Helga Kolb
1. General characteristics.
Over the last few years, psychophysicists, electrophysiologists, geneticists and anatomists have concluded that there is something unique about the short wavelength system compared with the two longer wavelength systems in the visual system.
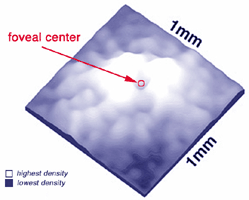
There are differences in the genetic structure and locus of the S-cone visual pigment compared with the L- and M-cone pigments (Nathans et al., 1986), yet the S-cones are common to all vertebrate retinas and always form a consistent 8-10% of the cone photoreceptor population (Marc, 1982; Kolb and Lipetz, 1991).The S-cones are however, very few in the fovea center so causing a so-called S-cone blind spot (Williams et al., 1981) but they peak in number on the foveal slope at about 12% of the population (Fig. 2).
The short wavelength system has a lower spatial and temporal resolution than the other two cone systems (Stockman et al., 1991; Humanski and Wilson, 1992) but is probably the only system to truly carry color information through the retina (Rodieck, 1991; Gouras 1992).
The pathways for transmitting information from the short wavelength cones to ganglion cells appears to be different from the midget pathways for the medium and long wavelength cones. As we have discussed in a previous chapter, the latter two chromatic pathways are via midget bipolar and midget ganglion cells connections related to a single spectral type of cone, either L- or M-cones.
2. Blue cones.
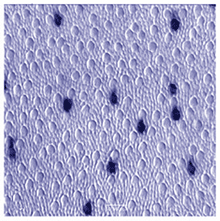
Of the three spectral types of cone found in the normal human retina, only the S-cone or blue cone can be distinguished from the others in the mosaic. Using special antibodies generated against cone opsins, which are the visual pigments contained within the cone, it is possible to selectively stain the short wavelength sensitive pigment- (or blue pigment) bearing cones (Fig. 3) (Szell et al., 1988; Ahnelt and Kolb, 2000).
Before the development of the antibody though, the S-cones were recognizable by staining with fluorescent dyes like Lucifer yellow (DeMonesterio et al. 1986) or from careful quantitative light microscopy of monkey and human retinas (Ahnelt et al., 1987).
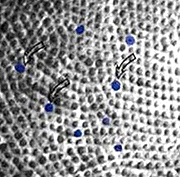
They are distinguished from other cones in the fovea by their larger inner segment diameter and their occurrence in a different mosaic than the the more numerous hexagonally packed L- and M-cones. Thus the S-cones seem to break up the regular hexagonal array into small distorted patches of the other cones (Fig. 1).
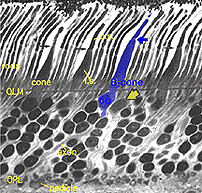 Fig. 4. Blue cone in a vertical section through human retina |
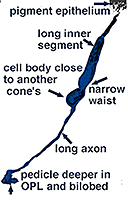 Fig. 5. Schematic drawing of a blue cone |
In vertical sections of human retina the S-cone is recognized by having a longer inner segment that sticks further into subretinal space than neighboring cones (Fig. 4). It also has a narrower “waist” at the outer limiting membrane than neighboring cones and often sits adjacent to another cone cell body without intervening rods. Pedicles belonging to S-cones project deeper into the outer plexiform layer (OPL) and have a smaller or unusual shaped pedicle compared with other cone pedicles (Figs. 4 and 5) (Ahnelt and Kolb, 1994; Kolb et al.,1997).
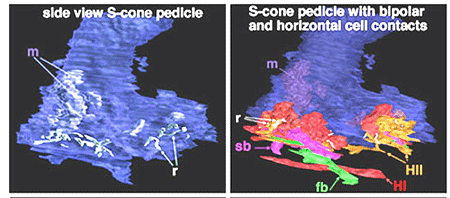
In peripheral retina. S-cone pedicles are smaller than M- and L-cone pedicles (Ahnelt et al., 1990), and sometimes in peripheral retina, they are bilobed in shape, with synaptic invaginations and ribbons separated to the two lobes (Kolb et al., 1997) (Fig. 6). They do not exhibit the long telodendria typical of other cone pedicles so they remain rather isolated from gap junction contacts with L- or M-cones at the level of the outer plexiform layer (Kolb et al., 1997).
Fig. 6. 3D computer reconstructions of an S-cone pedicle
3. S-cone bipolar cell.
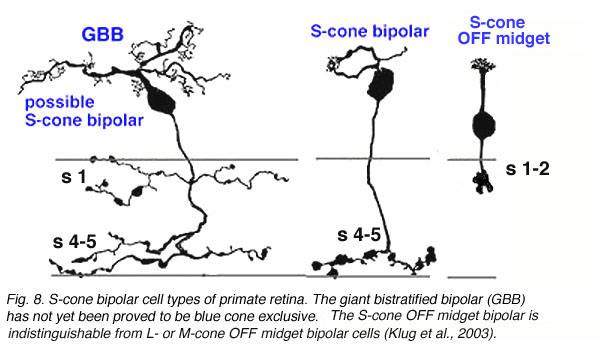
The S-cone pedicle is primarily contacted by a special S-cone bipolar cell, that was originally described by Mariani (1984) and shown later to be selectively stainable with antibodies to the peptide cholecystokinin (Kouyama and Marshak,1992). This bipolar is not like other midget bipolar cells, although in the central retina it is related mostly to a single S-cone (Kouyama and Marshak,1992). It differs primarily in its axonal ending in the inner plexiform layer, from the regular midget bipolar cells that contact M- and L-cones. The S-cone bipolar axon ends deep in the IPL with a long ranging terminal that runs amongst the rod bipolar axon terminals in stratum 5 (Fig. 7). Blue cone midget bipolar cells and associated midget ganglion cells have now also been discovered (Klug et al., 2003) (Fig. 7).
Blue cone specific diffuse bipolar cells are found in most mammalian retinas in both ON and OFF center varieties (rabbit retina; Liu and Chow, 2007) and (mouse; Haverkamp et al., 2005).
The wide axoned ON S-cone bipolar cell in primates makes invaginating ribbon junctions with the cone pedicle (Fig. 6, violet profile, sb; Fig. 7, S-cone bipolar). A small number of additional fine bipolar dendrites make basal junction contacts away from the ribbons (Fig. 6. green profile, fb), and may belong to some other kind of diffuse bipolar type, possibly one called GBB: a giant bistratified type (Fig. 7) (Mariani, 1983). In general though, diffuse bipolar cells in primate are biased against S-cones making very few contacts or even avoiding S-cones in their dendritic trees (Lee and Grunert, 2007). The newly described OFF S-cone midget bipolar makes many basal junction with the blue cone pedicle and these terminals appear to be located near the triad ribbon (Klug et al., 2003). The HII horizontal cells make many of the lateral element contacts with the S-cone in both lobes (Fig. 6, orange profiles, HII) and a fewer number of rather larger lateral elements are from HI cells (Fig. 6, red profiles, HI) (Ahnelt and Kolb, 1994).
4. S-cone horizontal cell
Originally there was thought to be only one type of horizontal cell in the primate retina; a long-axoned cell type that connected with cones at its dendrites and rods at its axonal endings (Polyak, 1941, Boycott and Dowling, 1969; Kolb, 1970). However, in 1980 a second type of horizontal cell was described (Kolb et al., 1980) and proposed to be more concerned with S-cones than the original long-axoned H1 type. The H2 cell was distinguished on its bushy dendritic tree with fine irregular dendrites and without clear clusters destined for cones (Fig. 8a, compare H1 and H2 cells morphology). Electron microscopy finally showed that the H2 type of horizontal cell indeed sent many dendritic processes to the few S-cones in its dendritic field and lesser concentrations of processes to overlying M- and L-cones (Fig. 8b). The short axons of these HII cells contact S-cones exclusively (Fig. 8b) (Ahnelt and Kolb, 1994). Intracellular recordings from H2 horizontal cells in monkey retina have proved conclusively that this horizontal cell is blue sensitive and an important element of the S-cone pathway in the primate retina (Dacey et al., 1996).
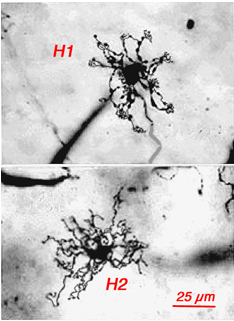 Fig. 8a. Comparisons of HI and HII cells in primate retina. Golgi staining |
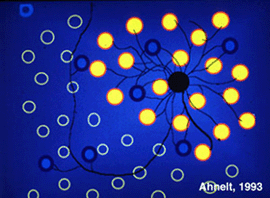 Fig. 8b. Schematic of the S-cone specific HII horizontal cell in primate retina |
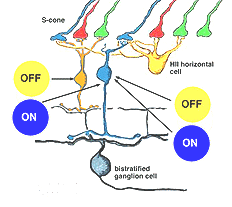
It has been demonstrated that horizontal cells produce negative feedback to cones (Baylor et al., 1971; Fuortes and Simon, 1974; Burkhardt, 1993) and are engaged in center surround generation in bipolar cells, and color opponent responses in horizontal cells of animals with good color vision (Fourtes and Simon 1974). In the case of the H1 and H2 horizontal cells of the primate retina. it has been difficult to demonstrated color opponency in intracellular recording and dye-markings (Dacey et al., 1996) although anatomically we know there is color selectivity of their contacts (Ahnelt and Kolb, 1994). Dacey and coauthors (1996) concluded that H2 cells, which of course make contact with L- and M-cones too, but are primarily concerned with S-cones, do not engage in feedback signals with the longer wavelength cones. However, now it has been shown that there is blue-yellow opponency in blue cones themselves (Packer et al., 2010). It seems likely then, that HII cells feed-back yellow (L + M cone sensitivity) to their own S-cones, thereby forming opponency in the S-cone bipolar cells. This should result in an opponent S-cone bipolar cell S-ON, L-/M-OFF (blue ON/yellow OFF) channel that is reflected in the blue-yellow ganglion cell receptive field (Fig. 9). This has indeed been shown to be the case in the recent findings of Crook and coworkers (Crook et al., 2009). An OFF yellow signal could also be provided by OFF center diffuse bipolar cell types (Fig. 9).
Fig. 9. Neurons of the S-cone pathways
5. S-cone ganglion cell.
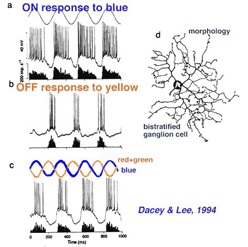
Early electrophysiological investigation of monkey retinal ganglion cells indicated that blue/yellow opponency was carried primarily by a S-cone ON center ganglion cell type with a much larger receptive field center than is typical of the L- or M-cone color and spatially opponent midget ganglion cells (Gouras 1984). Interestingly there are few if any recordings of the opposite type of ganglion cell i.e. yellow-ON and blue-OFF. There are now good morphophysiological studies that show the morphologies of several blue cone ganglion cells (Dacey et al., 2003). The best characterized is the small-field bistratified cell that is blue ON and yellow OFF (see below) (Dacey and Lee, 1994).
The morphology of the blue ON/yellow OFF ganglion cell has been known since Dacey and Lee (1994) made intracellular recordings and staining of these ganglion cells in monkey retina. It turns out to be a relatively small-field bistratified ganglion cell (Dacey, 1993) with its major dendritic branching in stratum 5 of the IPL and a smaller tier of dendrites in stratum 1 of the IPL (Fig. 10 d). It projects to the koniocellular layers of the LGN (Martin et al., 1997; Roy et al., 2009). As can be seen (Fig. 10 a) it gives ON responses to blue light stimulation and OFF responses to yellow (Fig. 10 b). There appears to be some disagreement over whether this blue-yellow ganglion cell has a spatially and color opponent surround (Field et al., 2007) or a coextensive ON blue and OFF yellow receptive field (Gouras, 1968; Crook et al., 2009). Field and co-authors (2007) claim that the center is pure blue ON and the yellow surround from L and M cones is probably not formed by OFF cone bipolar input because it is eliminated by APB (an ON bipolar cell pathway agonist). We look forward to new findings that might solve this disagreement.
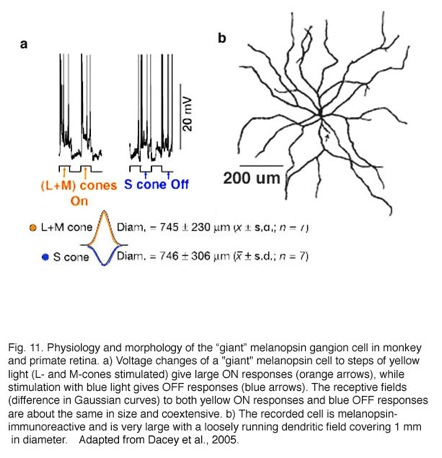
In 2005 Dacey and colleagues reported on a “giant” ganglion cell that expressed melanopsin, in monkey and human retinas, and gives a blue S-cone OFF response (Dacey et al., 2005). This melanopsin ganglion cell is intrinsically photoreceptive due to the melanopsin photopigment in its membrane but also is driven by both rod and cone inputs in an ON response (see chapter on melanopsin cells, webvision). Furthermore the cone input is S-cone (blue) OFF and M- and L-cone ON in response, with a coextensive receptive field organization (Fig. 11a, intracellular responses and receptive field difference of Gaussians). The morphology of the “giant” ganglion cells is shown in Fig. 11b. This cell type is many times larger in both cell body size and dendritic field diameter than the bistratified blue-ON, yellow-OFF bistratified ganglion cell described above (Fig. 11, dendritic tree covers a 1 mm area). Dendrites of the “giant” melanopsin cell stratify in stratum S1 right under the amacrine cell layer. And further dendrites run in S5 above the ganglion cell layer. There may be two varieties of this “giant type” GC (Dacey et al., 2005) or possibly one type that has dendrites in both strata of the IPL and often exhibits a displaced cell body. The “giant” blue-OFF GCs project to the parvo and magnocellar layers of the lateral geniculate nucleus (Dacey et al., 2005). This suggests that this ganglion cell is involved in color processing in vision.
6. Circuits for the S-cone pathways in the primate retina.
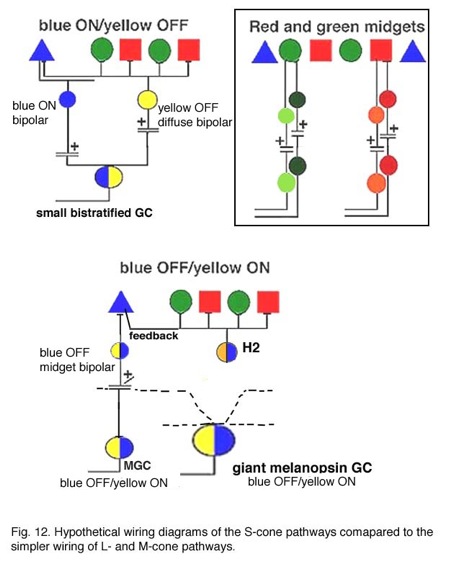
Hypothetical wiring diagrams of the S-cone pathway through the primate retina in comparison with the L- and M-cone midget pathways are shown in Figure 12. The S-cone bipolar would carry ON signals to the lower dendrites of the bistratified ganglion cell which may be the dominant input (Fig. 12). It is probable, though that the bipolar is already S-ON and yellow-OFF, in response due to the H2 cell feed-back influence as mentioned above (Field et al., 2007). Then both ON and OFF spectrally antagonistic components would drive the ganglion cell (Fig. 12 upper model). However, the yellow OFF response could also be complimented by an OFF bipolar, that contacts L- and M-cones as well, making synapses upon the upper dendrites of the bistratified ganglion cell (Crook et al., 2009) (Fig. 12).
The equivocal existence of the OFF S-cone channels (Gouras and Zrenner, 1981; Lennie, 1984) in the retina could be because only a small number of S-cone OFF ganglion cells are present and they are difficult to record. There is after all, a blue cone OFF midget bipolar and ganglion cell in the monkey retina (Figs. 7 and 12) (Klug et al., 2003) but it may not have been recorded from to date. This OFF blue midget cell is drawn into the wiring diagram of Figure 12 (lower model). Klug and coworkers (2003) suggest that the blue OFF midget does not contribute to blueness, but instead signals a short wavelength component to redness (Shinomori et al., 1999). However, the “giant” melanopsin ganglion cell seems to carry a true s-cone OFF signal to the color channels in the LGN (Dacey et al., 2005), and can be detected as the larger field S-cone (blue) OFF responding units in this nucleus (Tailby et al., 20078a). We can also hypothesize that the giant bistratifid bipolar of human and monkey retina (Fig. 7) is a large blue-cone specific bipolar and could have axonal inputs to both the upper and lower dendrites of the “giant” melanopsin cell. More discoveries concerning the blue pathways in the primate retina are clearly needed in the future.
7. References.
Ahnelt P, Kolb H. The mammalian photoreceptor mosaic-adaptive design. Prog Ret Eye Res. 2000;19:711–777.
Ahnelt PK, Kolb H, Pflug R. Identification of a subtype of cone photoreceptor, likely to be blue sensitive, in the human retina. J Comp Neurol.1987;255:18–34. [PubMed]
Ahnelt P, Kolb H. Horizontal cells and cone photoreceptors in human retina: a Golgi-electron microscopic study of spectral connectivity. J Comp Neurol.1994;343:406–427. [PubMed]
Baylor DA, Fuortes MGF, O’Bryan PM. Receptive fields of the cones in the retina of the turtle. J Physiol. 1971;214:265–294. [PubMed] [Free Full text in PMC]
Boycott BB, Dowling JE. Organization of the primate retina: light microscopy. Philos Trans R Soc B. 1969;255:109–184.
Burkhardt DA. Synaptic feedback, depolarization, and color opponency in cone photoreceptors. Vis Neurosci. 1993;10:981–989. [PubMed]
Dacey DM, Lee BB, Stafford DK, Pokorny J, Smith VC. Horizontal cells of the primate retina: cone specificity without spectral opponency. Science.1996;271:656–659. [PubMed]
Dacey DM, Lee BB. The “blue-on” opponent pathways in primate retina originates from a distinct bistratified ganglion cell. Nature. 1994;367:731–735.[PubMed]
Dacey DM. Morphology of a small-field bistratified ganglion cell type in the macaque and human retina. Vis Neurosci. 1993;10:1081–1089. [PubMed]
Dacey D.M., Peterson B.B., Gamlin P.D., Robinson F.R. Retrograde photofilling reveals the complete morphology of diverse new ganglion cell types that project to the lateral geniculate nucleus in macaque monkey. Invest Ophthal Vis Sci. 2001.
Dacey, DM, Liao, H-W, Peterson, BB, Robinson, FR, Smith, VC, Pokorny, J, Yau, K-W and Gamlin, PD (1005) Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature, 433, 749-754. [PubMed]
DeMonasterio FM, Schein SJ, McCrane EP. Staining of blue-sensitive cones of the macaque retina by fluorescent dye. Science. 1981;213:1278–1281.[PubMed]
Fuortes MGF, Simon EJ. Interactions leading to horizontal cellresponses in the turtle retina. J Physiol. 1974;240:177–199. [PubMed] [Free Full text in PMC]
Gouras P, Zrenner E. Color vision: a review from a neurophysiological perspective. In: Autrum H, Ottoson D, Perl ER, Schmidt RF, editors. Progress in sensory physiology. Vol. 1. Berlin: Springer-Verlag; 1981. p.139-179.
Gouras P. Progress in human visual evoked responses. J Clin Neurophysiol. 1984;1:77–82. [PubMed]
Gouras P. Retinal circuitry and its relevance to diagnostic psychophysics and electrophysiology. Curr Opin Ophthalmol. 1992;3:803–812.
Humanski RA, Wilson HR. Spatial frequency mechanisms withshort-wavelength-sensitive cone inputs. Vision Res. 1992;32:549–560. [PubMed]
Klug K, Herr S, Ngo IT, Sterling P and Schein S. Macaque retina contains an S-cone OFF midget pathway. J Neurosci, 2003. 23: 9881-9887. [PubMed]
Kolb H. Organization of the outer plexiform layer of the primate retina: electron microscopy of Golgi-impregnated cells. Philos Trans R Soc Lond B Biol Sci. 1970;258:261–283.
Kolb H, Mariani A, Gallego A. A second type of horizontal cell in the monkey retina. J Comp Neurol. 1980;189:31–44. [PubMed]
Kolb H, Lipetz LE. The anatomical basis for colour vision in the vertebrate retina. In: Gouras P, editor. Vision and visual dysfunction. Vol. 6. The perception of colour. London: Macmillan Press Ltd.; 1991. p. 128-145.
Kolb H, Goede P, Roberts S, McDermott R, Gouras P. Uniqueness of the S-cone pedicle in the human retina and consequences for color processing. J Comp Neurol. 1997;386:443–460. [PubMed]
Kouyama N, Marshak DW. Bipolar cells specific for blue cones in the macaque retina. J Neurosci. 1992;12:1233–1252. [PubMed]
Lennie P. Recent developments in the physiology of color vision. Trends Neurosci. 1984;5:243–248.
Marc RE. Chromatic organization of the retina. In: LaVail M, Hollyfield J, editors. Cell biology of the eye. New York: Academic Press; 1982. p.435-473.
Mariani AP. Giant bistratified bipolar cells in the monkey retina. Anat Rec. 1983;206:215–220.
Mariani AP. Bipolar cells in monkey retina selective for cones likely to be blue-sensitive. Nature. 1984;308:184–186. [PubMed]
Nathans J, Thomas D, Hogness DS. Molecular genetics of human color vision: the genes encoding the blue, green and red pigments. Science.1986;232:193–202. [PubMed]
Polyak SL. The retina. Chicago: University of Chicago Press. 1941.
Rodieck RW. In: Valberg A, Lee BB, editors. From pigments to perception. New York: Plenum Publishing Corp.; 1991. p. 83-94.
Stockman A, MacLeod DIA, DePriest DD. The temporal properties of the human short-wave photoreceptors and their associated pathways. Vision Res.1991;31:189–208. [PubMed]
Szel A, Diamanstein T, Röhlich P. Identification of blue-sensitive cones in the mammalian retina by antivisual pigment antibody. J Comp Neurol.1988;273:593–602. [PubMed]
Tailby, C, Solomon, SG and Lennie, P. (2008a) Functional asymmetries in visual pathways carrying S-cone signals in macaque. J Neurosci. 28, 4078-4087. [PubMed]
Williams DR, MacLeod DIA, Hayhoe M. Punctate sensitivity of the blue-sensitive mechanisms. Vision Res. 1981;21:1357–1375. [PubMed]
Last Updated: February 2014.