Ethan D. Cohen, Ph.D.,
Abstract
Retina prostheses try to reactivate the residual circuitry in a blind patient’s retina to produce a synthetic form of usable vision. Using an array of stimulus electrodes or light-sensitive proteins, the neurons in the degenerate retinal network are activated to elicit a series of light percepts termed “phosphenes”. If the patient’s phosphenes act as independent spatial percepts in their visual field, a crude type of form vision may be achieved. This form vision could improve a visually impaired patient’s ability to orient to landmarks in foreign visual environments, avoid obstacles, and improve social interactions. While most retinal prostheses in the current clinical trials rely on stimulation by electrical pulses from electrode arrays to locally excite the patient’s retinal neurons, several research groups are also trying more biologically compatible stimulation methods termed “optogenetics” which deploy viruses to transduce light-activated stimulator protein genes into select groups of retinal neurons which when expressed modulate their neural activity. Each technology presents different challenges and benefits for biological integration into the blind patient’s degenerate retina. Coupling and replacing the lost retinal neurons in the central fovea remains a challenge for both electronic and optogenetic combination therapies. There are many common issues involved in the real-world assessment of patient visual benefit from these novel medical technologies. Given the heterogeneity of RP as a disease, the availability of multiple therapies may be useful for patients, each offering different advantages for their type of retinal degeneration and its degree of progression.
Introduction
Fig. 1 Patients testing retinal prosthetic implants in human clinical trials. A. Subretinal implant patient using the Alpha IMS in a common object identification task. (Retinal Implant AG, retrieved from https://www.youtube.com/watch?v=WSdmWbItsvU). B. Suprachoroidal Australian retinal implant patient negotiating an obstacle avoidance course (Bionic Vision Australia, retrieved from https://www.youtube.com/watch?v=6EmleCs0KGY).
Retina prostheses try to reactivate the residual circuitry in a blind patient’s retina to produce a synthetic form of usable vision (Fig.1). Using an array of stimulus electrodes or light-sensitive proteins, the neurons in the degenerate retinal network are activated to elicit a series of light percepts termed “phosphenes” (Fig. 2). If the patient’s phosphenes act as independent spatial percepts in the patient’s visual field, a crude type of form vision may be achieved.
Fig. 2. Early examples of electrical phosphenes images resulting from gross stimulation of the eye. Drawings of self-induced phosphenes reported by Purkinje, [1] resulting from direct current stimulation across the eye between conductors in his mouth and forehead (Subfigures 15,16 inside Fig. 3.2). Similar electrical phosphenes evoked by transient current stimulation of the conjunctiva were also self-reported by Volta [2].
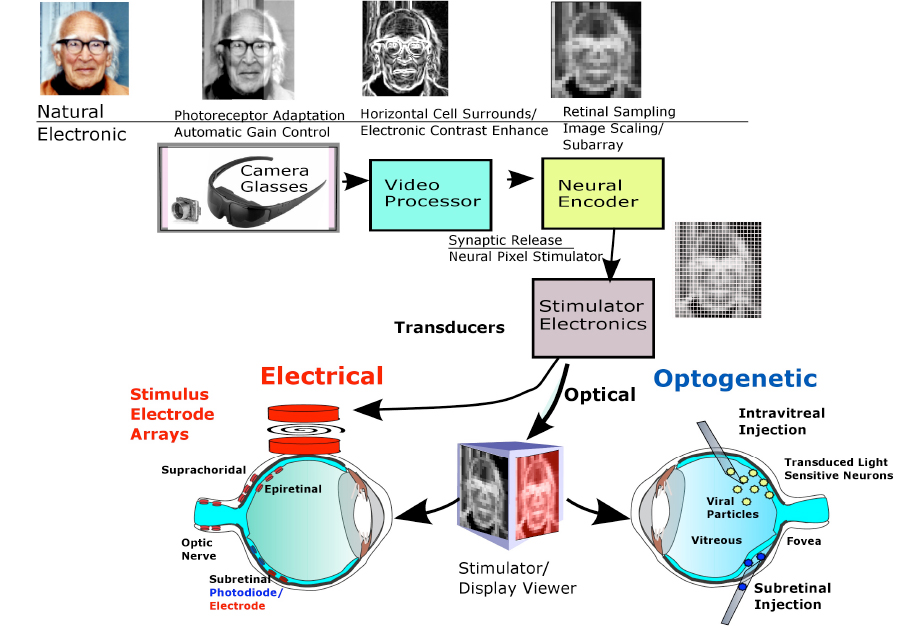
This form vision could improve a visually impaired patient’s ability to orient to landmarks in foreign visual environments, avoid obstacles, and improve social interactions. All prosthetic designs to date rely on the same general image encoding strategy to produce vision. The lost photoreceptor mosaic is replaced with an artificial array of silicon photosensors located in a pair of glasses or a photodiode chip (Fig. 3). Photoreceptor adaptation is replaced by an electronic automatic gain control. To simulate the antagonistic center-surround contrast enhancement of the ganglion cell receptive field by horizontal and amacrine cells, a video processor chip scales and edge enhances the veiwed image. Neural encoder circuitry then transforms the downsampled image into a limited set of discrete stimulation levels suitable for activating the retinal network (i.e., modulating spike frequencies for ganglion cells or graded potentials for inner retinal neurons) within its usable dynamic response range. Finally, the stimulator uses this level information to alter the firing patterns of retinal ganglion cells whose action potentials propagate up the optic nerve to generate phosphene patterns in the visual areas of the brain. While most retinal prostheses in the current clinical trials rely on stimulators producing electrical pulses from electrode arrays to locally excite the patient’s retinal neurons, several research groups are also trying more biologically compatible stimulation methods termed “optogenetics” which deploy patches of light-activated stimulator proteins expressed in groups of retinal neurons to selectively modulate their neural activity. To be effective, a retinal prosthesis must not only improve visual acuity on eye charts, but improve visual performance in real-world uncontrolled lighting environment tasks such as finding doors, navigating streets, socializing, and other activities of daily living. Patient performance test guidelines for these prosthetic devices are currently being formalized by the HOVER consortium to allow better device outcome comparisons [3]. For descriptions of earlier retinal prostheses see reviews [4], [5], [6], and [7].
Fig 3: Retinal prosthetic image encoding and stimulation schemes. The image boxes show the natural retinal mechanism and its prosthetic replacement. An example is shown of the conversion of a 250×250 pixel color image of a face for retinal stimulation using contrast enhancement methods. The camera-sampled color image is converted to a 256 grayscale b/w image, edge-detected with a Sobel algorithm [8]), descaled to a 125×125 pixel image, reduced to a 25×25 pixel stimulus pattern with 10 brightness levels similar to the abilities of Argus II patients,~6-10 levels [9], and sent to stimulate the retinal neurons using either external pulses of electrical current (via photodiode or electromagnetic coupling), or pulses of light on a display activating exogenously expressed photosensitive ion channels in transduced neurons (optogenetics). Image of George Wald kindly provided by Elijah Wald.
The retinal prosthetic patient:
A patient group commonly involved in visual prosthetic clinical trials has a severe form of a retinal degeneration termed “retinitis pigmentosa” (RP). With time, the patient’s peripheral rod and then cone vision is gradually lost resulting in a narrow central visual field some 3–10◦ in diameter. Upon funduscopic examination, pigmented lesions with the appearance of ‘spicules’ begin to appear in their peripheral retinae due to RPE cell migration into the retina. Disease progression in RP is highly variable, however a smaller cohort of patients progress to what is termed “late-stage RP” , characterized by nearly complete degeneration of both rod and cone photoreceptors resulting in bare or no light perception (See [10], [11],[12]). Currently, patients from this late-stage RP group are the main retinal prosthesis users.
RP is a genetically heterogeneous group of diseases which include rod-cone dystrophies, Usher’s syndrome, and Leber’s congenital amaurosis. The number of mutations involved in RP is very large, and a significant number of patients may clinically present with unknown genetic variants [13]. The types of RP inheritance can be autosomal dominant, X-linked, recessive, and simplex, from roughly 100 different gene families (RetNet, 3/2017). This heterogeneity presents a severe challenge to personalized gene therapies such as autologous stem cell transplants, CRISPR-Cas9 gene excision technology, or the recently approved biallelic RPE65 retinal dystrophy gene therapy which may require timely defect identification and selective treatment of each affected individual. Studies of gene therapy in dog models of some RP subtypes suggest an early genetic intervention is desirable, as the retinal degeneration may continue even after the gene therapy is delivered, [14]. In contrast, retinal prosthetic (electronic and optogenetics) therapies are not disease gene mutation dependent, which could potentially benefit a larger prosthetic patient cohort.
The end stage retina in RP patients also shows significant remodeling and reorganization of the anatomical organization, receptors, and function in the retinal network (See Marc et al Webvision entry for more details) [15],[16], which presents a challenge for prosthetic therapies replacing lost photoreceptors and foveal cones (ex.[17],[18, 19]). Bipolar cell dendrites retract their processes from the endings of dying central photoreceptors (See [5],[16, 19-22]). Bipolar and amacrine cells in the inner nuclear layer often survive by making abnormal synaptic contacts. RP patients experience on average a 30% decline in their number of retinal ganglion cells, whose axons are critical for sending phosphene-evoked spikes to the brain through the optic nerve [23]. Since all retinal prostheses rely on the presence of functioning retinal ganglion cells, prosthetic candidates are often evaluated for ganglion cell function using a corneal stimulation electrode to induce electrically-evoked phosphenes termed the “electrically-evoked response” (EER) ([24], [25],[26]). Currently, there are two main classes of retinal prostheses: electrical and optogenetic.
Retinal prostheses using electrical stimulation:
A large group of retinal prostheses elicit vision in blind patients by using electrical current pulses to depolarize and activate neurons in the retinal network. Retinal implants using electrical stimulation are classified according to the location of their stimulating electrodes in the eye. The prosthesis electrodes can be placed at the inner retinal surface or inner limiting membrane (ILM) (epiretinal), or in the subretinal space (subretinal), or in the sclera behind the choroidal vasculature (termed “suprachoroidal or episcleral”), or on/in the optic nerve (optic nerve) (Fig. 3). Each implant electrode location has different advantages and disadvantages for stimulating the retinal circuitry. Figs. 4,5.
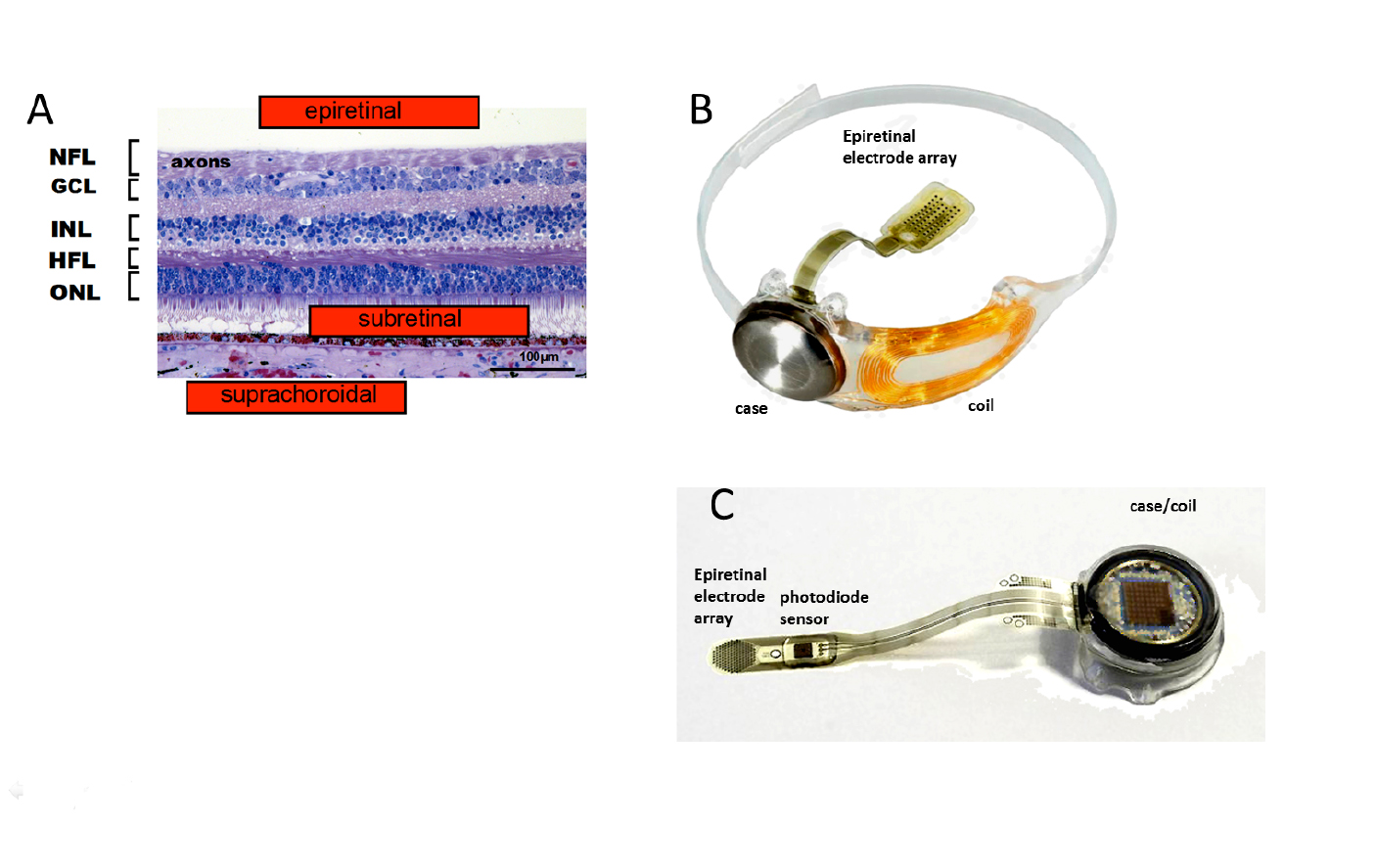
Fig. 4. Locations of electrically stimulating prosthetic stimulation electrode array electrodes and examples of epiretinal prostheses. A: Section of the human parafoveal retina showing the main stimulus electrode locations and the retinal layers: (from [5] with permission from IOP Publishing). Nerve fiber layer axons (NFL), Ganglion cell layer (GCL), Inner plexiform layer (IPL), Inner nuclear layer (INL), Henle fiber layer of synaptic extensions from foveal cones, and outer plexiform layer (HFL), Outer nuclear layer (ONL), and Pigment epithelium (PE). B,C: Examples of epiretinal types of retinal prostheses. B: Argus II retinal prosthesis (US FDA 2013). C: IRIS 2 retinal prosthesis. Images courtesy of Second Sight Medical Products, Pixium Vision, with permissions.
Epiretinal Electrode Prostheses
Epiretinal prostheses electrodes are placed on the inner limiting membrane of the retina, in order to locally stimulate the underlying retinal ganglion cells and inner retinal neurons. Through an incision in the ora serrata, the surgeon surgically implants a flexible stimulus electrode array close to the ILM surface; where it is held in place with one or more retinal tack(s). The array is connected by a cable to the stimulator case and coil which is sutured on the outside of the globe, and held in place by scleral bands. Telemetry coils mounted on the arm of the patient’s camera glasses deliver wireless power and data signals to the implant coil. A pocket visual processor/battery unit allows user selectable viewing modes. The first epiretinal prosthesis in a clinical trial, the Argus I used a modified cochlear implant stimulator and had a 4 x 4 array of 250-500µm diam. platinum (Pt) epiretinal electrodes [27]. For visual scale of electrode size at the retina, 1 degree visual angle is about 280µm on the retina. This is the visual field size of the nail of the index finger held at arm’s length [28]. Other early epiretinal prosthesis designs clinically tested included the Epi-Ret [29] and the IMI retinal implant [30]. The “Argus II Retinal Prosthesis System” is currently the only retinal prosthesis legally marketed in the U.S. It was approved by the U.S. Food and Drug Administration (FDA) in 2013 as a humanitarian use device, and was CE marked (EU) in 2011.
The Argus II uses a 6×10 array of 200µm diam Pt disc electrodes placed against the inner retinal surface (diagonal visual field of ~20°) (Fig. 2B) [31],[32]. Most Argus II subjects (~70%), can follow a white line on a black floor, and ~55% were able to find a black door on white wall (HDE FDA report, 2013 ), while a few (10%) are able to slowly read large white letters on black backgrounds [33].
A newer epiretinal prosthesis, the IRIS II sends event-based camera encoded images of visual scenes to a hexagonal array of 150 stimulus electrodes and is currently implanted in 10 patients in EU clinical trials (2017) (Fig. 3C). To avoid the data bottleneck of sending each electrode stimulus level as a radiofrequency (RF) signal through a magnetic coil, the IRIS II uses an IR LED mounted on the patient’s glasses to transmit the stimulation signal directly through the patient’s lens to a photodiode sensor decoder mounted on the intraocular cable of the implant’s stimulus electrode array.
Performance of Epiretinal Electrode Prostheses
The advantage of epiretinal prostheses is that they are minimally invasive to the retina, do not occlude the retinal vasculature, and are easily monitored through the patients lens using a funduscope or optical coherence tomography (OCT). In animal models, short current pulses (0.1-0.5msec) tend to activate action potentials directly at the ganglion cell. Longer duration pulses may produce delayed spikes due to synaptic transmission from activated bipolar or amacrine cells. ([34, 35],[36],[37]). Epiretinal electrode stimulation excites both ON- and OFF-center ganglion cells non-selectively. For Argus II users, phosphene thresholds to biphasic 0.45msec pulses averaged 206.5µA (93nC) [32]. Argus I subjects reported phosphene percepts from single stimulated electrodes as elliptical blobs (~4 x 10 degrees), or thin (1 degree wide) arcs some 7-15 degrees in length [38, 39], and Argus II subjects have reported similar shaped percepts [40]. Phosphene brightness perception of Argus II subjects is limited to some 6-10 stimulus current steps [9]. The Argus II’s camera glasses are fixed to the patient’s head but the electrode array is fixed on the retina in the eye. However, the brain maps the phosphenes induced by epiretinal stimulation electrodes based on the patient’s eye gaze position, so if the eyes move so do the phosphenes. Argus II subjects also report a perceptual fading of stimulated phosphenes due to a Troxler-like effect- in some cases in under 0.5 sec, so they often use head scans to study an object of interest [41].
For epiretinal prostheses, close conformation of the stimulus electrode array to the inner retinal surface is necessary to provide uniformly focused stimulation across the electrode array. While stimulus array electrodes near the retinal tack region lie directly against the retina, the proximity of stimulus electrodes of Argus II patients arrays averaged 179.6µm from the retinal surface [32]. Incomplete contact could be caused by residual vitreal cortex fibers, posterior hyaloid/epiretinal membranes, patient/gender differences in the retinal curvature, and a lack of conformation to the curved fovea. The closest structures to epiretinal stimulus electrodes are often arcuate nerve fiber bundles under the inner limiting membrane from the axons of peripheral retinal ganglion cells, which course around the fovea, and converge on the optic nerve head [42],[43],[20]. The thickness of the perifoveal nerve fiber layer bundles average 80 -120um [44], and the nerve fiber layer near the macula averages 33.9um [45]. Using submillisecond stimulus pulses, subjects often report seeing arc-like phosphene percepts to electrode stimulation (See [39], [40, 46] and [47] ). Recent stimulation strategies using long sinusoidal current pulses 10-20msec in duration may reduce this nerve fiber activation which could improve spatial percepts by individual electrodes [47], [48].
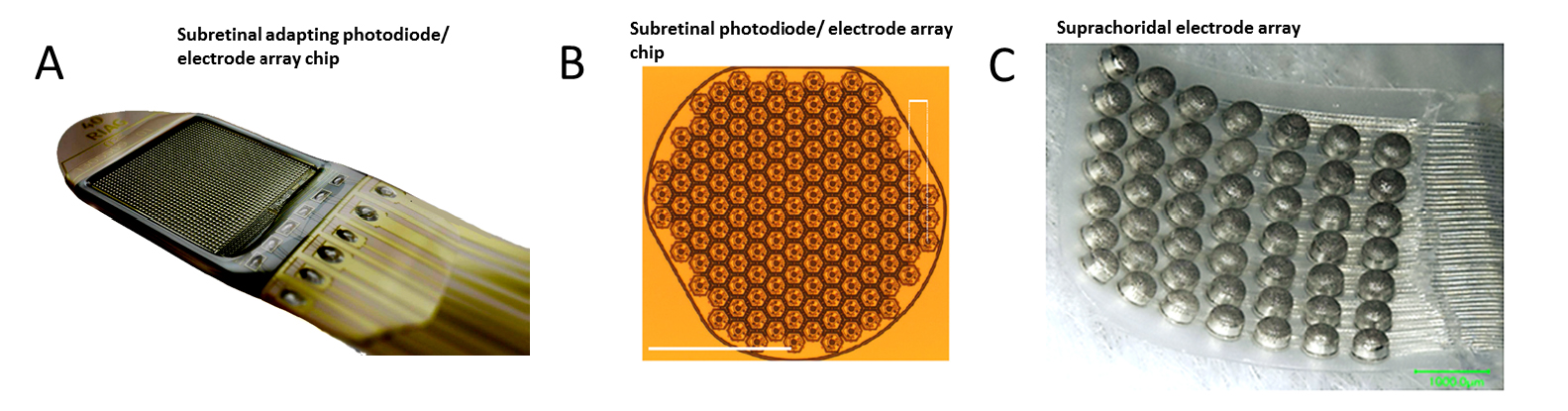
Fig. 5. Examples of electrically stimulating suprachoroidal and subretinal prosthetic stimulation electrode arrays. (A,B): Subretinal, and (C) suprachoroidal types of arrays. A: Alpha AMS photodiode/ stimulation electrode chip array and power cable, B: Prima multi-photodiode/stimulator array chip implant prototype. and C. Suprachoriodal stimulation electrode array [49]. IOVS (with permission). Scale bar 500µm. A, C: Implant case/coil not shown. Images courtesy of A: Retinal Implant AG, B: ref [50] Nature Pub. group with permissions, and C: ref [49] Invest. Opthalm. & Vis. Sci. (with permission).
Subretinal Electrode Prostheses
Subretinal Prostheses place electrode arrays in the subretinal space between the pigment epithelium and the degenerate photoreceptor layer (Fig. 5). This places the stimulus electrodes in close proximity to activate the remaining bipolar and amacrine cells of the blind patient, which theoretically could excite the remaining retinal circuitry in a more natural fashion. Early subretinal devices tested included an ambient light powered 5,000 microphotodiode-stimulator chip which performed poorly in US clinical trials [51]. However, actively-powered photodiode-based subretinal stimulator chips have been more successful in patient trials with some able to read large letters (5-10°) ([52], [53]). A second generation 40 × 40 stimulus electrode/photodiode array (1600 electrodes) chip termed the Alpha AMS was CE marked in the EU in 2016 with improved durability ([53, 54], [55]). This polyimide-encapsulated combination photodiode and stimulation electrode based chip (3.2x4mm, 70 ?m thick) allows subjects to use natural eye scanning with a synthetic visual field of ~13o to analyze objects. Active power to the AMS chip is provided by a cable from a coil-powered case sutured onto the skull similar to a cochlear implant. Patients with functional implants had grating acuities which ranged from 0.1 – 3.33 cycles/degree [55]. Several other subretinal stimulation implants are in active development by US/EU groups. Recently, the light-powered photodiode stimulation electrode chip concept has been revived by the Palanker group by using a chip whose pixels contain multiple photodiodes in series with a central stimulus electrode . When actively illuminated, each pixel generates enough voltage to excite the retinal network which altered ganglion cell firing in animal models [50]. Termed the “PRIMA” implant, a camera/viewscreen stimulator goggle generates IR light pulse “images” to power the implant chip array in the blind retina. A clinical trial for the PRIMA is currently started in the EU (11/2017). Other subretinal implant designs include a 256 electrode array by the Rizzo group with a more conventional globe-mounted titanium case/coil and camera glasses [56].
Performance of Subretinal Electrode Prostheses
The advantage of subretinal prostheses is that their electrodes stimulate the remaining neurons of the outer retina such as the bipolar cells. This could provide a more coordinated visual circuitry activation in blind patients which would propagate through the retinal network. In many subretinal designs, the photosensors are also located directly over the stimulation electrodes. An intraretinal mount allows blind subjects to use natural eye movements to scan objects of interest, and also helps prevent the perceptual fading reported for static retinal stimulation [57]. However, subretinal chips may also partially block oxygen diffusion from the choriocapillary bed, and some Alpha IMS implant patients exhibit mild leakage of the retinal vessels when studied with fluorescein angiography, [58], See [59]. Subretinal implantation devices need a thin package to fit in the subretinal space, and a durable near-hermetic encapsulation of the implant electronics.
Suprachoroidal Electrodes:
Suprachoroidal (or episcleral) prostheses place their stimulus electrodes in a scleral pocket directly behind the choroidal blood vessels (Fig. 5C). A suprachoroidal electrode array of 49 bullet shaped stimulus electrodes (0.5-mm diam.) was implanted behind the temporal retina in three subjects in Japan (Fujikado [49]). The array was connected to a multiplexing stimulator case (similar in form to a cochlear implant) which was surgically attached to the patient’s temporal bone, and controlled by a camera/processor. Using biphasic pulses (0.5msec duration), large phosphenes extending ≥10 degrees visual angle were reported ([60]). A second suprachoroidal design developed by the Bionic Vision Australia Consortium used a smaller array of 20 Pt electrodes (400-600um diam) which was implanted in the temporal suprachoroidal space near the fovea in 3 RP subjects. The cable ended in a percutaneous connector for interfacing with an external camera and stimulator [61]. Using either anodic or cathodic first biphasic pulses, 2 subjects reported phosphene shapes with average diameters of 8.4 and 9.0 degrees of visual angle, respectively [62].
The advantages of Suprachoroidal prostheses are that minimal surgery is needed for electrode array implantation which causes less risk of retinal detachment, and there is reduced risk of infection as the vitreal cavity of the eye is never entered. While suprachoroidal electrodes are located behind the retinal pigment epithelium/choroidal vessels which can spread the stimulus pulse currents at the retina, subjects can perceive simple phosphene shapes, suggesting these devices may have clinical utility for regaining peripheral vision commonly lost in most RP patients. However, strong electrical phosphene stimuli can occasionally evoke a tingling sensation in the trigeminal choroidal nerve [60].
Optic Nerve prostheses
Historically, a few groups have investigated stimulating the optic nerve, largely with surface electrodes [63], however each electrode appears to activate large numbers of axons, resulting in multiple phosphene percepts in the patients visual field.
Table I: Retinal Implants using Electrical Stimulation. Devices tested in human clinical trials are shown in bold. See also http://www.eye-tuebingen.de/zrenner/retimplantlist/, and http://www.io.mei.titech.ac.jp/research/retina/index.html
Neurotransmitter-releasing retinal prostheses:
The amino acid glutamate is the major excitatory neurotransmitter used in synaptic transmission from photoreceptor to bipolar, and bipolar to ganglion cell synapses. In theory, glutamate released onto bipolar cells would depolarize OFF-bipolar cells, while simultaneously hyperpolarizing ON-bipolar cells (OBCs). Conceivably, if a glutamate-releasing prosthesis could be placed at the level of the missing photoreceptor endings in RP patients, communication with the remaining bipolar cell dendrites could propagate the visual signal to ganglion cells. Several glutamate-releasing prosthetic designs have recently been proposed; one using optical waveguide uncaging of neurotransmitter near retinal neurons [65], and another using microfluidic neurotransmitter release through miniature orifices [66], [67]. The Pepperberg group has proposed stimulation using tethered neurotransmitters [68]. However it is unclear how these devices can attract the dendrites of retinal neurons, and replenish released neurotransmitter.
Retinal prostheses using optogenetic stimulation:
Optogenetics is a newer prosthetic stimulation technology that relies on viral expression (transduction) of light-sensitive ion channels in blind retinal neurons, enabling subsequent excitation of these neurons by light. This molecular technology has the potential to improve cellular targeting, increase spatial resolution and provide better coupling of the light stimulus to cellular excitation. Optogenetics originates from an 1865 observation by Ferdinand Cohn [69] that certain unicellular algae exhibit phototropism to blue but not red light. Mast (1916) localized this algal light behavior to an orange eye spot [70] (Fig. 6). Suction electrode recordings by Sineshchekov [71] [72] showed that light rapidly activated a current (submsec latency) in the algal membrane, with faster kinetics than rhodopsin. Hegemann and Nagel’s group expressed these algal membrane proteins in Xenopus oocytes and found one that acted as a direct light-activated mixed cation channel [73], which they termed “Channelrhodopsin” 2 (ChR). Boyden and Diesseroth expressed the ChR 2 protein in mammalian neurons and found they were able to evoke action potentials by single pulses of blue light [74].
Fig. 6. Location of the eyespot opsin pigment (arrow) which induces photaxis in the quadraflagellate freshwater algae Carteria. The flagellar attachment can be seen on the right. From the Connecticut College algae taxonomic database.
Given the large number of genetic mutations involved in RP, a disease-independent gene therapy such as optogenetics could benefit many blind patients. Currently, a variety of optogenetic protein stimulation technologies are currently being evaluated for modulating neurons including light-activated: ion channels, ion pumps (ex. bacteriorhodopsin, halorhodopsin), and G-proteins (ex. melanopsin, rhodopsin). Optogenetic proteins all incorporate a vitamin A /retinal analog in the binding pocket of their protein side chains (either all-trans 14-retinal or 13- retinal) [75]. Other light-activated proteins also under investigation include: N-ethyl maleimide modified glutamate channels (LiGluR) [76]), adenyl/guanyl cyclases [77], phosphodiesterases [78], and IR light-gated G-proteins (ex. snake TrpA1,) (often termed thermogenetics) [79]. However, it is not currently clear whether these mechanisms have the fast kinetics and higher quantal sensitivity needed for activating the retinal network.
All optogenetic proteins currently in development express the optoprotein solely in the neuronal membrane which severely reduces the quantal catch in comparison to retinal photoreceptors. For example, a foveal cone photoreceptor contains 1000-1200 photopsin-filled membrane infoldings which amplifies their quantal catch some 1000X [80], [81] and a similar number of rhodopsin-filled discs are found in rods [82]. Therefore, an intense light source is often required to supply the high irradiances (>1015-17quanta/cm2/sec) needed to adequately activate ChRs in the membrane to depolarize the retinal ganglion cells to action potential threshold [83], [84], [85]. Because these irradiance levels are rarely encountered in the office environment, most optogenetic prostheses are thought to be combination product technologies that rely on cameras and artificial light sources (i.e. projector glasses) for delivering high flux retinal activation, light adaptation, and scene contrast enhancement (i.e. Fig. 2.). Research is also progressing on the optical stimulus encoder paradigms for delivery of effective light stimuli for the ChR therapy to activate natural retinal network function (ex. Yan and Nirenberg [86]).
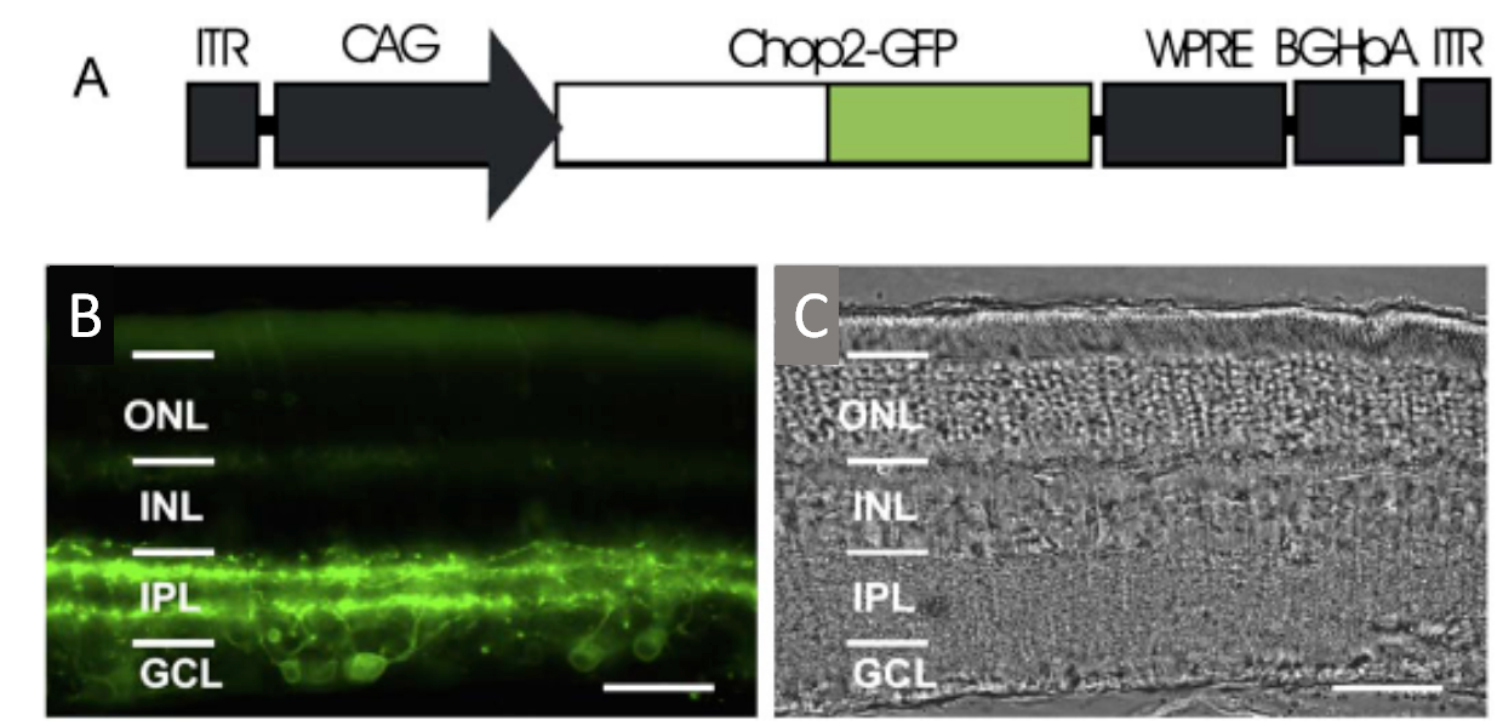
Fig. 7. Optogenetic transduction of retinal neurons by ChR2. A: AAV Promoter construct used to transduce retinal neurons using a cytomegalovirus beta actin (CAG) promoter, and a green fluorescent protein tag (GFP). Transduction pattern of the channelrhodopsin II construct in the rat retina. B: AAV CHr2 is transduced largely in inner retinal neurons including ganglion cells. C: Histology of same retina., Ganglion cell layer (GCL), Inner plexiform layer (IPL), Inner nuclear layer (INL), Outer nuclear layer (ONL). Bi et al., 2006 (Cell Press/Elsevier with permissions).
Light-activated depolarizing channels for optogenetic therapies:
ChR 2 was first virally transduced into blind rd1 mouse and rat inner retinal neurons by Bi et al. (2006) [83]. They used an adeno-associated virus (AAV) vector construct coupled to a powerful nonselective promoter to express the ChR2 GFP tag construct (Fig. 7). Injected into the vitreous humor, the AAV2 construct transduced many mouse and rat retinal ganglion cells and some inner retinal neurons. Blue light stimuli caused transduced retinal ganglion cells to depolarize and fire action potentials, which could be monitored as visual evoked potentials (VEPs). Currently, an early feasibility clinical trial of a ChR2 therapy has started in the US as of (8/2015), where it has been recently reported (11/17/2017) that some patients can sense the location of windows in rooms [87]. Red-shifted ChRs termed “ReaChR” or “Chrimsons” are also being explored in primate and human retinas for blindness indications also using a non-selective CAG promoter [88]. A recent (2017) EU clinical trial for retinitis pigmentosa patients uses the optogenetic combination product “ChrimsonR” ChR and biomimetic “Visual Interface Stimulating Glasses” (NCT03326336 Clinicaltrials.gov).
Like ChR2, OBCs normally produce a sustained depolarization to center light stimulation. In rodents, these cells can be genetically targeted using the Grm6 promoter [89]. Lagali et al. (2008) used in-vivo electroporation to transfect OBCs with a ChR2 lentiviral gene construct in the eyes of rd1 blind mouse neonates [84]. The transfected mice had a measurable optomotor response to moving gratings of up to 0.26 c/deg. Bipolar cell response thresholds as measured by ganglion cell firing to blue light stimulation were ~3x1015quanta/cm2/sec. Doroudchi et al (2011), used AAV Grm6 ChR2 constructs to transduce OBCs in a series of blind mouse lines (rd1, rd10, rd16) [85]. They showed that ChR2 expression was stable for up to one year, and found ganglion cell firing thresholds to blue light ~4 × 1016 quanta/cm2/sec. Transduced blind mice were able to discriminate escape platforms illuminated with blue light in a Morris water maze test [90].
Light-activated hyperpolarizing channels for optogenetic therapies:
Optogenetic hyperpolarizing mechansims are also useful for mimicking the light response of photoreceptors and OFF-center bipolar cells which normally hyperpolarize to light. Several pumps and channels that produce hyperpolarization have been discovered: (1) Halorhodopsin, a yellow light activated chloride pump hyperpolarizes cells; (2) Light-activation of proton pumps such as Chief or bacteriorhodopsin cause acid-sensing ion channels to open and hyperpolarize cells [91], [92], [93]. (3) A family of true light-gated chloride channels has recently been discovered, with increased light sensitivity and a green shifted activation spectrum (GtACR1) [94]; and (4) The chloride ChR, IC++ has been developed [95] . The Pan group transduced inner retinal neurons in rd1 blind mice with a viral combination construct of halorhodopsin and ChR2 for a dual blue light excitatory and yellow light inhibitory ion channel control of the neuron [96].
Degenerate cones have been known to survive in some blind mouse strains. Because Halorhodopsin activation hyperpolarizes neurons, similar to the light-evoked hyperpolarizations of photoreceptors, a second application for halorhodopsin was to restore the light response of degenerate cone photoreceptors. (See also Marc chapter). Busskamp et al, (2010) used a halorhodopsin EYFP construct and cone-specific promoters to transduce expression in two strains of blind mice [97]. Yellow light stimuli caused the transduced cones to hyperpolarize and synaptically activate the retinal network. Both ON- and OFF-center retinal ganglion cell light-responses could be recorded in Halorhodopsin-transduced cone retinas at thresholds of 1014quanta/cm2/sec. Improvements in cone-specific promoter transduction of primate and human foveal cones have recently been reported [98].
Light-activated G-protein gated mechanisms for optogenetic therapies:
The use of G-protein gated opsins for retinal prostheses, such as rod rhodopsin or melanopsin, employ energetically favorable biochemical amplification mechanisms which significantly increases their light sensitivity compared to their direct light-gated ion channel ChR counterparts. Early work by Kim et al (2005) showed light activation of G-protein chimaeras of rhodopsin could be coupled to adrenergic receptors [99], while Qiu et al., (2005) showed transfection of melanopsin caused light to depolarize kidney cells in culture [100]. The first retinal application by Lin et al. 2008 used an AAV cytomegalovirus (CMV) promotor-melanopsin construct to transduce retinal ganglion cells in rd1 blind mice[101]. The transduced blind mice were now able to detect light in a 2-channel water maze test.
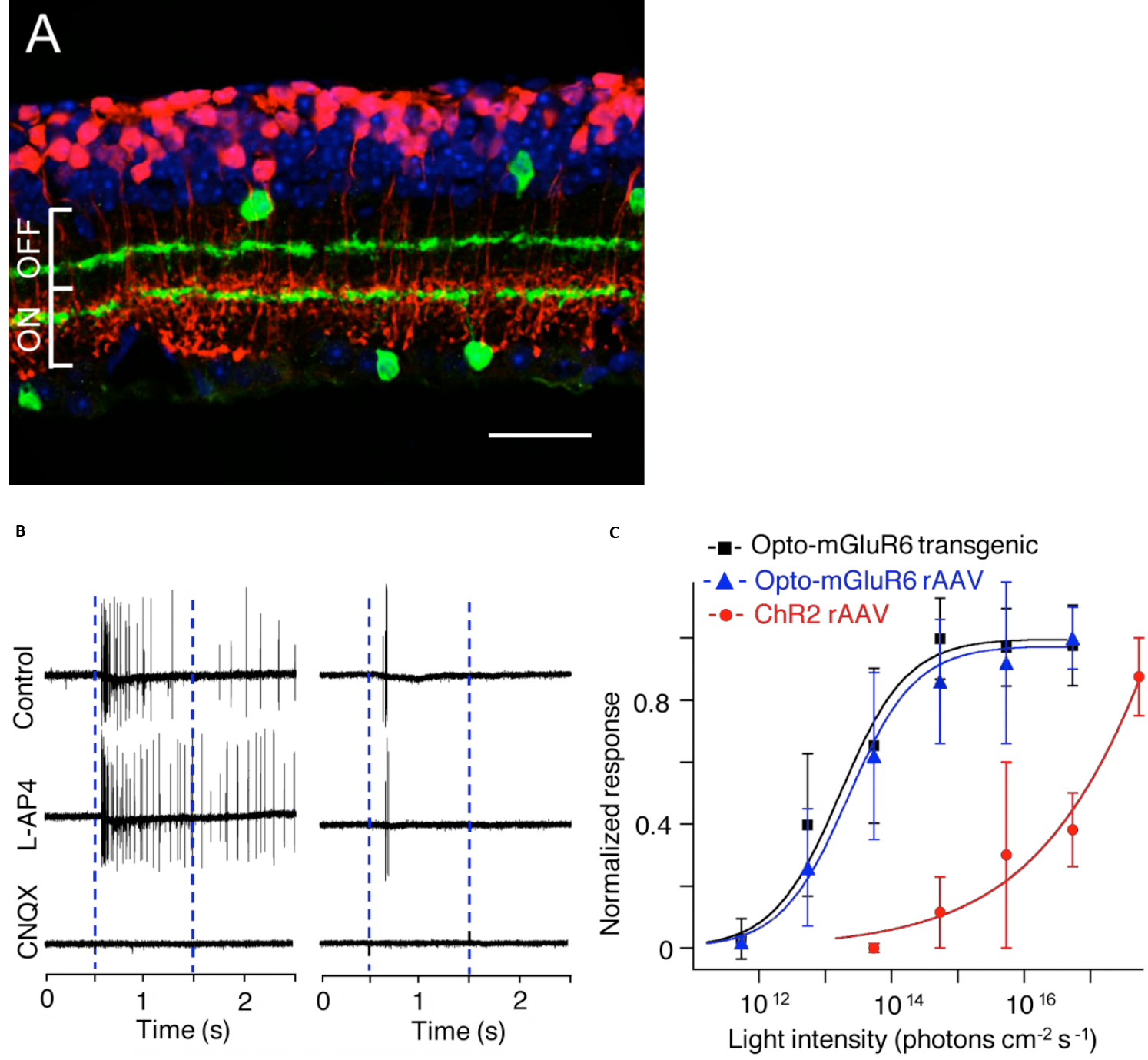
Currently the ON-center bipolar cell (OBC) is an active target area for optogenetic G-protein gated blindness therapies, due to the abundance of ON-center parafoveal rod and foveal cone bipolar cells, and the dependence of their On-response on G-protein activation of TrpM1 cation channels [102]. Recently, several groups found that transduction of melanopsin (Gq G-protein) or rhodopsin ( Gt) in OBCs could promiscuously activate the mGluR6 metabotropic receptor (Gq) G-protein. This foreign mechanism in OBC reverses the closure of the normally open TrpM1 excitatory channels to light, and causes the TrpM1 channel in the OBC to have a light-response which operates in reverse from normal. Van Wyk et al, (2015) used an AAV-Grm6 melanopsin-mGluR6 chimeras construct termed “Opto-mGluR6” to activate the TrpM1-gated excitatory ion channels in OBCs in Rd1 blind mice[103]. In Opto-mGluR6 Rd1 blind mice, light thresholds for ganglion cell activation were ~1000X lower than in their Rd1 ChR2 transduced littermates, and both light-ON- and OFF- retinal network responses appeared (Fig.8). A similar increase in light sensitivity in Rd1 blind mice was found using an AAV2-Grm6 rhodopsin construct by Gaub et al (2015) and also by Cehajic-Kapetanovic et al, (2015), who showed a behavioral increase in running distance when presented with a swooping owl movie [104, 105].
Fig. 8. Transduction pattern and light sensitivity of a melanopsin chimaera AAV construct targeted to ON-bipolar cells in a blind mouse model. A: Opto- Grm6 AAV transduction pattern for rod bipolar cells in an rd1 mouse retina. Anti-choline acetyltransferase in green, anti-melanopsin in red, DAPI nuclear stain, blue. (Scale bar 20um) B: Light stimulation causes melanopsin-mediated firing increases in an extracellularly recorded ganglion cell that is not blocked by APB (blue bars denote light onset/offset). C: Comparison of light-evoked ganglion cell firing thresholds of blind mice transduced with melanopsin chimaeras vs. ChR2:. AAV melanopsin, (blue), melanopsin transgenic, (black), ChR2 (red ) The G-protein gated opsin constructs are significantly more sensitive to light. From ref [103] with permission from PLOS.
Performance of the optogenetic therapies:
Optogenetic stimulation therapies have the advantage over electrical stimulation of being able to deliver photosensitive stimulation at near cellular resolution to a large retinal area, and to better spatially couple to the blind patient’s neurons in a retinal prosthesis. However, the injection of viral capsids into the central retinae of primates has revealed some unforeseen issues with the transduction of retinal neurons, particularly in the fovea, which is critically used for normal vision in humans. Foveal bipolar and ganglion cell bodies are displaced ~2 degrees from their cone photoreceptor inputs in the center [5, 21]. In the blind patient, viral transduction of ChRs into these surrounding bipolar and ganglion cell bodies when activated by light may elicit phosphenes that seem to originate from their central cones.
Finally, the primate retina has a series of membrane barriers that currently impede viral transduction, particularly to intravitreal injection of viral construct which is the preferred method in patients with advanced RP. Transduction of some AAV capsid constructs may be reduced in efficacy in primates when compared to mouse models [106], [107]. There may also be a modest host immune response to the viral capsid and or fluorescent protein tags, ex. [108], [109] . The ILM/Muller glia endfeet play a complex role in viral transduction, first by preventing diffusion of AAV viral proteins into the retina, but also by providing binding sites for viral attachment for retinal entry. In primates, the ILM appears to be thinnest near the perifovea and also in the retinal periphery [110]. The ILM is a basement membrane composed of several extracellular matrix proteins (laminin, agrin, perlecan, nidogen, collagen), nucleases and several heparan sulfate proteoglycans [111] [112].
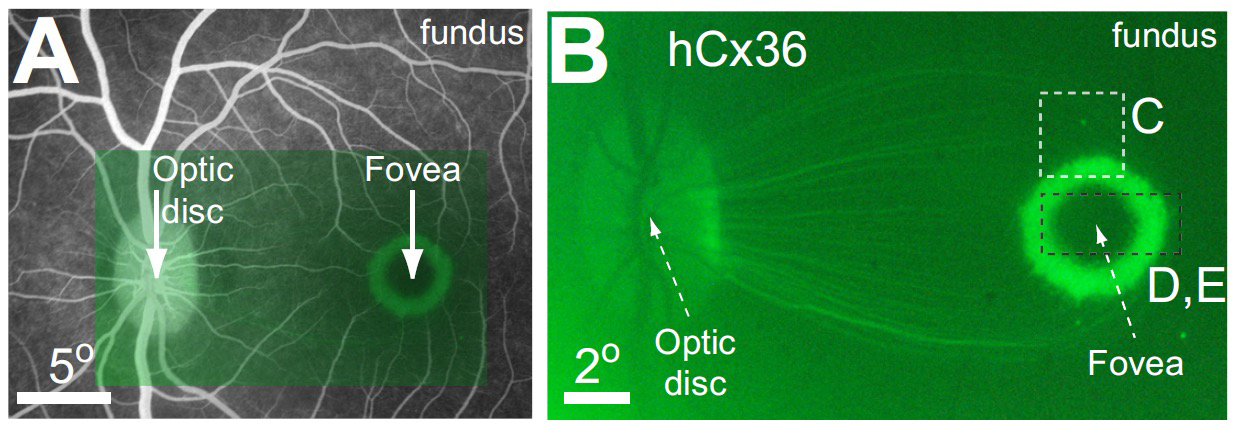
Yin et al, (2011,2014) and Ivanova et al (2010) both intravitreally injected AAV2-gene-GFP constructs with actin-based promoters in primate eyes and found that viral transduction of ganglion cell bodies was largely limited to a thin ring-like zone centered 2 degrees around the fovea where the ILM was thinnest, and a few isolated spots in peripheral retina Fig. 9 [113] [21, 114] (see also [106]). A similar ring-shaped foveal transduction pattern has also been reported with intravitreal injected constructs targeting bipolar cells in primates [107]. To reduce the ILM AAV diffusion barriers seen in old world primates eyes, injections of vitreolytic enzymes, vitrectomies, with or without inner limiting membrane peels have been proposed (See [113], [115], [116]), however, these treatments may also reduce AAV construct attachment. This has caused others to advocate limited subretinal injections (<100µL) of AAV constructs [117], [118], however these injections these may not be suitable for RP patients with advanced retinal disease [106]. However even effective optogenetic transduction of the parafoveal retina could provide significant visual benefit in severely blind patients.
Fig. 9. Viral transduction pattern by an intravitreal injection of an AAV capsid in the primate eye. A. Fluorescein angiogram showing retinal location of fluorescent image in B:. Transduction of the AAV2/2 hCx –GFP labeled construct only occurs to inner retinal neurons especially the ganglion cells. A ring-shaped transduction pattern is observed as the perifoveal ILM is thin allowing better capsid entry ( Connexin 36 (hCx) from Yin et al. 2011 IOVS[113] (with permission) .
Gene expression patterns in a degenerate RP retina may differ from normals, resulting in ineffective AAV therapies. RP patients experience long periods of gradual visual loss that may take many years, unlike the majority of blind rodent models. (See webvision section on retinal degeneration). Although RP has traditionally been represented as a disease that causes loss of photoreceptors, these retinal degenerations also affect retinal remodeling and synaptic reorganization of many inner retinal neurons [119], [120]. Inactive gene DNA may be methylated [121],[122] [123], which in the degenerate retina may produce gene inactivation/degradation in cells; reducing AAV promoter transduction efficiency, or cause off-target cellular gene expression [124] [103]. This is particularly true of AAV therapies for OBCs, where in cultured human retinal explants, transduction by an 4XGrm6 promoter construct was not OBC specific [124]. More specific OBC promoters may be required for transducing human retina [107]. How these promoter expression patterns change in OBCs in degenerate human RP patients is currently unknown, and may only be revealed by clinical trials.
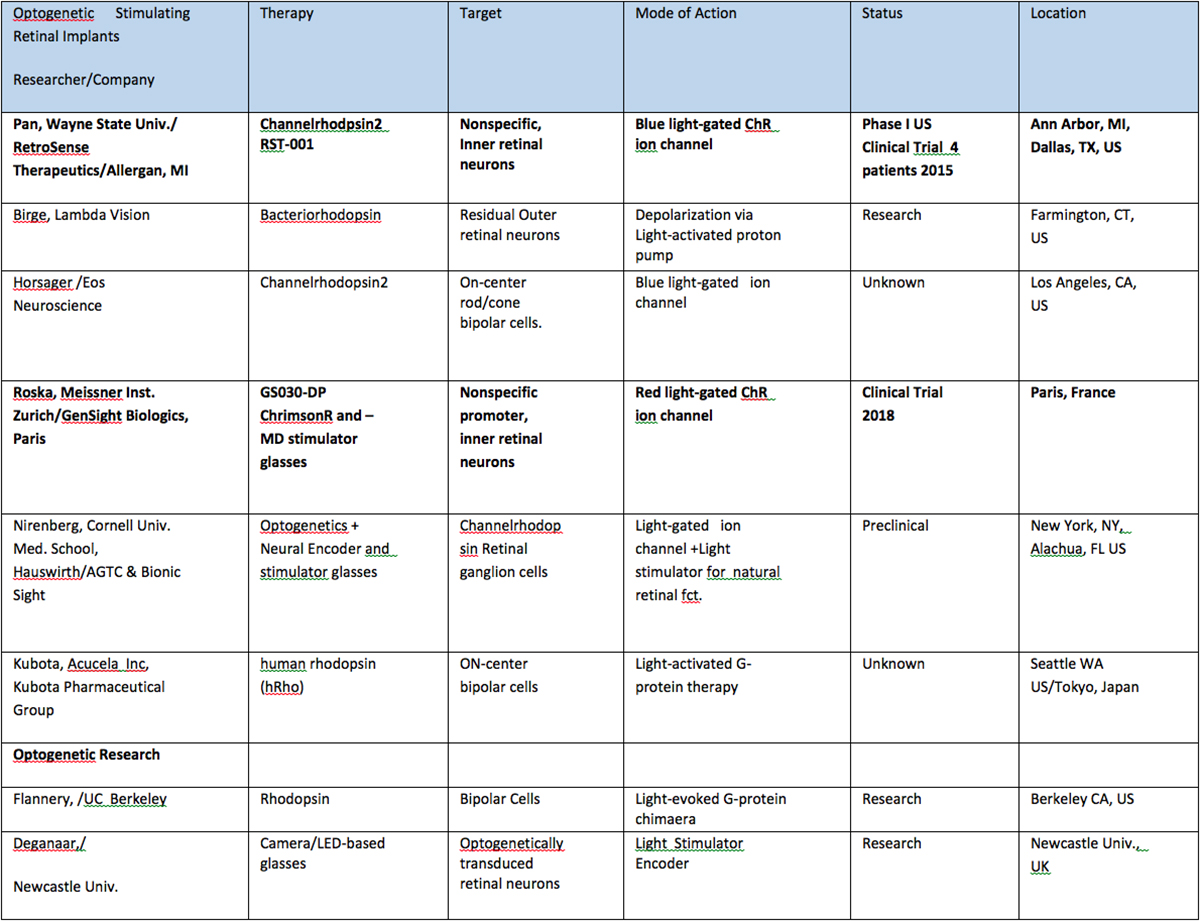
Table II: Retinal Implants using optogenetic combination therapies. Products tested in human clinical trials are shown in bold.
Retinal prosthetic stimulation safety:
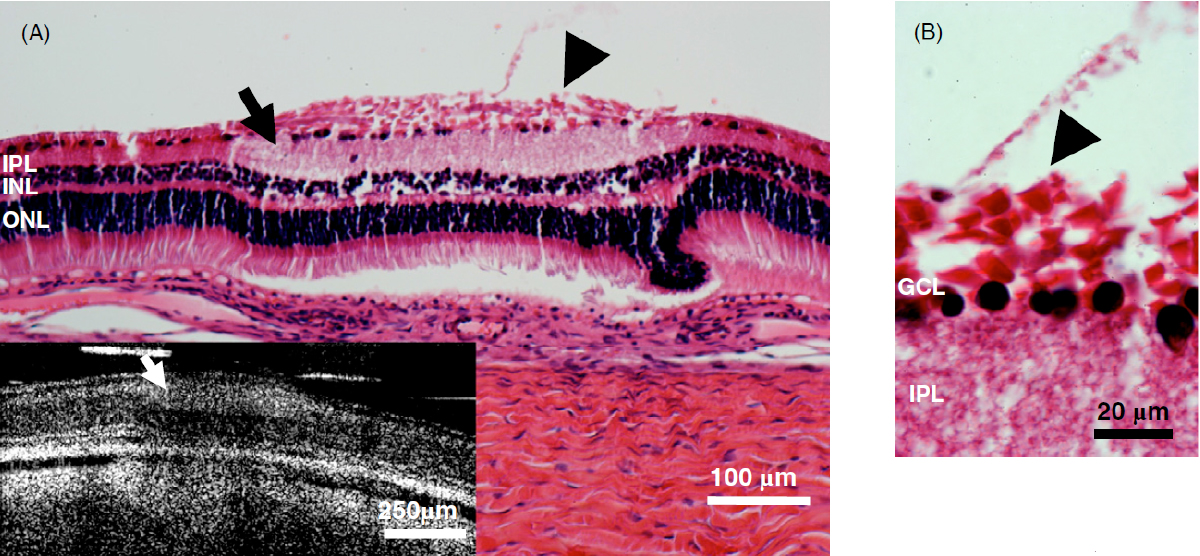
Fig. 10. Example of the use of optical coherence tomography (OCT) to image retinal damage under optically transparent stimulation electrodes in real time. Top: OCT time lapse movie of a 5 min period of retinal overstimulation (50Hz 749µC/cm2/ph) using a saline-filled transparent epiretinal electrode. B: Histological analysis under the stimulated zone post imaging shows a localized swelling of the ILM (arrowhead), IPL (arrow), and retinal detachment. C: Detail of swelling of the ILM Muller cell endfeet (arrowhead) and pyknotic ganglion cells (from ref. [125] with permission from IOP Publishing).
Patients using retinal prostheses in daily life expose their retinal tissue to chronic stimulation conditions. There are limits to the amount of electrical current and light that can be safely tolerated by the retinal tissue. A variety of methods have been developed to assess the retina under stimulation electrodes and to study retinal function by using funduscopes, fluorescence, optically transparent stimulus electrodes and/or optical coherence tomography (ex, [126], [125, 127]). Excessive electrical stimulation can cause electroporation of retinal neurons and glia cells. Overstimulation near the epiretinal surface can cause swelling of the Muller cell endfeet lining the ILM, and ganglion cell death (Fig. 10) ( [125]). However, chronic electrical stimulation damage limits (Hrs.) for the retina are less well defined, may be location specific, and sensitive to tissue proximity, (Ex. [128],[127], [129],[126]).
For prostheses using light stimulation to excite retinal neurons, standards exist to limit the retinal exposure of normal sighted patients to excessive UV and blue light. RP patients often complain of being sensitive to excess light and glare, while the retinae of RP animal models are very sensitive to light injury by long funduscopic illumination [130]. Many earlier ChR-based optogenetic therapies rely on high retinal fluxes of short wavelength light by the camera-projector glasses to provide adequate ChR channel activation of the transduced retinal neurons. However, newer ChRs and G-protein opsins have been developed with sensitivities shifted to longer wavelengths and with higher light sensitivity (ex. Fig. 8C).
Light can damage the retinal layers both thermally and photochemically. Standards for safe levels of illumination of healthy retina at different wavelengths were based on primate eye exposure data by Ham [131, 132] , and earlier studies by Noell in rats [133]. These studies and others have been adapted into a series of international standards (ANSI Z80.36, ISO15004, ISO 60825 , Rp27.1) among others for ophthalmic devices. Most of these light safety studies relied on photoreceptor-induced light injury as the damage endpoint [134, 135]. It is currently unresolved how much light can be safely tolerated by the remaining retinal neurons in endstage RP animal models and patients, with their degenerate photoreceptors and pigment epithelium cells.
Summary:
There are exciting new developments in retinal prosthetic therapies that use electrical and optogenetic stimulation that have the potential to provide better form vision and improve the quality of life for the severely visually impaired. Each technology presents different challenges and benefits for biological integration into the blind patient’s degenerate retina. Coupling and replacing the lost retinal neurons in the central fovea remains a challenge for both electronic and optogenetic combination therapies. There are many common issues involved in the real-world assessment of patient visual benefit from these novel medical technologies. Given the heterogeneity of RP as a disease, the availability of multiple therapies may be useful for patients, each offering different advantages for their type of retinal degeneration and its degree of progression.
Disclaimer: The opinions and/or conclusions expressed herein are solely those of the author and in no way imply a policy or position of the Food and Drug Administration. The mention of commercial products, their sources, or their use in connection with material reported herein is not to be construed as either an actual or implied endorsement of such products by the Department of Health and Human Services.
Ethan D. Cohen, Ph.D.,
Division of Biomedical Physics,
Office of Science and Engineering Labs,
Center for Devices and Radiological Health,
White Oak Federal Research Center. Silver Spring, Md, 20993.
EMAIL: ethan.cohen@fda.hhs.gov.
Acknowledgments: We thank Bruce Drum, Sunder Rajan, and Ksenia Blinova for critical manuscript comments.
About the author
Dr. Cohen received his Ph.D. in the retina lab of Dr. Peter Sterling at the University of Pennsylvania in 1987. After postdoctoral training in physiology at the University of Minnesota with Dr. Robert Miller, and at the UCLA Jules Stein Eye Institute with Dr. Gordon Fain, he joined the faculty of the Department. of Ophthalmology at Yale University Medical School as a retinal neurophysiologist in 1992. In 2000, he was a visiting professor in the Department of Molecular and Cellular Biology at Harvard University with John Dowling. Since 2003, he is a research scientist at the Office of Science and Engineering Labs at the Center for Devices and Radiological Health at FDA. His research interests include devising new methods to evaluate the safety and effectiveness of visual neurostimulation devices and improving the detection of neurotoxic drugs.
References
1. Purkinje, J., Beobachtungen und Versuche zur Physiologie der Sinne, Volume 1. Vol. 1. 1823, Prague: Calve. [Digital Book]
2. Volta, A., On the Electricity Excited by the Mere Contact of Conducting Substances of Different Kinds. Philosophical Transactions of the Royal Society of London, 1800. 90 p. 403- 431. [Digital Book]
3. Rizzo III, J.F. and L.N. Ayton, Psychophysical testing of visual prosthetic devices: a call to establish a multi-national joint task force. Journal of neural engineering, 2014. 11(2): p. 020301. [PubMed]
4. Margalit, E., et al., Retinal prosthesis for the blind. Survey of ophthalmology, 2002. 47(4): p. 335-356. [PubMed]
5. Cohen, E.D., Prosthetic interfaces with the visual system: biological issues. Journal of neural engineering, 2007. 4(2): p. R14. [PubMed]
6. Winter, J.O., S.F. Cogan, and J.F. Rizzo, Retinal prostheses: current challenges and future outlook. Journal of Biomaterials Science, Polymer Edition, 2007. 18(8): p. 1031-1055. [PubMed]
7. Weiland, J.D., A.K. Cho, and M.S. Humayun, Retinal prostheses: current clinical results and future needs. Ophthalmology, 2011. 118(11): p. 2227-2237. [PubMed]
8. Sobel, I., Camera models and machine perception. 1970, Stanford Univ Calif Dept of Computer Science. [Full Text Link]
9. Greenwald, S.H., et al., Brightness as a function of current amplitude in human retinal electrical stimulation. Investigative ophthalmology & visual science, 2009. 50(11): p. 5017-5025. [PubMed]
10. Grover, S., G.A. Fishman, and J. Brown, Patterns of visual field progression in patients with retinitis pigmentosa. Ophthalmology, 1998. 105(6): p. 1069-1075. [PubMed]
11. Fishman, G.A., Retinitis pigmentosa: visual loss. Archives of Ophthalmology, 1978. 96(7): p. 1185-1188. [PubMed]
12. Berson, E.L., et al., Disease progression in patients with dominant retinitis pigmentosa and rhodopsin mutations. Investigative Ophthalmology & Visual Science, 2002. 43(9): p. 3027-3036. [PubMed]
13. Daiger, S., L. Sullivan, and S. Bowne, Genes and mutations causing retinitis pigmentosa. Clinical genetics, 2013. 84(2): p. 132-141. [PubMed]
14. Cideciyan, A.V., et al., Human retinal gene therapy for Leber congenital amaurosis shows advancing retinal degeneration despite enduring visual improvement. Proc Natl Acad Sci U S A, 2013. 110(6): p. E517-25. [PubMed]
15. Stasheff, S.F., Emergence of sustained spontaneous hyperactivity and temporary preservation of OFF responses in ganglion cells of the retinal degeneration (rd1) mouse. Journal of neurophysiology, 2008. [PubMed]
16. Strettoi, E. and V. Pignatelli, Modifications of retinal neurons in a mouse model of retinitis pigmentosa. Proceedings of the National Academy of Sciences, 2000. 97(20): p. 11020-11025. [PubMed]
17. Marc, R.E. and B.W. Jones, Retinal remodeling in inherited photoreceptor degenerations. Molecular neurobiology, 2003. 28(2): p. 139-147. [PubMed]
18. Jones, B.W., et al., Retinal remodeling triggered by photoreceptor degenerations. Journal of Comparative Neurology, 2003. 464(1): p. 1-16. [PubMed]
19. Jones, B., et al., Retinal remodeling in human retinitis pigmentosa. Experimental eye research, 2016. 150: p. 149-165. [PubMed]
20. Sjöstrand, J., et al., Morphometric study of the displacement of retinal ganglion cells subserving cones within the human fovea. Graefe’s archive for clinical and experimental ophthalmology, 1999. 237(12): p. 1014-1023. [PubMed]
21. Yin, L., et al., Imaging light responses of foveal ganglion cells in the living macaque eye. J Neurosci, 2014. 34(19): p. 6596-605. [PubMed]
22. Yang, J., et al., Destructive Changes in the Neuronal Structure of the FVB/N Mouse Retina. PLoS One, 2015. 10(6): p. e0129719. [PubMed]
23. Santos, A., et al., Preservation of the inner retina in retinitis pigmentosa: a morphometric analysis. Archives of Ophthalmology, 1997. 115(4): p. 511-515. [PubMed]
24. Potts, A.M. and J. Inoue, The electrically evoked response (EER) of the visual system II. Effect of adaptation and retinitis pigmentosa. Investigative Ophthalmology & Visual Science, 1969. 8(6): p. 605-612. [PubMed]
25. Yanai, D., et al., The value of preoperative tests in the selection of blind patients for a permanent microelectronic implant. Transactions of the American Ophthalmological Society, 2003. 101: p. 223. [PubMed]
26. Naycheva, L., et al., Phosphene Thresholds Elicited by Transcorneal Electrical Stimulation in Healthy Subjects and Patients with Retinal DiseasesEPTs Elicited by Transcorneal Electrical Stimulation. Investigative ophthalmology & visual science, 2012. 53(12): p. 7440-7448. [PubMed]
27. Humayun, M.S., et al., Visual perception in a blind subject with a chronic microelectronic retinal prosthesis. Vision research, 2003. 43(24): p. 2573-2581. [PubMed]
28. O’Shea, R.P., Thumb’s rule tested: visual angle of thumb’s width is about 2 deg. Perception, 1991. 20(3): p. 415-8. [PubMed]
29. Roessler, G., et al., Implantation and explantation of a wireless epiretinal retina implant device: observations during the EPIRET3 prospective clinical trial. Investigative ophthalmology & visual science, 2009. 50(6): p. 3003-3008. [PubMed]
30. Keserü, M., et al., Acute electrical stimulation of the human retina with an epiretinal electrode array. Acta ophthalmologica, 2012. 90(1). [PubMed]
31. Humayun, M.S., et al., Interim results from the international trial of Second Sight’s visual prosthesis. Ophthalmology, 2012. 119(4): p. 779-88. [PubMed]
32. Ahuja, A., et al., Factors affecting perceptual threshold in Argus II retinal prosthesis subjects. Translational vision science & technology, 2013. 2(4): p. 1-1. [PubMed]
33. Da Cruz, L., et al., The Argus II epiretinal prosthesis system allows letter and word reading and long-term function in patients with profound vision loss. British Journal of Ophthalmology, 2013: p. bjophthalmol-2012-301525. [PubMed]
34. Jensen, R.J., O.R. Ziv, and J.F. Rizzo, Responses of rabbit retinal ganglion cells to electrical stimulation with an epiretinal electrode. Journal of neural engineering, 2005. 2(1): p. S16. [PubMed]
35. Jensen, R.J., O.R. Ziv, and J.F. Rizzo, Thresholds for activation of rabbit retinal ganglion cells with relatively large, extracellular microelectrodes. Investigative ophthalmology & visual science, 2005. 46(4): p. 1486-1496. [PubMed]
36. Margalit, E. and W.B. Thoreson, Inner retinal mechanisms engaged by retinal electrical stimulation. Investigative ophthalmology & visual science, 2006. 47(6): p. 2606-2612. [PubMed]
37. Fried, S.I., H.-A. Hsueh, and F.S. Werblin, A method for generating precise temporal patterns of retinal spiking using prosthetic stimulation. Journal of neurophysiology, 2006. 95(2): p. 970-978. [PubMed]
38. Nanduri, D., et al., Frequency and amplitude modulation have different effects on the percepts elicited by retinal stimulation. Investigative Ophthalmology & Visual Science, 2012. 53(1): p. 205-214. [PubMed]
39. Nanduri, D., et al. Retinal prosthesis phosphene shape analysis. in Engineering in Medicine and Biology Society, 2008. EMBS 2008. 30th Annual International Conference of the IEEE. 2008. IEEE. [PubMed]
40. Luo, Y.H., et al., Long-term repeatability and reproducibility of phosphene characteristics in chronically implanted Argus II retinal prosthesis subjects. American journal of ophthalmology, 2016. 170: p. 100-109. [PubMed]
41. Fornos, A.P., et al., Temporal properties of visual perception on electrical stimulation of the retinaperception upon electrical stimulation of the retina. Investigative ophthalmology & visual science, 2012. 53(6): p. 2720-2731. [PubMed]
42. Vrabec, F., The temporal raphe of the human retina. American journal of ophthalmology, 1966. 62(5): p. 926-938. [PubMed]
43. FitzGibbon, T. and S. Taylor, Mean retinal ganglion cell axon diameter varies with location in the human retina. Japanese journal of ophthalmology, 2012. 56(6): p. 631-637. [PubMed]
44. Paunescu, L.A., et al., Reproducibility of nerve fiber thickness, macular thickness, and optic nerve head measurements using StratusOCT. Investigative ophthalmology & visual science, 2004. 45(6): p. 1716-1724. [PubMed]
45. Tan, O., et al., Mapping of macular substructures with optical coherence tomography for glaucoma diagnosis. Ophthalmology, 2008. 115(6): p. 949-56. [PubMed]
46. Fine, I. and G.M. Boynton, Pulse trains to percepts: the challenge of creating a perceptually intelligible world with sight recovery technologies. Phil. Trans. R. Soc. B, 2015. 370(1677): p. 20140208. [PubMed]
47. Weitz, A.C., et al., Improving the spatial resolution of epiretinal implants by increasing stimulus pulse duration. Science translational medicine, 2015. 7(318): p. 318ra203-318ra203. [PubMed]
48. Freeman, D.K., et al., Selective activation of neuronal targets with sinusoidal electric stimulation. Journal of neurophysiology, 2010. 104(5): p. 2778-2791. [PubMed]
49. Fujikado, T., et al., One-Year Outcome of 49-Channel Suprachoroidal–Transretinal Stimulation Prosthesis in Patients With Advanced Retinitis PigmentosaOne-Year Outcome of 49-Channel STS Prosthesis in Advanced RP. Investigative ophthalmology & visual science, 2016. 57(14): p. 6147-6157. [PubMed]
50. Lorach, H., et al., Photovoltaic restoration of sight with high visual acuity. Nature medicine, 2015. 21(5): p. 476-482. [PubMed]
51. Chow, A.Y., et al., The artificial silicon retina microchip for the treatment of visionloss from retinitis pigmentosa. Archives of ophthalmology, 2004. 122(4): p. 460-469. [PubMed]
52. Zrenner, E., et al., Subretinal electronic chips allow blind patients to read letters and combine them to words. Proc Biol Sci, 2011. 278(1711): p. 1489-97. [PubMed]
53. Stingl, K., et al. Artificial vision with wirelessly powered subretinal electronic implant alpha-IMS. in Proc. R. Soc. B. 2013. The Royal Society. [PubMed]
54. Stingl, K., et al., Interim Results of a Multicenter Trial with the New Electronic Subretinal Implant Alpha AMS in 15 Patients Blind from Inherited Retinal Degenerations. Frontiers in neuroscience, 2017. 11: p. 445. [PubMed]
55. Edwards, T.L., et al., Assessment of the Electronic Retinal Implant Alpha AMS in Restoring Vision to Blind Patients with End-Stage Retinitis Pigmentosa. Ophthalmology, 2017. [PubMed]
56. Kelly, S.K., et al. Developments on the Boston 256-channel retinal implant. in 2013 IEEE International Conference on Multimedia and Expo Workshops (ICMEW). 2013. [IEEE Explore]
57. Wilke, R., et al., Spatial resolution and perception of patterns mediated by a subretinal 16-electrode array in patients blinded by hereditary retinal dystrophies. Invest Ophthalmol Vis Sci, 2011. 52(8): p. 5995-6003. [PubMed]
58. Kitiratschky, V.B., et al., Safety evaluation of “retina implant alpha IMS”—a prospective clinical trial. Graefe’s Archive for Clinical and Experimental Ophthalmology, 2015. 253(3): p. 381-387. [PubMed]
59. Linsenmeier, R.A. and H.F. Zhang, Retinal oxygen: from animals to humans. Progress in retinal and eye research, 2017. [PubMed]
60. Fujikado, T., et al., Testing of semichronically implanted retinal prosthesis by suprachoroidal-transretinal stimulation in patients with retinitis pigmentosa. Investigative ophthalmology & visual science, 2011. 52(7): p. 4726-4733. [PubMed]
61. Ayton, L.N., et al., First-in-human trial of a novel suprachoroidal retinal prosthesis. PLoS One, 2014. 9(12): p. e115239. [PubMed]
62. Sinclair, N.C., et al., The appearance of phosphenes elicited using a suprachoroidal retinal prosthesisphosphenes of a suprachoroidal retinal prosthesis. Investigative ophthalmology & visual science, 2016. 57(11): p. 4948-4961. [PubMed]
63. Delbeke, J., M. Oozeer, and C. Veraart, Position, size and luminosity of phosphenes generated by direct optic nerve stimulation. Vision research, 2003. 43(9): p. 1091-1102. [PubMed]
64. Sui, X., et al., Mechanical analysis and fabrication of a penetrating silicon microprobe as an artificial optic nerve visual prosthesis. Int J Artif Organs, 2012. 35(1): p. 34-44. [PubMed]
65. Safadi, M., et al., Development of a microfluidic drug delivery neural stimulating device for vision. Investigative Ophthalmology & Visual Science, 2003. 44(13): p. 5082-5082. [IOVS]
66. Peterman, M.C., et al., Localized chemical release from an artificial synapse chip. Proceedings of the National Academy of Sciences of the United States of America, 2004. 101(27): p. 9951-9954. [PubMed]
67. Inayat, S., et al., Chemical stimulation of rat retinal neurons: feasibility of an epiretinal neurotransmitter-based prosthesis. Journal of neural engineering, 2014. 12(1): p. 016010. [PubMed]
68. Vu, T.Q., et al., Activation of membrane receptors by a neurotransmitter conjugate designed for surface attachment. Biomaterials, 2005. 26(14): p. 1895-1903. [PubMed]
69. Cohn, F., Über die Gesetze der Bewegung der microscopischen Pflanzen und Thiere unter Einfluß des Lichtes. Jb. Schles. Ges. Vaterl. Kultur, 1865. 42: p. 35-37.
70. Mast, S.O., The process of orientation in the colonial organism, Gonium pectorale, and a study of the structure and function of the eye‐spot. Journal of Experimental Zoology Part A: Ecological Genetics and Physiology, 1916. 20(1): p. 1-17. [Wiley]
71. Litvin, F.F., O.A. Sineshchekov, and V.A. Sineshchekov, Photoreceptor electric potential in the phototaxis of the alga Haematococcus pluvialis. Nature, 1978. 271(5644): p. 476-8. [PubMed]
72. Sineshchekov, O.A., F.F. Litvin, and L. Keszthelyi, Two components of photoreceptor potential in phototaxis of the flagellated green alga Haematococcus pluvialis. Biophysical journal, 1990. 57(1): p. 33-39. [PubMed]
73. Nagel, G., et al., Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proceedings of the National Academy of Sciences, 2003. 100(24): p. 13940-13945. [PubMed]
74. Boyden, E.S., et al., Millisecond-timescale, genetically targeted optical control of neural activity. Nature neuroscience, 2005. 8(9): p. 1263-1268. [PubMed]
75. Bruun, S., et al., Light-Dark Adaptation of Channelrhodopsin Involves Photoconversion between the all-trans and 13-cis Retinal Isomers. Biochemistry, 2015. 54(35): p. 5389-400. [PubMed]
76. Szobota, S., et al., Remote control of neuronal activity with a light-gated glutamate receptor. Neuron, 2007. 54(4): p. 535-545. [PubMed]
77. Schröder-Lang, S., et al., Fast manipulation of cellular cAMP level by light in vivo. Nature methods, 2007. 4(1): p. 39-42. [PubMed]
78. Gasser, C., et al., Engineering of a red-light–activated human cAMP/cGMP-specific phosphodiesterase. Proceedings of the National Academy of Sciences, 2014. 111(24): p. 8803-8808. [PubMed]
79. Ermakova, Y.G., et al., Thermogenetic neurostimulation with single-cell resolution. Nat Commun, 2017. 8: p. 15362. [PubMed]
80. Dowling, J.E., Foveal receptors of the monkey retina: fine structure. Science, 1965. 147(3653): p. 57-59. [PubMed]
81. Hogan, M.J., J.A. Alvarado, and J. Weddell, Ciliary body and posterior chamber. Histology of the human eye: an atlas and textbook. Philadelphia: Saunders, 1971.
82. Cohen, A.I., New details of the ultrastructure of the outer segments and ciliary connectives of the rods of human and macaque retinas. The Anatomical Record, 1965. 152(1): p. 63-79. [PubMed]
83. Bi, A., et al., Ectopic expression of a microbial-type rhodopsin restores visual responses in mice with photoreceptor degeneration. Neuron, 2006. 50(1): p. 23-33. [PubMed]
84. Lagali, P.S., et al., Light-activated channels targeted to ON bipolar cells restore visual function in retinal degeneration. Nature neuroscience, 2008. 11(6): p. 667-675. [PubMed]
85. Doroudchi, M.M., et al., Virally delivered channelrhodopsin-2 safely and effectively restores visual function in multiple mouse models of blindness. Molecular Therapy, 2011. 19(7): p. 1220-1229. [PubMed]
86. Yan, B. and S. Nirenberg, An Embedded Real-time Processing Platform for Optogenetic Neuroprosthetic Applications. IEEE Trans Neural Syst Rehabil Eng, 2017. [PubMed]
87. Williams, S., Optogenetic therapies move closer to clinical use. , in The Scientist. 2017, The Scientist: New York. [TheScientist]
88. Sengupta, A., et al., Red‐shifted channelrhodopsin stimulation restores light responses in blind mice, macaque retina, and human retina. EMBO molecular medicine, 2016. 8(11): p. 1248-1264. [PubMed]
89. Kim, D.S., T. Matsuda, and C.L. Cepko, A core paired-type and POU homeodomain-containing transcription factor program drives retinal bipolar cell gene expression. Journal of Neuroscience, 2008. 28(31): p. 7748-7764. [PubMed]
90. Morris, R., Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods, 1984. 11(1): p. 47-60. [PubMed]
91. Chow, B.Y., et al., High-performance genetically targetable optical neural silencing by light-driven proton pumps. Nature, 2010. 463(7277): p. 98-102. [PubMed]
92. Wagner, N.L., et al., Directed evolution of bacteriorhodopsin for applications in bioelectronics. Journal of The Royal Society Interface, 2013. 10(84): p. 20130197. [PubMed]
93. Greco, J.A., et al., Stimulation of Retinal Ganglion Cells Using an Ion-Mediated, Protein-Based Retinal Implant. Investigative Ophthalmology & Visual Science, 2017. 58(8): p. 4184-4184. [PubMed]
94. Govorunova, E.G., et al., Natural light-gated anion channels: A family of microbial rhodopsins for advanced optogenetics. Science, 2015. 349(6248): p. 647-650. [PubMed]
95. Berndt, A., et al., Structural foundations of optogenetics: Determinants of channelrhodopsin ion selectivity. Proceedings of the National Academy of Sciences, 2016. 113(4): p. 822-829. [PubMed]
96. Zhang, Y., et al., Ectopic expression of multiple microbial rhodopsins restores ON and OFF light responses in retinas with photoreceptor degeneration. Journal of Neuroscience, 2009. 29(29): p. 9186-9196. [PubMed]
97. Busskamp, V., et al., Genetic reactivation of cone photoreceptors restores visual responses in retinitis pigmentosa. science, 2010. 329(5990): p. 413-417. [PubMed]
98. Khabou, H., et al., Noninvasive gene delivery to foveal cones for vision restoration. JCI Insight, 2018. 3(2). [PubMed]
99. Kim, J.-M., et al., Light-driven activation of β2-adrenergic receptor signaling by a chimeric rhodopsin containing the β2-adrenergic receptor cytoplasmic loops. Biochemistry, 2005. 44(7): p. 2284-2292. [PubMed]
100. Qiu, X., et al., Induction of photosensitivity by heterologous expression of melanopsin. Nature, 2005. 433(7027): p. 745-749. [PubMed]
101. Lin, B., et al., Restoration of visual function in retinal degeneration mice by ectopic expression of melanopsin. Proc Natl Acad Sci U S A, 2008. 105(41): p. 16009-14. [PubMed]
102. Koike, C., et al., TRPM1 is a component of the retinal ON bipolar cell transduction channel in the mGluR6 cascade. Proceedings of the National Academy of Sciences, 2010. 107(1): p. 332-337. [PubMed]
103. van Wyk, M., et al., Restoring the ON switch in blind retinas: opto-mGluR6, a next-generation, cell-tailored optogenetic tool. PLoS biology, 2015. 13(5): p. e1002143. [PubMed]
104. Gaub, B.M., et al., Optogenetic vision restoration using rhodopsin for enhanced sensitivity. Molecular Therapy, 2015. 23(10): p. 1562-1571. [PubMed]
105. Cehajic-Kapetanovic, J., et al., Restoration of vision with ectopic expression of human rod opsin. Current Biology, 2015. 25(16): p. 2111-2122. [PubMed]
106. Dalkara, D., et al., In vivo–directed evolution of a new adeno-associated virus for therapeutic outer retinal gene delivery from the vitreous. Science translational medicine, 2013. 5(189): p. 189ra76-189ra76. [PubMed]
107. Lu, Q., et al., AAV-mediated transduction and targeting of retinal bipolar cells with improved mGluR6 promoters in rodents and primates. Gene therapy, 2016. 23(8): p. 680-689. [PubMed]
108. Hadaczek, P., et al., Transduction of nonhuman primate brain with adeno-associated virus serotype 1: vector trafficking and immune response. Human gene therapy, 2009. 20(3): p. 225-237. [PubMed]
109. Okada, H., et al., Robust long-term transduction of common marmoset neuromuscular tissue with rAAV1 and rAAV9. Molecular Therapy—Nucleic Acids, 2013. 2(5): p. e95. [PubMed]
110. Matsumoto, B., J. Blanks, and S. Ryan, Topographic variations in the rabbit and primate internal limiting membrane. Investigative ophthalmology & visual science, 1984. 25(1): p. 71-82. [PubMed]
111. Halfter, W., et al., Embryonic synthesis of the inner limiting membrane and vitreous body. Invest Ophthalmol Vis Sci, 2005. 46(6): p. 2202-9. [PubMed]
112. Vacca, O., et al., Using adeno-associated virus as a tool to study retinal barriers in disease. Journal of visualized experiments: JoVE, 2015(98). [PubMed]
113. Yin, L., et al., Intravitreal injection of AAV2 transduces macaque inner retina. Investigative ophthalmology & visual science, 2011. 52(5): p. 2775-2783. [PubMed]
114. Ivanova, E., et al., Evaluation of AAV-mediated expression of Chop2-GFP in the marmoset retina. Investigative ophthalmology & visual science, 2010. 51(10): p. 5288-5296. [PubMed]
115. Tshilenge, K.-T., et al., Vitrectomy Before Intravitreal Injection of AAV2/2 Vector Promotes Efficient Transduction of Retinal Ganglion Cells in Dogs and Nonhuman Primates. Human gene therapy methods, 2016. 27(3): p. 122-134. [PubMed]
116. Takahashi, K., et al., Improved intravitreal AAV-mediated inner retinal gene transduction after surgical internal limiting membrane peeling in cynomolgus monkeys. Molecular Therapy, 2017. 25(1): p. 296-302. [PubMed]
117. De Silva, S.R., et al., Long-term restoration of visual function in end-stage retinal degeneration using subretinal human melanopsin gene therapy. Proceedings of the National Academy of Sciences, 2017. 114(42): p. 11211-11216. [PubMed]
118. Xue, K., et al., Technique of retinal gene therapy: delivery of viral vector into the subretinal space. Eye (Lond), 2017. 31(9): p. 1308-1316. [PubMed]
119. Calame, M., et al., Retinal degeneration progression changes lentiviral vector cell targeting in the retina. PloS one, 2011. 6(8): p. e23782. [PubMed]
120. Pignatelli, V., C.L. Cepko, and E. Strettoi, Inner retinal abnormalities in a mouse model of Leber’s congenital amaurosis. Journal of Comparative Neurology, 2004. 469(3): p. 351-359. [PubMed]
121. Farinelli, P., et al., DNA methylation and differential gene regulation in photoreceptor cell death. Cell death & disease, 2014. 5(12): p. e1558. [PubMed]
122. Arango-Gonzalez, B., et al., Identification of a common non-apoptotic cell death mechanism in hereditary retinal degeneration. PLoS One, 2014. 9(11): p. e112142. [PubMed]
123. Wahlin, K.J., et al., Epigenetics and cell death: DNA hypermethylation in programmed retinal cell death. PloS one, 2013. 8(11): p. e79140. [PubMed]
124. van Wyk, M., et al., Present Molecular Limitations of ON-Bipolar Cell Targeted Gene Therapy. Front Neurosci, 2017. 11: p. 161. [PubMed]
125. Cohen, E., et al., Optical coherence tomography imaging of retinal damage in real time under a stimulus electrode. Journal of neural engineering, 2011. 8(5): p. 056017. [PubMed]
126. Butterwick, A., et al., Tissue damage by pulsed electrical stimulation. IEEE Trans Biomed Eng, 2007. 54(12): p. 2261-7. [PubMed]
127. Cohen, E.D., Effects of high-level pulse train stimulation on retinal function. J Neural Eng, 2009. 6(3): p. 035005. [PubMed]
128. Nayagam, D.A.X., et al., Chronic electrical stimulation with a suprachoroidal retinal prosthesis: a preclinical safety and efficacy study. PloS one, 2014. 9(5): p. e97182. [PubMed]
129. McCreery, D.B., et al., Charge density and charge per phase as cofactors in neural injury induced by electrical stimulation. IEEE Transactions on Biomedical Engineering, 1990. 37(10): p. 996-1001. [PubMed]
130. De Vera Mudry, M.C., et al., Blinded by the light: retinal phototoxicity in the context of safety studies. Toxicologic pathology, 2013. 41(6): p. 813-825. [PubMed]
131. Ham, W.T., H.A. Mueller, and D.H. Sliney, Retinal sensitivity to damage from short wavelength light. Nature, 1976. 260(5547): p. 153-155. [PubMed]
132. Ham, W.T., et al., Sensitivity of the retina to radiation damage as a function of wavelength. Photochemistry and Photobiology, 1979. 29(4): p. 735-743. [PubMed]
133. Noell, W.K., et al., Retinal damage by light in rats. Investigative Ophthalmology & Visual Science, 1966. 5(5): p. 450-473. [PubMed]
134. Wenzel, A., et al., The Rpe65 Leu450Met variation increases retinal resistance against light-induced degeneration by slowing rhodopsin regeneration. Journal of Neuroscience, 2001. 21(1): p. 53-58. [PubMed]
135. Wenzel, A., et al., Molecular mechanisms of light-induced photoreceptor apoptosis and neuroprotection for retinal degeneration. Progress in retinal and eye research, 2005. 24(2): p. 275-306. [PubMed]