Helga Kolb
1. General characteristics.
Amacrine cells of the vertebrate retina are interneurons that interact at the second synaptic level of the vertically direct pathways consisting of the photoreceptor-bipolar-ganglion cell chain. They are synaptically active in the inner plexiform layer (IPL) and serve to integrate, modulate and interpose a temporal domain to the visual message presented to the ganglion cell. Amacrine cells are so named because they are nerve cells thought to lack an axon (Cajal, 1892). Today we know that certain large field amacrine cells of the vertebrate retina can have long “axon-like” processes which probably function as true axons in the sense that they are output fibers of the cell (see later section on dopaminergic amacrine cells). However these amacrine axons remain within the retina and do not leave the retina in the optic nerve as do the ganglion cell axons. Figure 1 shows one of the earliest depictions of the retinal cell types including amacrine cells drawn by Ramon y Cajal (circa 1890). These retinal cell types were visualized using the anatomical silver impregnation method devised by the Italian anatomist Camillo Golgi in the nineteenth century (Fig. 2).
Fig. 1. Drawing of the retina made by Cajal
Since the time of Cajal we have known that amacrine cells come in all shapes, sizes and stratification patterns. Since those days many more morphological subtypes have and continue to be described from further Golgi studies, intracellular recordings and immunocytochemical staining. Thus, we presently have a classification of amacrine cells consisting of about 40 different morphological subtypes.
Fig. 2. Picture of Camillo Golgi
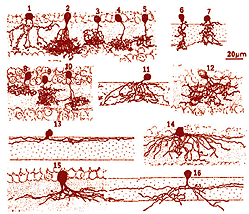
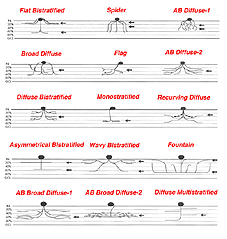
It is useful and most easily understandable to group the amacrine cell types into the general descriptors of narrow-field (30-150 um), small-field (150-300 um), medium-field (300-500 um) and wide-field (>500 um) based on a measurement of their dendritic field diameters (Kolb et al., 1981). Then the next most important criterion of classification involves knowing the cells stratification. It is generally agreed now that the IPL can be subdivided into five equi-thickness strata or sublayers (Cajal, 1892) into which amacrine, bipolar and ganglion cell processes can be assigned. All of these cell types are now classified primarily on the stratum or strata of the IPL in which their dendrites or axons are located. This is because, as mentioned in previous chapters, the IPL of vertebrate retinas can be divided up into areas of neuropil where specific cells are put into synaptic contacts and form circuits only with cells earmarked for a particular functional role.
Many varieties of amacrine cell are monostratified, restricted to a single stratum, while others are bi- or tri-stratified. When amacrine or ganglion cell processes pass through all the strata of the IPL from distal to proximal or vice versa, they are called diffuse cells. Superimposed upon Cajal’s five strata subdivision of the IPL, is a sublaminar division of the IPL. The first two strata, 1-2, are known as sublamina a of the IPL while strata 3-5 are known as sublamina b by this scheme (Famiglietti and Kolb, 1976). It will be remembered from previous chapters that sublamina a contains bipolar axons and ganglion cell connections that lead to OFF-center ganglion cell physiology, while sublamina b contains bipolar to ganglion cell connections resulting in ON-center ganglion cell physiology (Nelson et al., 1978).
Figure 3 shows drawings of some small field amacrine cells of the monkey retina as seen in vertical sections (Polyak, 1941). Small-field cells like these can be well visualized in section because their dendritic trees are contained within the depth of the section. However, large field cells are not so well described in section where their dendrites get cut off.
Fig. 3. Amacrine cells of the monkey retina. Adapted from Polyak, 1941.
It was only when wholemount preparations, from Golgi staining (Stell and Witkovsky, 1972; Boycott and Kolb, 1973) or immunocytochemical staining (Karten and Brecha, 1980) were attempted that we could classify such cells. Then the full extent of their dendritic trees which can be up to one millimeter in spread could be visualized (Fig. 4) and a whole new understanding of amacrine cells became available.
Fig.4. Amacrine cells as seen in wholemount cat retina
A new technique of intracellular staining by a photochemical method has been developed in Richard Masland’s group, as an alternative to the unreliable Golgi technique (MacNeil and Masland, 1998). Amacrine cells of the rabbit retina are labelled with the nuclear stain DAPI and then selected single nuclei are irradiated by a narrow beam of light to drive DAPI to the oxidation of non-fluorescent dihydrorhodamine 123 to the fluorescent rhodamine 123. The complete cell body and the dendritic tree is thus revealed under viewing in the fluorescence or confocal microscopes. By this method, 30 or so different varieties of amacrine cell can be photographed and drawn in full detail in the rabbit retina. 22 varieties of amacrine cell have been seen in Golgi preparations in cat and primate retinas so either some have been missed that were seen in rabbit, or else they are not as well deveoped in these less complex mammalian retinas. In any event the narrow field and medium field types revealed by MacNeil and Maslands work (1998) are shown in Figures 4a and b below. A further 5 different wide field monostratified types were also encountered in rabbit retina by this method (not illustrated). They correspond closely to the wide field types seen in monkey, cat and human (Fig. 4, above) (Mariani, 1990; Kolb et al., 1981, Kolb et al. 1992).
2. Amacrine cell circuitry as revealed by electron microscopy.
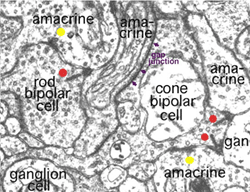
Kidd (1962) and later Dowling and Boycott (1966) were the first to identify the three types of profile that contribute to the IPL by electron microscopy. The electron micrograph (Fig. 5) below shows the cytological criteria on which we now recognize bipolar, amacrine and ganglion cell profiles in the neuropil. Thus bipolar cell axonal endings are recognized by being filled with synaptic vesicles and having a ribbon-shaped density (Fig. 5, red spots) pointing to two postsynaptic profiles (amacrine and ganglion). Amacrine profiles are also filled with synaptic vesicles but make synapses characterized by membrane densities at which the vesicles are particularly clustered (Fig. 5, yellow spots). Ganglion cell profiles are recognized as being only postsynaptic to either bipolar axons or amacrine processes, containing no vesicles but instead a content of neurotubules, ribosomes and glycogen granules.
Fig. 5. Electron micrograph of several profiles in the IPL
Amacrine cell synapses are frequently seen to be reciprocal to bipolar ribbon input, i.e. the amacrine returns a synapse in the vicinity of the ribbon input synapse (arrowheads). Most amacrine cells are inhibitory neurons in the vertebrate retina, containing the common inhibitory neurotransmitters GABA or glycine. GABAergic amacrine cells, in particular, typically make reciprocal synapses with bipolar cells. A17 is the most well studied of the GABAergic reciprocal amacrine cells in the retina and we shall return to this cell later.
We have learned much concerning the synaptic relationships of certain narrow-field amacrine cells as well as bipolar and small ganglion cell types such as midget ganglion cells of the primate retina, from reconstructions of serial-section electron micrographs. The circuitry of the AII amacrine cell in the cat retina was first appreciated by this means (Kolb and Famiglietti, 1974; Famiglietti and Kolb, 1975; Kolb, 1979). However, with the advent of intracellular dye injection of electron-dense materials (horseradish peroxidase, HRP, or the photoreduction of Lucifer yellow) after physiological recordings or the development of electron dense immunostains for electron microscopy, neurocircuitry was made easier for us. We could look at amacrine cells and their synaptic inputs by study of fewer sections and it was not as critical to photograph every single section in a series. The amacrine cell of interest would always be clearly marked black, and easily found in the synaptic neuropil. It is from this technique that we have learned most about amacrine cells and their circuitry in the mammalian retina. The remainder of this chapter will describe the morphology, circuitry and intracellular responses of the amacrine cells that are most completely understood at present.
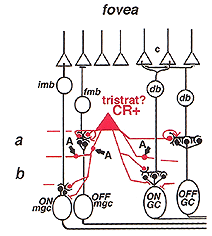
3. A2: narrow-field, cone pathway amacrine cell.
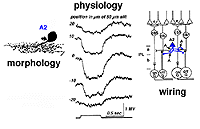
A2 is a narrow-field amacrine with a 20-60 um wide dendritic tree composed of multibranched, beaded and appendage-bearing dendrites mostly confined to stratum 2 of the IPL (Fig.6).
Fig. 6. Golgi drawings of A2 amacrine cells in cat and human retinas
Intracellular recordings from A2 cells (formerly called A4) indicate that these cells give true slow potential hyperpolarizing response to light (OFF-center) at all positions of the slit in their receptive fields and they have no sign of an inhibitory surround (Fig. 7) (Kolb and Nelson, 1984).
A2 cells receive bipolar input from OFF-center types of cone bipolar cell of sublamina a and make reciprocal synapses to these bipolar axons (Fig. 7). A2 amacrine cells then synapse upon OFF-center ganglion cell dendrites of sublamina a. The A2 cell makes an inhibitory synapses upon these ganglion cells, because it is thought to be a glycinergic cell type (Wassle et al., 2009 ).
A possible role for A2 amacrines is in disinhibition of the ganglion cells’ center responses. Alternatively, A2 cells, despite being small-field types, might have a role in the generation of antagonistic surrounds of ganglion cells (Kolb and Nelson, 1993). A2 cells receive a great many amacrine inputs to their dendritic trees which could be from wider field cells than they are are themselves so giving them a much larger receptive field size than their actual dendritic tree size would indicate.
Fig. 7. Summary diagram of A2 amacrine cell’s wiring pattern and physiological responses to light
A3, knotty Type 2 amacrine cells.
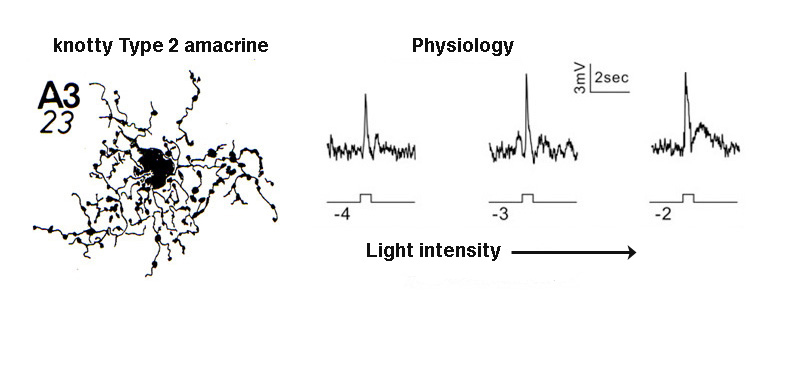
A small-field amacrine cell that branches in S2 and S3 (i.e. across the sublamina a/b border) is seen in cat and human retina with Golgi staining (Kolb et al., 1981; Kolb et al., 1992) (Fig. 7a). The same amacrine has been called a knotty Type2 amacrine cell in Golgi studies (Mariani, 1990) and immunostaining in the macaque retina (Klump et al., 2009). The A3 cell is clearly immunostained with parvalbumin (Fig. 7b) and has the typical A3 morphology and small field multibranched dendrites with large varicosities (Fig. 7a, b) (Klump et al., 2009). The varicose dendrites branch broadly through strata S2 and S3, so they are in a position to interact with both flat midget cone bipolars or other narrow field cone bipolars of the OFF sublamina a, and with cone bipolar cells of stratum 3 in the ON sublamina b.
A3 or the PARV+ amacrine can be seen to be glycinergic when double immunostained for either glycine (Wassle et al., 2009) or the glycine transporter Glyt-1 (Fig. 7b, the PARV+, A3 colocalizes parvalbumin and Glyt-1, a-d). This small field A3 amacrine has been intracellulaly recorded from by Klump and co-authors (2009) and proves to give an ON response to light stimulation (Fig. 7a, right traces). The PARV+ amacrine is situated amongst the axon terminals of flat (OFF) midget bipolar cells in S2 of the IPL and appears to be either pre or postsynaptic where apposing immunostained profiles occur (Fig. 7b, e and f). The A3 PARV+ cell is also know to be presynaptic to OFF parasol ganglion cells in sublamina a and interact with AII amacrine lobular appendages and starburst amacrine cells of sublamina a (Bordt et al., 2006). A3 cells are also extensively coupled into a network of the same cell type by gap junctions (Klump et al., 2009). The latter authors suggest that A3, knotty Type2 amacrines are driven by ON pathway cone bipolars and inhibit OFF pathways and through synapses upon AII amacrine cells inhibit the transmission of rod signals to these same OFF pathways.
4. AII: a bistratified rod amacrine cell.
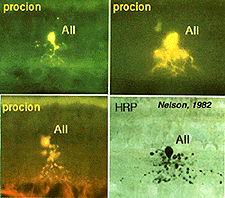
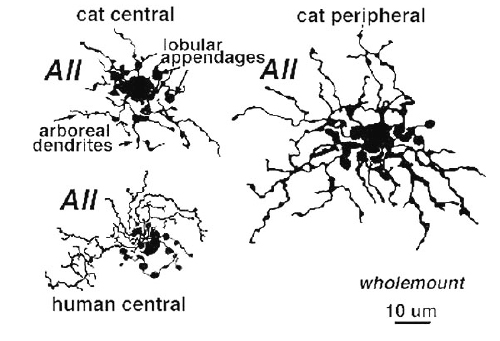
Above are shown four examples of the best studied amacrine of all in the vertebrate retina: the AII “rod amacrine” of the mammalian retina. These cells have been recorded from by microelectrodes and dyes have been iontophoresed into the cell after the intracellular recordings (Nelson, 1982). The AII cell, was first described from Golgi staining and electron microscopic examination (Famiglietti and Kolb, 1975; Kolb and Famiglietti, 1974).
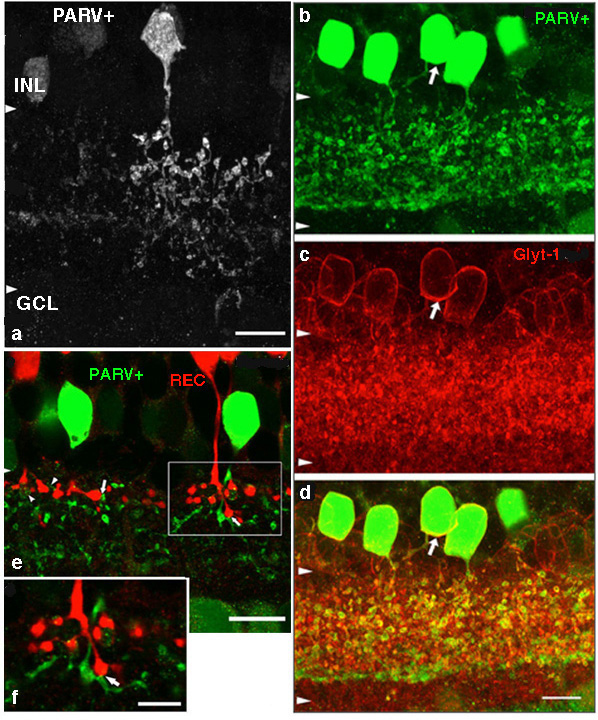
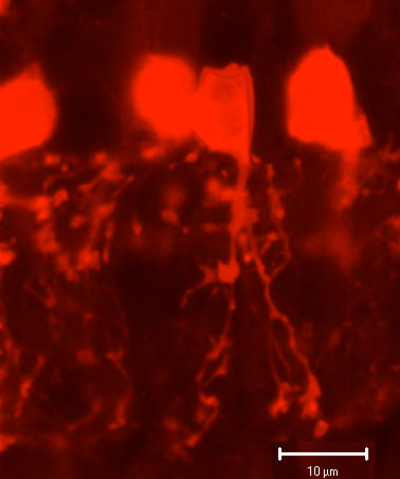
AII is a narrow field amacrine (dendritic tree diameter typically 30-70 um) with a bistratified morphology: the mitral shaped cell body gives off a single, stout apical dendrite and a cluster of lobular appendages (round blobs just below the cell body, Fig. 9) arise from the main dendrite in sublamina a of the IPL (Fig 9). The finer “arboreal dendrites” (Vaney et al., 1991) penetrate down into sublamina b to end close to the ganglion cell layer (Fig. 9). AII amacrine cells are glycine-immunoreactive (Pourcho and Goebel, 1985; Crooks and Kolb, 1992) and contain the calcium binding proteins parvalbumin, calbindin and calretinin (Wässle et al., 1995). Figure 10 shows a Golgi stained AII amacrine cell in cat and human retinas as seen in a surface view of a wholemount.
Fig. 9. Parvalbumin staining of AII amacrine cells in hamster retina.
Fig. 10 Golgi stained AII amacrine cells seen in wholemount.
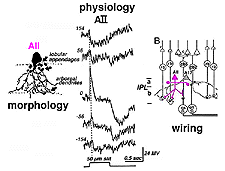
In cat and rabbit retinas where AIIs have been recorded from, the AII cell is a rod-dominated depolarizing (ON-center) cell (Bloomfield, 1992; Dacheux and Raviola, 1986; Nelson, 1982). Thus, in the center of its receptive field the cell gives a transient depolarizing response with a pronounced sustained plateau (ON-center) and a long drawn out hyperpolarization after light off (Fig. 11). By 140 um to either side of the center, the response to a light flash is now an inverted response indicating a hyperpolarizing surround (OFF-surround) (Fig. 11) (Nelson, 1982).
Fig. 11. Schematic diagram of the morphology, physiology
and wiring pattern of the AII amacrine cell
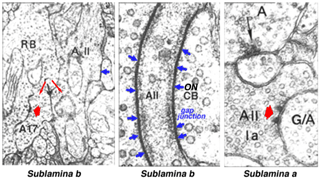
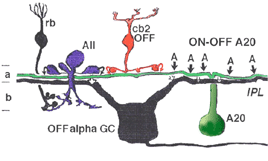
Electron microscopy has shown that the AII, is primarily postsynaptic to rod bipolar axon terminals in lower sublamina b of the IPL (30% of its input, Strettoi et al., 1992) (Fig. 12, left). Some OFF cone bipolar input is directed at the AII’s lobular appendages in sublamina a (Fig. 13). AII’s major output is upon ganglion cells that have dendrites only in sublamina a. AII cell lobular appendages synapse upon OFF-center ganglion cells (Fig. 12, right) and to OFF-center cone bipolar cell axons (possibly cb1 and cb2 types) in sublamina a (Fig. 13) (Kolb, 1979).
Fig. 12. Electron micrographs of AII amacrine synapses
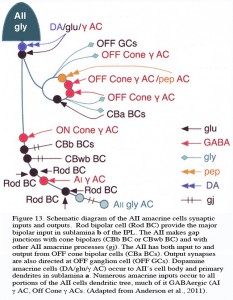
The AII also passes rod-driven information through the ON-center cone bipolar axons in sublamina b to ON-center ganglion cells by means of gap junctions (Fig. 12, center panel) (Kolb and Famiglietti, 1974; Famiglietti and Kolb, 1975; Kolb, 1979). Several, if not all, cone bipolar axons of sublamina b have gap junctions with AII cell dendrites. A new finding is that even the blue-cone bipolar receives rod signals through this gap junction pathway (Field et al., 2009). AII cells also join with other AII cells by gap junctions in sublamina b of the IPL (Fig. 13, lowest gj) (Famiglietti and Kolb, 1975; Nelson, 1982; Vaney, 1994a). Summary Figure 13 shows the major input and output circuitry of the AII amacrine cell.
Fig. 13. Schematic drawing of the wiring pattern of the AII amacrine cell
It is clear that both OFF and ON GABAergic amacrines amongst them the A17 (Kolb et al., 1981) called AI by others (Anderson et al., 2011) have synapses upon AII cells in both sublaminae of the IPL (Fig. 13. γ AC). Dopamine containing cells provides synapse on the AII cell (Fig. 13, DA), either directly upon its cell body or upon its lobular appendages (Voigt and Wässle, 1987; Kolb et al, 1991; Anderson et al., 2011) (see later section on dopamine amacrine cells, A18). Dopamine cells are thought to have a function in the inner retina to uncouple AII amacrine cells from both their contacts with the depolarizing cone bipolar and the AII amacrine coupled network (Daw et al., 1990; Vaney, 1994a).
Thus the AII amacrine cells are the major carriers of rod signals to the ganglion cells in the retina. As such they play a role in speeding up the slow potential rod messages for presentation to ganglion cells (Nelson, 1982; Smith, 1994). Their distribution in the retina suggests that they tile the complete retina (Vaney, 1990). AII amacrine cells peak in density at 1.5 mm from the foveal center in monkey and at the area centralis in cat (Vaney, 1984). In addition, because of their high density across all parts of the retina and their synaptic involvement with millions of rod bipolar cells, they may contribute in a major way to the pattern ERG (Zrenner, 1990).
Click here to see an animation of the wiring pattern of the AII amacrine cells
(Quicktime movie)
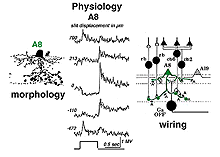
5. A8: a bistratified cone amacrine cell.
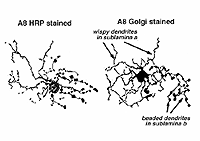
A8 is a bistratified, narrow-field amacrine cell which is easy to confuse with AII in wholemount, stained retina (Fig. 14). It actually looks like an upside-down AII cell. A8 has short, wispy processes coming from the apical dendrite to ramify in sublamina a of the IPL whereas a small number of heavily-beaded dendrites penetrate down to sublamina b, to run in strata 4 and 5. This cell type may correspond to the DAPI-3 of the rabbit retina, described by Vaney (1990) and Bloomfield (1992). It is a glycinergic amacrine cell (Pourcho and Goebel, 1985; Crooks and Kolb,1992; Wassle et al., 2009; Neumann and Haverkamp, 2012; Lee et al., 2015).Very recently the A8 amacrine cell population has been demonstrated to immunostain for the protein synaptotagmin-2 (Syt2) in mouse and macaque monkey retina (Neumann and Haverkamp, 2012; lee et al., 2015). Syt2 cells peak at a density of 1400/mm2 at 1-2 mm and subside to 22/mm2 at 9-10 mm of eccentricity (Neumann and Haverkamp, 2012).
Fig. 14. Golgi and HRP appearance of A8 amacrine cells
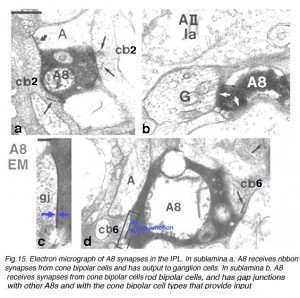
Fig.15. Synapses in the IPL of a HR-peroxidase injected A8 cell
The A8 cell has been intracellularly recorded and studied by electron microscopy after iontophoresis of horseradish peroxidase. In Fig. 15 we can see the synapses of this cell type.
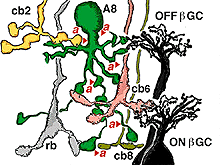
The A8 amacrine cell is involved in the cone pathways of the cat retina, rather than the rod pathways, that the AII is committed to. Thus, in sublamina a, excitatory cone driven signals come from cone bipolar cells like cb2 which we know are OFF center in physiology (Fig. 15a), and in sublamina b from cb6, another OFF-center bipolar cell (Fig. 15d) (Nelson and Kolb, 1983). Altogether cone bipolar synapses account for 42% of the input to A8 cells. Lesser rod bipolar input (20%) also occurs to the lower dendrites in sublamina b of the IPL. Like AII amacrine cells, A8 cells also engages in gap junctions with a cone bipolar type of sublamina b, but the bipolar is a different type, possibly cb6, and in addition to the gap junction makes the common ribbon synapse to A8 dendrites (Figs. 15c and d). A8’s major output is to OFF-center beta ganglion cell dendrites in sublamina a of the IPL (Fig. 15b). We have not seen A8 synapses to OFF-center alpha cells in the cat retina (Kolb and Nelson, 1996). The input and output synapses for A8 amacrine cells are seen in the summary diagram of Figure 16 and the animation below.
Fig. 16. Summary diagram of the wiring pattern of the A8 amacrine cell
Click here to see an animation of the wiring pattern of the A8 amacrine cells
(Quicktime movie)
The intracellular response of A8 indicates a hyperpolarization to light at the receptive field center with a rather transient OFF-response to light. The transientness could reflect the amacrine synapses (38% of input) occurring over all parts of its dendritic tree (Fig. 16, red arrowheads). (0, response is at largest magnitude).
Both rod driven and cone driven signals contribute to the response but the cone-driven response is largest. Its receptive field extent can be mapped with a slit of light. As the slit is stepped some distance to either side of the center of the receptive field the response of the cell inverts and a depolarizing or ON-surround appears by 700 um from the central position (top and bottom trace) (Kolb and Nelson, 1996).
Fig. 17. Summary diagram of the A8 amacrine cell
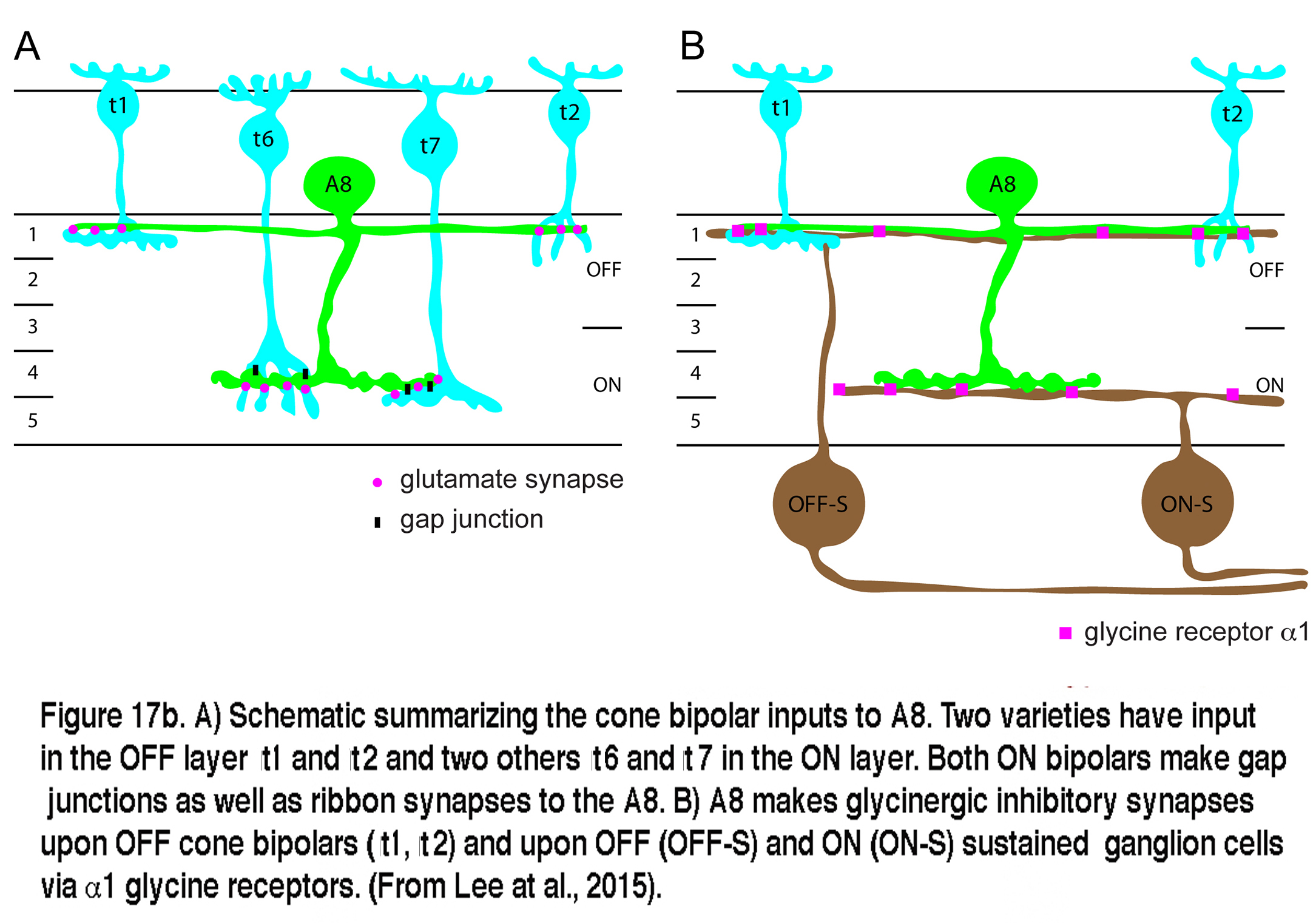
Very recent studies have identified the A8 in both monkey and mouse retinas (Neumann and Haverkamp, 2012; Lee et al., 2015). The morphology of the A8 in these species is very similar to the A8 of cat and the wiring diagram is essentially the same as well. Glutamatergic input comes from both OFF and ON cone bipolar cells with a minor rod bipolar cell contribution. The A8 cells in mouse similarly make gap junctions with the ON cone bipolar that have ribbon input to it. Output of the mouse A8 is to OFF cone bipolar cells via glycine receptor subunit α1, and to both OFF and ON sustained ganglion cell types (Lee et al., 2015). A copy of Lee and coworkers wiring diagram for the mouse A8 is shown in Figure 17b.
Some part of the amacrine input to A8, particularly that upon its cell body and proximal dendrites in stratum 1 of the IPL, may be from dopaminergic amacrine cells (A18) (Kolb et al., 1991). So it is probable that this cell type is also under the control of the dopaminergic amacrine cells, like the AII amacrine cell (see above). Thus, the dopamine cell may control A8’s spatial characteristics through gating its gap junctions in light and dark. We have suggested that A8 cells also function in the disinhibition of ganglion cell receptive field centers (Kolb and Nelson, 1996) in a manner that has been called “crossover inhibition” (Werblin, 2010).
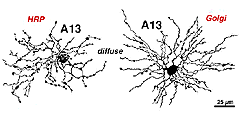
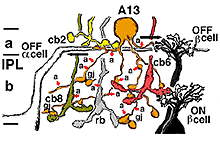
6. A13: a small-field diffuse amacrine cell of the cone system.
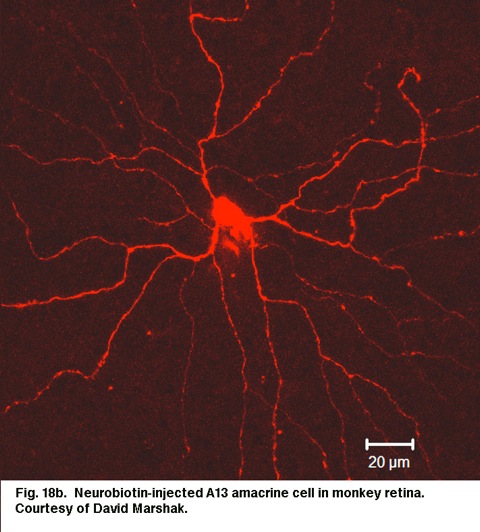
Cell A13 is a diffusely branched cell with a large cell body (12 um diameter) and fine dendrites bearing distinct beads at regular intervals that run through mostly strata 3-5 of sublamina b of the IPL to end up along the top of ganglion cell bodies (Figs 18a and 18b). The complete tree covers a 100-150 um area.
Fig. 18. HRP injected and Golgi appearance of A13 amacrine cells
Fig. 18b. Neurobiotin-injected A13 amacrine cell in monkey retina. Courtesy of David Marshak.
Electron microscopy (not shown) indicates that A13 cells, like A8 cells, get only a minor rod-bipolar-cell (12%) input while three different types of cone bipolar cell have major synaptic input (28%). The cone bipolar inputs are from axons in sublamina a, stratum 3-4 of sublamina b and stratum 5 of sublamina b. A13 cells appear to make reciprocal synapses upon cone bipolar cells and possibly also upon rod bipolar cells. Amacrine cells provide much more synaptic input than bipolar cells (60%). A13 makes synaptic output to OFF-center ganglion cells, of both alpha and beta types, in sublamina a. Gap junctions link A13 cells at their beaded dendrites (Kolb and Nelson, 1996). The summary diagram of A13 is shown in Fig. 19 and in the animation below.
Fig. 19. Wiring pattern of the A13 amacrine cell
Click here to see an animation of the wiring pattern of the A13 amacrine cell
(Quicktime movie)
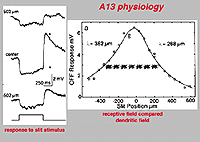
The intracellular response of A13 is a slow potential hyperpolarizing response, looking very like a horizontal cell response in the cat retina. Mixtures of rod and cone signals contributed to A13 cells intracellular response. Its receptive field is large and does not appear to exhibit a surround (Fig. 20). The spatial extent of the receptive field is very suggestive of a coupled electrical syncytium of cells of at least 7 cells wide (Fig. 20) (Kolb and Nelson, 1996).
Fig. 20. Physiology of the A13 amacrine cell
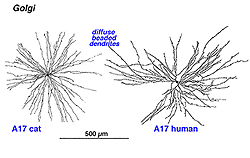
7. A17: the wide-field reciprocal rod amacrine cell.
A17 is a wide-field diffuse amacrine. Its dendritic tree can span close to a millimeter of retinal surface. Its very fine dendrites bear pronounced beads at regular intervals along their length (Fig. 21). The majority of the dendritic tree runs in sublamina b of the IPL, along the top of the ganglion cell layer in stratum 5 as a dense network of fine fibers. Over 1000 beads have been counted on such A17 amacrine cells in the cat retina (Nelson and Kolb, 1985), and, as we shall see later, the beads are the synaptic points where reciprocal synapses with rod bipolar cells occur (Nelson and Kolb, 1985; Sandell et al., 1987).
Fig. 21. Golgi staining of A17 amacrine cells
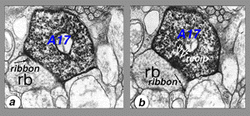
Electron microscopy shows that A17 cells are the predominant reciprocal amacrine cell at the rod bipolar cell axon terminals in sublamina b of the IPL (Fig. 22). In addition, their fine dendrites in sublamina a receive some synapses from the dopaminergic amacrine cell A18 (see section on A18). However, there has not yet been discovered any synaptic output of the A17 except the reciprocal synapses with the rod bipolar axon (Fig. 22, white arrows recip).
Fig. 22. Electron micrograph of reciprocal synapse
A17 cells are driven almost exclusively by rod-matched stimulating conditions. In the intensity series (Fig. 23) using wavelengths of light matched for rods at rod and cone stimulating wavelengths. The responses at each light intensity superimpose, indicating that only a single receptor mechanism is present, that due to the rods. At all intensities the response is a depolarization and up till the highest intensities (Fig. 23, bottom trace) essentially a slow potential response. At the highest light intensity the response becomes slightly transient although there is a depolarizing plateau phase. The response now resembles that of its input neuron, the rod bipolar cell (Dacheux and Raviola, 1986). A17 cells do not appear to have inhibitory surrounds although they exhibit spatially dependent characteristics to their response amplitudes with far surround stimulation (Nelson and Kolb, 1985).
An A17 cell wiring diagram is shown in Figure 23. A17’s huge coverage of the IPL neuropil by its up to a millimeter dendritic spread, allows it to collect scotopic rod signals from several thousand rod bipolar axons. Its high sensitivity to scotopic conditions (rod driven light intensities) suggests that this amacrine plays a role in converging rod signals from huge areas of retina and to amplify them at very low light intensities. A17 is known to accumulate serotonin in rabbit retina (Vaney, 1986) but is thought to be a GABA-ergic neuron in terms of neurotransmission, in all mammalian retinas (Pourcho and Goebel, 1983).
Fig. 23. Summary diagram of the morphology, physiology and
wiring pattern of the A17 amacrine cell
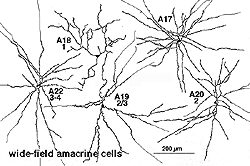
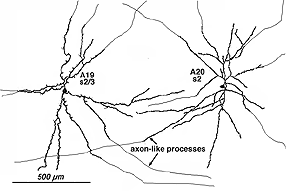
8. A19 and A20: ON-OFF wide-field amacrine cells.
A19 and A20 are similar appearing wide-field radiate amacrine cells with 300-500 um diameter fields. However, their fields are further expanded to several mm by the presence of fine axon-like processes coming either from the ends of tapering dendrites (A19, A20) or from primary dendrites or even from the cell body.
Fig. 24. A19 and A20 amacrine cell subtypes
They differ in stratification levels levels in the IPL though. A19 stratifies on the sublamina a/b border and A20, just above, in stratum 2 of sublamina a. A19 has distinctly spiny dendrites while A20 has long, smooth, spineless but beaded dendrites.
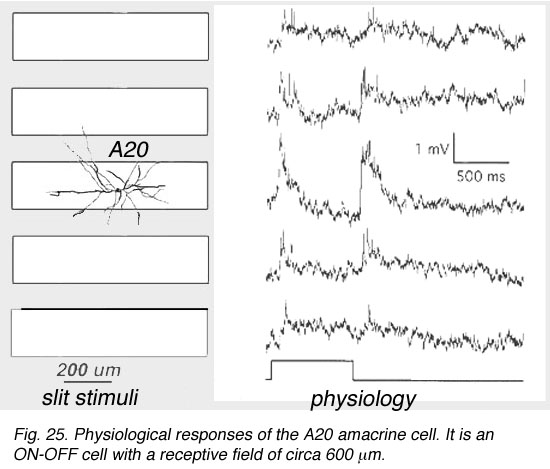
A19 and A20 are both transient depolarizing with responses at ON and at OFF of the light sstimulus. The are thus known as ON-OFF amacrine cells. Such cells have been difficult to record long-term responses from in a mammalian retina (Freed et al., 1996) and the example illustrated in Figure 25 of an A19 cell, is one of the longest held and thus a receptive field could be mapped.
Fig. 25. Physiological responses of the A20 amacrine cell
The receptive fields are large i.e >550 um radius, rather larger than their immediate dendritic tree sizes, and undoubtedly due to their axon-like extensions (Freed et al., 1996). Neither A19 nor A20 exhibited antagonistic surrounds.
Electron microscopy for circuitry of HRP injected A19 and 22 has been performed. A19 receives two types of cone bipolar input (24%): the first is from OFF-center cb2 cone bipolar cells of sublamina a, while the other is ON-center input from cb5 at the sublamina a/b border where cb5 passes into sublamina b (Fig. 26). OFF- and ON-center bipolar input could account for this cell’s ON-OFF physiology. The predominant input is from amacrine cells of sublamina a though (76%). Some of the amacrine inputs could be from rod amacrine AII cells, others from A2 cells while some looked like they could be from other A19 cells (Fig. 26). A19’s output is reciprocal synapses to both types of bipolar (40%), to unknown dark amacrines (40%) and possibly some fine diameter ganglion cell dendrites (20%) (Freed et al., 1996).
Fig. 26. Wiring diagram of the ON-OFF A19 amacrine cell
Gap junctions have been seen in electron microscopy. Gap junctions probably connect A19 dendrites one to another (Fig. 26, A19, gj). A19 is known to be GABA-ergic (Pourcho and Goebel, 1983).
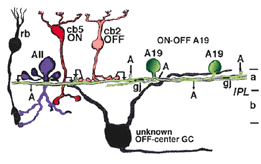
A20 cells, running in upper stratum 2 of sublamina a have slightly different synaptic relationships with other nerve cells in the neuropil, from A19, which will be remembered, sits on the sublamina a/b border (Fig. 26). Thus A20 receives some cone bipolar inputs in stratum 1/2 and 2 (7%) but they are dominated by amacrine cell inputs (93%) (Fig. 27). Much of this input is recognizable as being from lobular appendages of rod AII cells. A20’s output is about equally as reciprocal synapses to bipolar cells (5%) and synapses to amacrine cells (5%), but the cells have major output to OFF-center Alpha ganglion cells of sublamina a (90%) (Fig. 27).
Fig. 27. Wiring diagram of the ON-OFF A20 amacrine cell
Both A19 and A20 cells are probably involved in the transfer of fast messages from one part of the retina to another. They may be the basis of the proximal negative response recordable in the intraretinal ERG and in the shift effect in Y type ganglion cells.
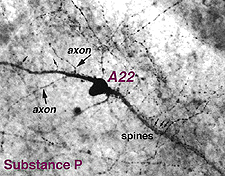
9. A22: A putative substance P containing, ON-OFF neuron of the cone system.
Immunocytochemical staining with antibodies against the neuropeptide substance P (SP) reveal a large-field amacrine cell in human retina (Cuenca et al., 1994). The SP containing amacrine is probably equivalent to a wide-field A22 cell of cat that has been briefly intracellularly recorded as an ON-OFF cell (no EM of A22 was possible)(Freed et al., 1996).
SP-containing and A22 cells are wide-field amacrine cells (dendritic trees of 500 um span), often having large cell bodies (14-16 um) displaced to the ganglion cell layer, with their major dendritic stratification in strata 3 and 4 of sublamina b of the IPL (Fig. 28). Both cells have long axon-like processes that pass up into sublamina a to run for up to a mm and also down into stratum b to run over ganglion cell bodies. Their major dendrites are covered with conspicuous spines (Fig. 28).
Fig. 28. Substance P containing amacrine cell in human retina
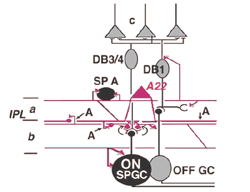
Electron microscopy of substance P immunoreative cells (Cuenca et al., 1994) shows that the dendritic spines are postsynaptic with reciprocal synapses to cone bipolar cells (Fig. 29). They also receive synapses from unidentified amacrine cells and other SP-IR cell dendrites. They are presynaptic to ganglion cell dendrites and directly to ganglion cell bodies in sublamina b (possibly some of the latter are of SP-IR ganglion cells) (Fig. 29).
Their axonal processes are presynaptic to amacrine and ganglion cells in stratum 1 and in stratum 5 of the IPL. The ganglion cell in stratum 1 would be OFF-center. They receive cone bipolar input from a cone bipolar type in stratum 1 of the IPL. Some substance P axons even reach into the OPL and synapse upon bipolar cells (Fig. 29). Almost certainly A22 cells or SP-IR amacrine cells of cat and human are GABA-ergic (Porcho and Goebel, 1988). This ON-OFF cell probably plays a similar role as the A19 and A20 cells in being recordable in the proximal negative response (PNR) (Burkhardt, 1970) and being involved in fast moving aspects of visual coding in the retina.
Fig. 29. Diagram of the organization of the A22 amacrine cell
10. A18: the dopaminergic amacrine cell .
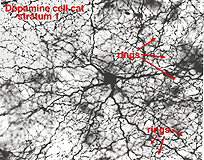
The dopaminergic amacrine cell types have been revealed by immunostaining with an antibody directed against tyrosine hydroxylase (TOH)(the rate limiting enzyme for dopamine), is a wide-field cell that stratifies almost exclusively in stratum 1 of the IPL (under the amacrine cell bodies) (Fig. 30). It has been identified with A18 cells or Type 1CA (CA=catecholamine) of the Golgi studies (Kolb et al., 1981, 1992; Mariani, 1990). Dopamine amacrines also label with glutamate antibodies (Marc, personal communication).
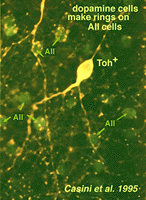
Their dendrites form a densely packed network of processes leaving only few “rings” for other amacrine cell bodies and major dendrites to pass through (Figs. 30 and 31). The rings are beaded dendrites that appear to be synaptic points on amacrine cells, particularly the AII and A8 cells. Another feature of dopamine cells is only seen after dye injection (Dacey, 1990) or immunostaining (Kolb et al., 1990), and this is that their dendrites emit long axon-like processes running in different strata of the IPL, in the ganglion cell layer and some into the outer plexiform layer (OPL).
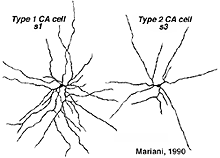
A second type of dopaminergic cell has been described in the monkey and human retinas (Hokoc and Mariani, 1987; Crooks and Kolb, 1992), in the rabbt retina (Tauchi et al., 1990) and in the mouse retina (Zhang et al., 2004; Zhang et al., 2007; Contini et al., 2010). This Type 2 CA cell is described as a “wispy” cell in Golgi studies in monkey (Fig. 32a) (Mariani, 1990). In mouse retina they are characterized by a smaller cell body and have ramifications in stratum 3 of the IPL like the one in the monkey. Interestingly in mouse CAType2 cells do not stain well for TH and are only visible because of genetic labeling for catecholamines (Zhang et al., 2004; Contini et al., 2010). There is also evidence for the two types of dopamine cells from electrophysiology (Zhang et al., 2007). The one is probably the CA Type 1 (The DA cell) and gives ON-sustained responses to light, while the other is probably CA Type 2 and gives ON-transient responses to lighted (Zhang et al., 2007).
Fig. 32. Types of dopaminergic cells
In turtle and fish retinas the equivalent to type 1 CA cells is an ON-center cell. In turtle the cell is tristratified in the inner plexiform layer, and gives a slight ON transient which continues as a sustained depolarization during the light on. At light off, it has a very distinct slow sustained hyperpolarization that lasts for several seconds. The increasing spot sizes bring out the depolarizing ON-plateau while with an annulus, a sustained OFF-surround is evident (see Fig. 33A) (Ammermüller and Kolb, 1995).
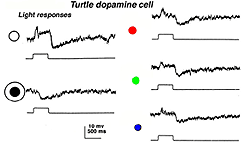 Fig. 33a. Light responses of the dopaminergic amacrine cell in the turtle retina (Ammermuller and Kolb et al. 1995) |
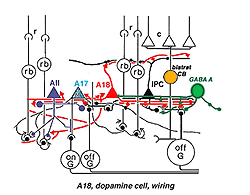 Fig. 33b. Wiring diagram of the A18 amacrine cell |
Synaptic relations of Type 1 CA cells have bee studied by electron microscopy (Kolb et al., 1991). This amacrine makes most of its synaptic arrangements in stratum 1 of the IPL as would be predicted from its branching pattern (see summary Diagram 33b). Sparse cone bipolar input occurs to the cell’s primary dendrites in stratum 1. The cone bipolar input is proposed to be from giant bistratified cone bipolar cells known to be present in cat and monkey retinas and thought to be a blue cone pathway bipolar cell. Both the cell body and primary dendrites in stratum 1 of the IPL also receive GABAergic and glycinergic amacrine cell inputs. It has been suggested (Critz and Marc, 1992) that GABAergic amacrine cells act as intermediaries between OFF center bipolar cells of sublamina a and the dopamine cell, thereby contributing to the dopamine cell’s ON center response (Fig. 33b). The major output of the A18 cell is in the fine network of dendrites and axon terminals surrounding the cell bodies and apical dendrites of AII and A8 cells (Kolb et al., 1991). We also saw by EM (Kolb et al., 1991) that the dopamine cell in the mammal makes ring contacts upon both gaba-ergic A17 and A13 cell bodies in stratum 1 of the IPL (Fig. 33b). Some dopamine cell axons contact AII dendrites in sublamina b of the IPL and the few processes that run into the OPL make synapses upon the GABAergic interplexiform cell (Kolb et al., 1991). The latest evidence from transgenic mouse retinas is that Type 1 CA cells (A18 cells) get ON center sustained bipolar input to the sublamina b processes (Zhang et al., 2007; Contini et al., 2010). Figure 33b is a proposed summary diagram of Type 1 CA cell (A18) and its input and output relationships in the mammalian retina.
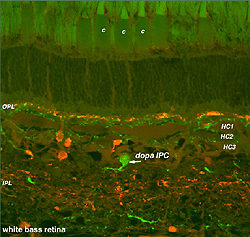
Dopamine cells in the fish retina are true interplexiform cells (IPCs) and have profuse arborations in the inner nuclear layer where they make extensive synapses upon the 3 types of horizontal cell axons and receive synapses from the HC cell bodies (Marshak and Dowling, 1987) (Fig. 34A). In the outer plexiform layer fish dopamine cells make synapses upon photoreceptors. In the inner plexiform layer of the fish, the dopamine IPCs makes some synaptic connections with the Mb (rod-dominated) bipolar axon terminals (Yazulla et al. 2001).
Fig. 34A. The dopaminergic interplexiform cell of the fish retina
Dopamine IPCs are thought to be influential upon the horizontal cells by decreasing their light responsiveness (Hedden and Dowling, 1978) and modulating their spatial extent by uncoupling their gap junctions (Teranishi et al., 1983). Horizontal cells in all retinas are joined by gap junctions in a strong syncytium (Kaneko, 1971; reviewed in Negishi et al., 1990). Dopamine uncouples horizontal cells from the syncytium by interacting with D1 receptors, on the horizontal cell bodies and dendrites, linked to the second messenger cyclic AMP (see Dowling, 1987 and Witkovsky and Dearry 1991 for reviews on dopamine effects in the retina). In the mammalian retina a similar but lesser amount of uncoupling of horizontal cells due to dopamine has been reported (Pflug and Nelson, 1989; Bloomfield, 1993).
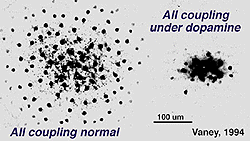
Fig. 34. Effects of dopamine on AII amacrine cell coupling
The dopamine cell is, however, primarily an amacrine cell and not an interplexiform cell, in the mammalian retina, thus their effect on inner retina circuitry would be expected to be more pronounced than on outer retina. We known that ganglion cells have their spiking properties variously altered by dopamine and by agonists and antagonists of dopamine and D1 receptors (Ikeda et al., 1986; Jensen and Daw, 1984, 1986; Thier and Alder, 1984). These neuromodulatory effects are thought to occur via amacrine intermediaries, particularly the AII cell, or by diffusion of dopamine transmitter from a distance to ganglion cell bodies and dendrites because there are no direct contacts of dopamine amacrine cells and ganglion cell dendrites. Both D1 and D2 receptors have been seen upon some ganglion cell bodies (Dearry et al., 1991; Veruki and Wassle, 1995; Wagner et al., 1993). The second effect of dopamine in the inner retina is thought to be upon amacrine cell coupling via gap junctions, somewhat analogous to the effect upon gap junction between horizontal cells in the outer plexiform layer (Fig. 34). Dopamine uncouples AII amacrine cells, in the mammalian retina, mediated through a D1 receptor (see Fig. 34) (Hampson et al., 1992; Vaney, 1994).
11. ACh amacrines: mirror symmetric starburst cells.
Golgi studies and intracellular Lucifer yellow staining, has revealed amacrine cells that are known to use acetylcholine as a neurotransmitter (Masland and Tauchi, 1986). These cells have also been called “starburst” amacrine cells and are particularly striking in appearance in turtle, rabbit and ground squirrel retinas (Figs. 35 and 36) (Famiglietti, 1983; Tauchi and Masland, 1984; Vaney, 1990; Linberg et al., 1996; Cuenca et al., 2003).
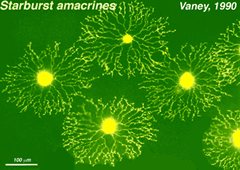 Fig. 35. Starburst amacrine cells in rabbit retina |
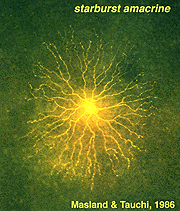 Fig. 36. Lucifer yellow labeling of a starburst amacrine cell |
Both Golgi staining and immunolabelling of ACh amacrines clearly shows that they occur as two mirror symmetric pairs of cells. One type, ACh-a type, has its cell body in the amacrine cell layer and its dendrites stratify in stratum 2 of sublamina a. The other type, ACh-b type, has a cell body displaced to the ganglion cell layer and its dendrites stratify in stratum 4 of sublamina b. ACh-b cells are medium-field in size but have a tremendous overlap of their dendritic trees such that as many as 70 cells overlap a single central cell in peripheral retina (Tauchi and Masland, 1984; Vaney, 1984). ACh-a type cells have slightly larger dendritic tree sizes (13% larger) and their overlap values can reach 90 or more (Vaney, 1990a). The ACh cells in the human retina are similar to those in rabbit, but less dramatic in shape and branching pattern (Fig. 37a). In all retinas studied ACh starburst cells contain GABA inhibitory neurotransmitter as well as the excitatory neurotransmitter acetylcholine (ACh) (Vaney and Young, 1988). Cholinergic starburst amacrine cells are particularly striking in the turtle retina where the mirror symmetric pairs are arranged in an essentially one to one relationship (Fig. 37b) and see movie below.
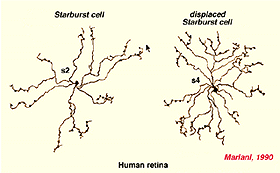 Fig. 37a. Golgi staining of ACh containing amacrine cells in human retina |
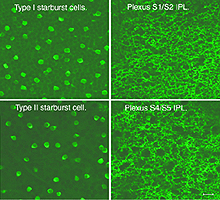 Fig. 37b. Immunioabeling of a starburst amacrine cell with ChAT in turtle whole mount retina. |
Golgi stained ACh containing, starburst amacrine cells of the rabbit retina have been extensively studied by electron microscopy (Famiglietti, 1991; 1992; 2002; 2005). Cone bipolar and amacrine cell inputs are distributed irregularly over the entire dendritic tree, but the proximal dendrites to the cell body, containing small spines, are particularly choice for bipolar input. A small amount of AII amacrine input may occur to the proximal dendrites of the ACh-a type cell. The varicosities on the distal dendrites (Figs. 35 and 36) are the only sites of synaptic output to ganglion cells. The postsynaptic ganglion cells for both ACh-a and ACh-b type cells, are thought to be ON-OFF directional selective bistratified ganglion cells (Amthor et al., 1984, 1989; Famiglietti, 1987, 1991; Tauchi and Masland, 1984; Vaney, 1990, 1994b). Additionally, the monostratified ON-directionally selective ganglion cell may be postsynaptic to the ACh-b type cell (Famiglietti, 1991) but proving the existence of synapses of starburst processes to DS ganglion cells has been problematic by EM techniques. The most recent paper by Briggman and co-authors (2011) using new techniques of blockface EM and 2 photon calcium imaging, has shown that starburst amacrines (ACh amacrines) make very selective synapses upon DS ganglion cells, with an indication of a structural asymmetry of this input depending on the ganglion cell’s preferred direction. Remarkable new experiments in transgenic mice show that the asymmetry of excitatory inputs (via the release of acetlcholine from the cells) and inhibitory inputs from the same starburst cell (via the GABA release synapses) proves to reorganize during deveopment of the retina (Wei et al., 2011; Yonehara et al., 2011). Apparently starburst amacrine cells are excitatory with ACh release early in development of the retina and this release is necessary for developmet of retinal waves. Later in development the starburst cells use inhibitory GABA release to influence directional selectivity in the DS ganglion cells (Zheng et al., 2004; Masland, 2005).
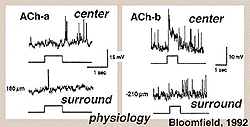
Fig. 38. Intracellular recordings of ACh containing amacrine cells
Both mirror symmetric pairs of starburst cells of the rabbit retina have been intracellularly recorded from and dye-marked by Bloomfield (1992) (Fig. 38). The a-type of the pair is an OFF-center cell giving a transient burst of small spikes at light off while the ACh-b type is an ON-center cell (top traces, Fig. 38). Both types have antagonistic surrounds, (bottom trace, Fig. 38).
A wiring diagram (Fig. 39), adapted from Famiglietti’s findings (1991) shows the proposed interaction of the two types of ACh amacrine cells with bipolars and ganglion cells. They get input from OFF (ACh-a type) or ON cone bipolar cells (ACh-b type) and probably rod bipolar input through the AII amacrine cell. They output to directionally selective ganglion cells of both the ON-type and the bistratified ON-OFF type.
Fig. 39. Wiring diagram of the two types of ACh containing amacrine cells
12. DAPI-3 cells in the rabbit retina.
In 1997, another bistratified medium field amacrine cell that’s nucleus stained with DAPI was revealed by intracellular dye injection of Lucifer yellow. It was given the name DAPI-3 (Wright et al., 1997). Since then the DAPI-3 cell has also been revealed by the fluoresecent rhodamine probe used by MacNeil and Masland (1998) (Fig. 40, right side) and glycine immunoreactivity in the rabbit and groundsquirrel retinas (Zucker and Ehinger, 1998; Cuenca et al., 2002). DAPI-3 cells are medium field in size i.e. have a 100 um field diameter and a profusion of overlapping dendrites as seen in wholemount views (Fig. 40 b) that prove to be the two tiers of branches of a bistratified cell (Fig. 40 d). One of the tiers of dendrites in sublamina a run just below the ACh a type (starburst a type) and the other tier run in sublamina b just above the ACh b type (starburst b type) cells (Fig. 40 d).
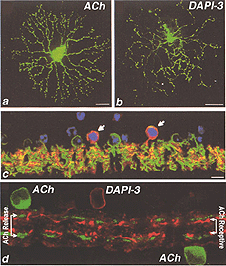 Fig. 40. A comparison between DAPI-3 cells and cholinergic ACh cells (Adapted from Zucker and Ehinger, 2001) |
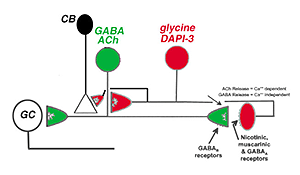 Fig. 41. Wiring diagram of the DAP-3 cell and its relationship to the ACh amacrine cell. |
The DAPI-3 cell proves to be glycinergic and can be stained with the glycine transporter (Fig. 40 c, green). It is also immunopositive for GABAA receptors (Fig. 40 c, red). Thus, the cell appears to be orange in the figure (40 c, red and green combine to make orange stained cells with a blue DAPI nucleus, arrows). The starburst ACh cells stains with CHAT in Fig. 40 d and the overlapping dendrites of the DAPI-3 cell stained for GABAA receptors are juxtaposed to the cholinergic cell’s dendrites.
Zucker and Ehinger’s (1998) nice study on the DAPI-3 cells and immunostaining with the glycine and GABAA transporter/receptors allowed them to draw a summary diagram of the probably manner on which the DAPI-3 amacrine cells interact with the starburst ACh cells (Fig. 41). The DAPI-3 cell is acetylcholine receptive at nicotinic and muscarinic synapses and feeds back upon the starburst cell via a glycinergic synapse (glycine receptors have been identified on starburst cells). The DAPI-3 cell also contacts the cone bipolar cell that has input to the starburst cell. We know the starburst cell contains GABA in addition to acetylcholine and the starburst cell can presumably feed forward to the DAPI-3 cell at a GABAergic synapse with GABAA receptors on the DAPI-3 cells. The starburst cell is known to synapse upon a directionally selective ganglion cell type in rabbit retina (Fig. 41). It is not clear yet whether the DAPI-3 cell exists in other mammals that do not have as pronounced a visual streak as does the rabbit.
13. Midget system amacrine cell.
The calcium binding protein calretinin is found in three types of amacrine cell in the monkey and human retinas (Kolb et al., 2002). The most numerous population are the AII amacrine cells but a small- to medium-field diffuse amacrine cell and a large-field, stratified A19 type are also calretinin-immunoreactive (IR) (Fig. 42). Of these non-AII cell types, the small diffuse (or tristratified cell type, difficult to know which) is of particular interest.
Its appearance is shown in figure 42 (A, arrow) in immunostained slice preparations. The cell has a large cell body but unlike the AII cells (Fig. 42, AII) many fine dendrites are given off the cell body instead of the typical AII thick primary dendrite. The dendrites pass down to all levels of the IPL as thin, beaded and branched processes (Fig. 42, fine arrows). This small-field diffuse calretinin-IR cell is found in high numbers in the foveal, rod-free area of the retina, and in lesser numbers in the peripheral retina. Electron microscopic examination of foveal diffuse calretinin-IR cells has shown that they get synaptic input from ON- and OFF- midget bipolar terminals, reciprocate synapses to these bipolars and have synaptic output to ON center midget ganglion cells (Fig. 43, wiring diagram). In more central to peripheral retina where rods are present, the diffuse calretinin-IR amacrines are probably also in synaptic interplay with both diffuse bipolar cells and larger parasol ganglion cells (Fig. 43). Because these calretinin-IR amacrines are found in the fovea and have particular relationships with the midget bipolar and ganglion cell systems, we have suggested that they may have a role in the antagonistic surround generation for midget ganglion cells. Moreover, they could also contribute to color opponency in these ganglion cells (Kolb et al., 2002).
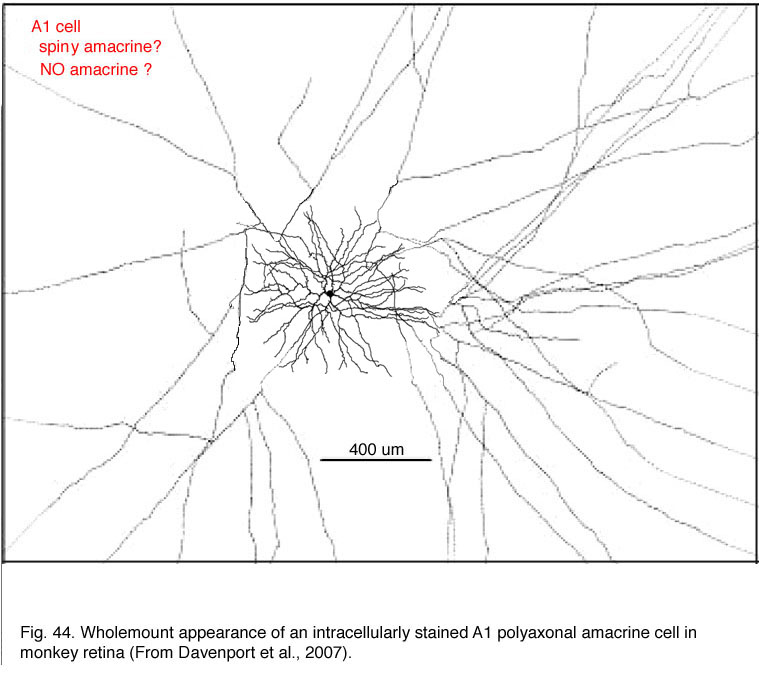
14. Spiny Polyaxonal A1 Cell.
The polyaxonal amacrine cell type described in primate retinas (Dacey, 1989; Stafford and Dacey, 1997; Davenport et al., 2007) is probably the same cell that has been described as a “spiny” amacrine cell type in Golgi studies (Mariani, 1990; Kolb et al., 1992). The cells shown by Dacey and co-authors were revealed by more sophisticated and complete intracellular staining techniques than the Golgi techniques, and thus the spectacular axonal field has been more completely stained. The cell is described as diffuse (Dacey, 1989; Stafford and Dacey, 1997; Davenport et al., 2007) although Golgi techniques suggested the major part of the dendritic field was running down to stratum 3 of the IPL where it branched into a spine-bearing radiate overlapping dendritic tree of circa 400 um diameter (Fig. 44). From the dendrites fine axons emerge bearing varicosities and radiate out for up to 4 mm of coverage of the IPL (Fig. 44). Neurobiotin injection shows that A1 cells are extensively coupled to neighboring A1 amacrine cells, other amacrine cell types, and to a ganglion cell type. This amacrine cell type is very similar to an amacrine staining for nitric oxide content with NADPH-diaphorase in monkey retina (see chapter on neurotransmitters).
These amacrine cells have been intracellulary recorded from and prove to have ON-OFF transient responses to light and generate action potentials in the radiating axonal field. They have an antagonistic center surround organization, the ON component of the center can be blocked by L-AP4 (an ON bipolar cell antagonist). The action potentials in the axons can be blocked by TTX (calcium transient blocker) (Davenport et al., 2007). So this amacrine cell is thought to receive input to the dendrites from ON and probably OFF bipolar pathways and propogate inhibitory output to other parts of the retina via the large axonal field of influence (Davenport et al., 2007).
12. References.
Ammermüller J, Kolb H. The organization of the turtle inner retina. I. The organization of on- and off-center pathways. J Comp Neurol. 1995;358:1–34.[PubMed]
Amthor FR, Oyster CW, Takahashi ES. Morphology of on-off direction-selective ganglion cells in the rabbit retina. Brain Res. 1984;298:187–190.[PubMed]
Amthor FR, Takahashi ES, Oyster CW. Morphologies of rabbit retinal ganglion cells with complex receptive fields. J Comp Neurol. 1989;280:97–121.[PubMed]
Anderson, J.R, Jones, B.W., Warr, C.B., Shaw, M.V., Yand, Y-W., DeMill, J., Lauritzen, J.S., Lin, Y-H., Rapp, K.D., Mastronarde, D., Koshevoy, P. Grimm, B., Tasdizen, T., Whitaker, R. and Marc, R.E. (2011) Exploring the retinal connectome. Mol. Vis. 17, 355-379. [PubMed]
Bloomfield SA. Relationship between receptive and dendritic field size of amacrine cells in the rabbit retina. J Neurophysiol. 1992;68:711–725. [PubMed]
Bloomfield SA, Xin D, Persky SE. A comparison of receptive field and tracer coupling size of horizontal cells in the rabbit retina. Vis Sci. 1995;12:985–999.[PubMed]
Briggman KL, Helmstaedter M and Denk W (2011) Wiring specificity in the direction-selective circuit of the retina. Nature 471, 183-8.
Bordt AS, Hoshi H, Yamada ES, Perryman-Stout WC and Marshak, DW. Synaptic input to OFF parasol ganglion cells in macaque retina. (2006) J. Comp. Neurol. 498, 46-57. [PubMed]
Boycott BB, Kolb H. The horizontal cells of the rhesus monkey retina. J Comp Neurol. 1973;148:115–140. [PubMed]
Burkhardt DA. Proximal negative response of frog retina. J Neurophysiol. 1970;33:405–420. [PubMed]
Cajal SR. In: Thorpe SA, Glickstein M., translators. 1892. The structure of the retina. Springfield (IL): Thomas; 1972.
Casini G, Rickman DW, Brecha NC. AII amacrine cell population in the rabbit retina identified by parvalbum in immunoreactivity. J Comp Neurol.1995;356:132–142. [PubMed]
Contini M, Lin B, Kobayashi K, Okano H, Masland RH and Raviola E. (2010) Synaptic input of ON-bipolar cells onto the dopaminergic neurons of the mouse retina. J Comp Neurol, 518, 2035-50.
Critz SD, Marc RE. Glutamate antagonists that block hyperpolarizing bipolar cells increase the release of dopamine from turtle retina. Vis Neurosci.1992;9:271–278. [PubMed]
Crooks J, Kolb H. Localization of GABA, glycine, glutamate and tyrosine hydroxylase in the human retina. J Comp Neurol. 1992;315:287–302. [PubMed]
Cuenca N, De Juan J, Kolb H. Substance P-immunoreactive neurons in the human retina. J Comp Neurol. 1995;356:491–504. [PubMed]
Cuenca N, Deng P, Linberg K, Lewis GP, Fisher SK, Kolb H. The neurons of the ground squirrel retina as revealed by immunostaining for calcium binding proteins and neuro transmitters. J Neurocytol. 2002; 31:649–666. [PubMed]
Cuenca N, Deng P, Linberg K, Fisher SK, Kolb H. Acetylcholine is found in a second type of amacrine cell in the ground squirrel retina. Brain Res.2003; 964:21–30. [PubMed]
Dacey DM (1989) Axon-bearing amacrine cells of the macaque monkey retina. J. Comp. Neurol 284, 275-293.
Dacey DM. The dopaminergic amacrine cell. J Comp Neurol. 1990;301:461–489. [PubMed]
Dacheux RF, Raviola E. The rod pathway in the rabbit: a depolarizing bipolar and amacrine cell. J Neurosci. 1986;6:331–345. [PubMed]
Davenport CM, Detwiler PB and Dacey DM (2007) Functional polarity of dendrities and axons of primate A1 amacrine cells. Vis. Neurosci. 24, 449-57.
Daw NW, Jensen RJ, Brunken WJ. Rod pathways in mammalian retinae. Trends Neurosci. 1990;13:110–115. [PubMed]
Dearry A, Falardeau P, Shores C, Caron MG. D2 dopamine receptors in the human retina: cloning of cDNA and localization of mRNA. Cell Mol Neurobiol.1991;11:437–453. [PubMed]
Dowling JE, Boycott BB. Organization of the primate retina: electron microscopy. Proc R Soc Lond B Biol Sci. 1966;166:80–111. [PubMed]
Dowling JE. . The retina: an approachable part of the brain. Cambridge (MA): The Belknap Press, Harvard University Press. 1987.
Famiglietti EV, Kolb H. A bistratified amacrine cell and synaptic circuitry in the inner plexiform layer of the retina. Brain Res. 1975;84:293–300. [PubMed]
Famiglietti EV, Kolb H. Structural basis for ON- and OFF-center responses in retinal ganglion cells. Science. 1976;194:193–195. [PubMed]
Famiglietti EV. Starburst” amacrine cells and cholinergic neurons: mirror-symmetric ON and OFF amacrine cells of rabbit retina. Brain Res. 1983;261:138–144. [PubMed]
Famiglietti EV. Starburst amacrine cells in cat retina are associated with bistratified, presumed directionally selective, ganglion cells. Brain Res.1987;413:404–408. [PubMed]
Famiglietti EV. Synaptic organization of starburst amacrine cells in rabbit retina: analysis of serial thin sections by electron microscopy and graphic reconstruction. J Comp Neurol. 1991;309:40–70. [PubMed]
Famiglietti EV (1992) Dendritic co-stratification of ON and ON-OFF directionally selective ganglion cells with starburst amacrine cells in rabbit retina J Comp Neurol 324, 322-35. [PubMed]
Famiglietti EV (2002) A structural basis for omnidirectional connections between starburst amacrine cells and directionally selective ganglion cells in rabbit retina, with associated bipolar cells. Vis. Neurosci 19 145-162. [PubMed]
Famiglietti EV (2005) Synaptic organization of complex ganglion cells in rabbit reina: type and arrangement of inputs to directionally selective and local edge-detector cells. J Comp Neurol 484, 357-91. [PubMed]
Field GD, Greschner M, Gauthier JL, Rangel C, Shlens J, Sher A, Marshak DW, Litke AM, and Chichilnisky EJ (2009) High sensitivity rod photoreceptor input to the blue-yellow color opponent pathway in macaque retina. Nature Neuroscience 12, 1159 – 1164. [PubMed]
Freed MA, Pflug R, Kolb H, Nelson R. ON-OFF amacrine cells in cat retina. J Comp Neurol. 1996;364:556–566. [PubMed]
Hampson ECGM, Vaney DI, Weiler R. Dopaminergic modulation of gap junction permeability between amacrine cells in mammalian retina. J Neurosci.1992;12:4911–4922. [PubMed]
Hedden WL, Dowling JE. The interplexiform cell system. II. Effects of dopamine on goldfish retinal neurones. Proc R Soc Lond B Biol Sci. 1978;201:27–55. [PubMed]
Hokoc JN, Mariani AP. Tyrosine hydroxylase immunoreactivity in the rhesus monkey retina reveals synapses from bipolar cells to dopaminergic amacrine cells. J Neurosci. 1987;7:2785–2793. [PubMed]
Ikeda H, Priest TD, Robbins J, Wakakuwa K. Silent dopaminergic synapses at feline retinal ganglion cells? Clin. Vis Res. 1986;1:25–38.
Jensen RJ, Daw NW. Effects of dopamine antagonists on receptive fields of brisk cells and directionally selective cells in the rabbit retina. J Neurosci.1984;4:2972–2985. [PubMed]
Jensen RJ, Daw NW. Effects of dopamine and its agonists and antagonists on the receptive field properties of ganglion cells in the rabbit retina.Neuroscience. 1986;17:837–855. [PubMed]
Kaneko A. Elecrical connections between horizontal cells in the dog fish retina. J Physiol. 1971;213:95–105. [PubMed] [Free Full text in PMC]
Karten HJ, Brecha N. Localization of substance P immunoreactivity in amacrine cells of the retina. Nature. 1980;283:87–88. [PubMed]
Kidd M. Electron microscopy of the inner plexiform layer of the retina in the cat and the pigeon. J Anat. 1962;96:179–187. [PubMed]
Klump KE, Zhang AJ, Wu SM, Marshak DW (2009) Parvalbumin-immunoreactive amacrine cells of macaque retina. Vis Neurosci. 26):287-96.
Kolb H., Famiglietti E. V. Rod and cone pathways in the inner plexiform layer of the cat retina. Science. 1974;186:47–49. [PubMed]
Kolb H. The inner plexiform layer in the retina of the cat: electron microscopic observations. J Neurocytol. 1979;8:295–329. [PubMed]
Kolb H, Nelson R, Mariani A. Amacrine cells, bipolar cells and ganglion cells of the cat retina: a Golgi study. Vision Res. 1981;21:1081–1114. [PubMed]
Kolb H, Nelson R. Neural architecture of the cat retina. Prog Ret Res. 1984;3:21–60.
Kolb H, Cuenca N, Wang HH, Dekorver L. The synaptic organization of the dopaminergic amacrine cell in the cat retina. J Neurocytol. 1990;19:343–366.[PubMed]
Kolb H, Cuenca N, DeKorver L. Post embedding immunocytochemistry for GABA and glycine reveals the synaptic relationships of the dopaminergic amacrine cell of the cat retina. J Comp Neurol. 1991;310:267–284. [PubMed]
Kolb H, Linberg KA, Fisher SK. The neurons of the human retina: a Golgi study. J Comp Neurol. 1992;318:147–187. [PubMed]
Kolb H, Nelson R. Off-alpha and off-beta ganglion cells in the cat retina. II. Neural circuitry as revealed by electron microscopy of HRP stains. J Comp Neurol. 1993;329:85–110. [PubMed]
Kolb H, Nelson R. Hyperpolarizing, small-field amacrine cells in cone pathways of cat retina. J Comp Neurol. 1996;371:415–436. [PubMed]
Kolb H., Zhang L., DeKorver L, Cuenca N. A new look at calretinin-immunoreactive amacrine cell types in the monkey retina. J Comp Neurol.2002;453:168–184. [PubMed]
Lee SC, Meyer A, Schubert T, Hüser L, Dedek K, Haverkamp S. (2015) Morphology and connectivity of the small bistratified A8 amacrine cell in the mouse retina. J Comp Neurol., 523(10): 1529-47. [PubMed]
Linberg KA, Suemune S, Fisher SK. Retinal neurons of the Californian ground squirrel, Spermophilus beecheyi: a Golgi study. J Comp Neurol.1996;365:173–216. [PubMed]
Mariani AP. Amacrine cells of the rhesus monkey retina. J Comp Neurol. 1990;301:382–400. [PubMed]
MacNeil MA, Masland RH. Extreme diversity among amacrine cells: implications for function. Neuron. 1998;20:971–982. [PubMed]
Marshak, DW and Dowling, JE. (1987) Synapses of cone horizontal cell axons in goldfish retina. J Comp Neurol. 256, 430-43. Masland, R.H.and Tauchi, M. (1988) The cholinergic amacrine cells. TINS, 9 : 218-223.
Masland RH, Tauchi M. The cholinergic amacrine cells. Trends Neurosci. 1988;9:218–223.
Negishi K, Teransihi T, Kato S. The dopamine system of the teleost fish retina. Prog Ret Res. 1990;9:1–48.
Nelson R, Famiglietti EV, Kolb H. Intracellular staining reveals different levels of stratification for on- and off-center ganglion cells in cat retina. J Neurophysiol. 1978;41:472–483. [PubMed]
Nelson R. AII amacrine cells quicken the time course of rod signals in the cat retina. J Neurophysiol. 1982;47:928–947. [PubMed]
Nelson R, Kolb H. Synaptic patterns and response properties of bipolar and ganglion cells in the cat retina. Vision Res. 1983;23:1183–1195. [PubMed]
Nelson R, Kolb H. A17: a broad-field amacrine cell of the rod system in the retina of the cat. J Neurophysiol. 1985;54:592–614. [PubMed]
Neumann S. and Haverkamp S. Synaptotagmin-2 labels a small bitratified amacrine cell in the macaque retina. J. Comp. Neurol. 2012. Jul 23. [PubMed]
Pflug R, Nelson R. Dopaminergic effects on response kinetics of rabbit and cat horizontal cells Invest Ophthalmol Vis Sci 1989. 30Suppl.18.
Polyak SL. (1941) The retina. Chicago: University of Chicago.
Pourcho RG, Goebel DJ. Neuronal subpopulations in cat retina which accumulate the GABA agonist (3H) muscimol: a combined Golgi and autoradiographic study. J Comp Neurol. 1983;219:25–35. [PubMed]
Pourcho RG, Goebel DJ. A combined Golgi and autoradiographic study of 3(H) glycine-accumulating amacrine cells in the cat retina. J Comp Neurol.1985;233:473–480. [PubMed]
Pourcho RG, Goebel DJ. Co-localization of substance P and GABA in amacrine cells of the cat retina. Brain Res. 1988;447:164–168. [PubMed]
Sandell JH, Masland RH, Raviola E, Dacheux RF. Connections of indole amine-accumulating cells in the rabbit retina. J Comp Neurol. 1989;283:303–313.[PubMed]
Smith RG, Vardi N. Simulation of the AII amacrine cell of mammalian retina: functional consequences of electrical coupling and regenerative membrane potentials. Vis Neurosci. 1995;12:851–860. [PubMed]
Stafford DK and Dacey DM (1997) Physiology of the A1 amacrine; A spiking, axon-bearing interneuron of the macaque monkey retina. Vis. Neurosci. 14, 507-522.
Strettoi E, Raviola E, Dacheux RF. Synaptic connections of the narrow-field, bistratified rod amacrine cell (AII) in the rabbit retina. J Comp Neurol.1992;325:152–168. [PubMed]
Stell WK, Witkovsky P. Retinal structure in the smooth dogfish, Mustelus canis: light microscopy of photoreceptor and horizontal cells. J Comp Neurol.1973;148:33–46. [PubMed]
Tauchi M, Masland RH. The shape and arrangement of the cholinergic neurons in the rabbit retina. Proc R Soc Lond B Biol Sci. 1984;223:101–119.[PubMed]
Tauchi M, Madigan NK and Masland RH (1990) Shapes and distributions of catecholamine-accumulating neurons in the rabbit retina. J Comp Neurol, 293, 178-89.
Teranishi T, Negishi K, Kato S. Dopamine modulates S-potential amplitude and dye-coupling between external horizontal cells in carp retina. Nature.1983;301:243–246. [PubMed]
Thier P, Alder V. Action of iontophoretically applied dopamine on cat rerinal ganglion cells. Brain Res. 1984;292:109–121. [PubMed]
Vaney DI. The mosaic of amacrine cells in the mammalian retina. Prog Ret Res. 1990;9:49–100.
Vaney DI, Gynther IC, Young HM. Rod-signal interneurons in the rabbit retina: 2. AII amacrine cells. J Comp Neurol. 1991;310:154–169. [PubMed]
Vaney DI. The morphology and topographic distribution of AII amacrine cells in the cat retina. Proc R Soc Lond B Biol Sci. 1985;224:475–488. [PubMed]
Vaney DI. Morphological identification of serotonin-accumulating neurons in the living retina. Science. 1986;233:444–446. [PubMed]
Vaney DI. Patterns of neuronal coupling in the retina. Prog Ret Res. 1994;13:301–389.
Vaney DI. Territorial organization of direction-selective ganglion cells in rabbit retina. J Neurosci. 1994;14:6301–6316. [PubMed]
Veruki ML, Wässle H. Localization and function of dopamine D1 receptors in rat retina. Soc Neurosci Abstr. 1995;21:900.
Voigt T, Wässle H. Dopaminergic innervation of AII amacrine cells in mammalian retina. J Neurosci. 1987;7:4115–4128. [PubMed]
Wagner H-J, Luo B-G, Ariano MA, Sibley DR, Stell WK. Localization of D2 dopamine receptors in vertebrate retinae with anti-peptide antibodies. J Comp Neurol. 1993;331:469–481. [PubMed]
Wässle H, Grünert U, Chun M-H, Boycott BB. The rod pathways of the macaque monkey retina: identification of AII-amacrine cells with antibodies against calretinin. J Comp Neurol. 1995;361:537–551. [PubMed]
Wässle, H., Heinze, L., Ivanova, E., Majumdar, S., Weiss, J., Harvey, R.J. and Haverkamp, S. (2009) Glycinergic transmission in the mammalian retina . Frontiers in Molecular Neuroscience, www.frontiersin.org.
Wei W, Hamby AM, Zhou K and Feller MB (2011) Development of asymmetric inhibition underlying direction selectivity in the retina. Nature 469, 402-6.
Witkovsky P, Dearry A. Functional roles of dopamine in the vertebrate retina. Prog Ret Res. 1991;11:247–292.
Wright LL, Macqueen CL, Elston GN, Young HM, Pow DV, Vaney DI. The DAPI-3 amacrine cells of the rabbit retina. Vis Neurosci. 1997;14:473–492.[PubMed]
Yazulla S, Studholme KM, Fan S-F, Mora-Ferrer C. Neuromodulation of voltage-dependent K+ channels in bipolar cells: immunocytochemical and electrophysiological studies. In: Kolb H, Ripps H, Wu S, editors. Concepts and challenges in retinal biology: a tribute to John E. Dowling. 2001. p. 201-213.
Yonehara K, Balint K, Noda M, Nagel G, Bamberg E and Roska B (2011) Spatially asymmetric reorganization of inhibition establishes a motion sensitive circuit. Nature 469 407-10.
Zrenner E. The physiological basis of the pattern electroretinogram. Prog Ret Res. 1990;9:427–464.
Zhang DQ, Stone JF, Zhou T, Ohta H and McMahon DG (2004) Characterization of genetically labeled catecholamine neurons in the mouse retina. Neuroreport 15, 1761-5.
Zhang DQ, Zhou TR and McMahon DG (2007) Functional heterogeneity of retinal dopaminergic neurons underlying their multiple roles in vision. J Neurosci 27, 692-99.
Zheng JJ, Lee S, Zhou ZJ. (2004) A developmental switch in the excitability and function of the starburst network in the mammalian retina. Neuron. 44, 851-64.
Zucker CL, Ehinger BE. Distribution of GABAA receptors on a bistratified amacrine cell type in the rabbit retina. J Comp Neurol. 1998;393:309–319.[PubMed]
Zucker CL, Ehinger BE. Complexities of retina circuitry revealed by neurotransmitter receptor localization. In: Kolb H, Ripps H, Wu S, editors. Concepts and challenges in retinal biology: a tribute to John E. Dowling. Progress in Brain Research, Vol. 131. 2001. p. 71-81.
Last Updated: October 15, 2015.