber HP, Ferrara N, Crouch RK, Olsen BR. Vascular endothelial growth factor expression in the retinal pigment epithelium is essential for choriocapillaris development and visual function. Am J Pathol. 2005;167:1451-9.
Marzi CA, Antonini A, Di Stefano M, Legg CR. Callosum-dependent binocular interactions in the lateral suprasylvian area of Siamese cats which lack binocular neurons in areas 17 and 18. Brain Res. 1980;197:230-235.
McCulloch DL, Marmor MF, Brigell MG, Hamilton R, Holder GE, Tzekov R, Bach M. ISCEV Standard for full-field clinical electroretinography (2015 update). Doc Ophthalmol. 2015;130:1-12. doi: 10.1007/s10633-014-9473-7. Epub 2014 Dec 14. PubMed PMID: 25502644.
McAllister JT, Dubis AM, Tait DM, Ostler S, Rha J, Stepien KE, Summers CG, Carroll J. Arrested development: high-resolution imaging of foveal morphology in albinism. Vision Res. 2010;50:810-817.
McKetton L, Kelly KR, Schneider KA. Abnormal lateral geniculate nucleus and optic chiasm in human albinism. J Comp Neurol. 2014;522:2680–2687.
Meckel JF. Handbuch der Pathologischen Anatomie. Halle:Kummer 1812.
Montoliu L, Grønskov K, Wei A-H, Martínez-García M, Fernández A, Arveiler B, Morice-Picard F, Riazuddin S, Suzuki T, Ahmed ZM, Rosenberg T, Li W. Increasing the complexity: new genes and new types of albinism. Pigment Cell Melan Res. 2014;27:11-18.
Moore DR, Kowalchuk NE. Auditory brainstem of the ferret: Effects of unilateral cochlear lesions on cochlear nucleus volume and projections to the inferior colliculus. J Comp Neurol. 1988;272:503–515.
Morland AB, Baseler HA, Hoffmann MB, Sharpe LT, Wandell BA. Abnormal retinotopic representations in human visual cortex revealed by fMRI. Acta Psychol (Amst). 2001;107:229-247.
Muntz WRA, Sutherland NS. The role of crossed and uncrossed optic nerve fibers in the visual discrimination of shape by rats. J Comp Neurol. 1964;122:69–77.
Murillo-Cuesta S, Contreras, J, Zurita E, Cediel R, Cantero M, Varela-Nieto I, Montoliu L. Melanin precursors prevent premature age-related and noise-induced hearing loss in albino mice. Pig Cell Melan Res. 2010;23:72–83.
Mustari MJ, Ono S, Vitorello KC. How disturbed visual processing early in life leads to disorders of gaze-holding and smooth pursuit. Prog Brain Res. 2008;171:487–495.
Neveu, MM, Jeffery, G., Burton, LC, Sloper, JJ and Holder, GE. Age-related changes in the dynamics of human albino visual pathways. Eur J Neurosci. 2003;18:1939–1949.
Neveu MM, Holder GE, Ragge NK, Sloper JJ, Collin JR, Jeffery G. Early midline interactions are important in mouse optic chiasm formation but are not critical in man: a significant distinction between man and mouse. Eur J Neurosci. 2006;23:3034-3042.
Neveu MM and Jeffery G. Chiasm formation in man is fundamentally different from that in the mouse. Eye. 2007;21:1264–1270.
Nouvian R, Beutner D, Parsons TD, Moser T. Structure and function of the hair cell ribbon synapse. J Membrane Biol. 2006;209:153-165.
Oetting WS, Fryer JP, Shriram S, King RA. Oculocutaneous albinism type 1: The last 100 years. Pigment Cell Res. 2003;16:307-311.
Oetting WS, Stine OC, Townsend D, King RA. Evolution of tyrosinase related gene (TYRL) in primates. Pigment Cell Res. 1993;6:171-177.
Opitz JM. 2011 William Allan Award: Development and Evolution. American Journal of Human Genetics. 2012;90(3):392-404. doi:10.1016/j.ajhg.2011.12.025.
Oster SF, Bodeker MO, He F, Sretavan DW. Invariant Sema 5A inhibition serves an ensheathing function during optic nerve development. Development. 2003;130:775–784.
Petros TJ, Rebsam A, Mason CA. Retinal axon growth at the optic chiasm: To cross or not to cross. Annu. Rev. Neurosci. 2008;31,295-315.
Phillips MJ, Perez ET, Martin JM, Reshel ST, Wallace KA, Capowski EE, Singh R, Wright LS, Clark EM, Barney PM, Stewart R, Dickerson SJ, Miller MJ, Percin EF, Thomson JA, Gamm DM. Modeling human retinal development with patient-specific induced pluripotent stem cells reveals multiple roles for visual system homeobox 2. Stem Cells. 2014;32:1480-92.
Piatigorsky J, Kozmik Z. Cubozoan jellyfish: an Evo/Devo model for eyes and other sensory systems. Int J Dev Biol. 2004;48(8-9):719-29.
Pietrasanta M, Restani L, Caleo M. The corpus callosum and the visual cortex: plasticity is a game for two.Neural Plasticity Epub 2012 Jun 21, Review.
Plachetzki DC, Fong CR, Oakley TH. Cnidocyte discharge is regulated by light and opsin-mediated phototransduction BMC Biol. 2012;10:17-26.
Plump AS, Erskine L, Sabatier C, Brose K, Epstein CJ, Goodman CS, Mason CA, Tessier-Lavigne M. Slit1 and Slit2 cooperate to prevent premature midline crossing of retinal axons in the mouse visual system. Neuron. 2002;33:219–232.
Polyak S. The vertebrate visual system. University of Chicago Press, Chicago 1957.
Poulter JA, Al-Araimi M, Conte I, van Genderen MM, Sheridan E, Carr IM, Parry DA, Shires M, Carrella S, Bradbury J, Khan K, Lakeman P, Sergouniotis PI, Webster AR, Moore AT, Pal B, Mohamed MD, Venkataramana A, Ramprasad V, Shetty R, Saktivel M, Kumaramanickavel G, Tan A, Mackey DA, Hewitt AW, Banfi S, Ali M, Inglehearn CF, Toomes C. Recessive mutations in SLC38A8 cause foveal hypoplasia and optic nerve misrouting without albinism. Am J Hum Genet. 2013;93:1143-50.
Prabhakar S, Noonan JP, Pääbo S, Rubin EM. Accelerated evolution of conserved noncoding sequences in humans. Science. 2006;314:786-791.
Rebsam A, Bhansali P, Mason CA. Eye-specific projections of retinogeniculate axons are altered in albino mice. J. Neurosci. 2012;32: 4821-4826.
Rijn LJ van, Simonsz HJ, Tusscher ten MPM. Dissociated vertical deviation and eye torsion: relation to disparity induced vertical vergence. Strabismus. 1997;5,225-226.
Roffler-Tarlov S, Liu JH, Naumova EN, Bernal-Ayala MM, Mason CA. L-Dopa and the albino riddle: content of L-Dopa in the developing retina of pigmented and albino mice. PLoS One. 2013;8(3):e57184. doi: 10.1371/journal.pone.0057184.
Sanderson KJ. Retinogeniculate projections in the rabbits of the albino allelomorphic series1. J Comp Neurol. 1975;159:15-27.
Schmitz B, Schaefer T, Krick CM, Reith W, Backens M, Kasmann-Kellner B. Configuration of the optic chiasm in humans with albinism as revealed by magnetic resonance imaging. Invest Ophthalmol Vis Sci. 2003;44:16-21.
Schmolesky MT, Wang Y, Creel DJ, Leventhal AG. Abnormal retinotopic organization of the dorsal lateral geniculate nucleus of the tyrosinase-negative albino cat. J Comp Neurol 2000;427:209-219.
Shatz CJ. Anatomy of interhemispheric connections in the visual system of Boston Siamese and ordinary cats. J Comp Neurol. 1977;173:497-518.
Sheridan CL. Interocular transfer of brightness and pattern discriminations in normal and corpus callosum sectioned rats. J Comp Physiol Psychol. 1965;59:292-294.
Smuckler SR, Runciman SB, Xu S, van der Kooy D. Embryonic stem cells assume a primitive neural stem cell fate in the absence of extrinsic influences. J Cell Biol. 2006;172:79–90.
Spitzweck, B., M. Brankatschk, and B.J. Dickson, Distinct protein domains and expression patterns confer divergent axon guidance functions for Drosophila Robo receptors. Cell. 2010;140(3):409-420.
St John R, Timney B. Sensitivity deficits consistent with aberrant crossed visual pathways in human albinos. Invest Ophthal Vis Sci. 1981;21:873-877.
Summers CG. Vision in albinism. Trans Am Ophthalmol Soc. 1996;94:1095-155.
Summers CG. Albinism: classification, clinical characteristics, and recent findings. Optom Vis Sci. 2009;86:659-62.
Summers CG, Creel D, Townsend D, Handoko H, King RA. Variable expression of vision in sibs with albinism. Am J Med Genetics 1991;40:327-331.
Sutter E E. Noninvasive Testing Methods: Multifocal Electrophysiology. In: Darlene A. Dartt, editor. Encyclopedia of the Eye, Vol 3. Oxford: Academic Press; 2010. pp. 142-160.
Taylor WO. Edridge-Green Lecture, 1978. Visual disabilities of oculocutaneous albinism and their alleviation. Trans Ophthalmol Soc U K. 1978;98(4):423-445. PubMed PMID: 115122.
Thaler L, Arnott SR, Goodale MA. Neural correlates of natural human echolocation in early and late blind echolocation experts. PLoS ONE 2011;6: e20162. doi:10.1371/journal.pone.0020162
Tremblay F, De Becker I, Cheung C, LaRoche GR. Visual evoked potentials with crossed asymmetry in incomplete congenital stationary night blindness. Invest Ophthalmol Vis Sci. 1996;37:1783-1792.
Trousse F, Marti E, Gruss P, Torres M, Bovolenta P. Control of retinal ganglion cell axon growth: a new role for Sonic hedgehog. Development. 2001;128:3927–3936.
Tusscher ten MPM, Rijn van JL. A hypothetical mechanism of Dissociated Vertical Divergence: unbalanced Cortical Input to Subcortical Pathways. Strabismus. 2010;18:98-103
Tusscher ten MPM. A neural model for cyclovertical eye movements and their disorders. Strabismus. 2011;19: 162–165.
Tusscher ten MPM, Rijn van JL. There is nothing fishy about the cerebral cortex. Strabismus. 2011;19:69-70.
Tusscher ten MPM. Dissociated deviation in the infantile strabismus syndrome. Strabismus. 2012;20:33-34.
Tusscher ten M. How brain pathology may cause infantile strabismus. Transections ISA Kyoto 2014.
Tusscher ten MP. Does dominance of crossing retinal ganglion cells make the eyes cross? The temporal retina in the origin of infantile esotropia – a neuroanatomical and evolutionary analysis. Acta Ophthalmol. 2014;92:419-423.
Ung T, Allen LE, Moore AT, Trump D, Zito I, Hardcastle AJ, Yates J, Bradshaw K. Is optic nerve fibre mis-routing a feature of congenital stationary night blindness? Doc Ophthalmol. 2005;111:169-78.
van Genderen MM, Riemslag FC, Schuil J, Hoeben FP, Stilma JS, Meire FM. Chiasmal misrouting and foveal hypoplasia without albinism. Br J Ophthalmol. 2006;90:1098-1102.
Van Rijn LJ, Simonsz HJ, Tusscher MP. Dissociated vertical deviation and eye torsion: relation to disparity-induced vertical vergence. Strabismus. 1997;5:13-20.
Vici CD, Sabetta G, Gambarara M, Vigevano F, Bertini E, Boldrini R, Parisi SG, Quinti I, Aiuti F, Fiorilli M, Optiz, JM. Reynolds JF Agenesis of the corpus callosum, combined immunodeficiency, bilateral cataract, and hypopigmentation in two brothers. Am J Med Genet. 1988;29:1–8.
von dem Hagen EA, Houston GC, Hoffmann MB, Jeffery G, Morland AB. Retinal abnormalities in human albinism translate into a reduction of grey matter in the occipital cortex. Eur J Neurosci. 2005;22:2475-2480.
von dem Hagen EA, Hoffmann MB, Morland AB. Identifying human albinism: a comparison of VEP and fMRI. Invest Ophthalmol Vis Sci. 2008;49:238-249.
von dem Hagen EA, Houston GC, Hoffmann MB, Morland AB. Pigmentation predicts the shift in the line of decussation in humans with albinism. Eur J Neurosci 2007;25:503–511.
Vopalensky P, Pergner J, Leigertova M, Benito-Gutierrez E, Arendt D, Kosmik Z. Molecular analysis of the amphioxus frontal eye unravels the evolutionary origin of the retina and pigment cells of the vertebrate eye. Proc Natl Acad Sci. 2012;109:1583-1588.
Wässle H, Illing RB. The retinal projection to the superior colliculus in the cat: a quantitative study with HRP. J Comp Neurol. 1980;190:333-356.
Williams SE, Mann F, Erskin L, Sakurai, T, Wei S, Rossi DJ, Gale NW, Holt CE, Mason CA, Henkemeyer M. Ephrin-B2 and EphB1 mediate retinal axon divergence at the optic chiasm. Neuron. 2003;39:919–935.
Wilson HR, Mets MB, Nagy SE, Kressel AB. Albino spatial vision as an instance of arrested visual development. Vision Res. 1988;28:979-90.
Wolynski B, Kanowski M, Meltendorf S, Behrens-Baumann W, Hoffmann MB. Self-organisation in the human visual system–visuo-motor processing with congenitally abnormal V1 input. Neuropsychologia. 2010;48:3834-3845.
Woolfe A, Elgar G. Organization of conserved elements near key developmental regulators in vertebrate genomes. Adv Genet. 2008;61:307-38.
Woolfe A, Goodson M, Goode DK, Snell P, McEwen GK, Vavouri T, Smith SF, North P, Callaway H, Kelly K, Walter K, Abnizova I, Gilks W, Edwards YJ, Cooke JE, Elgar G. Highly conserved non-coding sequences are associated with vertebrate development. PLoS Biol. 2005;3:0116-0131.
Yang J. Usher syndrome: genes, proteins, models, molecular mechanisms, and therapies. Hearing Loss, S. Naz (Ed.), Intech 2012:293-328.
Yin TCT, Carney LH, Joris PX. Interaural time sensitivity in the inferior colliculus of the albino cat. J Comp Neurol. 1990;295:438-448.
Zeki S, Fries W. A function of the corpus callosum in the Siamese cat. Proc Royal Soc London – Biological Sciences. 1980;207:249-258.
Zhang HY, Hoffmann KP. Retinal projections to the pretectum, accessory optic system and superior colliculus in pigmented and albino ferrets. Eur J Neurosci. 1993;5:486-5001.
Zhang B, Stevenson SS, Cheng H, Laron M, Kumar G, Tong J, Chino YM. Effects of fixation instability on multifocal VEP (mfVEP) responses in amblyopes. J Vis. 2008;8:16.1-14.
Zhu M, Bai Y, Zheng L, Le YZ. Presence of RPE-produced VEGF in a timely manner is critical to choroidal vascular development. Adv Exp Med Biol. 2012;723:299-304.
Zou C, Levine EM. Vsx2 controls eye organogenesis and retinal progenitor identity via homeodomain and non-homeodomain residues required for high affinity DNA binding. PLoS Genet. 2012;8(9):e1002924. doi: 10.1371/journal.pgen.1002924.
Updated September 7, 2014
The author
Dr. Donnell J. Creel was born in Kansas City, Missouri. He received his B.A and M.A. from the University of Missouri at Kansas City, and his Ph.D. from the University of Utah in 1969. In 1971 Don first made the connection that visual anomalies in Siamese cats was associated with albinism and hypothesized that all albino mammals likely have optic misrouting, and published first visual evoked potential studies in human albinos in 1974 and ocular albinos in 1978. Don has been the Director of Clinical Electrophysiology at the Moran Eye Center since its inception in 1993. e-mail Don at donnell.creel@hsc.utah.edu
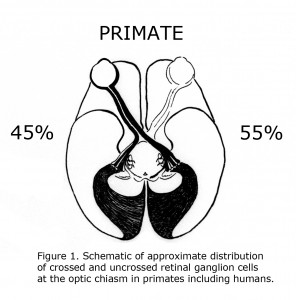
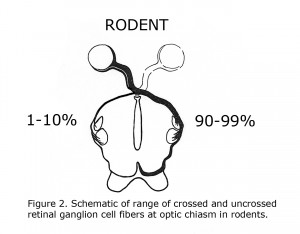 Figure 2. Schematic of range of crossed and uncrossed retinal ganglion cell fibers at optic chasm in rodents.
Figure 2. Schematic of range of crossed and uncrossed retinal ganglion cell fibers at optic chasm in rodents.