By Helga Kolb, Ralph Nelson, Peter Ahnelt, Isabel Ortuño-Lizarán and Nicolas Cuenca
Abstract
We summarize the development, structure, different neural types and neural circuitry in the human fovea. The foveal pit is devoid of rod photoreceptors and of secondary and tertiary neurons, allowing light to directly stimulate cones and give us maximal visual acuity. The circuitry underlying the transmission to the brain occurs at the rim of the fovea. The predominant circuitry is concerned with the ‘private’ cone to midget bipolar cell and midget ganglion cell pathways. Every cone drives two midget bipolar cells and two midget ganglion cells so that the message from a single cone is provided to the brain as a contrast between lighter signals (ON pathways) or darker signals (OFF pathways). The sharpening of this contrast message is provided by horizontal-cell feedback circuits and, in some pathways by amacrine circuitry. These midget pathways carry a concentric color and spatially opponent message from red and green cones.
Blue cones are sparse, even largely missing in the foveal center while occurring at somewhat higher density than elsewhere in the cone mosaic of the foveal slope. Signals from blue cones have different pathways to ganglion cells. The best understood is through an ON-type blue-cone-selecting bipolar cell to a non-midget, small bistratified ganglion cell. An OFF-center blue midget bipolar is known to be present in the fovea and connects to a blue OFF midget ganglion cell. Another OFF blue message is sent to a giant melanopsin ganglion cell that is present in the foveal rim area, but the circuitry driving that is less certain and possibly involves an intermediate amacrine cell. The H2 horizontal cells are thought to be feedback neurons primarily of the blue cone system.
Amacrine cells of the fovea are mostly small-field and glycinergic. The larger field GABAergic amacrines are present but more typically surround the fovea in a ring of processes, with little or no penetration into the foveal center. Thus, the small field glycinergic amacrines are important in some sort of interplay with the midget bipolar–midget ganglion cell channels. We have anatomical descriptions of their synaptology but only a few have been recorded from physiologically. Both OFF pathway and ON pathway amacrines are present in the fovea.
Introduction
The central point of the visual field ahead of us is the image falling on the fovea in the human retina. This is the area of our visually sensitive retina where the cone photoreceptors are tightly packed, where rod photoreceptors are excluded and where all intervening layers of the retina are pushed aside concentrically to allow light to reach the densely packed sensory cones with minimum scatter from overlying tissues. The fovea is where focusing on fine detail in the image is perfected, allowing us to read, discriminate colors well and sense three-dimensional depth.
General features of the fovea
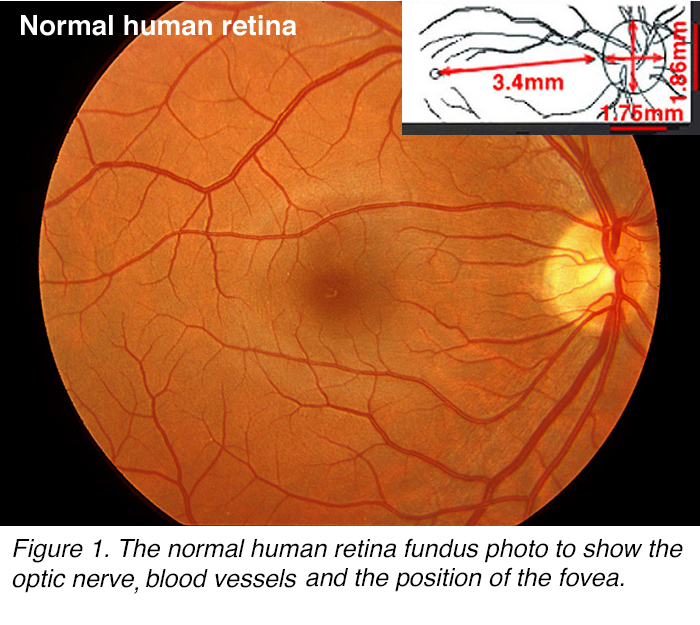 Figure 1. The normal human retina fundus photo shows the optic nerve (right), blood vessels and the position of the fovea (center).
Figure 1. The normal human retina fundus photo shows the optic nerve (right), blood vessels and the position of the fovea (center).
Looking at the retina lining the back of the eyeball in a human, we can see the clear landmark of the optic nerve head (papilla) and radiating blood vessels (Figure 1). Temporal to the optic nerve head at a distance approximately 2.5 optic nerve (disk) diameters at roughly 3.4 mm distance lies a dark brown-yellowish area (Figure 1), in the center of which is the tiny circular fovea. The position of the fovea can be seen clearly in the retina illustrated in Figure 2A. This eye was treated with RNA-later for preservation, allowing for a clear view of a yellow macula lutea area and including the brown central point, (foveal pit) (Figure 2A).
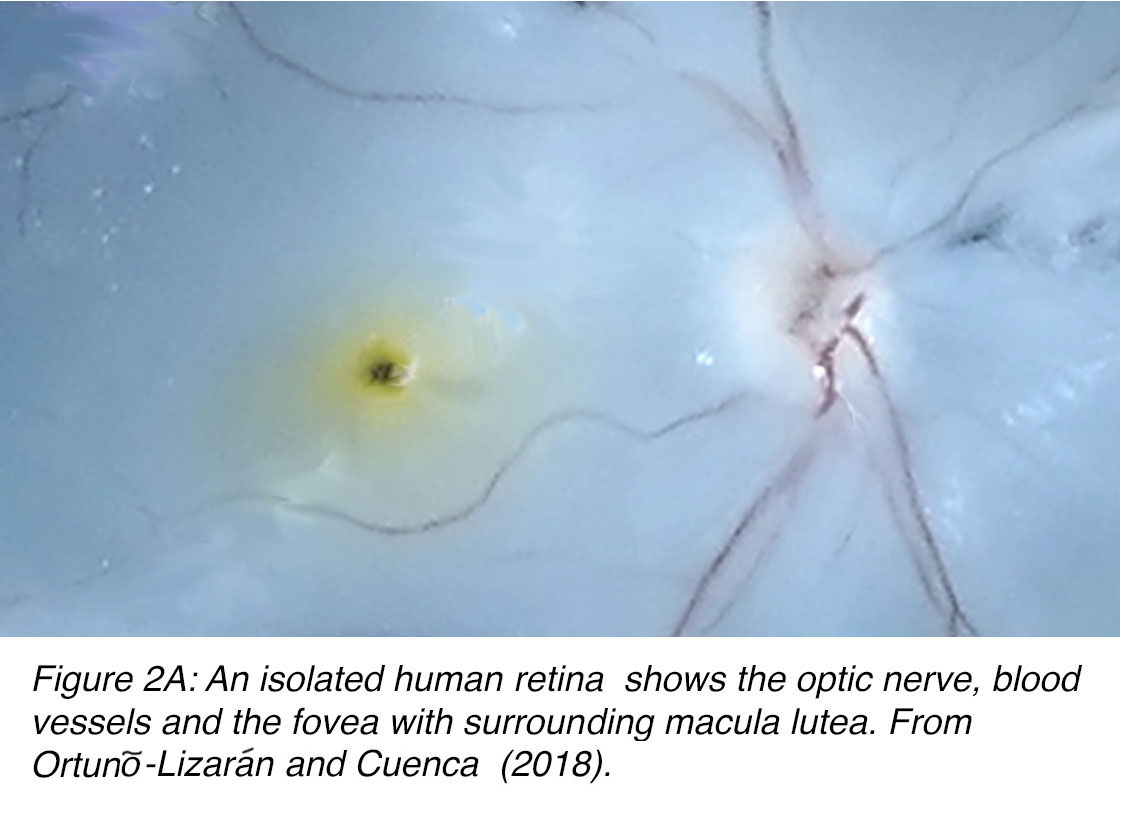 Figure 2A. An isolated human retina shows the optic nerve (right), blood vessels and the fovea (center) with surrounding macula lutea (yellow). Cuenca et al, prepublication.
Figure 2A. An isolated human retina shows the optic nerve (right), blood vessels and the fovea (center) with surrounding macula lutea (yellow). Cuenca et al, prepublication.
The area called the macula by ophthalmologists is a circular area around the foveal center of approximately 5.5 mm diameter (Figure 2B) The macula lutea with the yellow pigmentation extends across the fovea into the parafoveal region and a little beyond. This area is about 2.5 mm in diameter (Figure 2B). The actual fovea is about 1.5 mm in diameter and the central fovea consists of a foveal pit (umbo) that is a mere 0.15 mm across (Figure 2B). This foveal pit is almost devoid of all layers of the retina beneath the cone photoreceptors. On the edges of the foveal pit the foveal slope is still mainly devoid of other layers but some cell bodies of retinal interneurons, bipolar and horizontal cells and even some amacrine cell processes are becoming evident. By the 0.35 mm diameter circular area the first ganglion cell bodies, the retinal neurons sending signals to the brain, are beginning to appear. All the central fovea that measures 0.5 mm across is avascular (FAZ).
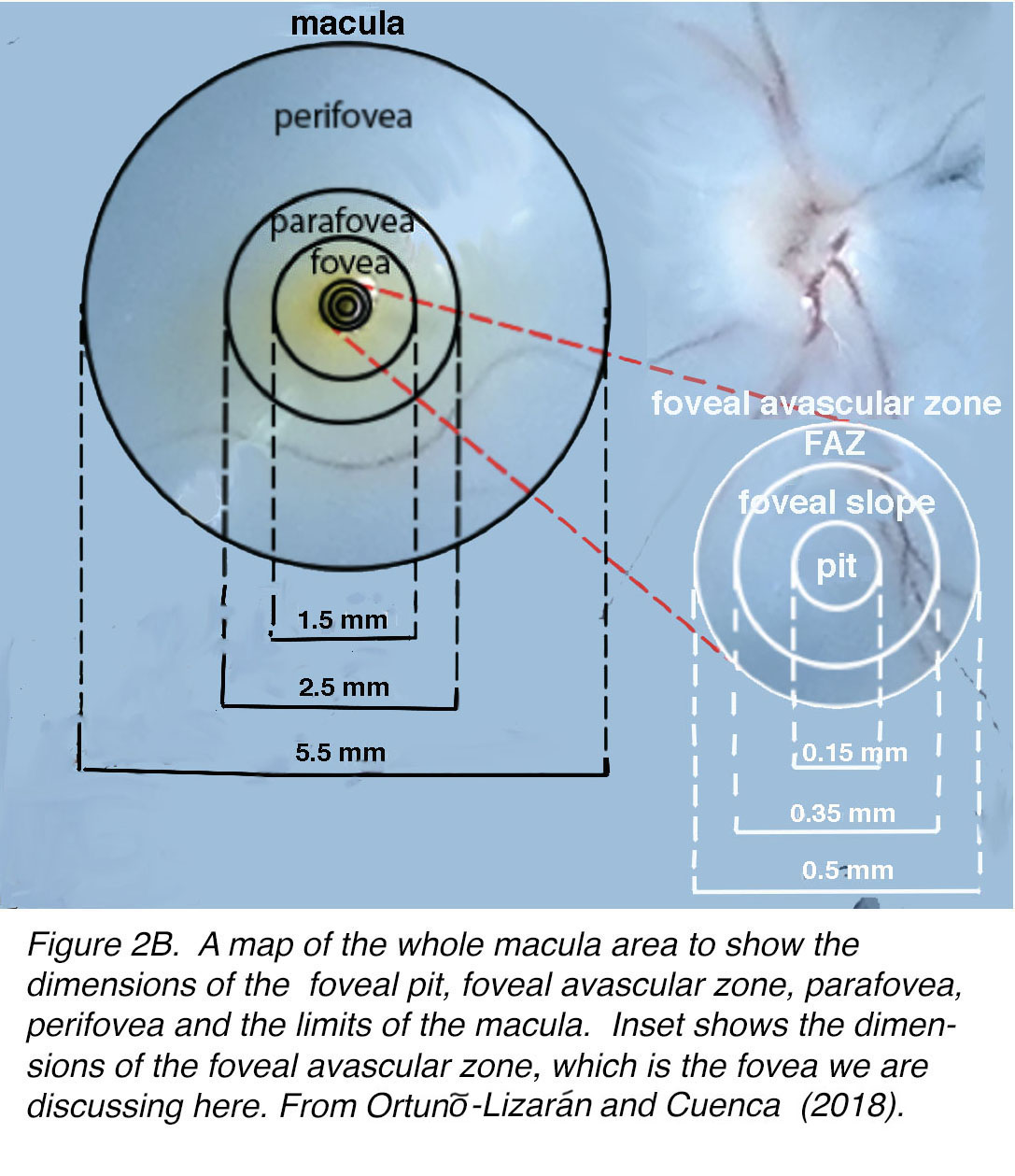 Figure 2B. A map of the whole macular area to show the dimensions of the foveal pit, foveal avascular zone, parafovea, perifovea, and the limits of the macula. Inset shows the dimensions of the foveal avascular zone, which is the fovea we are discussing here.
Figure 2B. A map of the whole macular area to show the dimensions of the foveal pit, foveal avascular zone, parafovea, perifovea, and the limits of the macula. Inset shows the dimensions of the foveal avascular zone, which is the fovea we are discussing here.
The avascular nature of the central fovea is depicted in Figure 3. A human retina wholemount has the blood vessels immunostained with antibodies against Collagen IV and is photographed by stacked images in a confocal microscope. It is absolutely clear that the smallest capillaries even, do not intrude into the foveal center (Figure 3, f) of 500 µm diameter, thereby known as the avascular zone.
Figure 3. Wholemount of human retina with blood vessels immunostained with Collagen IV. The confocal microscopy of stacked images clearly shows the optic nerve head (ON) and all the blood vessels to the smallest capillaries. The capillaries surround the fovea (f), but do not enter it, thereby making the fovea avascular.
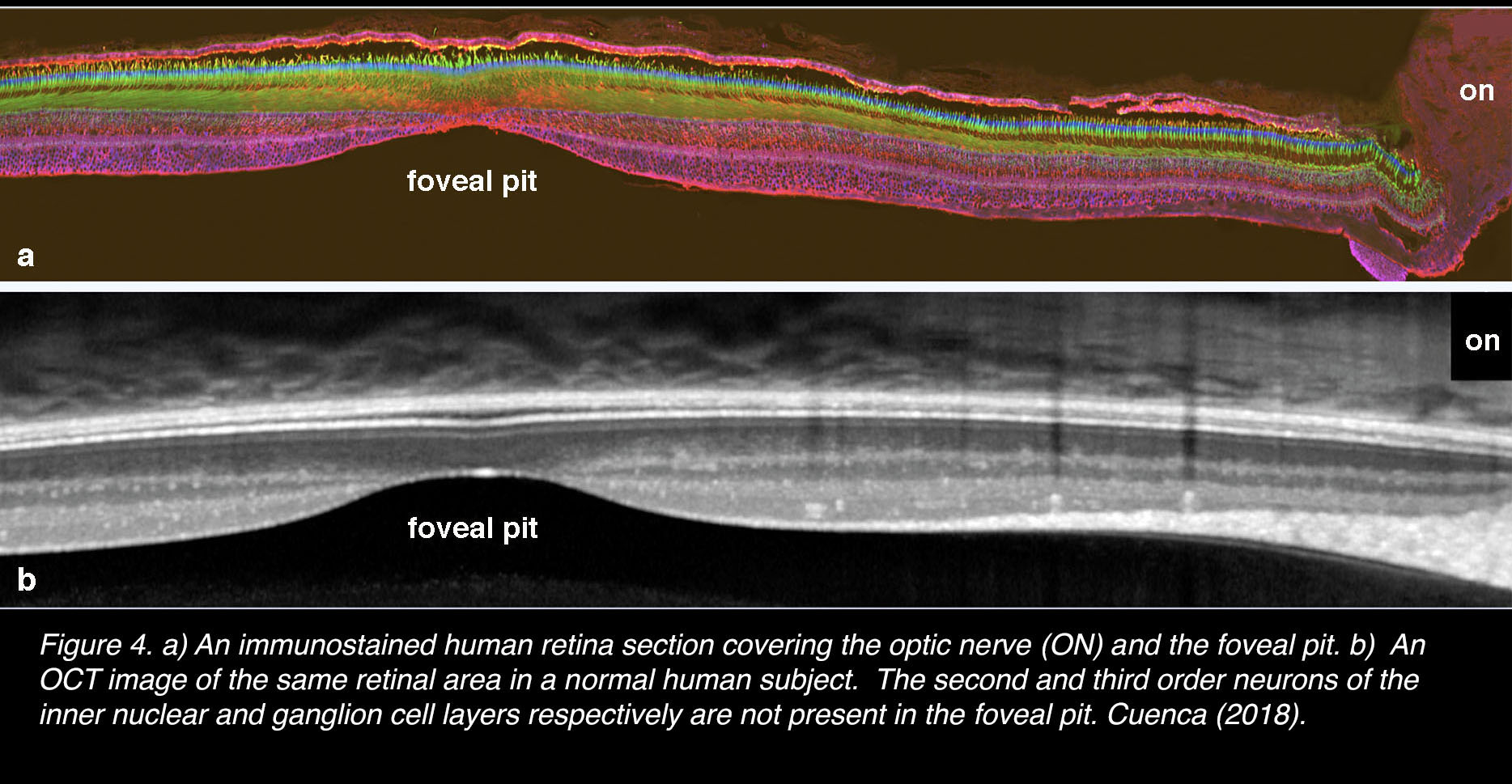
In vertical section of the human retina from the optic nerve head through the foveal pit and beyond (Figure 4), it is clear where the fovea is located relative to the nerve head (on). Figure 4 (a) is a confocal image after immunostaining with antibodies that are specific for cone photoreceptors [arrestin antibodies for cones, green; cytochrome C antibodies for mitochondria, blue; and for Müller glial cells and RPE, antibodies against cytoplasmic retinaldehyde binding protein (CRALBP), red]. In comparison is seen an optical coherence tomography (OCT) picture in Figure 4 (b) of exactly the same area of human retina. In both images it is clear that the second and third order neurons of the inner nuclear and ganglion cell layers respectively are not present in the foveal pit.
Figure 4. (a) An immunostained human retina section covering the optic nerve (ON) and the foveal pit. Cones, anti-arrestin (green); pigment epithelial and Müller cells, anti CRALPB (red) (109); mitochondria, anti-cytochrome C (blue). (b) An OCT image of the same retinal area in a normal human subject. The second and third order neurons of the retinal inner nuclear and ganglion cell layers respectively are not present in the foveal pit. Adapted from Cuenca, Ortuño-Lizarán and Pinilla 2018 (110).
In the foveal pit the only neurons are cone photoreceptors, all with slim inner segments, packed cell bodies, up to 6 layers deep reaching to the floor of the foveal pit (Figure 5, green cells). However, there are many expanded-looking Müller glia surrounding these cones (Figure 5, red profiles). A central bouquet of cones has their synaptic pedicles ending at the foveal pit floor (Figure 5, green spots, arrows), whereas the cones surrounding them stretch their axons (known as Henle fibers) and presynaptic pedicles away from the center of the foveal pit to the foveal slope area (Figure 5, green spots form a continuous line, arrows). The lack of blood vessels in the central pit can be seen by the absence of the blue circular profiles there (Figure 5, bv).
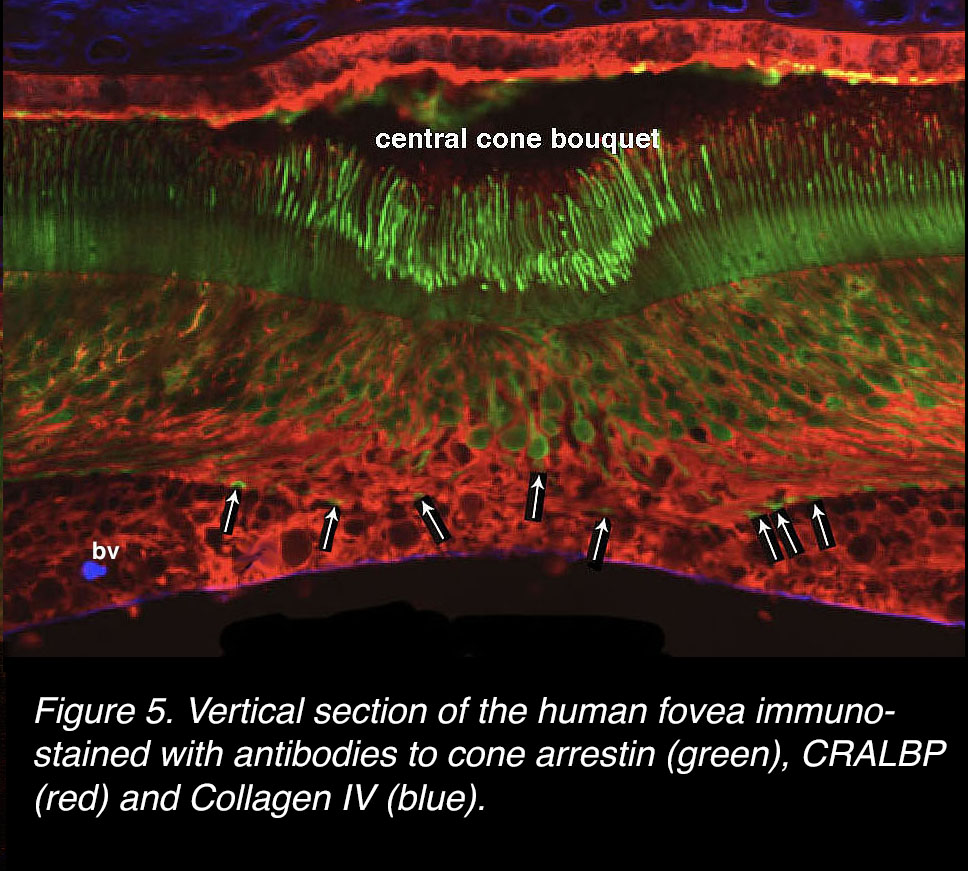 Figure 5. Vertical section of the human fovea immunostained with antibodies to cone arrestin (green), CRALBP (red) and Collagen IV (blue).
Figure 5. Vertical section of the human fovea immunostained with antibodies to cone arrestin (green), CRALBP (red) and Collagen IV (blue).
O’Brien and colleagues (1) very elegantly illustrated the cone axons radiating out from the foveal pit forming the Henle fiber layer and terminating in distant pedicles in a whole mount monkey retina (Figure 6). The picture would be very similar in a human retina. The Henle fiber layer is a combination of outward radially directed axons of the cones, and where rods begin to appear, also rod axons, and Müller cell processes. It is interesting to note in Figure 5 that the pedicles of the very central bouquet of cones are widely spaced ending on the foveal pit floor. We know from Figure 5 that these central bouquet cone pedicles are separated by voluminous Müller cell elements.
 Figure 6. A wholemount monkey fovea immunostained with cone arrestin. The axons of the cones radiate out to a ring of cone pedicles. Central bouquet cone axons stay in the foveal pit. From O’Brien et al., 2012 (1).
Figure 6. A wholemount monkey fovea immunostained with cone arrestin. The axons of the cones radiate out to a ring of cone pedicles. Central bouquet cone axons stay in the foveal pit. From O’Brien et al., 2012 (1).
Development of the fovea
Understanding how the primate fovea develops from fetal to adult stage of the retina has been a very difficult task in vision research. This has, of course been due to the difficulty of obtaining retinas from human pre-birth and baby eyes. Even fetal monkey material has been scarce to obtain. Dr. Anita Hendrickson (Figure 7) at the University of Washington, Seattle, spent most of her career pursuing this subject of retinal research, and has contributed almost all we know.
 Figure 7. A young Anita Hendrickson at her microscope. From her obituary in 2017 (111).
Figure 7. A young Anita Hendrickson at her microscope. From her obituary in 2017 (111).
The earliest fetal retinas examined (2) were from a week-22 eye. The fovea is not recognizable at this stage, because the central region of the retina, where the fovea will develop, consists primarily of several layers of ganglion cell bodies and inner nuclear layer cells (INL), presumably amacrine and bipolar cells (Figure 8, a). A single layer of developing cones stretches from outer plexiform layer (OPL) to pigment epithelium and choroid (Figure 8, a, right inset). A hint of a developing cone pedicle is seen (Figure 8, right red arrow) but there is no sign of outer segments of cones (Figure 8, right, apposing red arrowheads). By fetal week 28, an indentation of the retina at the thickest ganglion cell layer appears and can be considered the earliest sign of the foveal pit (Figure 8, b, P). The inner nuclear layer has become thinner and appears pushed out of the pit (P) but a kind of split is occurring in the middle of the INL known as the transient layer of Chievitz (TC, Figure 8, c) (3). By fetal week 37 (Figure 8, c) a pronounced foveal pit is evident (P), the ganglion cells are thinned to 2 or 3 deep and the TC area in the INL appears like a sheared, radially projecting area of probable Müller cell fibers. Through the latter two fetal stages, where the foveal pit is becoming obvious, the cones are still immature, arranged in a single layer and have no visible outer segments (Figure 8, b and c). However, there is the first suggestion of the cone axons being tilted away from their cell bodies to form the early Henle fiber layer.
Figure 8. Foetal human retina at (a) foetal week (Fwk) 22, (b) Fwk 28, and (c) Fwk 37. The foveal position is not noticed at week 22 but in later weeks becomes dimpled as ganglion cells become displaced out radially from the developing foveal pit. In the beginning the retina is thick, multilayered and cones are undeveloped with no outer segments or visual pigment (a: right enlarged photo, red arrow heads point to a cone nucleus, a stubby inner segment, and a developing cone pedicle). From Hendrickson et al., 2012 (26).
It is interesting to closely examine the cone photoreceptors in the fetal 35-to-37-week retinas as illustrated by Hendrickson and coauthors (2). Figure 9 shows how immature the cones of the foveal pit are compared with those of the cones at some distance from the fovea (Figure 9. 2 mm from fovea). At the foveal pit area, the cones are just stubby cells with a synaptic pedicle, little to no lengthened inner segment and zero outer segments (Figure 9, fovea). By 800 µm to 2 mm from the developing foveal pit, the cones become elongated vertically and have definite cone pedicles. Most cell bodies descend away from the external limiting membrane and have elongating axons that are angled away from the foveal pit, forming the early Henle fiber layer. Inner segments are long, but the outer segments are still not formed. (Figure 9, 800 µm and 2 mm).
 Figure 9. Sections of the retina of a human foetus at 25 weeks gestation. The cones of the fovea are still undeveloped with no outer segments, and a synaptic area with no axon. From 800 µm to 2 mm from the foveal center there are clear elongated inner segments but still no outer segments. The slanting of the cone axons out radially is beginning to be evidence of a developing Henle fiber layer. From Hendrickson et al., 2012 (26).
Figure 9. Sections of the retina of a human foetus at 25 weeks gestation. The cones of the fovea are still undeveloped with no outer segments, and a synaptic area with no axon. From 800 µm to 2 mm from the foveal center there are clear elongated inner segments but still no outer segments. The slanting of the cone axons out radially is beginning to be evidence of a developing Henle fiber layer. From Hendrickson et al., 2012 (26).
At birth of the human baby the retina in the eye is looking recognizably foveate (Figure 10, a). The foveal pit now contains a very thin, only one layer thick, ganglion cell layer, a thin inner plexiform layer (IPL) but a prominent inner nuclear layer (INL) (Figure 10, a). The cones are now evident as straight vertical cones with synaptic pedicles, cell bodies and inner segments. There are probably developing cone outer segments too (not easy to see at this magnification). But the pit is still several cell layers thick with only the cones on the foveal slope beginning to angle away from the pit. Further out on the foveal slope the cone Henle fiber layer is obvious now (Figure 10, a). By 15 months after birth, the baby retina has a definite fovea and even the central cones are angling out to the foveal slope. Inner and outer segments are well developed in the pit and no other layers of the retina are here anymore (Figure 10, b and c). By 13 years the fovea is completely developed (Figure 10, d) (2).
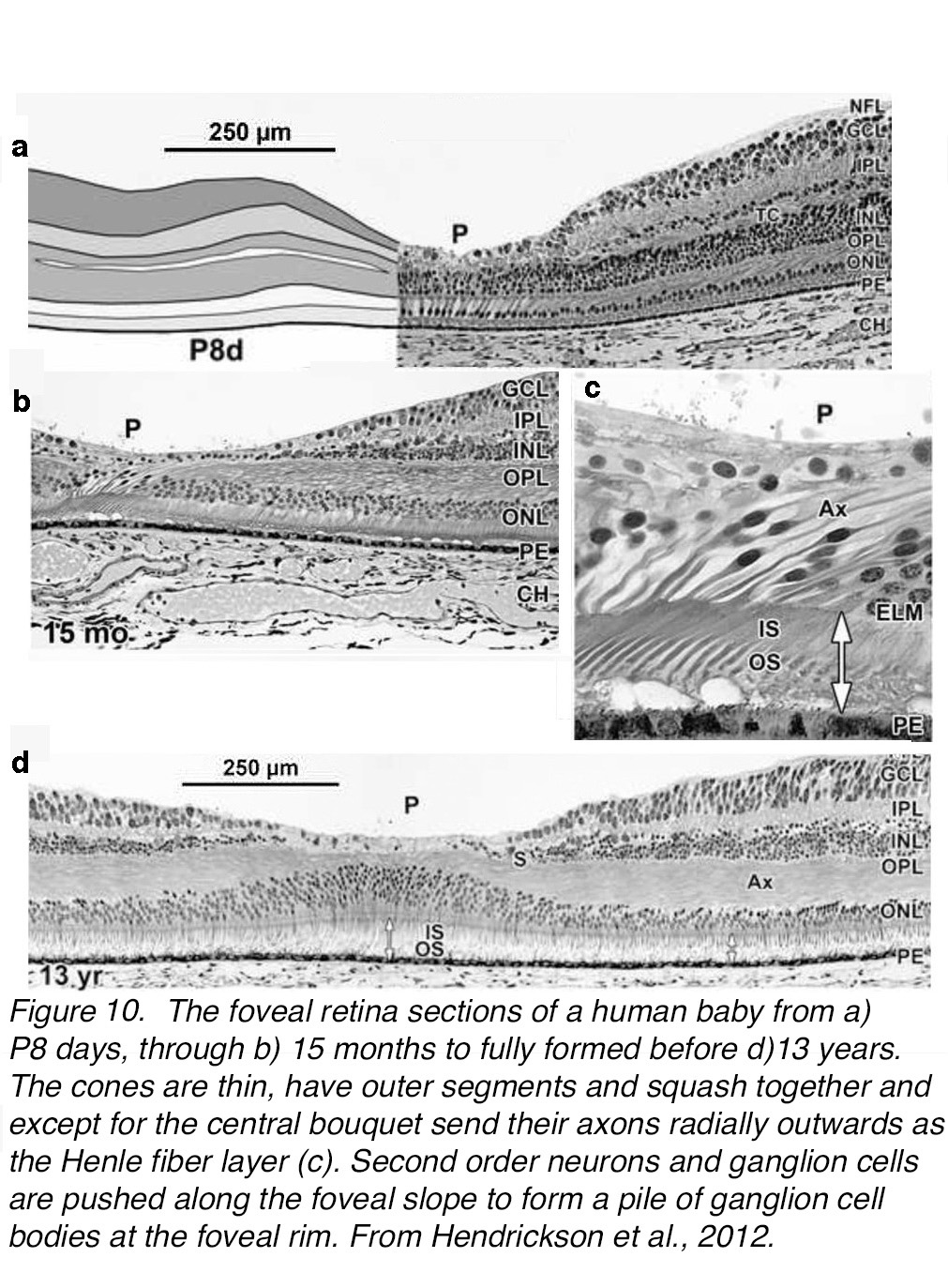 Figure 10. The foveal retina sections of a human from (a) postnatal 8 days (P8d), through (b) 15 months, to fully formed (d) 13 years. (c) At 15 months the cones are thin, have outer segments and squash together and, except for the central bouquet, send axons radially outwards as the Henle fiber layer. Second order neurons and ganglion cells are pushed along the foveal slope to form a pile of ganglion cell bodies at the foveal rim. From Hendrickson et al., 2012 (26).
Figure 10. The foveal retina sections of a human from (a) postnatal 8 days (P8d), through (b) 15 months, to fully formed (d) 13 years. (c) At 15 months the cones are thin, have outer segments and squash together and, except for the central bouquet, send axons radially outwards as the Henle fiber layer. Second order neurons and ganglion cells are pushed along the foveal slope to form a pile of ganglion cell bodies at the foveal rim. From Hendrickson et al., 2012 (26).
What forces could cause this remarkable transformation of an evenly thick multi-cell, layered retina to become concavely dimpled, buckled up and stretched outwards to form a single layered pit at the fovea and a high sided sloping tissue with the highest concentration of cell layers at the foveal rim. The developmental effort is to ensure that a central area of the retina is concentrated with the slimmest packed cones with no obstruction of incoming light by secondary and tertiary cell layers.
The most recent investigations on this developmental phenomenon in the human (primate) retina provide evidence that the radial retinal glia – the Müller cells and possibly the astrocytes of the ganglion cell layer – are instrumental in this process (4). The Müller cells of the foveal pit are closely associated with the cone fibers and together they make up the Henle fibers layer (Figure 11A, red profiles). Bringmann and colleagues suggest that the Müller cells exert tractional forces onto cone axons fibers by a vertical contraction of the central most Müller cells and cones so they become elongated and very thin (Figure 11, B, blue arrows). After widening of the foveal pit by elimination of astrocytes in the pit and ganglion cell layers, the Henle fibers are forced, by horizontal contraction of their surrounding Müller cell processes in the outer plexiform layer, to pull the cone and then rod photoreceptor centrifugally away from the pit (Figure 11, B, orange arrows).
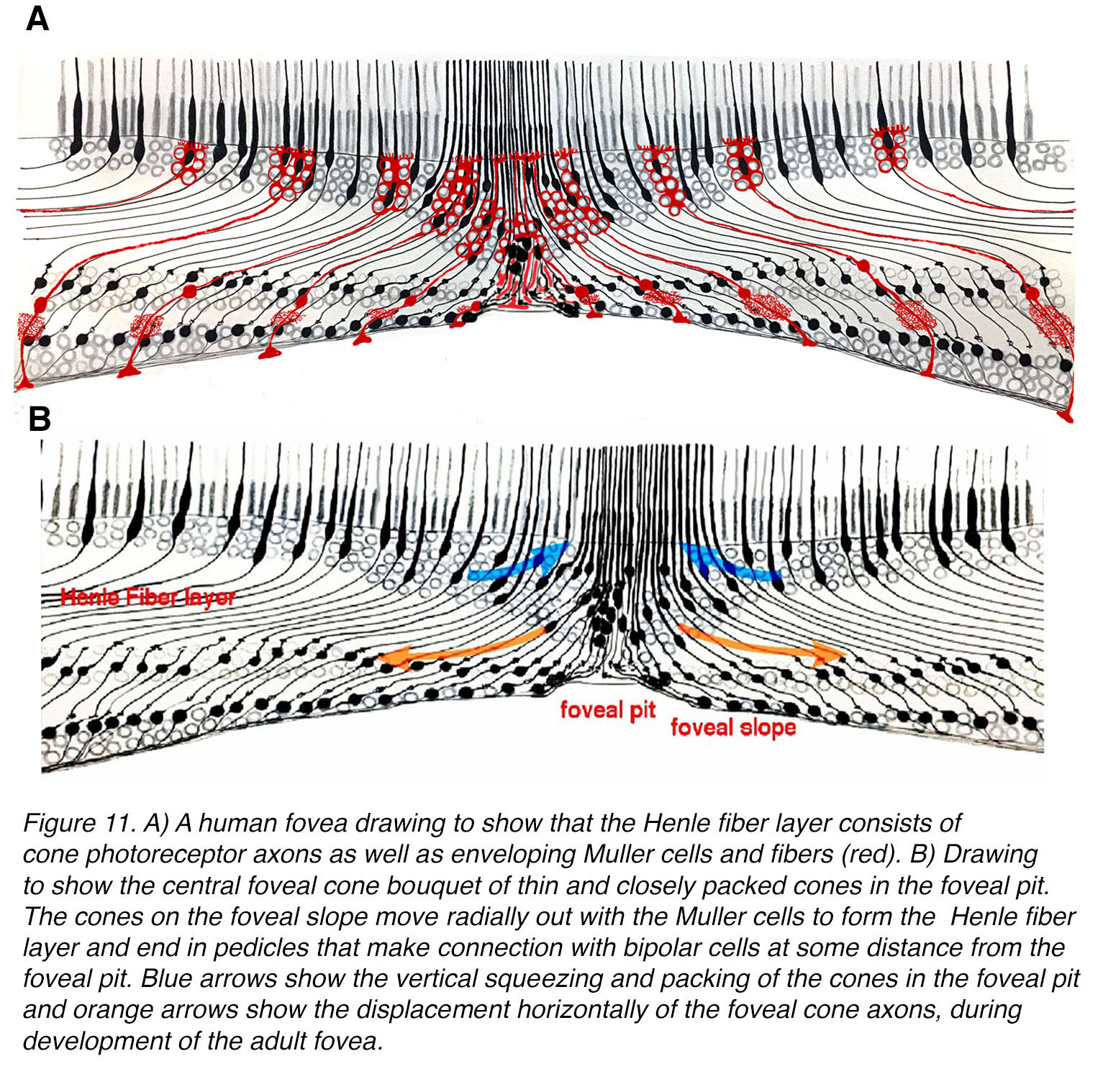 Figure 11. (A) A human fovea drawing to show that the Henle fiber layer consists of cone photoreceptor axons as well as envelopingMüller cells and fibers (red). B) Drawing to show the central foveal cone bouquet of thin and closely packed cones in the foveal pit. The cone axons on the foveal slope move radially out with the Müller cells to form the Henle fiber layer and end in pedicles that make connection with bipolar cells at some distance from the foveal pit. Blue arrows show the vertical squeezing and packing of the cones in the foveal pit and orange arrows show the displacement horizontally of the foveal cone axons, during development of the adult fovea.
Figure 11. (A) A human fovea drawing to show that the Henle fiber layer consists of cone photoreceptor axons as well as envelopingMüller cells and fibers (red). B) Drawing to show the central foveal cone bouquet of thin and closely packed cones in the foveal pit. The cone axons on the foveal slope move radially out with the Müller cells to form the Henle fiber layer and end in pedicles that make connection with bipolar cells at some distance from the foveal pit. Blue arrows show the vertical squeezing and packing of the cones in the foveal pit and orange arrows show the displacement horizontally of the foveal cone axons, during development of the adult fovea.
The mosaic of cones in the fovea
The term “foveal cone mosaic” generally refers to the strikingly regular patterns of condensed cone inner and outer segments with largely triangular crystalline organization, which nevertheless includes non-randomly distributed discontinuities (5, 6). The less familiar and less understood part of foveal cones is the further course towards their synaptic terminals. It includes a two-step transition. From a two-dimensional mosaic for image reception it is rearranged into to a three-dimensional somata tiling, which then again spreads out to establish the concentric monolayered pedicle meshwork (7-9).
The mature human fovea consists of 3 spectral types of cone: red or long wavelength sensitive cones, L-cones; green or medium wavelength cones, or M-cones; and blue or short wavelength cones, S-cones. These three types of cone are tightly packed and at their most concentrated (up to 200,000/mm2 in the fovea (8, 10) (see Webvision Facts and Figures). Rods are not present in the foveal pit, appearing first halfway into the foveal slope, beyond the 300 µm diameter area (see Figure 2B).
It is extremely difficult to get a horizontal section through the central fovea particularly including the central bouquet of cones because of the concave nature of the fovea. Figure 12.1 manages to get such a view of a horizontal slice through the inner segments of the cones of a human fovea (7). The tiniest central cones in the center of the photograph (Figure 12.1) are very slim at 2.5-3 µm in diameter and become progressively larger as they move along a radial gradient from the central bouquet. It is noticeable that the cones are not uniformly distributed in a hexagonal mosaic. Small patches of cones are hexagonal and then the patch is interrupted and shifts the surrounding patches slightly (Figure 12.1). Ahnelt and coauthors (11) noticed that these shifts in the mosaic usually were associated with the position of a slightly larger diameter cone. They proposed that these larger cones were the short wavelength cones, the S-cones, and described their morphological differences from the surrounding, more common L- and M-cones (11).
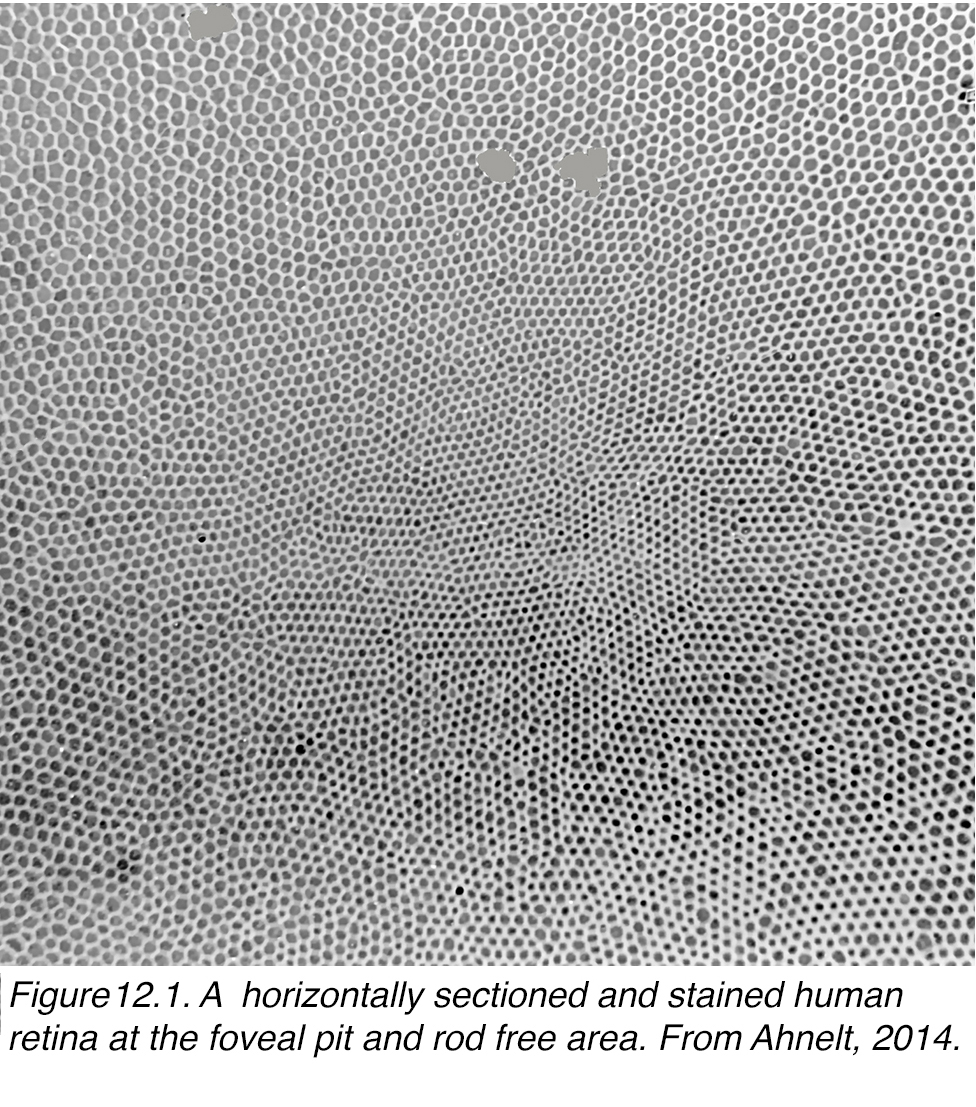 Figure 12.1. A horizontally sectioned and stained human retina at the foveal pit and rod free area. From Ahnelt et al, 1987 (11).
Figure 12.1. A horizontally sectioned and stained human retina at the foveal pit and rod free area. From Ahnelt et al, 1987 (11).
S-cones are relatively rare in the retina compared with the much more dominant L- and M- cones. The S-cones are, however, ubiquitous in all vertebrate retinas, with the exception of cetaceans (12). As far as other mammals are concerned S-cones are commonly paired with L-cones to give them a dichromatic color sense. These L-cones vary in spectral peak, and the more mid-spectral types are called M-cones. In old world monkeys and apes, and in man an L-opsin gene duplication and further mutation produced an extra mid-spectral L-cone opsin subtype, M-cone opsin. The combination of L-cones, M-cones and S-cones provides trichromacy. This trichromacy allows discrimination of green, yellow and blue/purple hues.
There are differences in the genetic structure and locus of the S-cone visual pigment compared with the M- and L-cone pigments (13), yet the S-cones always form a consistent 8-10% of the mammalian cone photoreceptor population (14, 15). In primates and humans of course, the S-cones are rather scarce in the foveal pit. Some authors suggest that there is a so-called blue cone blind spot (16). However, S-cones peak in number on the foveal slope of the human retina and here form about 12% of the population. Figure 12.2, (a) shows the peak S-cone distribution on the foveal slope in a human retina as identified by the larger size and arrangement in the mosaic breaking up the regular hexagonal pattern distribution of the other cone types. In Figure 12.2, (b) the S-cones have been colored in for clarity.
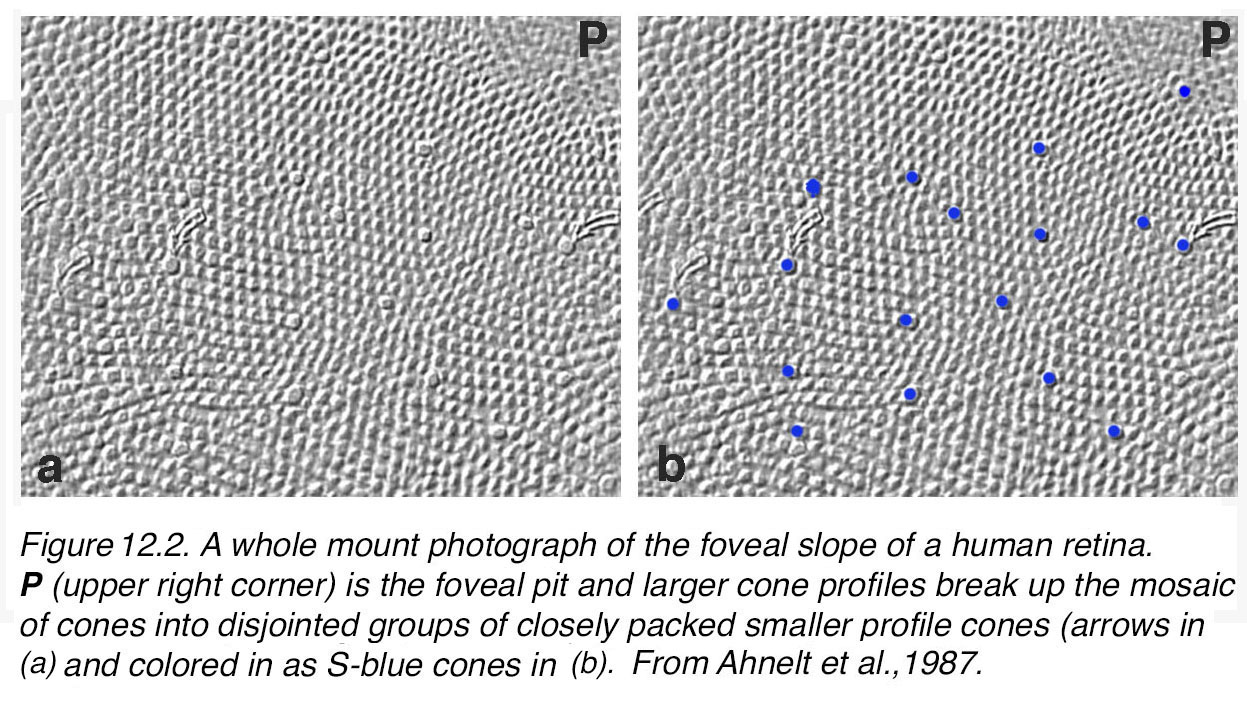 Figure 12.2. A whole-mount photograph of the foveal slope of a human retina. P (upper right corner) is the foveal pit. Larger cone profiles break up the mosaic of cones into disjointed groups of closely packed smaller profile cones [arrows in (a, b) and colored in as S-blue cones in (b)]. From Ahnelt et al., 1987 (11).
Figure 12.2. A whole-mount photograph of the foveal slope of a human retina. P (upper right corner) is the foveal pit. Larger cone profiles break up the mosaic of cones into disjointed groups of closely packed smaller profile cones [arrows in (a, b) and colored in as S-blue cones in (b)]. From Ahnelt et al., 1987 (11).
Since these earlier identifications of foveal S-cones on morphological criteria (11), antibodies against the S-cone pigments in the cone outer segments have been developed and are able to positively identify the S-cones in the overall population by immunocytochemical methods. In figure 13, the human foveal pit (FP) and foveal slope are immunostained with an S-cone antibody and illustrate the S-cones as black spots and angled black cone outer segments. In the foveal pit only a few S-cones appear interspersed in the mosaic of highest density (Figure 13). However, their proportion increases in surrounding areas and are at their highest density on the foveal slope (Figure 13 brown spots, top and right-hand side).
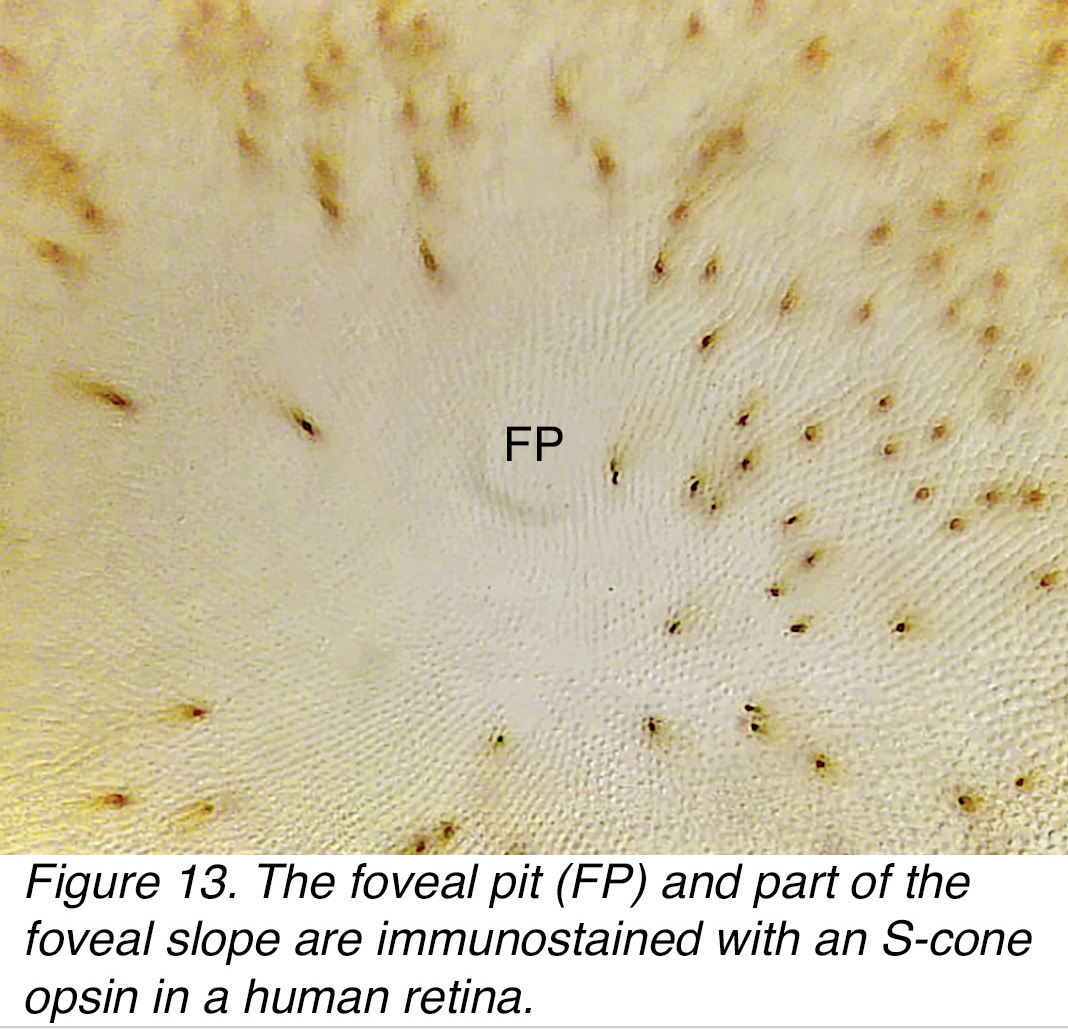 Figure 13. The foveal pit (FP) and part of the foveal slope are immunostained with an S-cone opsin in a human retina.
Figure 13. The foveal pit (FP) and part of the foveal slope are immunostained with an S-cone opsin in a human retina.
Figure 14 illustrates immunostaining in vertical section and the scarcity of S-cones in the foveal pit compared to the increase in number of this population of cones on the foveal slope, of a human retina. A map of the S- cone distribution in another human fovea is shown in Figure 15. The lighter to darker blue shading indicates less dense to denser S- cone presence. Note in both images (Figs. 14 and 15) there are very small numbers of S-cones in the foveal pit.
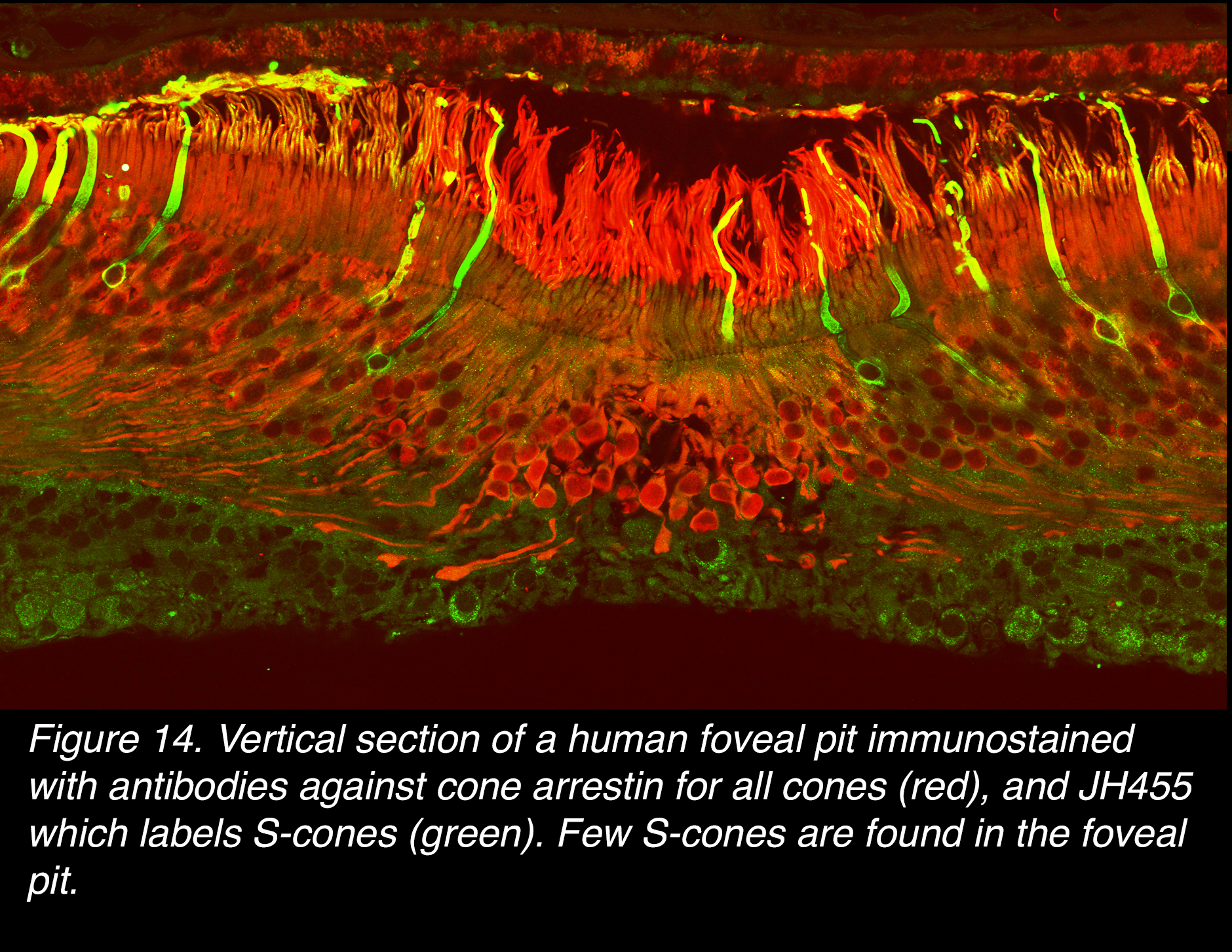 Figure 14. Vertical section of a human foveal pit immunostained with antibodies against cone arrestin for all cones (red), and JH455, which labels S-cones (green). Few S-cones are found in the foveal pit.
Figure 14. Vertical section of a human foveal pit immunostained with antibodies against cone arrestin for all cones (red), and JH455, which labels S-cones (green). Few S-cones are found in the foveal pit.
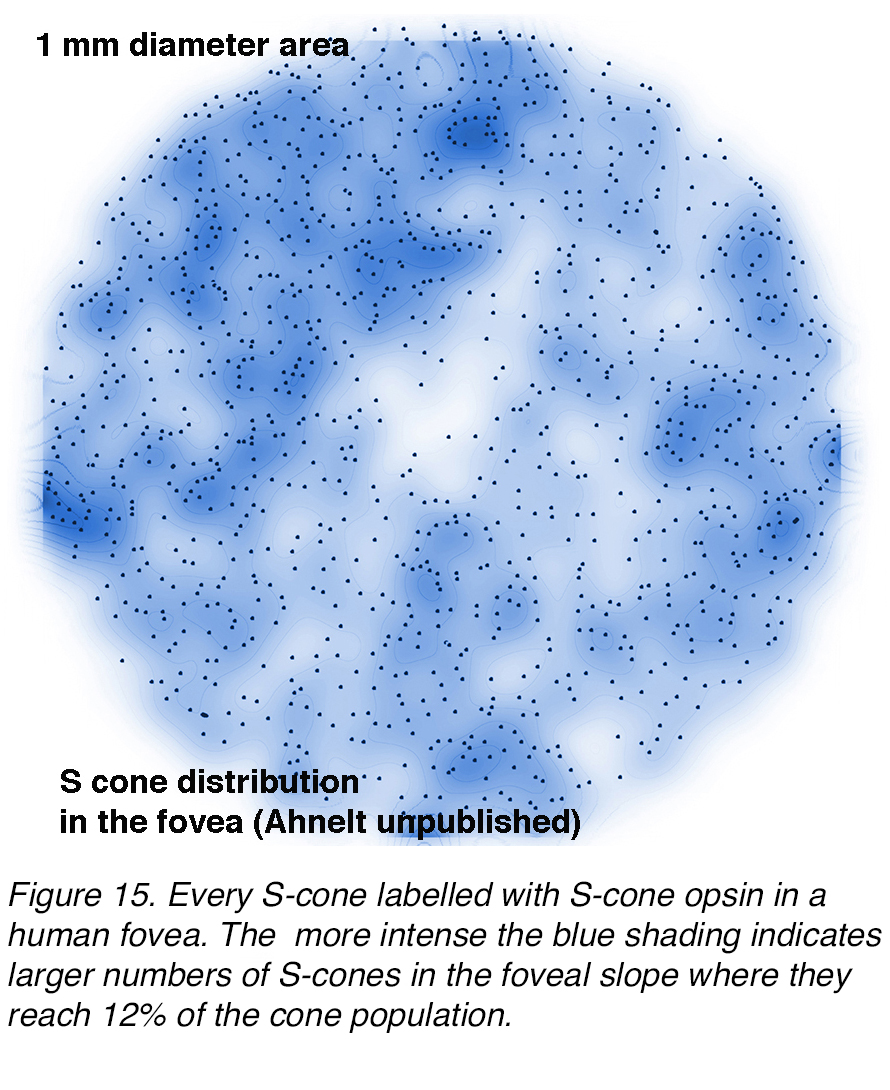 Figure 15. Every S-cone is labelled with S-cone opsin antibody in a human fovea. The more intense blue shading indicates greater densities of S-cones in the foveal slope where they reach 12% of the cone population.
Figure 15. Every S-cone is labelled with S-cone opsin antibody in a human fovea. The more intense blue shading indicates greater densities of S-cones in the foveal slope where they reach 12% of the cone population.
It has been rather easy to identify S-cones in the human fovea and the rest of the retina by these immunocytochemical techniques where S- cones can be visualized and distinguished from the surrounding L- or M-cones. Figure 16 shows a spectacular confocal image of the cones in near peripheral human retina by immunolabeling with cone arrestin, and by the HJ455 antibody to S-cones, that shows up the S-cone opsin both in the outer and inner segments.
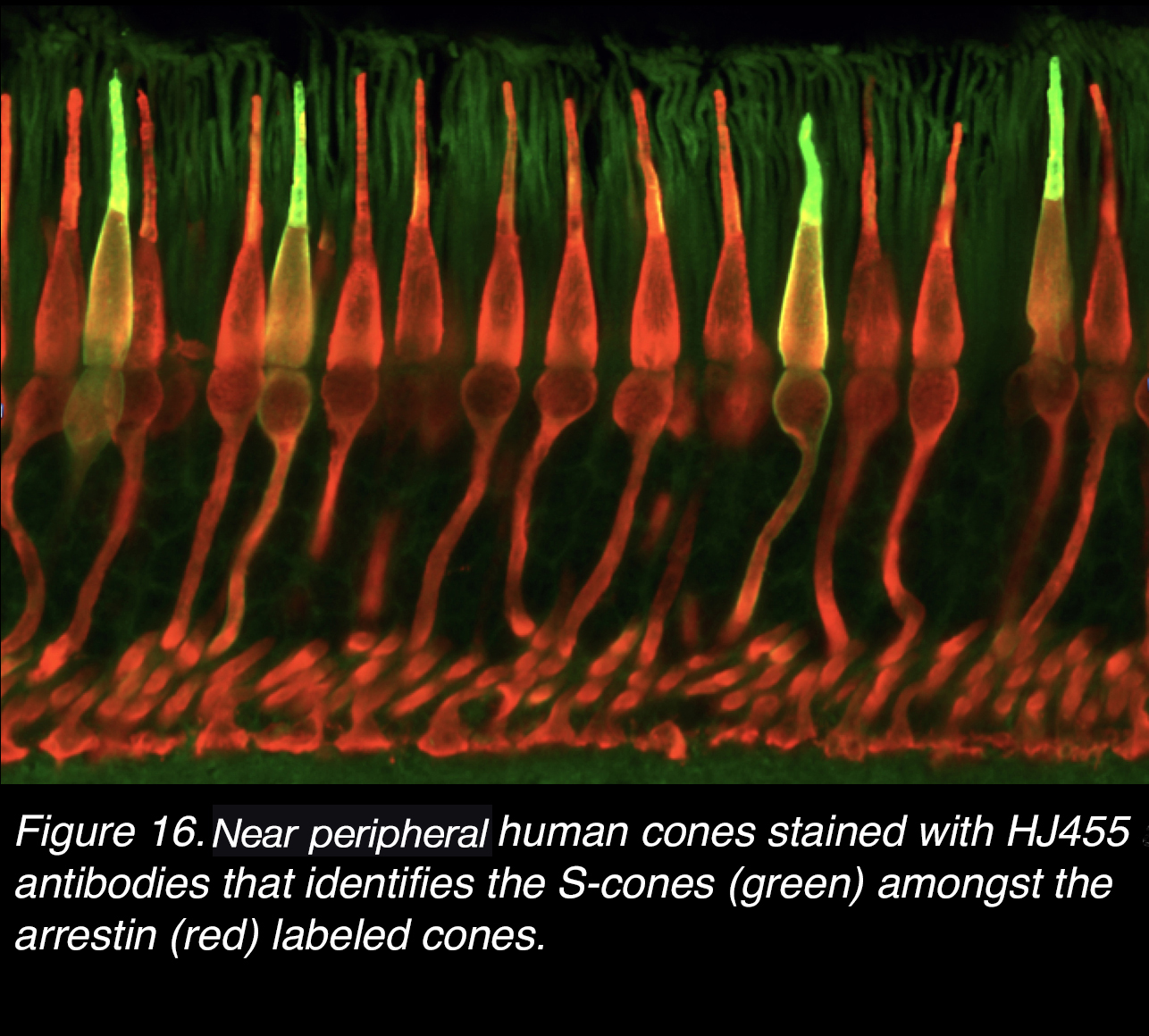 Figure 16. Near peripheral retinal human cones stained with HJ455 antibody that identifies the S-cones (green) amongst the arrestin (red) labeled cones.
Figure 16. Near peripheral retinal human cones stained with HJ455 antibody that identifies the S-cones (green) amongst the arrestin (red) labeled cones.
Sadly, the L-cones and M-cones are not distinguishable on immunostaining techniques because their visual pigments are so close in structure. There is presently no antibody developed to separately mark them into L- or M- cone types. So, to identify L- and M-cones in the human fovea we must go to other more sophisticated techniques. Psychophysical measurements have suggested that L- cones usually outnumber M-cones by 2:1 in the human fovea (17). Microspectrophotometry of all cones in small patches of cones in the fovea of monkeys, has revealed that L- and M-cones occur in about equal proportion (18).
Newer techniques, introduced by Roorda and Williams (19), use adaptive optics to make direct measurements of spectral sensitivity of foveal cones in the living human eye (Figure 17). They found that humans varied greatly in the proportions of L-cones to M-cones: some individuals have almost equal proportions while others have a higher proportion of L-cones, even to the extreme of 16 L-cones to every M-cone (Figure17, BS). While the sparser S-cones are spaced regularly, the L- and M-cones lie randomly in the mosaic meaning that clusters of cones of the same spectral type will occur together as suggested from Mollon and Bowmaker’s paper (18). Roorda and coauthors (20) concluded that L- and M-cones are in a random distribution in the foveal center (21). Nevertheless, the human subjects HS and BS in Figure 17 would seem intuitively to have a different perception of color. But both subjects were reported to have normal color vision (19). A single cone is achromatic, and its stimulation doesn’t result in color vision unless there is comparison to stimulation of a neighbouring cone with different opsin (22). This comparison is done by retinal and brain neural circuitry (see later section on horizontal cell roles in spectral antagonism). Some elegant recent human adaptive optics studies and psychophysical reporting found that 79% of targeted cones in the foveal center, tested for color perception, correctly identified the color (hue) (22). Interestingly, others, using similar techniques of adaptive optics and human reports of hue for single cone stimulation with colored light in the fovea, found a considerable proportion of cones produced only white sensations (21).
 Figure 17. Method of adaptive optics shows mosaics of L (red), M (green) and S (blue) cones in four human subjects with normal color vision. The ratio of S to L and M cones is constant, but that of L to M cones varies from 2.7:1 (L:M) to 16.5:1 (L:M). Adapted from Roorda and Williams, 1999 (19).
Figure 17. Method of adaptive optics shows mosaics of L (red), M (green) and S (blue) cones in four human subjects with normal color vision. The ratio of S to L and M cones is constant, but that of L to M cones varies from 2.7:1 (L:M) to 16.5:1 (L:M). Adapted from Roorda and Williams, 1999 (19).
The architecture of foveal fibres of Henle and pedicles
The process of centrifugal displacement by the Henle layer affects cone pedicles in different ways, depending on their eccentricity (Figure 18).
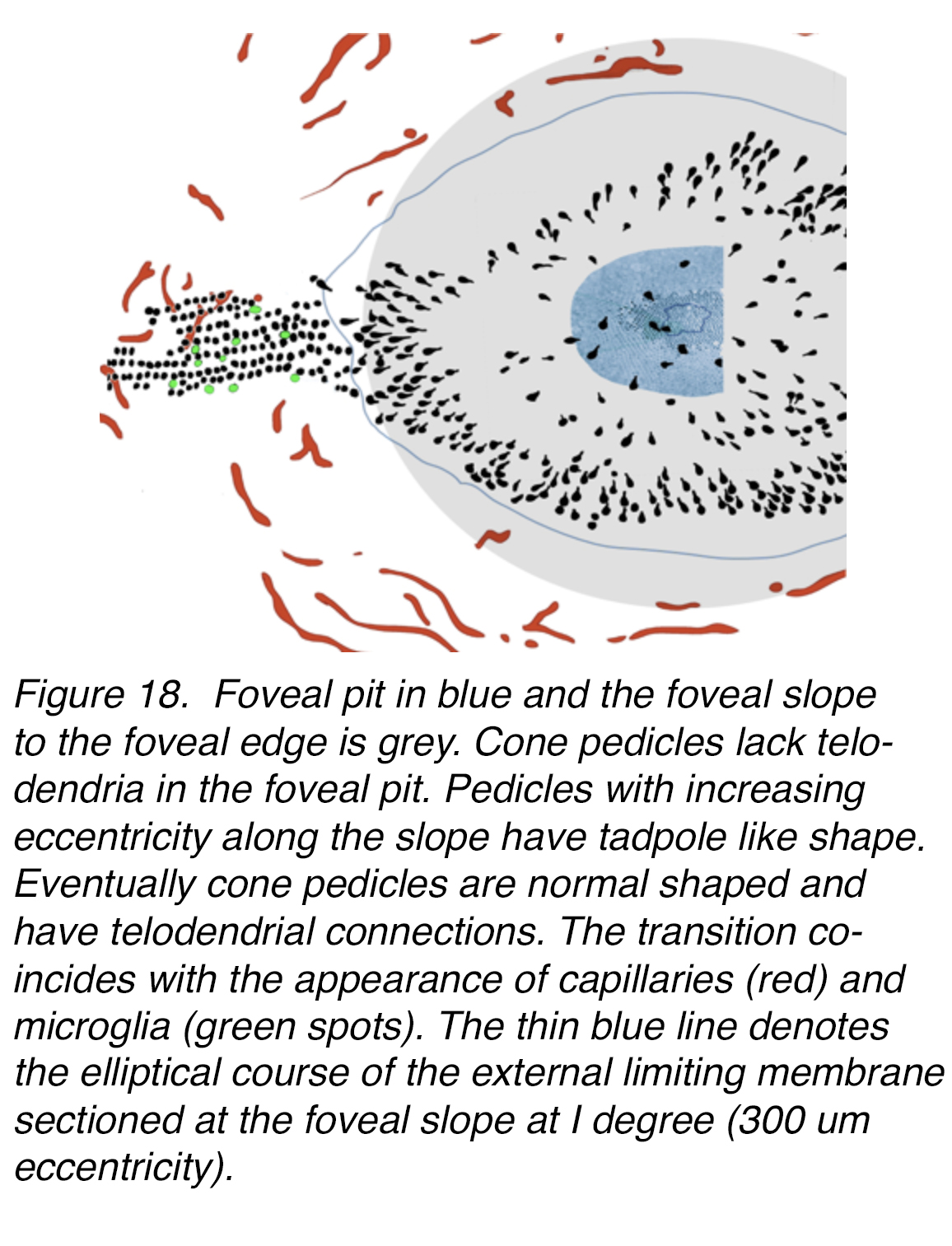 Figure 18. Foveal pit in blue and the foveal slope to the foveal edge in grey. Cone pedicles lack telodendria in the foveal pit. Pedicles with increasing eccentricity along the slope have tadpole-like shape. More peripherally cone pedicles are round in shape and have telodendrial interconnections. The transition coincides with the appearance of capillaries (red) and microglia (green spots). The thin blue line denotes the elliptical course of the external limiting membrane sectioned at the foveal slope at 1 degree (300 µm eccentricity).
Figure 18. Foveal pit in blue and the foveal slope to the foveal edge in grey. Cone pedicles lack telodendria in the foveal pit. Pedicles with increasing eccentricity along the slope have tadpole-like shape. More peripherally cone pedicles are round in shape and have telodendrial interconnections. The transition coincides with the appearance of capillaries (red) and microglia (green spots). The thin blue line denotes the elliptical course of the external limiting membrane sectioned at the foveal slope at 1 degree (300 µm eccentricity).
In the central bouquet of cones in the foveal pit, the pedicles appear to stay in place (Figure 18). In serial semithin (Figure 19, a) and electron microscopic (Figure 19, b) sections, a few roundish pedicles can be found at the foveal floor (Figure 19, a-c, circles). They are isolated from each other, thus lacking any connections to other cones via telodendria. Still they are contacted by dendritic processes running horizontally from a few interneurons (presumably bipolar and horizontal cells) from the foveal slope or even those neurons lying embedded in voluminous Müller cell processes (Figure 19 b-c, red circles around pedicles).
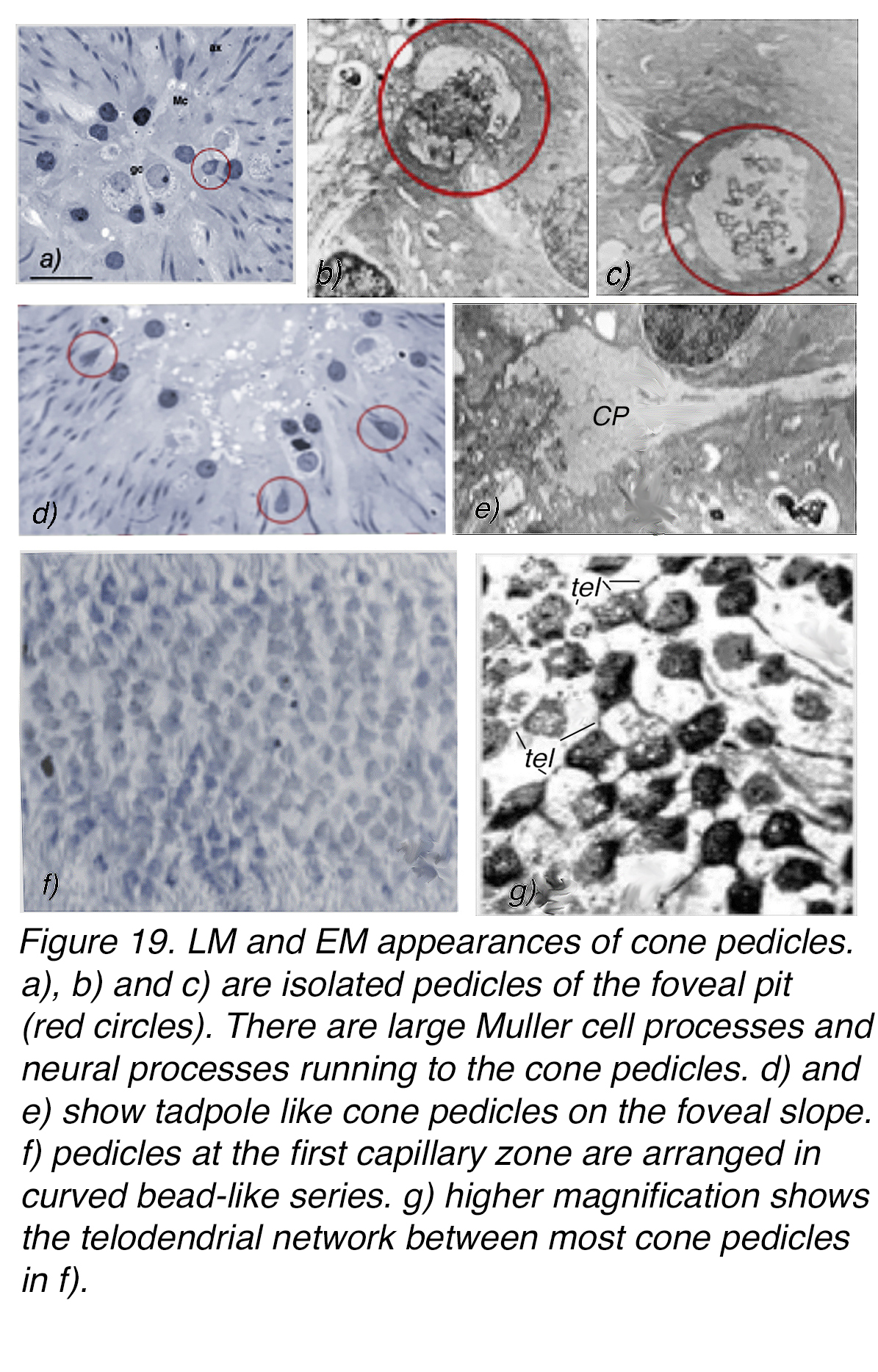 Figure 19. LM and EM appearances of cone pedicles. (a), (b) and (c) are isolated pedicles of the foveal pit (red circles). There are large Müller-cell processes and neural processes running to the cone pedicles. (d) and (e) show tadpole-like cone pedicles on the foveal slope. (f) Pedicles at the first capillary zone are arranged in curved, bead-like series. (g) Higher magnification shows the telodendrial network between most cone pedicles in (f). (a) is from Ahnelt, 1998 (112), ganglion cell (gc), Müller cell (Mc), cone axon (ax), scale bar 50 µm. (g) is from Ahnelt and Pflug 1986 (113).
Figure 19. LM and EM appearances of cone pedicles. (a), (b) and (c) are isolated pedicles of the foveal pit (red circles). There are large Müller-cell processes and neural processes running to the cone pedicles. (d) and (e) show tadpole-like cone pedicles on the foveal slope. (f) Pedicles at the first capillary zone are arranged in curved, bead-like series. (g) Higher magnification shows the telodendrial network between most cone pedicles in (f). (a) is from Ahnelt, 1998 (112), ganglion cell (gc), Müller cell (Mc), cone axon (ax), scale bar 50 µm. (g) is from Ahnelt and Pflug 1986 (113).
From the outer central cones, Henle fibers of short length terminate in peculiar tadpole-like pedicles (Figure 18, Figure 19, d-e). They too are largely isolated from neighboring terminals and are characteristic of the cone pedicles until about 1° or 288 µm out (23). Beyond this zone – still almost entirely established by cone terminals only – the pedicles make up a patchy mosaic (Figure 19, f-g). These terminals elaborate telodendrial networks that end on neighboring cone pedicles at gap junction connections (1, 24). This pedicle mosaic tends to establish radial arrays yet is locally influenced by interspersed glia (Figure 19, g).
The cones of the foveal pit project vertically downwards (Figure 20, a). As the concentrated central cones have to extend their axons radially out of the pit they, together with Müller cells, become the Henle fibers. The cone axons become longer and longer as they project onto the foveal slope and into the parafovea (Figure 20, b, 200-400 µm long). From then on, further out into the perifovea, the axons begin to shorten and by 3 mm eccentricity from the foveal pit axons are essentially no length at all (Figure 20, c-d, 4000 µm periphery). The Henle fiber layer is over as is the macula lutea (Figure 2A, Figure 2B).
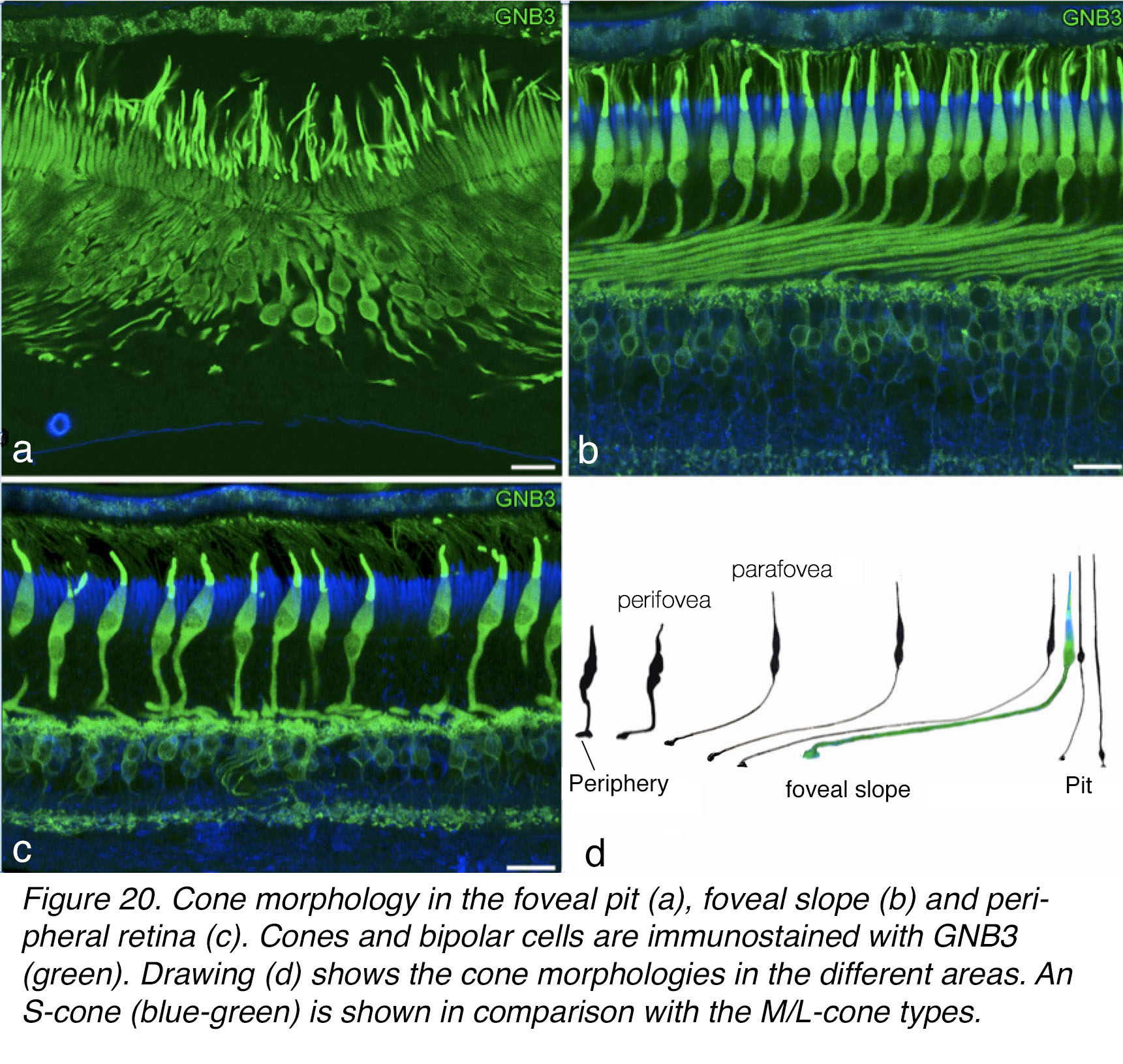 Figure 20. Cone morphology in the foveal pit (a), foveal slope (b) and peripheral retina (c). Cones and ON bipolar cells are immunostained with GNB3 (green). Drawing (d) shows the cone morphologies in the different areas. An S-cone (blue-green) is shown in comparison with the M/L-cone types.
Figure 20. Cone morphology in the foveal pit (a), foveal slope (b) and peripheral retina (c). Cones and ON bipolar cells are immunostained with GNB3 (green). Drawing (d) shows the cone morphologies in the different areas. An S-cone (blue-green) is shown in comparison with the M/L-cone types.
Substructure of the foveal cone architecture
S-cones and M/L-cones differ in the time course of mitotic differentiation and expression of opsins. According to Xiao and Hendickson (25), S-opsin and various synaptic proteins are detectable at fetal week 11, while various synaptic and transduction proteins appear in M/L cone subclasses before their opsin visual pigments are detected at fetal week 13 (26). It is clear that S-cones develop in a different mosaic than M/L-cones. Ahnelt and coworkers (7) have noted that cones likely to be short wavelength sensitive tend to occur in irregular positions in both, foveal and peripheral areas. Figure 21A shows an opsin labeled S-cone (asterisk) positioned between seemingly linear series of unlabeled M/L-cone inner segments. Thus in the foveal all-cone mosaic, S-cones appear to interrupt the linear beads of L/M cone-cell inner segments and clearly do not belong to the mosaic of M- and L-cones (6).
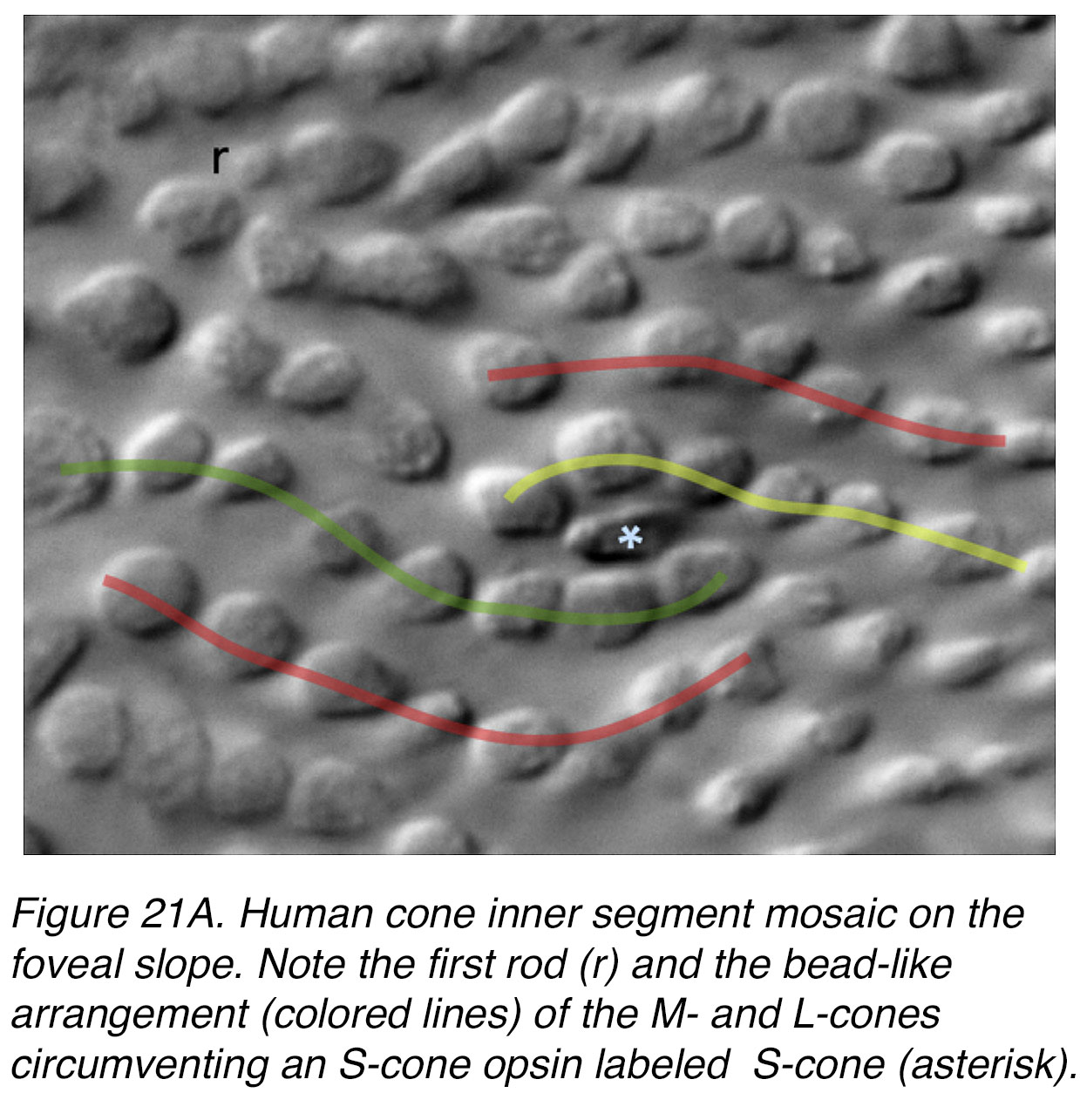 Figure 21A. Human cone inner segment mosaic on the foveal slope. Note the first rod (r), and the bead-like arrangement (colored lines) of the M- and L-cones circumventing an S-cone labeled by an S-opsin antibody (asterisk).
Figure 21A. Human cone inner segment mosaic on the foveal slope. Note the first rod (r), and the bead-like arrangement (colored lines) of the M- and L-cones circumventing an S-cone labeled by an S-opsin antibody (asterisk).
The S-cones form a random mosaic like the M/L cones except at the foveal slope area where they are at highest concentration. Here they approach a non-random distribution (25).
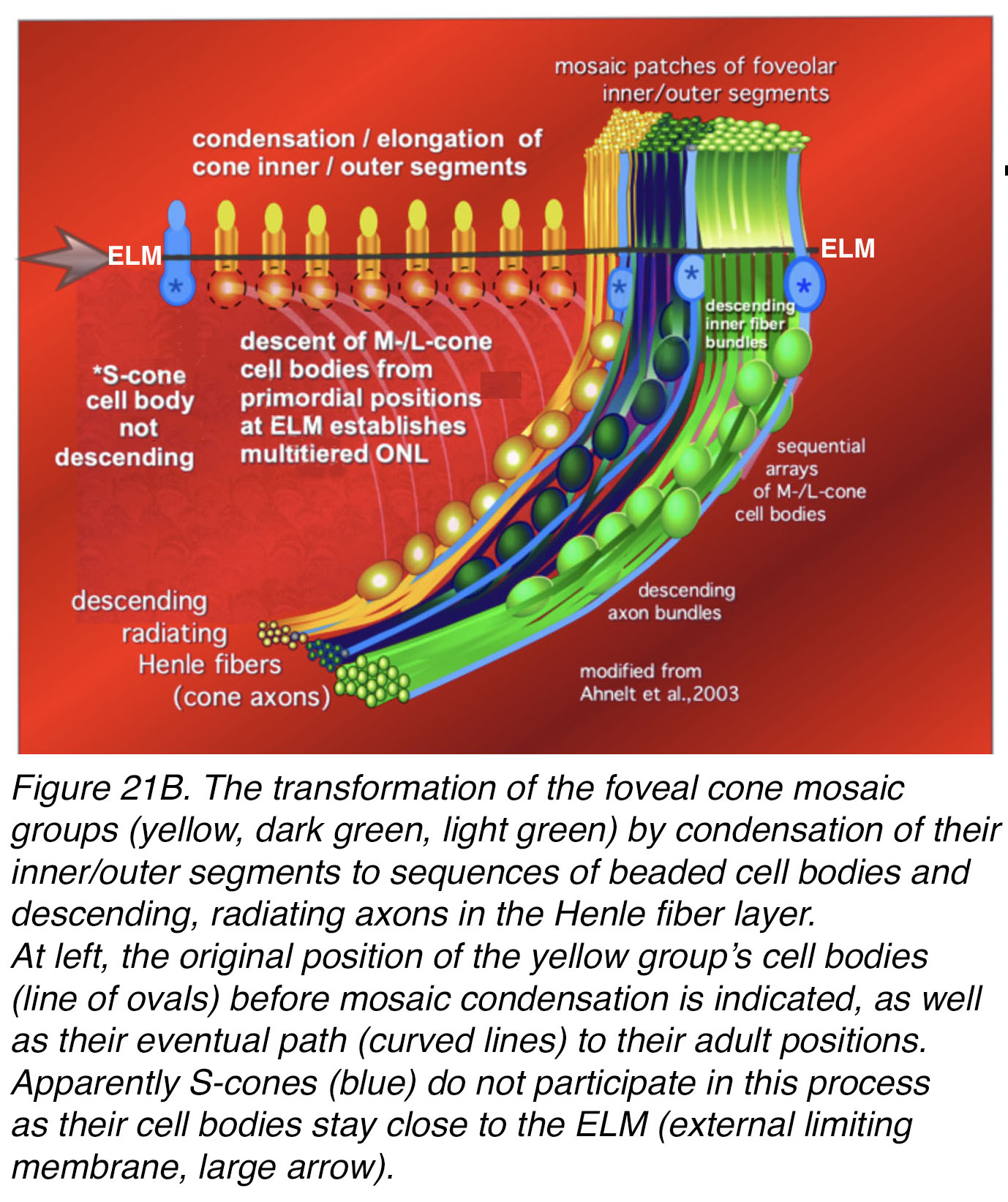
Figure 21B shows a schematic summary (7) of cone arrangement in the mosaic of the foveal slope area where the S-cones develop first and reach the non-random mosaic arrangement (25, 27). Three L/M cone patches are exemplified with false colors (yellow, dark blue green and light green). These have migrated downward from an initial position near the external limiting membrane (ELM) to form bead-like arrangements of M/L cone cell bodies in the depths of the outer nuclear layer (ONL). Their axons (Henle fibers) emerge from the cone nuclear layer and radiate centrifugally towards their pedicles. At the intersection of the L/M patches sits an S-cone always with its cell body, unmigrated, up at the outer limiting membrane. Figure 21B left top, indicates the original position (transparent ovals) of M/L cell bodies before mosaic condensation and their presumed path (tapered rays) to their adult positions.
Figure 21B. The transformation of the foveal cone mosaic groups (yellow, dark green, light green) by condensation of their inner/outer segments to vertical sequences of beaded cell bodies and descending, radiating axons in the Henle fiber layer. At left, the original position of the yellow group’s cell bodies (line of ovals) before mosaic condensation is indicated, as well as their eventual path (curved lines) to their adult positions. Apparently, S-cones (blue) do not participate in this process, as their cell bodies stay close to the ELM (external limiting membrane, large arrow). Adapted from Ahnelt et al, 2004 (7).
Horizontal cells of the fovea
As we have illustrated in Figure 2B, the whole fovea is roughly 1.5 mm across and so any cell found within 750 µm of the foveal center is considered a foveal associated cell. It has been hard to get good staining of horizontal cells (HC) of the fovea but some Golgi impregnated human retinas in our possession did allow us to see a few within the 750 µm of eccentricity around the central foveal pit (Figure 22) (28).
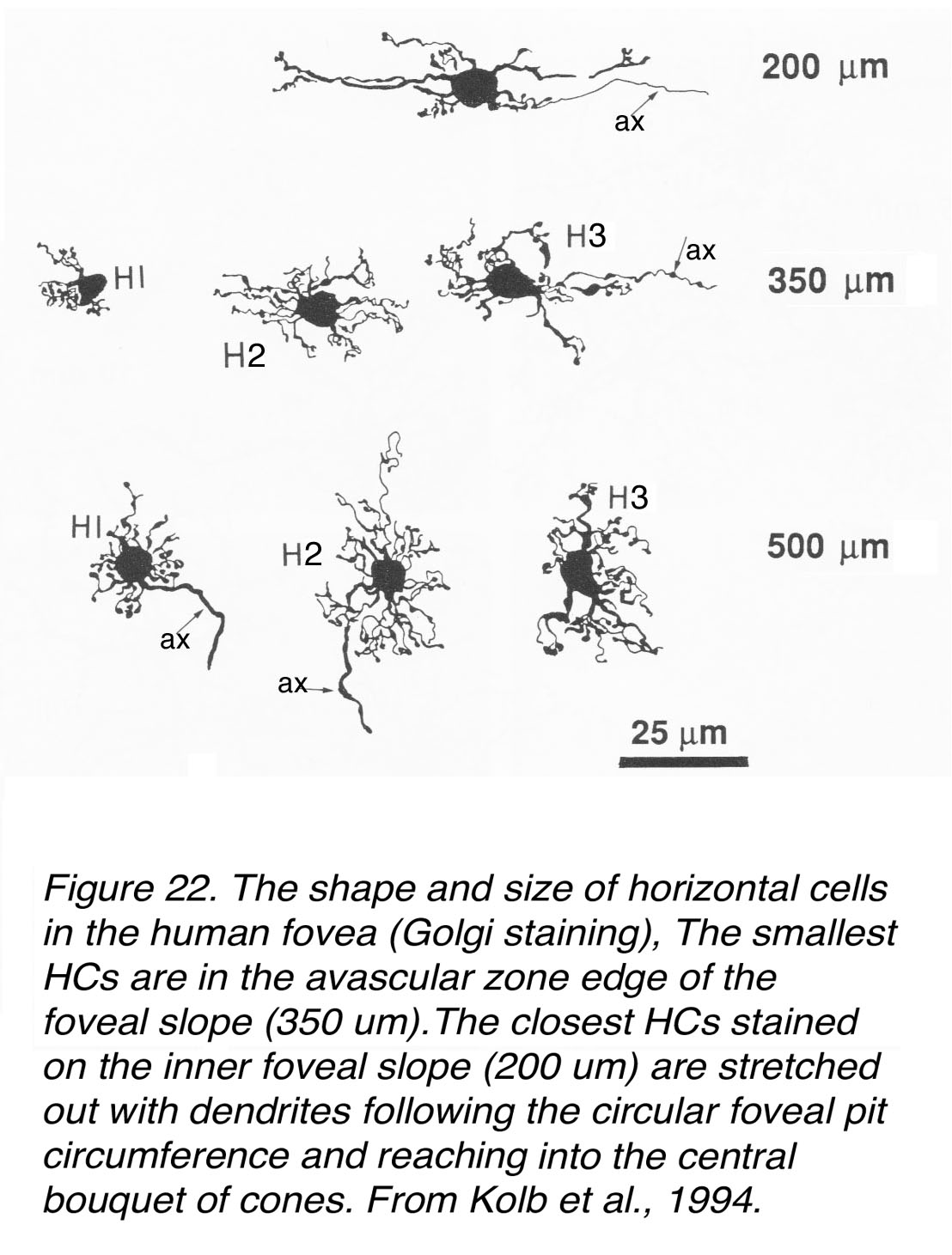 Figure 22. The shape and size of horizontal cells in the human fovea (Golgi staining). The smallest HCs are in the avascular zone edge of the foveal slope (350 µm). The closest HCs stained on the inner foveal slope (200 µm) are stretched out, with dendrites following the circular foveal pit circumference and reaching into the central bouquet of cones. From Kolb et al., 1994 (28).
Figure 22. The shape and size of horizontal cells in the human fovea (Golgi staining). The smallest HCs are in the avascular zone edge of the foveal slope (350 µm). The closest HCs stained on the inner foveal slope (200 µm) are stretched out, with dendrites following the circular foveal pit circumference and reaching into the central bouquet of cones. From Kolb et al., 1994 (28).
The closest to the foveal center, which is of course cell free except for cone photoreceptors and some dendrites running up to synapse with the central cones, would be the HC at 200 µm from the foveal center (Figure 22, top cell). These horizontal cells are elongated and arranged concentrically in a circle around the foveal center and on the far edge of the foveal pit. The area could still be in the avascular zone. Note the dendrites are reaching quite far to contact central cones. The cells are axon bearing, but morphologically it is difficult to judge of which type. The cells at 350 µm (Figure 22) are much smaller than the foveal edge HC but now recognizable as H1, H2 and H3 cell types (28). The smallest are the H1 cells that appear to contact about 4-5 cones, judging by their dendritic clusters. H2 cells are wirier and more irregular than H1 and H3 cells but have quite closely packed and profuse dendrites (Figure 22). These H2 cells would be reaching into the foveal slope area, where we know there is the highest density of S-cones, to contact the latter cone type. H3 cells may also be reaching into the foveal slope but we know from previous data they do not receive synapses from S-cones (29, 30). There are no evident axons on these Golgi stained horizontal cells (Figure 22, 350 µm), which probably reflects understaining.
The three horizontal cells at 500 µm from the foveal center (Figure 22) would also be foveal HCs but in an area where blood vessels occur and the first rod photoreceptors are present. As can be seen they are a little larger in dendritic field size (Figure 22). The H1 cell contacts 6 cones and the H3 about 8-9 cones (Figure 22). H1 and H2 types here have axons (small arrows in Figure 22), which will expand into axon terminals in contact with rods in the case of H1, and with S-cones in the case of H2 cells (31).
By confocal microscopy the central human fovea can be seen to contain parvalbumin immunoreactive horizontal cells (Figure 23, a-b; green cells under the cone pedicles). Parvalbumin identifies H1/H3 horizontal cell types and it is likely that the Golgi staining at the 200 µm distance from the central foveal pit is therefore of these types. They are elongated and not closely packed. Their dendrites would be reaching to contact central foveal bouquet cones (Figure 23, b). In contrast, the H1s of the foveal slope are closely packed with vertically squashed cell bodies and small bushy dendrites reaching to the closely packed cone pedicles at the ends of the Henle-fiber-layer cone axons (Figure 23, c). These HCs are clearly the same as those in the Golgi preparations at 300-500 µm (Figure 22).
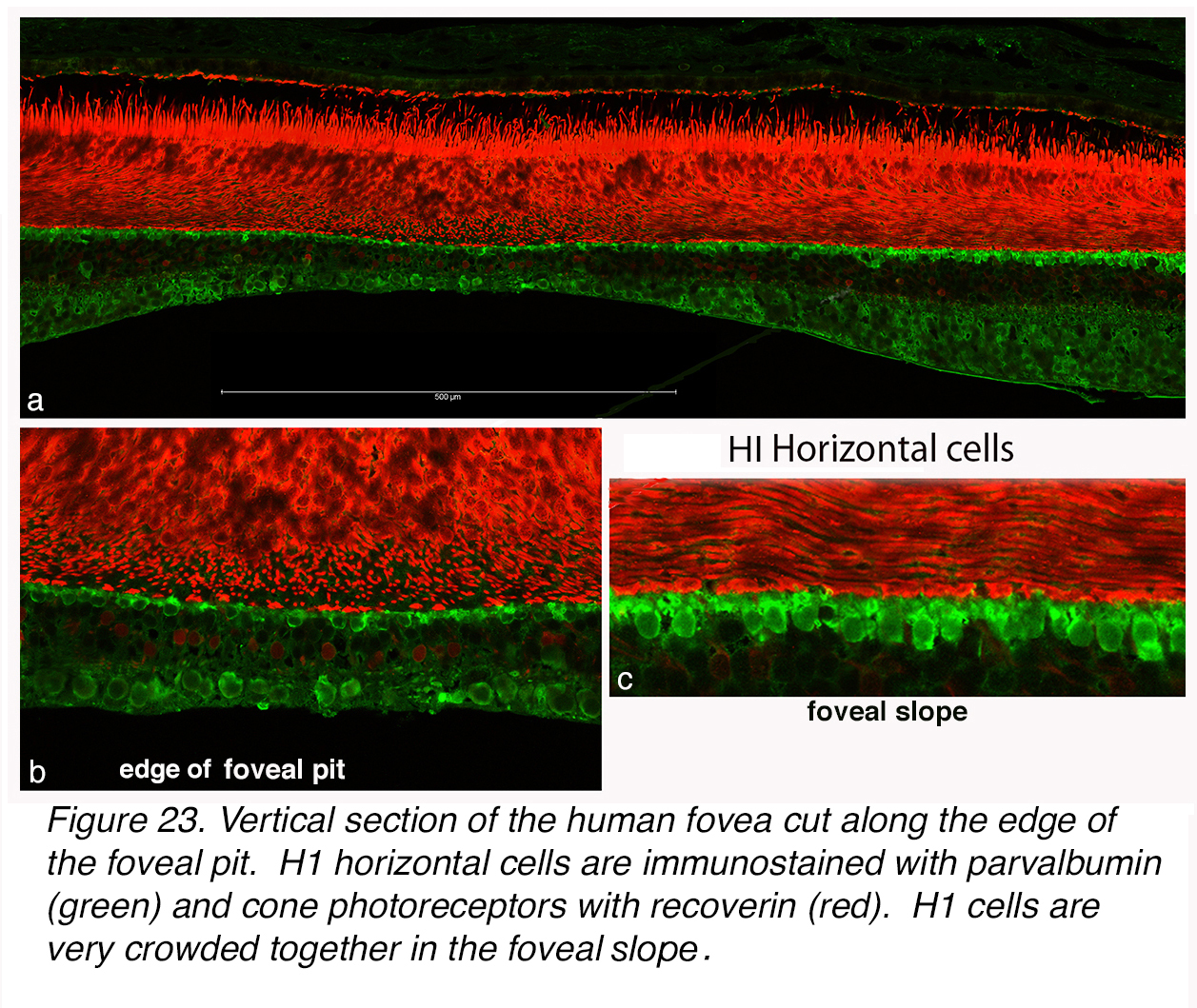 Figure 23. Vertical section of the human fovea cut along the edge of the foveal pit. H1 horizontal cells are immunostained with anti-parvalbumin (green) and cone photoreceptors with recoverin (red). H1 cells are very crowded together in the foveal slope.
Figure 23. Vertical section of the human fovea cut along the edge of the foveal pit. H1 horizontal cells are immunostained with anti-parvalbumin (green) and cone photoreceptors with recoverin (red). H1 cells are very crowded together in the foveal slope.
The H2 cells of the human retina are known to be particularly associated with the S-cone (blue) photoreceptors (see Webvision chapter on S-cone pathways). We know that H2 cells stain with antibodies to calbindin in the human retina as compared to parvalbumen staining for H1/H3 cells. Figure 24 (white arrows) shows a few calbindin positive HCs (red cells, arrows) on the foveal slope in human retina. In addition to the H2 cells with cell bodies close to the OPL, there are diffuse cone bipolar cells contacting several cones, and amacrine cells stained with calbindin. These red, diffuse bipolar cells have cell bodies lower in the inner nuclear layer and long slanted single apical dendrites as compared to the red H2 cells. Note in this section of human fovea the first rods are present on the foveal slope and the first rod bipolar cells are staining for the antibody to PKC (Figure 24, green cells).
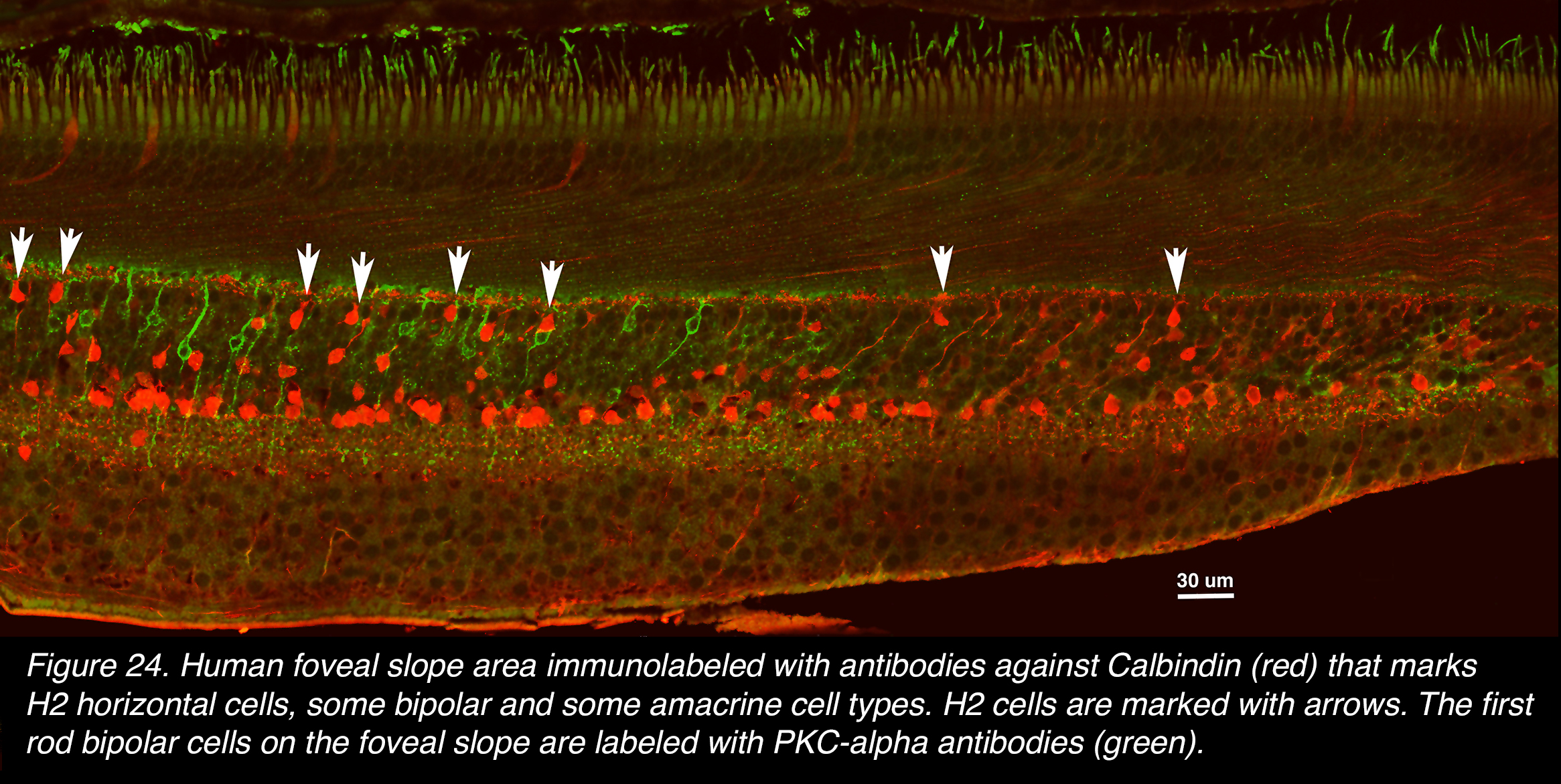 Figure 24. Human foveal slope area immunolabeled with antibodies against calbindin (red) that marks H2 horizontal cells, some bipolar and some amacrine cell types. H2 cells are marked with arrows. The first rod bipolar cells on the foveal slope are labeled with PKC-alpha antibodies (green).
Figure 24. Human foveal slope area immunolabeled with antibodies against calbindin (red) that marks H2 horizontal cells, some bipolar and some amacrine cell types. H2 cells are marked with arrows. The first rod bipolar cells on the foveal slope are labeled with PKC-alpha antibodies (green).
Horizontal cells of the vertebrate retina are known to have important roles in sharpening and scaling of responses from photoreceptors through the subsequent retinal pathways to influence the ganglion cell output (32). At the first level of the outer plexiform layer, horizontal cells are involved in feedback of signal from surrounding cones to each individual cone’s receptive field. This surround input is expanded well beyond the horizontal cell’s dendritic connectivity field by virtue of gap junctions that join the dendrites of many horizontal cells of the same type together. i.e. in human retina the H1-H1 cells would be joined in gap junctions and the H2 cells would likewise be joined to other H2 cells (See the Webvision chapter Myriad roles for gap junctions in retinal circuits). This large feedback effect provokes an expanded region of antagonistic signal compared with the central cone signal. In the case of M- or L-cones the antagonistic surround is a mixed M- and L-cone signal. In other words, individual M- and L-cones do not show classic spectral opponency just mixed M- / L-cone surround antagonism (33). The feedback in the case of an S-cone would come from H2 cells, whose contacts include surrounding M- and L-cones. Indeed S-cones have been recorded from in monkey retina and found to have blue–yellow spectral opponency as well as center-surround organization (34, 35). Presumably spatial opponency would be transmitted from the M- and L-cones to their respective bipolar cell connections, and in the case of the S-cone, a true spectral opponency has been proven to be transmitted as well (34). No recordings have been made in foveal cones to really see if an M- or L-cone has a spectrally opponent surround like that of (albeit peripheral) S-cones (35).
Midget bipolar cells of the fovea
A long time ago the great Spanish anatomist, Santiago Ramón y Cajal described the neurons of the different vertebrate retinas as seen by sectioned Golgi-stained material. He noted many different types of bipolar cells in the various species and that there were particularly tiny dendritic spreads for some bipolar cells in the bird retina (36). He suggested that these bipolar cells contacted single cones.
In 1941, Stephen Polyak (Figure 25) published books on the neural cell types revealed by Golgi and other silver methods in monkey and human retinas and brain. In central monkey and human retinas Polyak observed and illustrated several types of bipolar cells, but he was very concentrated on the remarkably small dendritic tops of some types that he construed as contacting single cones. He named these bipolar cells, midget bipolar cells (mbc).
 Figure 25. Steven Polyak circa 1940.
Figure 25. Steven Polyak circa 1940.
Figure 26 shows Polyak’s original drawing of these midget bipolar cells and larger dendritic field size bipolar cells that would appear to contact several cones (Figure 26, imb, fmb and dfb). Polyak also drew and commented briefly that the midget bipolar cells appeared to be of two varieties, one that had a long axon to the inner plexiform layer, and the other a much shorter axon ending higher in the inner plexiform layer. At the same time, there were midget ganglion cells that had small dendritic trees that came in the two varieties possibly reaching to the axon terminals of the two types of midget bipolar cells (Figure 26, mgcs).
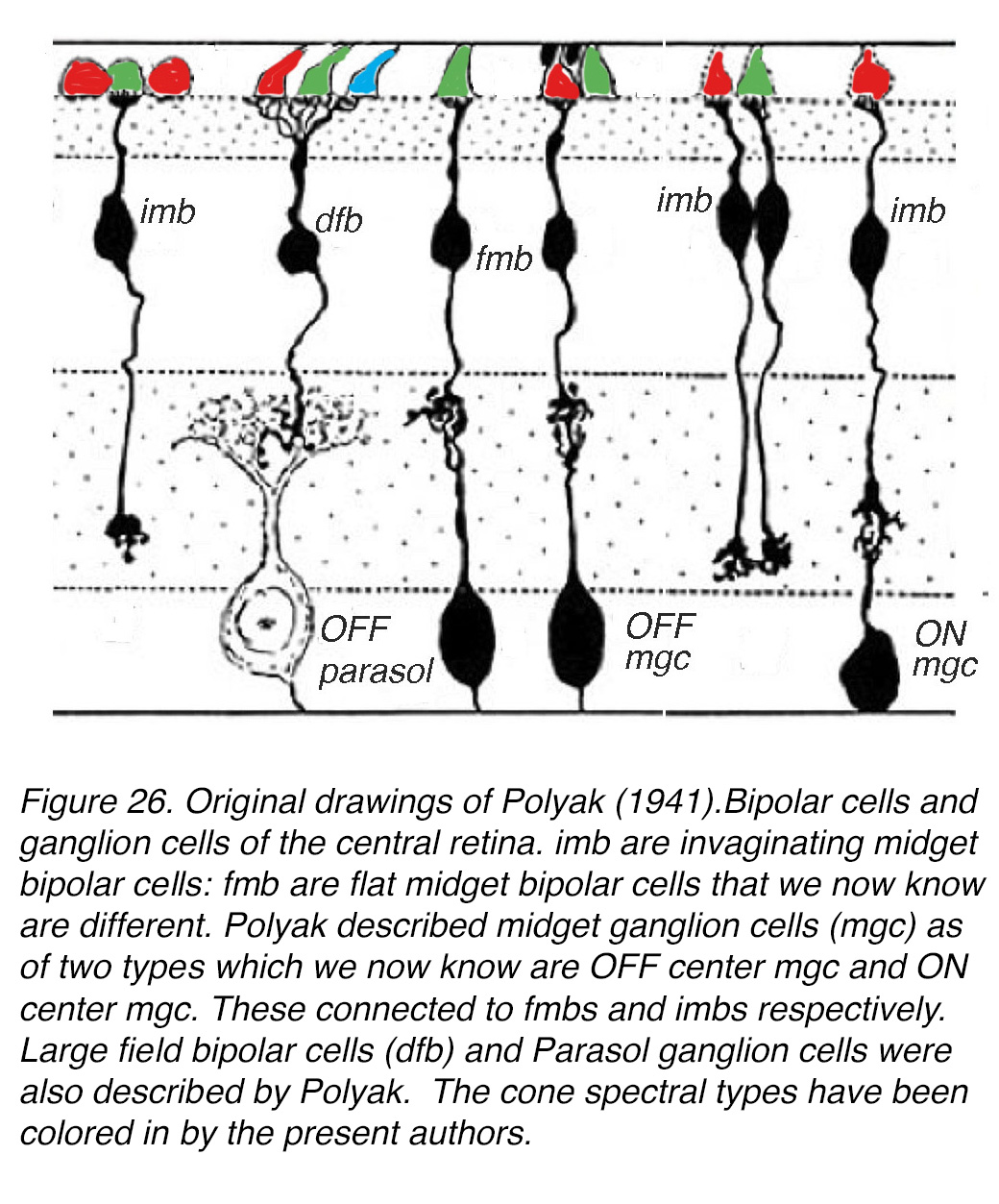 Figure 26. Original drawings of Polyak (90). Bipolar cells and ganglion cells of the central retina. We now know that the invaginating midget bipolar cells (imb) and flat midget bipolar cells (fmb) are physiologically different. Polyak described midget ganglion cells (mgc) as of two types, which we now know are OFF mgc and ON mgc. These connect to fmbs and imbs respectively. Large field bipolar cells (dfb) and parasol ganglion cells were also described by Polyak. The cone spectral types have been colored in by the present authors.
Figure 26. Original drawings of Polyak (90). Bipolar cells and ganglion cells of the central retina. We now know that the invaginating midget bipolar cells (imb) and flat midget bipolar cells (fmb) are physiologically different. Polyak described midget ganglion cells (mgc) as of two types, which we now know are OFF mgc and ON mgc. These connect to fmbs and imbs respectively. Large field bipolar cells (dfb) and parasol ganglion cells were also described by Polyak. The cone spectral types have been colored in by the present authors.
Some years later, we set out to see whether Polyak was correct in assuming midget bipolar dendrites synapsed only with one cone pedicle (37, 38) using the technique of performing electron microscopy on Golgi impregnated cells located by light microscopy (39). It turned out that, indeed midget bipolar cells contacted a single cone, but unexpectedly, there were two types of midget bipolar cells each having different synaptic connections with their cone pedicles (Figure 27). One type, that we called flat midget bipolar cells, had a particularly horizontally orientated and flattened dendritic top and made contacts with the cone pedicle on either side of the central dendrite at the synaptic ribbon triad of processes (Figure 27, a-c). The other type of midget bipolar that we called an invaginating midget bipolar cell had a spikey dendritic top with small finger like projections (tiny arrow, Figure 27, d) that contacted the cone pedicle as the central element of the ribbon triad of processes (Figure 27, e-f).
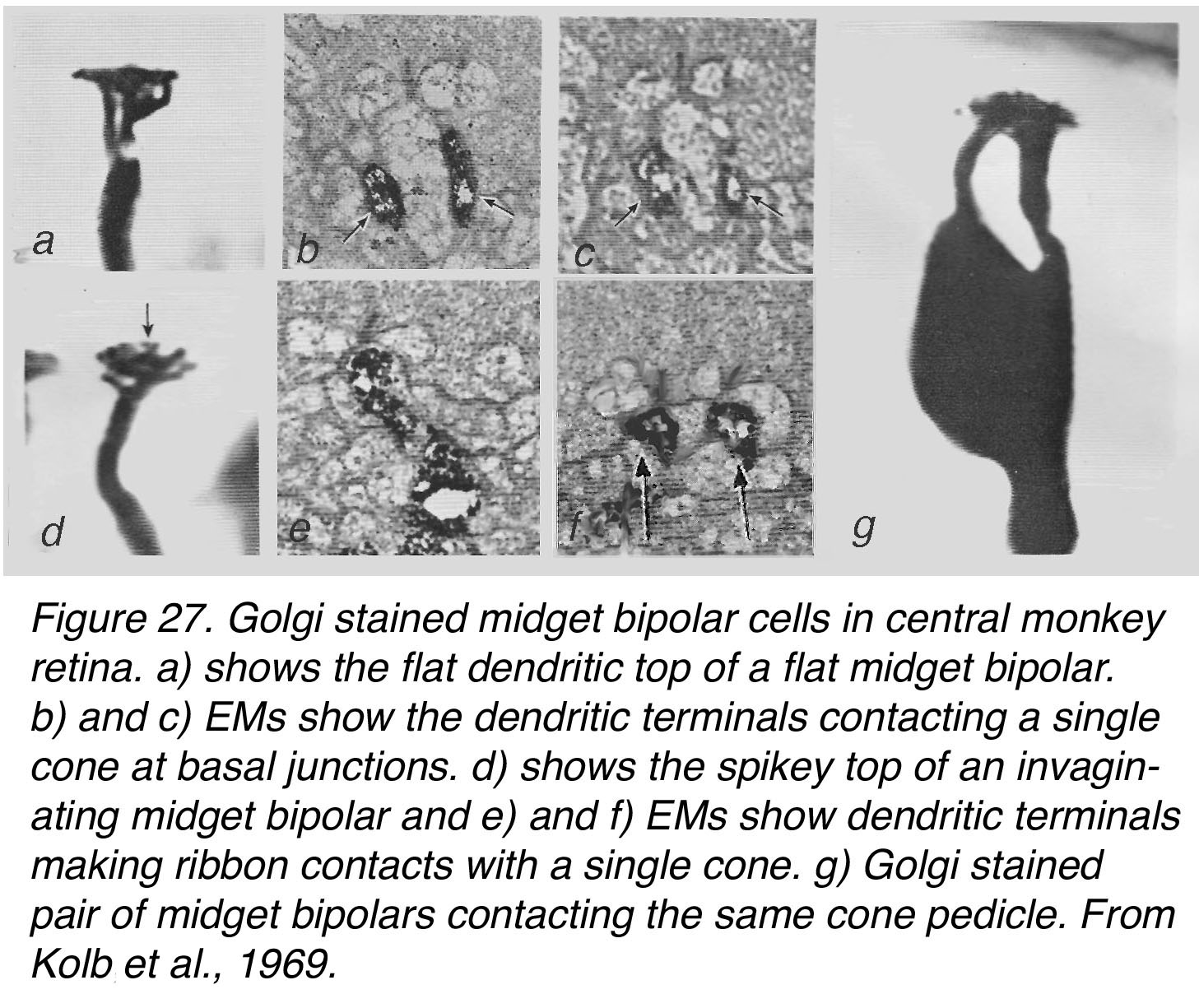 Figure 27. Golgi-stained midget bipolar cells in central monkey retina. (a) shows the flat dendritic top of a flat midget bipolar. (b) and (c) electron micrographs (EMs) show the dendritic terminals contacting a single cone at basal junctions. (d) shows the spikey top of an invaginating midget bipolar and (e) and (f) EMs show dendritic terminals making ribbon contacts with a single cone. (g) Golgi-stained pair of midget bipolar cells contacting the same cone pedicle. From Kolb, 1970 (37).
Figure 27. Golgi-stained midget bipolar cells in central monkey retina. (a) shows the flat dendritic top of a flat midget bipolar. (b) and (c) electron micrographs (EMs) show the dendritic terminals contacting a single cone at basal junctions. (d) shows the spikey top of an invaginating midget bipolar and (e) and (f) EMs show dendritic terminals making ribbon contacts with a single cone. (g) Golgi-stained pair of midget bipolar cells contacting the same cone pedicle. From Kolb, 1970 (37).
By careful examination of our Golgi stained monkey and human retinas we could occasionally observe that the two Golgi stained midget bipolar cells types converged on the same cone (40) (Figure 27, g).
Confocal imaging of the human foveal slope and rim can distinguish the two types of midget bipolar cell by using different immunostains. Figure 28 illustrates the GNB3 stained cones, Henle fibers and the invaginating midget bipolar cells (in green) contacting single cone pedicles and sending axonal endings to the lower (more vitread) strata of the inner plexiform layer to end close to the multitiered ganglion cell layer of predominantly midget ganglion cells. GNB3 is a G-protein subunit common to both cones and ON bipolar cells (41). The invaginating midget bipolar cells are also reaching into the foveal center to contact the central isolated cones of the cone bouquet (Figure 28, c, inset).
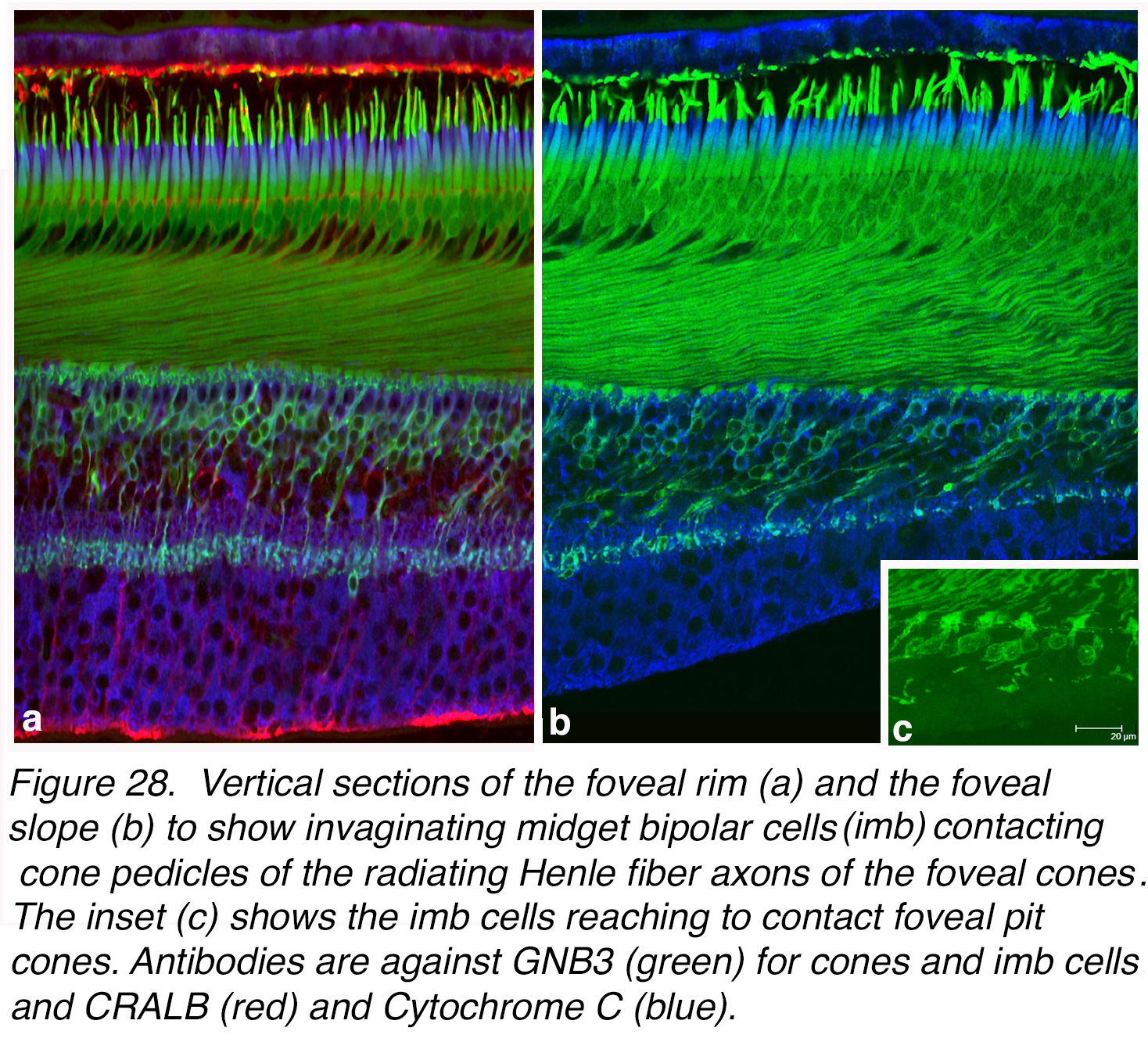 Figure 28. Vertical sections of the foveal rim (a) and the foveal slope (b) to show invaginating midget bipolar cells (imb) contacting cone pedicles of the radiating Henle fiber axons of the foveal cones. The inset (c) shows the imb cells reaching to contact foveal pit cones. Antibodies are against GNB3 (green) for cones and imb cells, CRALBP (red) marks Müller cells and pigment epithelial cells, and Cytochrome C stains mitochondria (blue).
Figure 28. Vertical sections of the foveal rim (a) and the foveal slope (b) to show invaginating midget bipolar cells (imb) contacting cone pedicles of the radiating Henle fiber axons of the foveal cones. The inset (c) shows the imb cells reaching to contact foveal pit cones. Antibodies are against GNB3 (green) for cones and imb cells, CRALBP (red) marks Müller cells and pigment epithelial cells, and Cytochrome C stains mitochondria (blue).
In contrast, Figure 29 shows a section of the human fovea immunostained with recoverin antibodies (red) known to reveal flat midget bipolar cells specifically (42), as well as photoreceptors (43). These flat midget bipolar cells are contacting each a single cone pedicle and their axons are angled with the foveal slope direction to their axon terminals ending high (more sclerad) in the outer strata of the IPL. The more isolated cone pedicles of the foveal pit (Figure 29, b, inset) are clearly being contacted by these flat midget bipolar cells too.
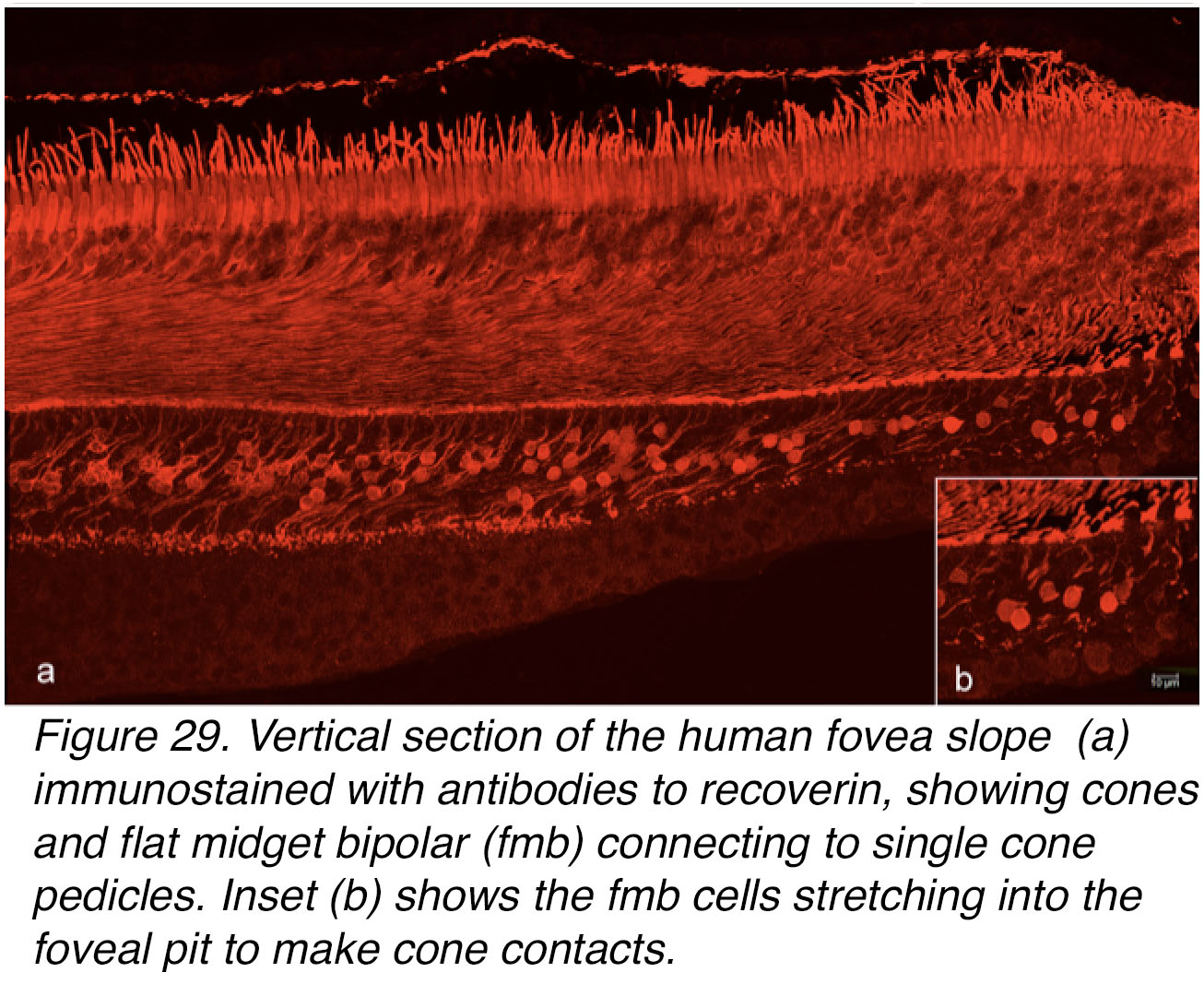 Figure 29. Vertical section of the human fovea slope (a) immunostained with antibodies to recoverin, showing cones, and flat midget bipolar cells (fmb) connecting to single cone pedicles. Inset (b) shows the fmb cells stretching into the foveal pit to make cone contacts.
Figure 29. Vertical section of the human fovea slope (a) immunostained with antibodies to recoverin, showing cones, and flat midget bipolar cells (fmb) connecting to single cone pedicles. Inset (b) shows the fmb cells stretching into the foveal pit to make cone contacts.
So, we understand now that each foveal cone photoreceptor synapses with two midget bipolar cells. We also now know that there are four or five other types of cone bipolar cells (Figure 26, dfb) that overlap to contact many cones in primate retina, as in other vertebrate retinas (see Websion chapter on Outer Plexiform Layer). Midget bipolar cells are only found in primate and bird retinas though, and these are the only retinas that have a fovea. Hence the very special nature of the foveal midget system in high visual acuity and color processing.
Midget ganglion cells and the midget system for red L and green M-cones in the fovea
We have seen how every cone of the foveal area contacts two types of midget bipolar cell which in turn contact a midget ganglion cell each. The flat midget bipolar cell (fmb) contacts the type of midget ganglion cell that reaches high into the IPL, while the invaginating midget bipolar (imb) cell contacts the type of midget ganglion cell that has dendrites in the lower IPL close to its cell body in the ganglion cell layer, as was first suggested by Polyak’s drawing (Figure 26). Figure 30 illustrated this finding for Golgi stained midget bipolar cells (mBC) and ganglion cells (mGCs) as seen in the human fovea itself and at 2 mm distance from the fovea i.e. in perifovea. In the fovea where rods are absent (Figure 30, a, foveal cone mosaic) the midget bipolar cells and midget ganglion cells have very small axon terminals and dendrites respectively (less than 10 µm span) and in one image can be seen to stain clasped together (Figure 30, c left cell couple). In the perifovea the cones are now separated by rings of rods (Figure 30, b, receptor mosaic) and the related mb axon terminals and mGC dendrites are slightly wider spread (10-12 µm) (Figure 30, d).
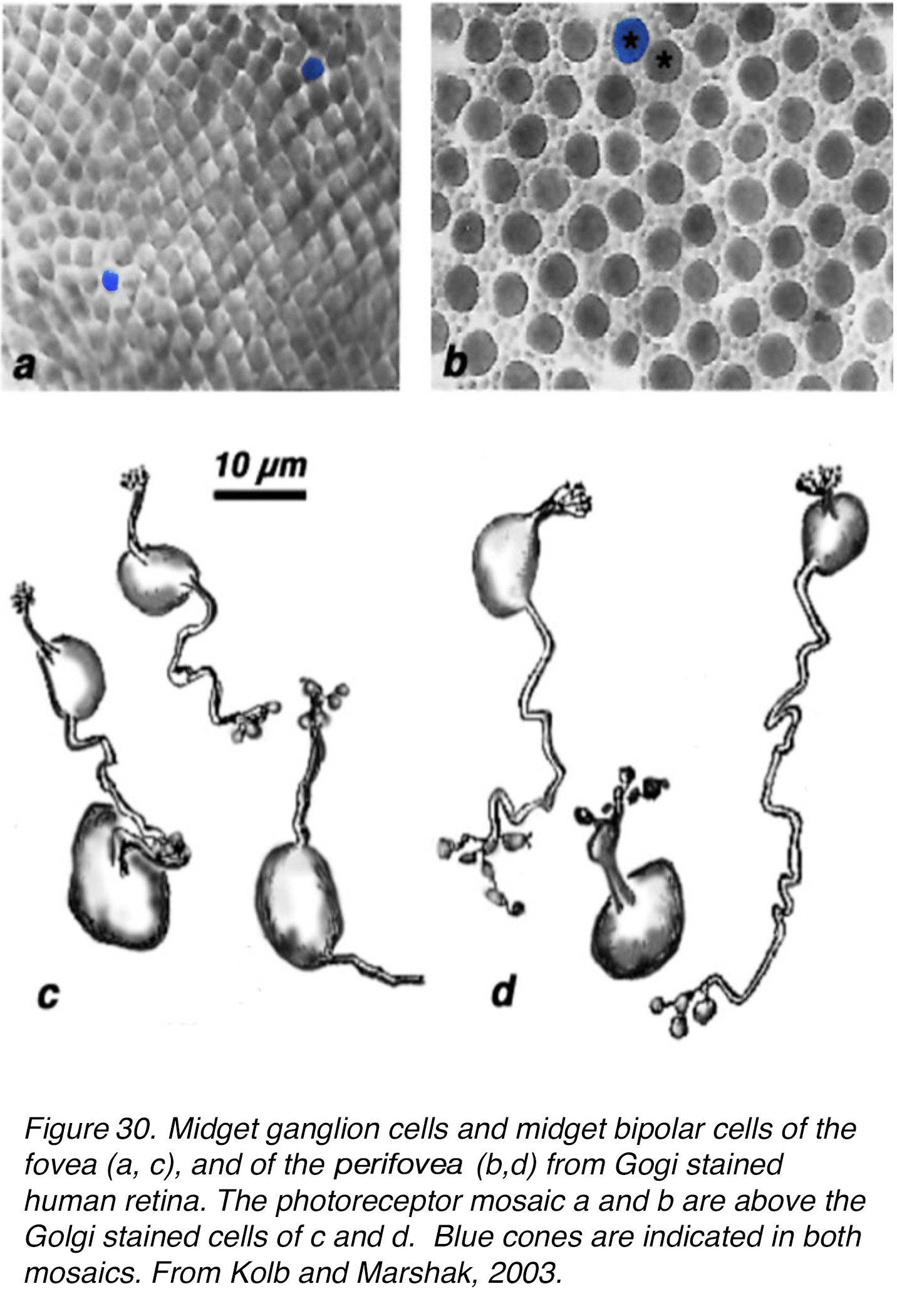 Figure 30. Midget ganglion cells and midget bipolar cells of the fovea (c), and of the perifovea (d) from Golgi-stained human retina. The photoreceptor mosaics (a, b) are above the Golgi-stained cells of (c) and (d). Blue cones are indicated in both mosaics. Midget ganglion cells (mGC), midget bipolar cells (mBC). From Kolb and Marshak, 2003 (46).
Figure 30. Midget ganglion cells and midget bipolar cells of the fovea (c), and of the perifovea (d) from Golgi-stained human retina. The photoreceptor mosaics (a, b) are above the Golgi-stained cells of (c) and (d). Blue cones are indicated in both mosaics. Midget ganglion cells (mGC), midget bipolar cells (mBC). From Kolb and Marshak, 2003 (46).
We have been able to reconstruct the midget bipolar and midget ganglion cell synaptic connections by serial section electron microscopy in the fovea and perifovea, in order to see whether there is indeed a one to one relationship (44, 45). Figure 31 shows some sample micrographs from flat-midget-ganglion-cell (mgc) contacts with flat midget bipolar cells (fmb) in the foveal slope (rod free area) and both types of bipolar to ganglion cell contacts in the perifoveal (as in the area of Figure 30, b). All our EM images show all ribbon contacts from one mbc axon to dendrites of a single mGC in the perifoveal material. Typically, there is an amacrine cell companion at the postsynaptic dyad (Figure 31, a-d). Frequently the amacrine cells make reciprocal (feedback) synapses to the midget bipolar terminal, and occasionally to the midget GC involved as well (Figure 31, d). The amacrines are probably of at least two types involved in these synaptic exchanges (see later section on amacrines of the fovea).
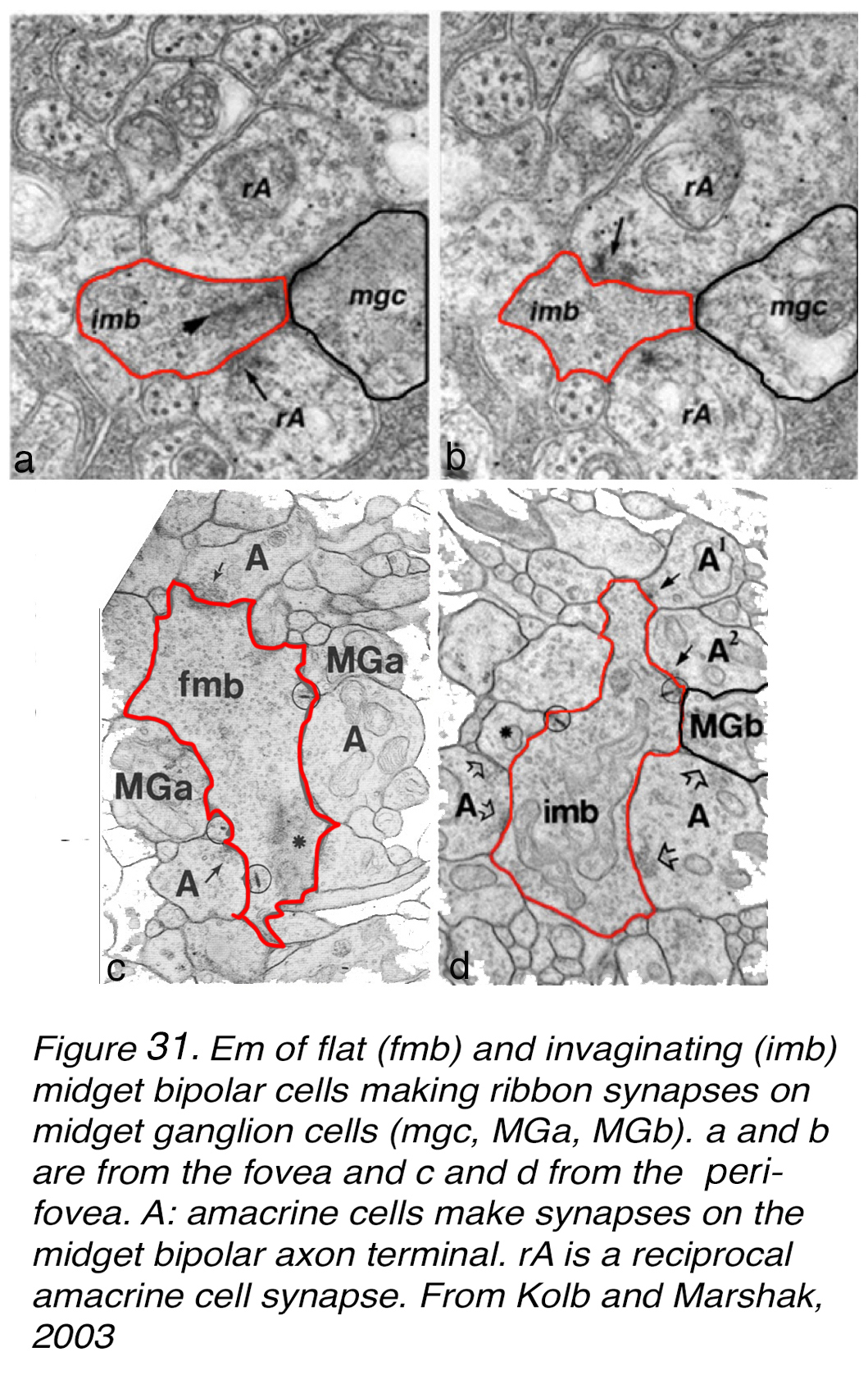
Figure 31. EM of flat (fmb) and invaginating (imb) midget bipolar cells making ribbon synapses on midget ganglion cells (mgc, MGa, MGb). (a) and (b) are from the human fovea and (c) and (d) from the parafovea. A: amacrine cells make synapses on the midget bipolar cell axon terminals. rA is a reciprocal amacrine cell synapse. From Kolb and Marshak, 2003 (46).
The reconstructions of the EM serial photographs are shown in Figure 32 (44, 45). The reconstructed perifoveal invaginating and flat midget bipolar cells with their respective ganglion cells showed almost exclusive interaction between a bipolar axon and a ganglion cell dendritic tree. There were 2 or so ribbon synapses that could have been directed to another ganglion cell dendrite in the 4 pairs of cells reconstructed (Figure 32, Perifovea). In the case of the reconstruction of midget bipolar cells and midget ganglion cells of the fovea (Figure 32, Fovea at 570 µm) we determined that there were 3 imbcs to 2 mgcs (Figure 32, fovea, very left-hand cells) and 2 imbcs to a single midget GC (Figure 32, Fovea, right side). However, the limits of our series of the reconstruction probably mean that we missed some adjoining mGCs in this tightly packed neuropil of very small dendritic and axonal profiles. We suggested (46) that each midget bipolar type directed the majority of its synapses to a single midget ganglion cell top, but some synapses probably got shared with a neighboring midget ganglion cell too.
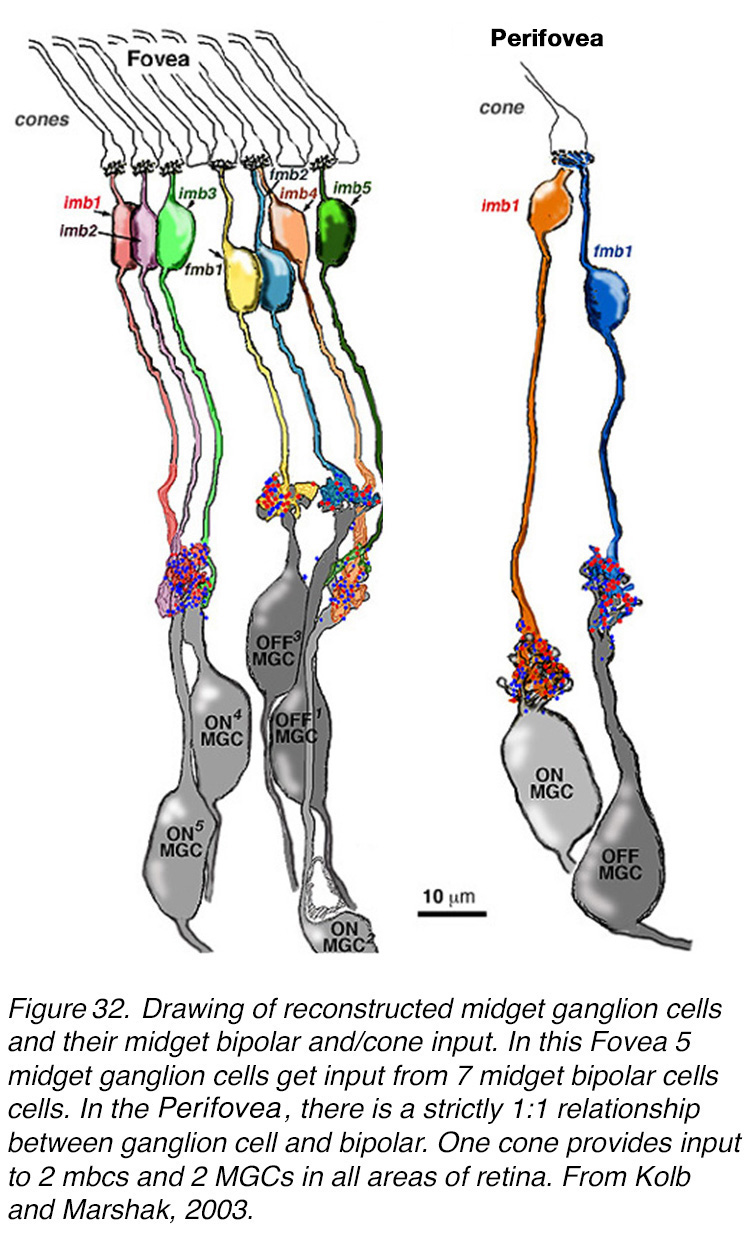
Figure 32. Drawing of reconstructed human midget ganglion cells with their cone input as routed through midget bipolar cells. Fovea: 5 midget ganglion cells get input from 7 midget bipolar cells. Perifovea: there is a strictly 1:1 relationship between ganglion cells and bipolar cells. One cone provides input to 2 mbcs and 2 MGCs in all areas of retina outside the fovea. From Kolb and Marshak, 2003 (46).
In sum, the relationship between cones and their midget ganglion cells appears to be one cone to two midget bipolar cells and two midget ganglion cells in the foveal and perifoveal regions of the human retina. The same applies to macaque and new world monkey retinas (47).
Our understanding of the significance of every cone photoreceptor having two bipolar and two ganglion cell channels through the primate retina became clear in the 1970s when it was proved unequivocally that the IPL was divided into an OFF-center neural processing in the upper half of the IPL and an ON-center neural processing in the lower half of the IPL (48) (see Webvision chapter on Inner Plexiform Layer). The bipolar cells that make central ribbon-related contacts give ON center (depolarizing signals to light flashes) while the bipolar cells with flat (superficial junction) contacts with the cone pedicles give OFF center (hyperpolarizing signals to light flashes). So, the invaginating midget bipolar cells are ON center and connect with the lower-IPL-branching ON-center midget ganglion cells (Figure 32, Figure 33). In contrast the flat midget bipolar cells are OFF center and connect exclusively with the OFF-center midget ganglion cells of the upper IPL (Figure 32, Figure 33). The messages from a single cone can be transmitted to the brain via exclusive (private) channels for either brighter than background light (ON center message) or darker than background light (OFF center message). Figure 33 shows diagrammatically these private pathways for the midget system in the fovea and the perifovea.
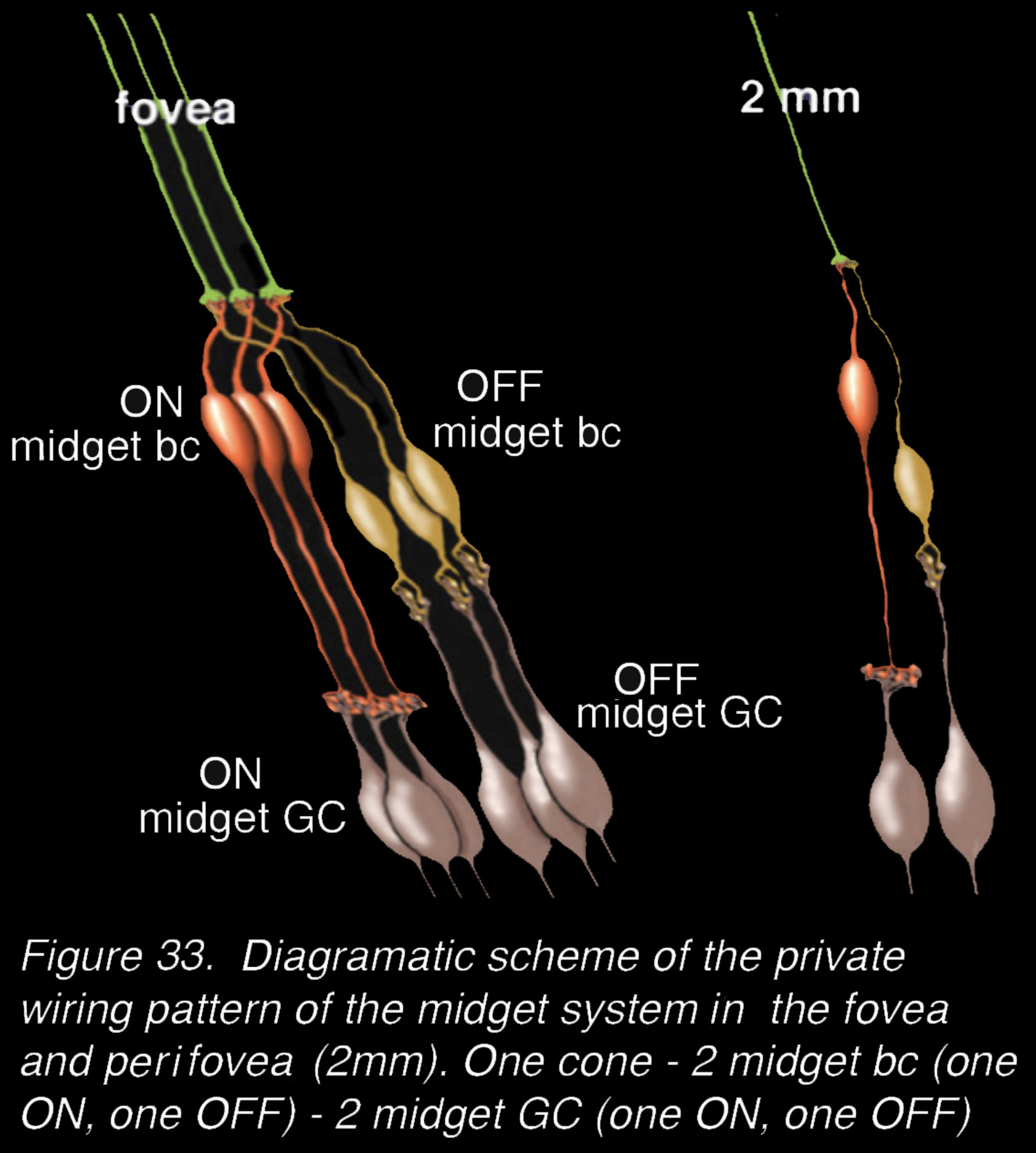
Figure 33. Diagrammatic scheme of the private wiring pattern of the midget system in the fovea and perifovea (at 2 mm). One cone – 2 midget bc’s (one ON, one OFF) – 2 midget GC (one ON, one OFF).
This ON and OFF segregation of the light signal to the two parallel pathways is basic to the organization of light perception of both intensity and hue in the retina and brain (49). In the human fovea and perifovea the private midget pathways dominate the architecture probably forming about 80% of the neural channels. The density of the midget bipolar cells and their midget ganglion cells bodies stacked 6-8 cells deep on the edge of the fovea can be seen in Figure 24, Figure 28, and Figure 29. Furthermore, as we saw in the mapping of cone spectral types in the fovea (19), the vast majority are red L-cones or green M-cones in the fovea and elsewhere in the retina. And these red/green cone pathways are thought to be using the midget bipolar and midget ganglion cell systems to ensure private lines for high acuity function as well as hue (see Webvision chapter on Midget Pathways).
The one-cone-to-two-ganglion-cell midget pathways break down beyond 10 degrees from the fovea in the retina. Outside of the fovea the midget system is multicone connected (50-52).
It is well established that the midget chain of neurons for M- and L-wavelength detection in the midget ganglion cells has a spectrally opponent center-surround organization (Figure 34) (53, 54), reviewed in (55). Thus, midget ganglion cells of central retina (not fovea though), when recorded from electrophysiologically, have the smallest receptive fields, and are organized as L-cone ON or OFF center, and M-cone ON or OFF center. Each midget ganglion cell type has a larger surround of the opposite polarity and of the opponent color (Figure 34). The centers of the midget ganglion cells in peripheral retina, as Chichilnisky’s group have elegantly shown (50), are comprised of a predominant numerical cone type, say L-or M-cones, but many more M-, L-, and even S-cones in the larger surround receptive fields. This suggests a more mixed spectral input to surrounds. The retinal pathways making opponent color surrounds are still not clearly understood for the red and green midget ganglion cells although the predominant theory is that HC feedback at the outer plexiform layer makes for a center surround organization in the bipolar cells (34, 56). Dennis Dacey’s group (57) have recently measured the strength and opponent color of the surrounds in peripheral midget ganglion cells and using a difference of Gaussian receptive field model predicted the color opponent surround that is recorded. But as stated above the midget bipolar cells of the fovea have not been recorded from to understand whether the midget bipolar is already color opponent due to horizontal cell feedback.
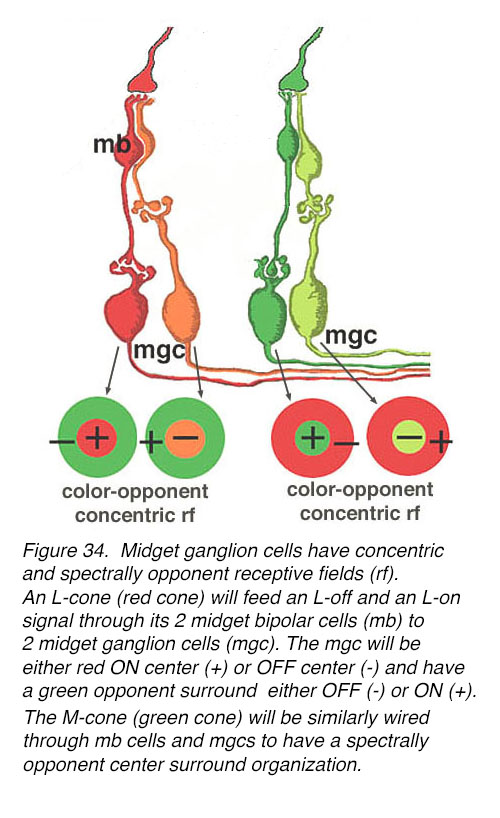 Figure 34. L/M midget ganglion cells have concentric and spectrally opponent receptive fields (rf). An L-cone (red cone) will feed an L-off and an L-on signal through its 2 midget bipolar cells (mb) to 2 midget ganglion cells (mgc). The mgcs will be either red ON center (+) or red OFF center (-) and have a green opponent surround either OFF (-) or ON (+). The M-cone (green cone) is similarly wired through mb cells and mgcs to generate spectrally opponent center surround organizations.
Figure 34. L/M midget ganglion cells have concentric and spectrally opponent receptive fields (rf). An L-cone (red cone) will feed an L-off and an L-on signal through its 2 midget bipolar cells (mb) to 2 midget ganglion cells (mgc). The mgcs will be either red ON center (+) or red OFF center (-) and have a green opponent surround either OFF (-) or ON (+). The M-cone (green cone) is similarly wired through mb cells and mgcs to generate spectrally opponent center surround organizations.
There are also more diffuse channels mixing red and green cone signals in the fovea. Figure 24, in the horizontal cell section, showed a diffuse type of cone bipolar cell called db3 that immunostains with calbindin in the fovea. Its axon terminals are seen positioned in the center of the IPL (Figure 24). The diagram of Figure 35 illustrates ON and OFF pairs of diffuse bipolar cells with contacts to ON and OFF parasol ganglion cells (also shown in Polyak’s drawing of the foveal bipolar and ganglion cells in Figure 26 and see later drawings of wide-field bipolar cells). Parasol ganglion cells are thought to be achromatic (53) but motion sensitive (58). See more details of glutamate channels of the two ON and OFF bipolar connections (59) and types of bipolar cells in vertebrate retinas in the Webvision chapter on Bipolar Cell Pathways in the Vertebrate Retina by Nelson and Connaughton.
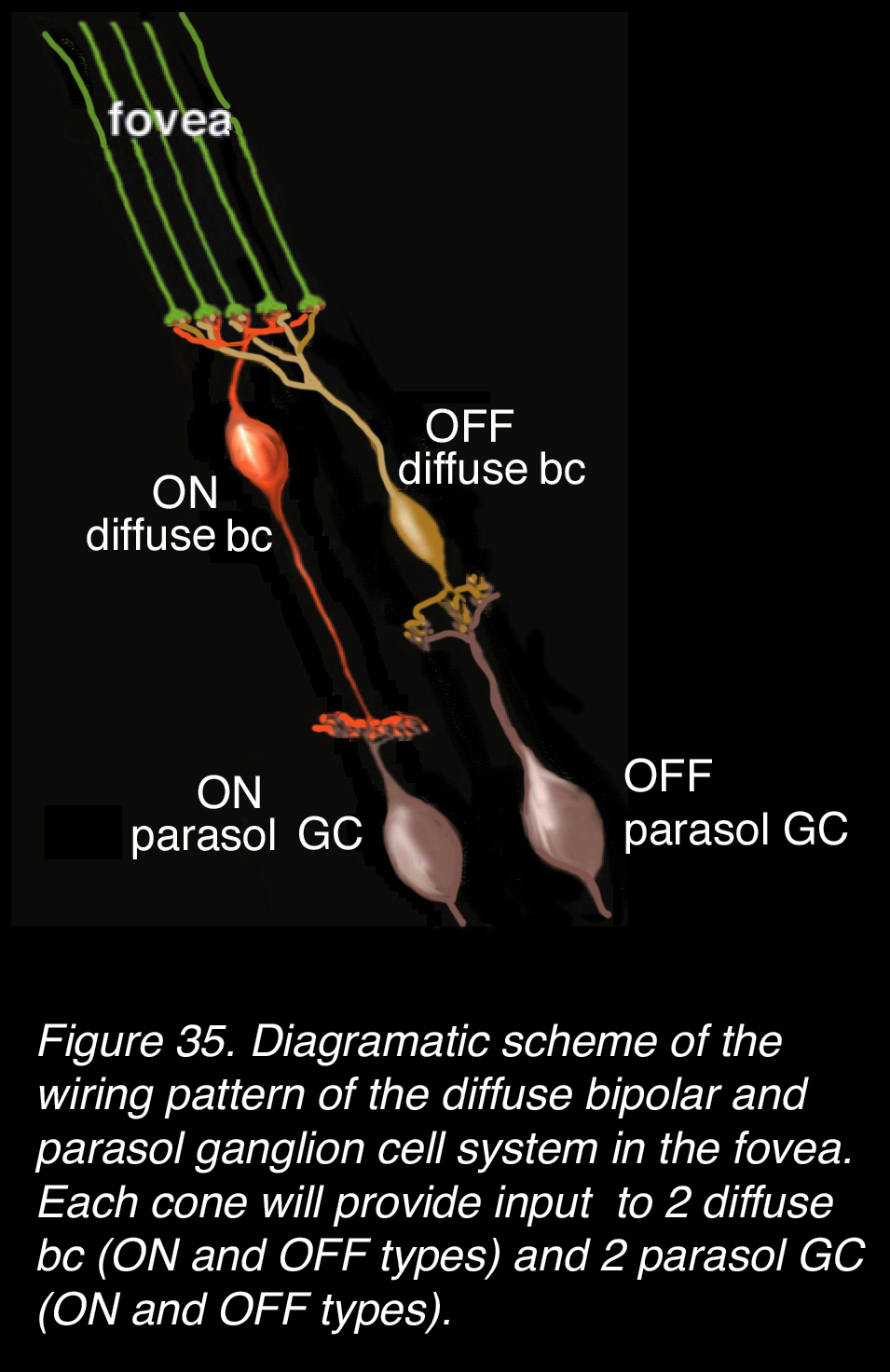 Figure 35. Diagrammatic scheme of the wiring pattern of the diffuse bipolar cell (bc) and parasol ganglion cell (GC) system in the fovea. Each cone (either L, M, or S type) will provide input to two diffuse bcs (ON and OFF types) and two parasol GCs (ON and OFF types).
Figure 35. Diagrammatic scheme of the wiring pattern of the diffuse bipolar cell (bc) and parasol ganglion cell (GC) system in the fovea. Each cone (either L, M, or S type) will provide input to two diffuse bcs (ON and OFF types) and two parasol GCs (ON and OFF types).
Counts of midget ganglion cells in the fovea
As we have seen above, the high acuity red and green channels in the retina are processed through parallel single-channel midget bipolar cells and midget ganglion cells to the brain. These high acuity circuits mean that every cone of the fovea will transmit an ON and an OFF signal via two midget bipolar cells and two midget ganglion cells. Every cone in the fovea must have at least two ganglion cells to contact. Are there enough ganglion cells in the foveal slope and foveal rim to ensure these parallel pathways in human retina? Curcio and Allen’s (10) quantitative counts on human retinas indicate that there are.
Maps of the ganglion cell numbers counted at every 400 µm from the foveal pit (Figure 36, a, foveal pit is black) show the peak ring of ganglion cell (GC) bodies (Figure 36 orange patchy red ring) at about 800 µm to 1 mm from the central pit. There are large numbers of GCs up to this ring, consistent with the increasing piling up of GC bodies into 6 rows deep at maximum. This piling up of the GCs is clearly seen in the previous confocal images of the foveal rim of human retinas (Figure 24, Figure 28).
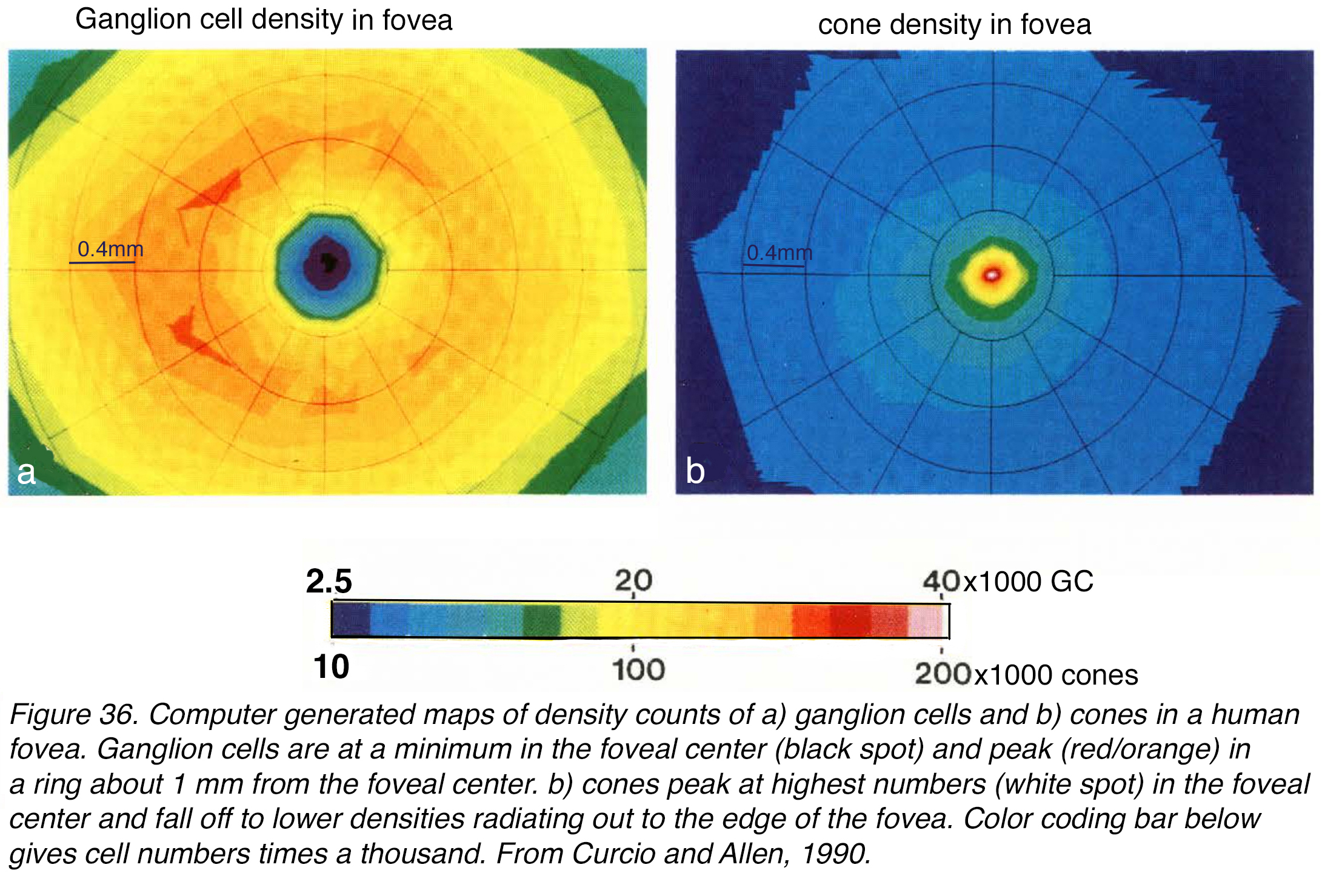 Figure 36. Computer generated heat maps of density counts of (a) ganglion cells, and (b) cones in a human fovea. Ganglion cells are at a minimum in the foveal center (black spot) and peak (red/orange) in a ring about 1 mm from the foveal center. (b) Cones peak at the highest numbers (white spot) in the foveal center and fall off to lower densities radiating out to the edge of the fovea. Color coding bar below gives cell density per mm2 times a thousand. From Curcio and Allen, 1990 (10).
Figure 36. Computer generated heat maps of density counts of (a) ganglion cells, and (b) cones in a human fovea. Ganglion cells are at a minimum in the foveal center (black spot) and peak (red/orange) in a ring about 1 mm from the foveal center. (b) Cones peak at the highest numbers (white spot) in the foveal center and fall off to lower densities radiating out to the edge of the fovea. Color coding bar below gives cell density per mm2 times a thousand. From Curcio and Allen, 1990 (10).
The cone densities are highest in the foveal pit and rod free area (Figure 36, b) and the Henle fiber lengths (cone axons) allow the foveal cones to reach the midget and other cone bipolar cells, which are also eccentrically directed to final connections with the midget and a few other GC types (see later section on blue or S-cone pathways). The counts reveal that between 2 and 3 GCs are present in the foveal area for each cone photoreceptor (10).
Things are different in peripheral retina where single cones have input to midget cone bipolar cells with several dendritic clusters for up to 3 cones per bipolar, and many midget bipolar cells converge onto a single midget ganglion cell with a wide dendritic tree (46). The one-cone-to-two-ganglion-cell channel is not available for high acuity vision in the periphery.
A demonstration of single cones mapping onto single midget ganglion cells with either ON or OFF light responses was elegantly demonstrated in the fovea of a living monkey retina by William’s group (60). Using their signature methodology of adaptive optics to image the cones (as in Figure 17), spatial white noise stimulation of central foveal cones, and responses of ganglion cells with calcium signaling, this team of researchers was able to relate a single cone to its ON or OFF centered midget ganglion cell at the foveal rim 300-400 µm distance (Figure 37, ON mgcs circled in red and OFF mgcs in green).
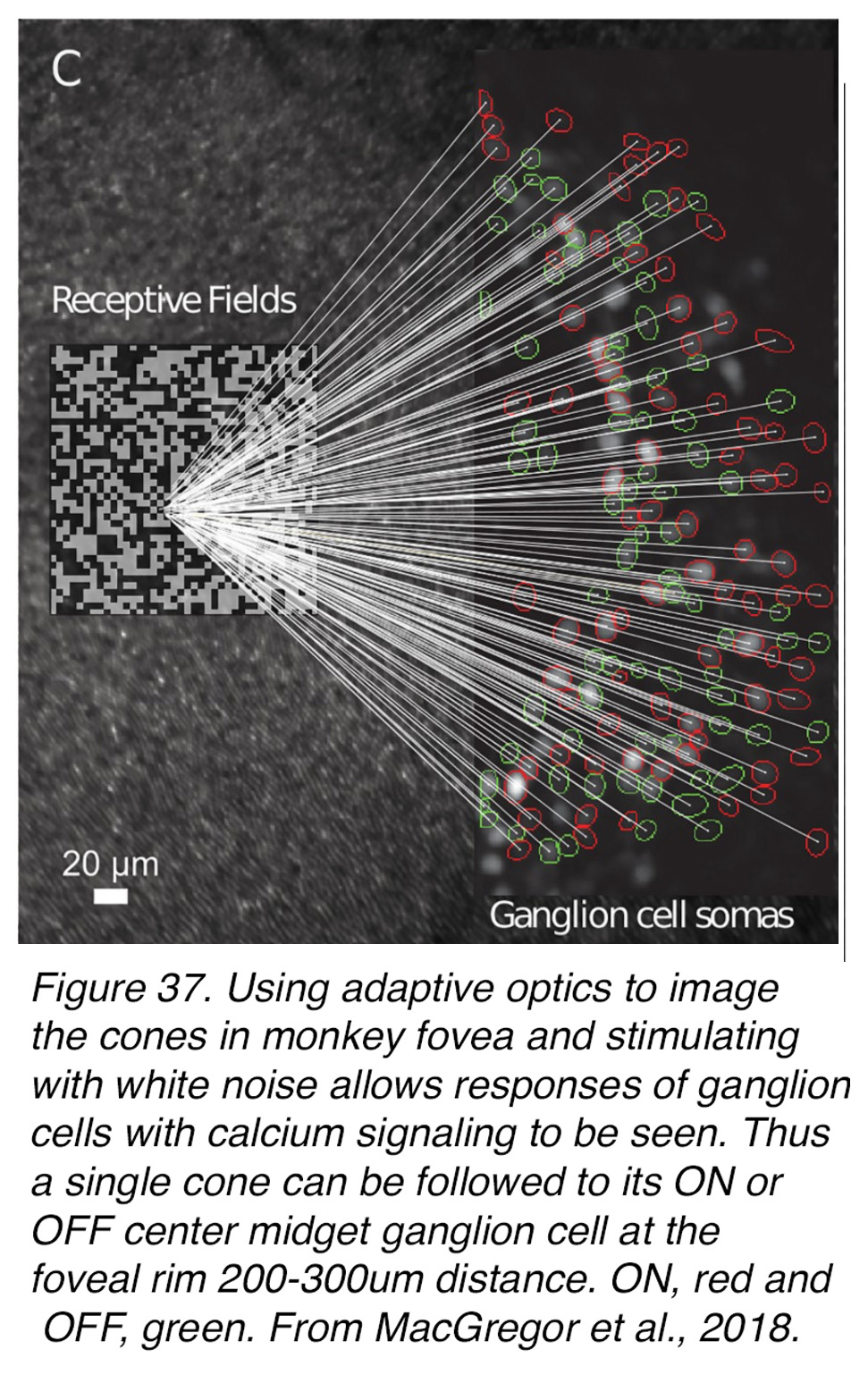 Figure 37. Using adaptive optics to image the cones in monkey fovea and stimulating with spatial white-noise checkerboards allows responses of ganglion cells to be seen by calcium signaling. Thus, a single cone can be followed to its ON- or OFF-center midget ganglion cell at the foveal rim 200-400 µm distant. ON, red and OFF, green. From MacGregor et al., 2018 (60).
Figure 37. Using adaptive optics to image the cones in monkey fovea and stimulating with spatial white-noise checkerboards allows responses of ganglion cells to be seen by calcium signaling. Thus, a single cone can be followed to its ON- or OFF-center midget ganglion cell at the foveal rim 200-400 µm distant. ON, red and OFF, green. From MacGregor et al., 2018 (60).
In this way this team of researchers was able to map the distribution of the midget ganglion cells in a ring around the fovea at between 300 and 400 µm from the cones central to their receptive fields. Interestingly the ganglion cells with deep cell bodies closer to the outer plexiform layer were closer to the central fovea i.e that 300 µm ring, whereas ganglion cells in the superficial ganglion cell layer were between 400 and 500 µm from the central cones, so that one point in space at the ganglion-cell layer represents more than one point in visual space. This all reflects the Henle axon layer and the pulling of the inner retinal cells obliquely away from the center with the superficial cells being pulled further out radially in development (see section of development of the fovea).
Blue S-cone pathways in the fovea
So far, we have dealt with the architecture of the predominant cone spectral and high acuity pathways in the fovea namely the midget pathways for the green M-cones and red L-cones. What of the blue S-cone pathways? As we have seen above the S-cones have a small presence in the foveal cone mosaic (3% of cones generally) but reach their peak number for the whole retina at 12% of cones in foveal slope and rim at approximately 1 degree of eccentricity (Figure 13, Figure 14, Figure 15).
Mariani (61) described from a Golgi staining study of rhesus monkey retina, a bipolar cell that appeared to be selective for cone types that he proposed were the blue or S-cones. These cone bipolar cells were not midget per se. They appeared to be contacting 1-3 cones situated far apart and their axon terminals were always in the ON layer deep in the IPL neuropil (Figure 38, A). Axon terminals were rather wide-spread compared with the restricted narrow axon terminals of M- or L-cone connecting midget bipolar cells of the central retina. Kouyama and Marshak (62) later confirmed that this type of bipolar was selective for S-cones by an immunostaining light microscope study. The cells were selectively immunoreactive for glycine-extended precursors of cholecystokinin. Later an electron microscope study of the fovea with serial sections and reconstructions (63) showed that this type of S-cone bipolar (Figure 38, A) was probably an ON center type due to its invaginating synapses with the S-cone (not shown in Figure 38), and its axonal terminal ending deep in the IPL neuropil to contact the lower branches of a small bistratified ganglion cell (Figure 38, B-C, Figure, 39).
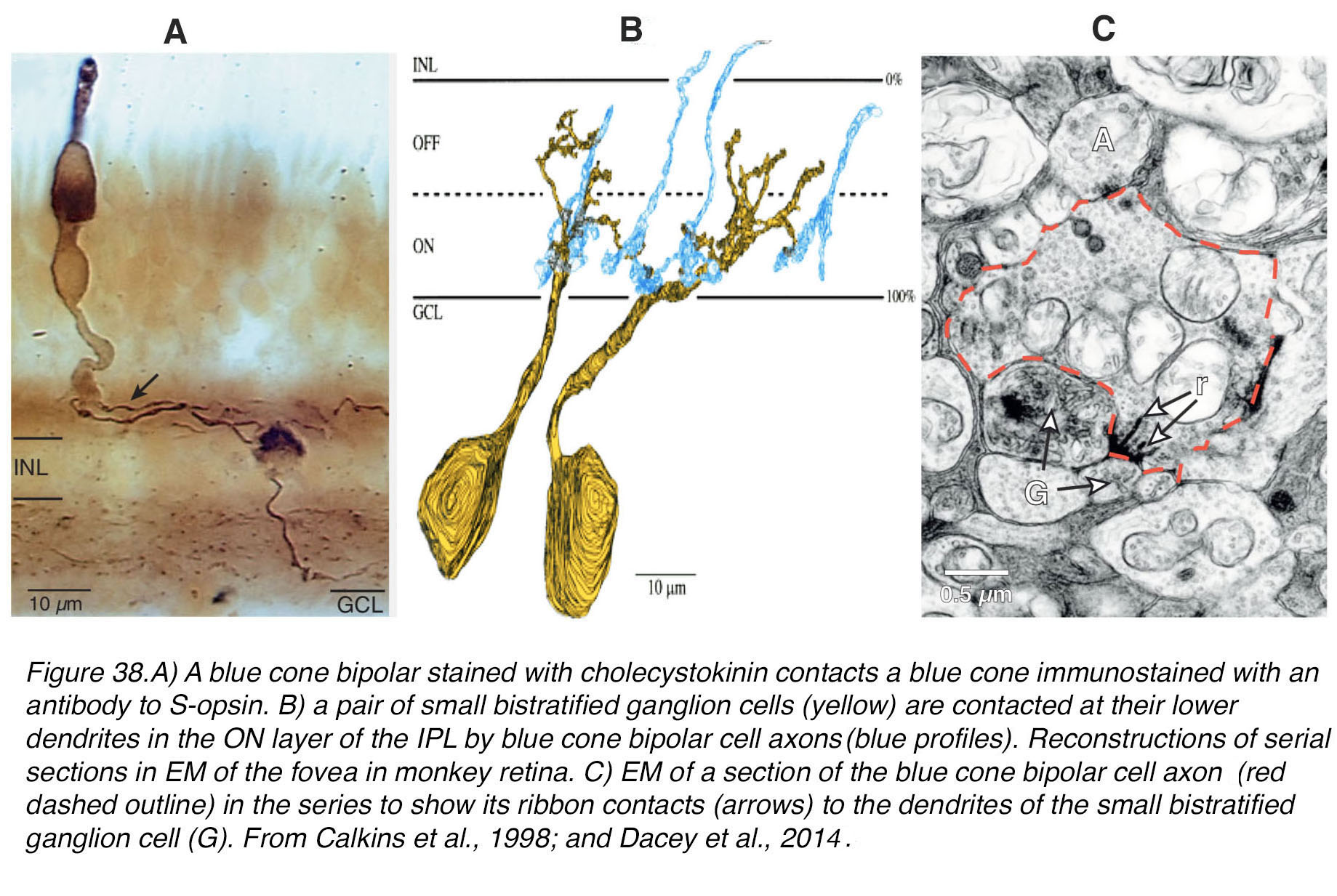 Figure 38. (A) A blue cone bipolar stained with anti-cholecystokinin contacts a blue cone, immunostained with an antibody to S-opsin. (B) A pair of small bistratified ganglion cells (yellow) are contacted at their lower dendrites in the ON layer of the IPL by blue cone bipolar cell axons (blue profiles). Reconstructions of serial sections in EM of the fovea in monkey retina. (C) EM of a section of the blue cone bipolar cell axon (red dashed outline) in the series to show its ribbon contacts (arrows) to the dendrites of the small bistratified ganglion cell (G). From Calkins et al. (63), 1998; and Dacey et al., 2014 (34).
Figure 38. (A) A blue cone bipolar stained with anti-cholecystokinin contacts a blue cone, immunostained with an antibody to S-opsin. (B) A pair of small bistratified ganglion cells (yellow) are contacted at their lower dendrites in the ON layer of the IPL by blue cone bipolar cell axons (blue profiles). Reconstructions of serial sections in EM of the fovea in monkey retina. (C) EM of a section of the blue cone bipolar cell axon (red dashed outline) in the series to show its ribbon contacts (arrows) to the dendrites of the small bistratified ganglion cell (G). From Calkins et al. (63), 1998; and Dacey et al., 2014 (34).
In the nineties Dacey and Lee (64) first described the morphology and the intracellular responses of the small bistratified ganglion cell as blue ON and yellow OFF. Figure 39 shows this blue-ON yellow-OFF bistratified ganglion cell stained with neurobiotin and recorded for light response and receptive field structure in the monkey peripheral retina (34). The bistratified cells of the fovea are presumed to have a similar dendritic tree but much smaller of course. The estimate from Calkins and coauthors (63) was that this ganglion cell type on the foveal rim, where they are at their highest numbers, forms 3% the ganglion cell population.
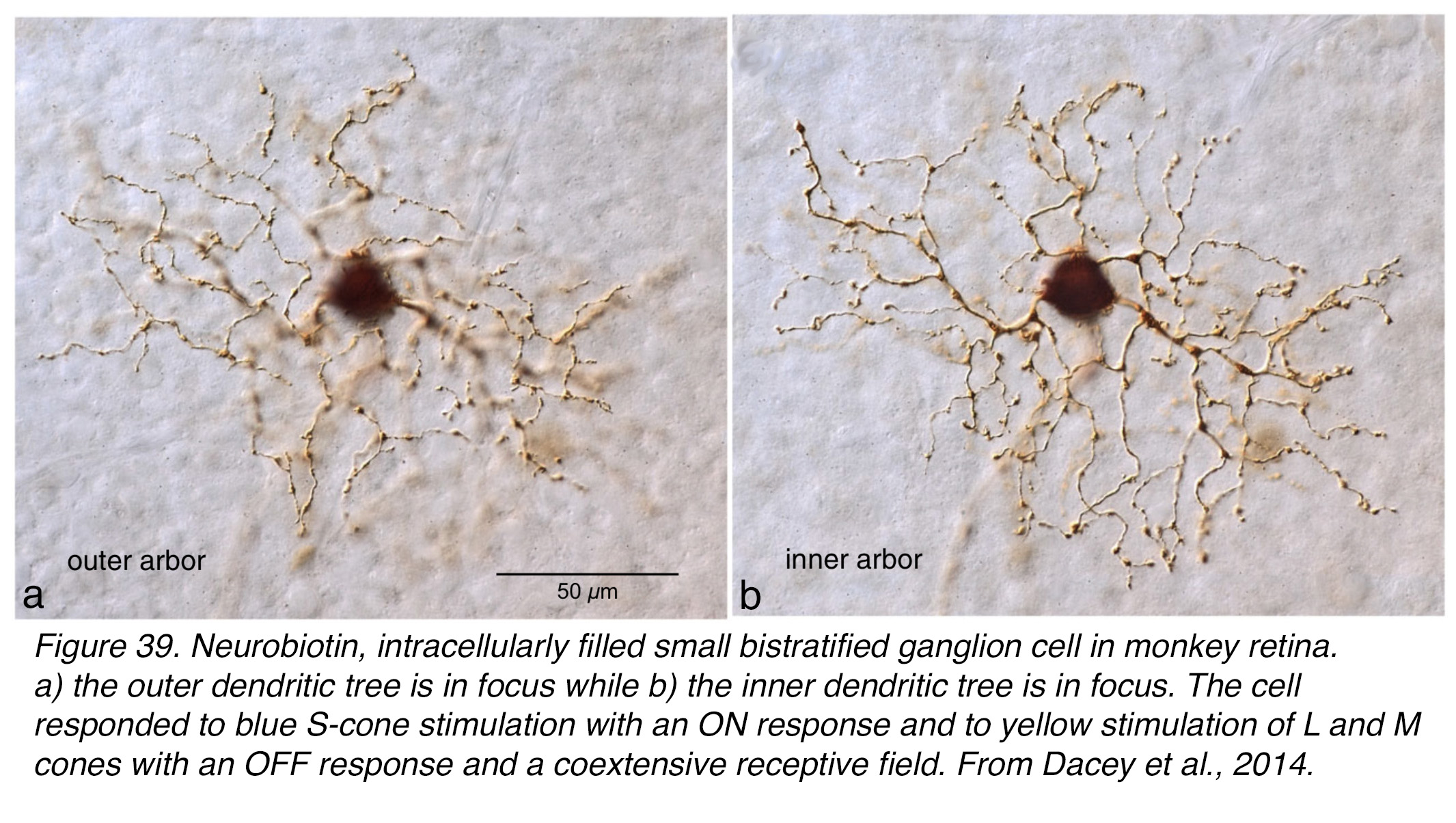 Figure 39. Neurobiotin, intracellularly filled, small bistratified ganglion cell in monkey retina. (a) The outer dendritic tree is in focus while (b) the inner dendritic tree is in focus. The cell responded to blue S-cone stimulation with an ON response and to yellow stimulation of L and M cones with an OFF response. Both receptive fields were coextensive. From Dacey et al., 2014 (34).
Figure 39. Neurobiotin, intracellularly filled, small bistratified ganglion cell in monkey retina. (a) The outer dendritic tree is in focus while (b) the inner dendritic tree is in focus. The cell responded to blue S-cone stimulation with an ON response and to yellow stimulation of L and M cones with an OFF response. Both receptive fields were coextensive. From Dacey et al., 2014 (34).
Apparently, humans can discriminate blue–yellow gratings of about 7–14 cycles/degree (16, 65-67). This limit corresponds to the Nyquist limit for the densest region of the S cone mosaic at 1° eccentricity (16, 68). The B–Y ganglion cell mosaic would support discrimination of the finest blue–yellow grating. These ON blue, yellow OFF GCs project to the koniocellular layers of the lateral geniculate body in the brain. It is now accepted that this blue-yellow ganglion cell circuit is the major pathway from blue S-cones to signal the brain about blue hue discrimination (34).
So far in this chapter only an ON center bipolar and ganglion cell for the S-cones have been described. Are there any OFF-center S-cone pathways? An OFF S-cone midget bipolar making basal junctions with the blue cone pedicle near the triad ribbon synapses, like in red and green midget bipolar cells, has been reconstructed by serial section electron microscopy in the monkey fovea (Figure 40; A, A) (69, 70). Its axon terminal contacts the small dendritic tree of a midget ganglion cell (69-71) (Figure 40; B, B).
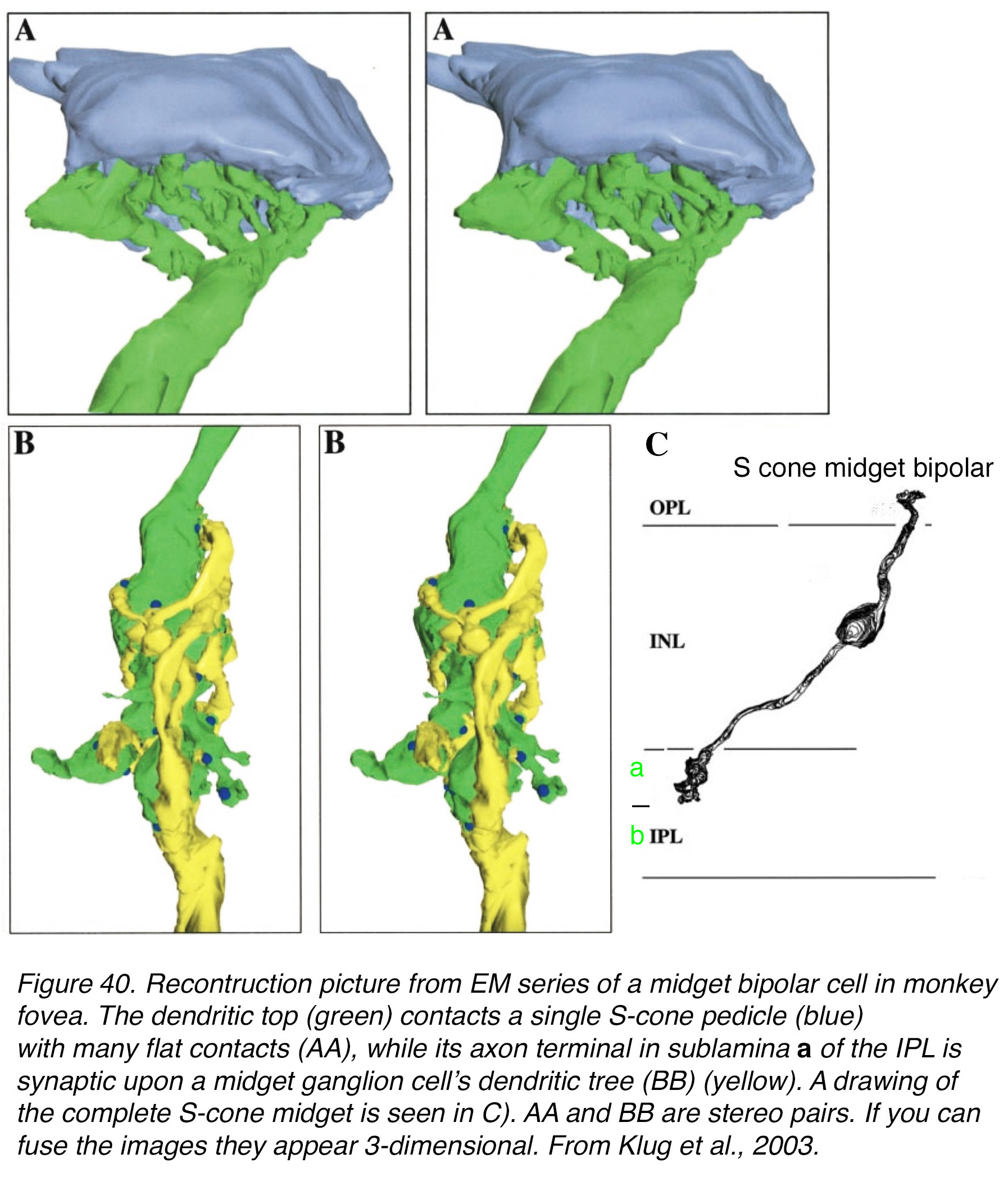 Figure 40. Reconstruction picture from EM series of a midget bipolar cell in monkey fovea. The dendritic top (green) contacts a single S-cone pedicle (blue) with many flat contacts (A, A), while its axon terminal in sublamina a of the IPL is synaptic upon a midget ganglion cell’s dendritic tree (B, B) (yellow). A drawing of the complete S-cone midget is seen in (C). A, A and B, B are stereo pairs. If you can fuse the images, they appear 3-dimensional. From Klug et al., 2003 (69).
Figure 40. Reconstruction picture from EM series of a midget bipolar cell in monkey fovea. The dendritic top (green) contacts a single S-cone pedicle (blue) with many flat contacts (A, A), while its axon terminal in sublamina a of the IPL is synaptic upon a midget ganglion cell’s dendritic tree (B, B) (yellow). A drawing of the complete S-cone midget is seen in (C). A, A and B, B are stereo pairs. If you can fuse the images, they appear 3-dimensional. From Klug et al., 2003 (69).
Very recently S-cone OFF type of responses have been recorded from S-OFF midget ganglion cells (mgc) in the monkey macular area (70). This S-OFF mgc had a blue-OFF center and blue-ON surround organization. Its receptive field was interpreted by the authors to have a role in edge detection similar to L/M mgcs (70). Previously in monkey lateral geniculate nucleus, S-OFF responses had been recorded (72). To date there is no evidence for a matching S-ON midget ganglion cell in primate retina.
In 2005 Dacey and colleagues reported on “giant” ganglion cells that gave blue S-cone OFF responses in monkeys (73). These ganglion cells are intrinsically photoreceptive ON-types (iPRGCs) due to melanopsin photopigment in their membranes but also they are driven by both rod and L/M cone inputs in their ON responses (see Webvision chapter on Melanopsin-Expressing, Intrinsically Photosensitive Retinal Ganglion Cells). The overall cone input is S-cone OFF and M/L-cone ON, with a coextensive receptive field organization. In human retina multielectrode array studies also showed the ON responses of iPRGCs integrated rod and cone signals with intrinsic melanopsin photosensitivity. The human melanopsin signals came in at least three photoresponse variants (74). The morphologies of the “giant” melanopsin ganglion cells are shown in Figure 41.
 Figure 41. (a) A giant melanopsin-IR ganglion cell of peripheral human retina (green). (b) inner and outer melanopsin-IR ganglion cells in vertical section with dendrites in S5 and S1 of the IPL respectively. From Dacey et al., 2005 (73). (c) Giant melanopsin cells immunostained with melanopsin antibody in peripheral human retina. Their dendritic trees cover one mm area of retina.
Figure 41. (a) A giant melanopsin-IR ganglion cell of peripheral human retina (green). (b) inner and outer melanopsin-IR ganglion cells in vertical section with dendrites in S5 and S1 of the IPL respectively. From Dacey et al., 2005 (73). (c) Giant melanopsin cells immunostained with melanopsin antibody in peripheral human retina. Their dendritic trees cover one mm area of retina.
These cell types are many times larger in both cell body size and dendritic field diameter than the bistratified S-ON, yellow-OFF ganglion cell described above (Figure 39). The giant cells have dendritic trees covering 1 mm in area (Figure 41, a, c). There are two stratification types of giant melanopsin ganglion cells. One of the giant melanopsin cell types (outer type) stratifies in stratum S1 right under the amacrine cell layer. The other (inner type) has dendrites running in S5 just above the ganglion cell layer (Figure 41, b) (73). The dendritic trees of “giant” melanopsin types are rather large in the fovea too (500-1000 µm diameter), forming a distinct ring of cells around the fovea (Figure 42). The “giant” S-OFF melanopsin GCs project to the parvo and magnocellular layers of the lateral geniculate nucleus (73). Furthermore, there is now thought to be to be a third type of ip-melanopsin cell in the human retina and all are densely distributed in perifoveal retina (74, 75).
 Figure 42. Melanopsin-expressing cells in the human foveal region. A single image from a stack of images is shown. The dendritic processes of the foveal cells wrap around the foveal pit. Scale bar = 100 µm. From Nasir-Ahmad et al., 2019 (114).
Figure 42. Melanopsin-expressing cells in the human foveal region. A single image from a stack of images is shown. The dendritic processes of the foveal cells wrap around the foveal pit. Scale bar = 100 µm. From Nasir-Ahmad et al., 2019 (114).
The question arises as to what bipolar or amacrine cell would have input to S-cone-OFF, L/M-cone-ON melanopsin cells, particularly those that branch in the lower or inner IPL. The S-OFF midget bipolar cell could contact the outer giant melanopsin cell, but it is so far only known to contact the S-cone OFF midget ganglion cell. Most of the diffuse cone bipolar cells of the primate retina have contacts with an S-cone in their dendritic coverage of the overlying cones, as summarized by Dacey (34) (Figure 43). These types of bipolar have not been recorded from to know how much OFF- S-cone signal would come through in their possible input to the outer type giant melanopsin ganglion cell dendritic trees (i.e. from DB1 and DB2 in Figure 43). Those bipolar cells with minority S-cone contacts that branch in the area of the inner types of giant melanopsin ganglion cells would potentially have minimal S-cone ON input but not S-cone OFF input (Figure 43). For these reasons the S-cone OFF signal in the giant melanopsin ganglion cells is now theorized to be via an intervening inhibitory amacrine cell circuit from the ON blue bipolar (B, B, Figure 43) to the dendrites of the melanopsin GCs (76, 77). An S-cone amacrine circuit from S-ON bipolar cells to M1 melanopsin iPRGCs has been found in dense reconstructions of monkey perifovea (77).
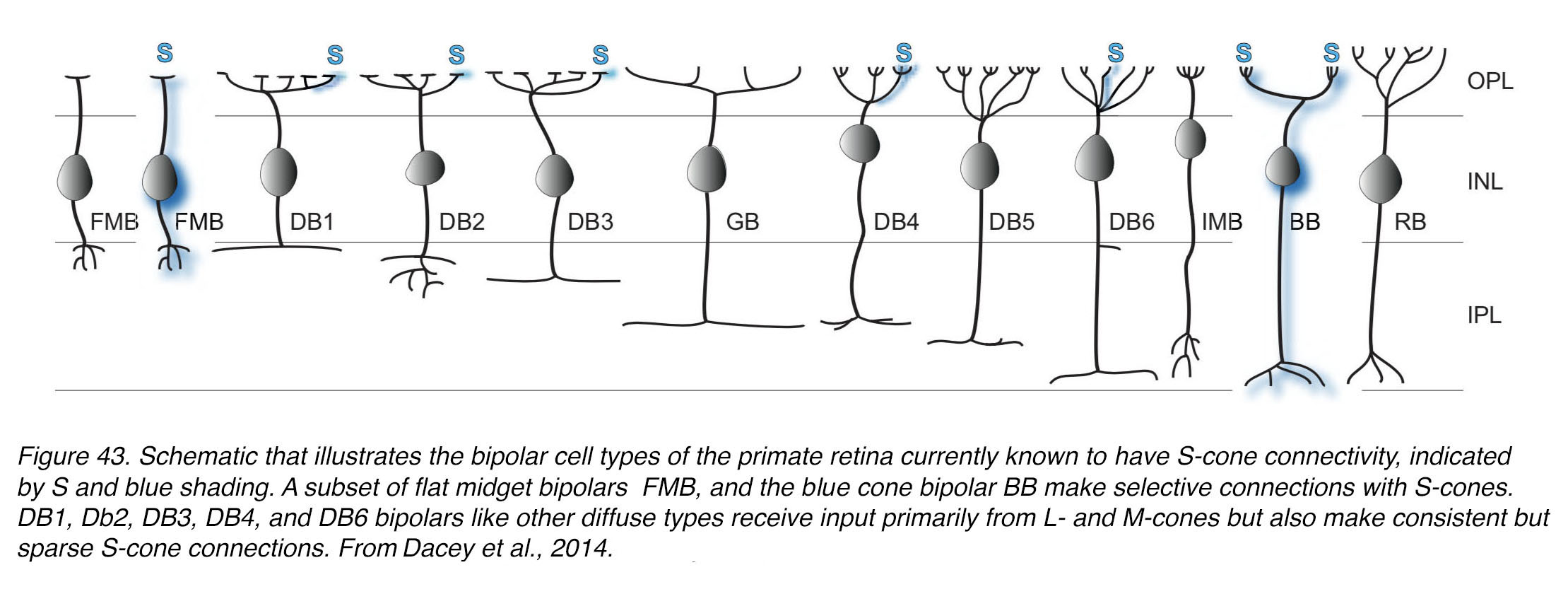 Figure 43. Schematic that illustrates the bipolar cell types of the primate retina currently known to have S-cone connectivity, indicated by S and blue shading. A subset of flat midget bipolar cells (FMB), and the blue cone bipolar (BB) make selective connections with S-cones. DB1, DB2, DB3, DB4, and DB6 bipolar cells, like other diffuse types, receive input primarily from L- and M-cones, but also make consistent but sparse S-cone connections. From Dacey et al. 2014 (34).
Figure 43. Schematic that illustrates the bipolar cell types of the primate retina currently known to have S-cone connectivity, indicated by S and blue shading. A subset of flat midget bipolar cells (FMB), and the blue cone bipolar (BB) make selective connections with S-cones. DB1, DB2, DB3, DB4, and DB6 bipolar cells, like other diffuse types, receive input primarily from L- and M-cones, but also make consistent but sparse S-cone connections. From Dacey et al. 2014 (34).
There is one last cone bipolar cell that has been all but ignored in the human retina. This is a giant bistratified bipolar cell that was described first by Mariani (78) in monkey retina and later by Kolb and coauthors (79) in a Golgi study of human retina. This bipolar type has the widest dendritic span of all cone bipolar cells, exceeding 50 µm in the central retina and as much as 100 µm in the periphery. The dendrites are searching for occasional cones to contact as would be expected if they were synapsing with the occasional S-cone in the dendritic field spread. The contacts are basal junctions, typically associated with OFF-bipolar physiology. The wide spreading axon terminal stratifies in both stratum 1 and stratum 5 of the IPL viz, right in the same strata of the dendritic spreads of the ON-layer and OFF-layer giant melanopsin ganglion cells (Figure 44). This giant bipolar has recently been mentioned in a paper on synaptic connectivity to melanopsin-containing ganglion cells in primate retinas (80). Putative and confirmed S-cone bipolar cells and their S-cone driven ganglion cells for the human fovea are illustrated in the summary diagram of Figure 44.
 Figure 44. Drawing of the neurons involved in transmitting S-cone signals through the retina. The ON S-cone bipolar cell and the S-cone OFF midget bipolar cell are known to contact bistratified ON blue/yellow ganglion cells (Bistrat ON B/Y GC) and OFF S-cone midget ganglion cells (OFF S-cone mgc) respectively. The OFF yellow diffuse bipolar cells (dfb) contact all spectral cone types, and possibly contact the bistratified ON B/Y GC to provide OFF yellow opponency. The inner giant melanopsin GC could receive some ON input from the S-cone bipolar cells, but a dominant OFF S-cone input is not fully explained yet. An S-cone amacrine cell selective for ON-S-cone bipolar cells (not shown) likely provides S-cone OFF input to the outer giant melanopsin M1 GC. A giant bistratified bipolar cell (GBB, possible OFF S-cone bipolar) is theorized by us to be available to both inner and outer giant intrinsically photosensitive melanopsin ganglion cells.
Figure 44. Drawing of the neurons involved in transmitting S-cone signals through the retina. The ON S-cone bipolar cell and the S-cone OFF midget bipolar cell are known to contact bistratified ON blue/yellow ganglion cells (Bistrat ON B/Y GC) and OFF S-cone midget ganglion cells (OFF S-cone mgc) respectively. The OFF yellow diffuse bipolar cells (dfb) contact all spectral cone types, and possibly contact the bistratified ON B/Y GC to provide OFF yellow opponency. The inner giant melanopsin GC could receive some ON input from the S-cone bipolar cells, but a dominant OFF S-cone input is not fully explained yet. An S-cone amacrine cell selective for ON-S-cone bipolar cells (not shown) likely provides S-cone OFF input to the outer giant melanopsin M1 GC. A giant bistratified bipolar cell (GBB, possible OFF S-cone bipolar) is theorized by us to be available to both inner and outer giant intrinsically photosensitive melanopsin ganglion cells.
Amacrine cells of the fovea
There are about 30 different amacrine cell types in the human retina (79). All those with small dendritic spreads are known to use glycine as a neurotransmitter, while the larger field amacrine cells use GABA as a transmitter. It is assumed that all the medium to larger field GABAergic amacrine cells would be at their densest and form a ring around the fovea much like the larger field ganglion cell types as in Figure 42. Densities and distributions of amacrine cells are very difficult to assess, except with mixtures of immunostains for different putative amino-acid or peptide neurotransmitters, enzymes for pathways involved in catecholamine synthesis, or characteristic calcium-binding proteins. So, it has been a challenge to categorize the different amacrine cell populations particularly among the small-field amacrine cells that are crowded together in the fovea and tile the retinal IPL. Larger field amacrines are more widely spaced but have considerable overlap of their dendritic fields.
Data concerning amacrine cells in human retina, and fovea in particular, are very scarce. Golgi studies have revealed the different types of amacrine cell in the monkey and human retinas (79, 81) and immunostaining studies have given us details of acetylcholine containing amacrines (starburst cells) (82), dopamine containing amacrine cells (61), peptides (83) and AII amacrines (84-87) (see Webvision chapter on Roles of Amacrine Cells). The paper by Rodieck and Marshak (82) gives the only quantification we have for starburst amacrine cells which contain acetylcholine in the human retina (Figure 45). Clearly, they peak in density at the fovea between 0- and 2-mm eccentricity. The two types of starburst cells are plotted separately in Figure 45, i.e. amacrine cells in the ganglion cell layer (GCL) and those in the amacrine cell layer (ACL). The ACL starburst cells are not counted any closer than 1.5 mm eccentricity. However, the ACL cells are always lower in number compared with the GCL-occurring cells in all areas of retina, i.e. foveal, temporal, nasal, upper and lower quadrants (Figure 45).
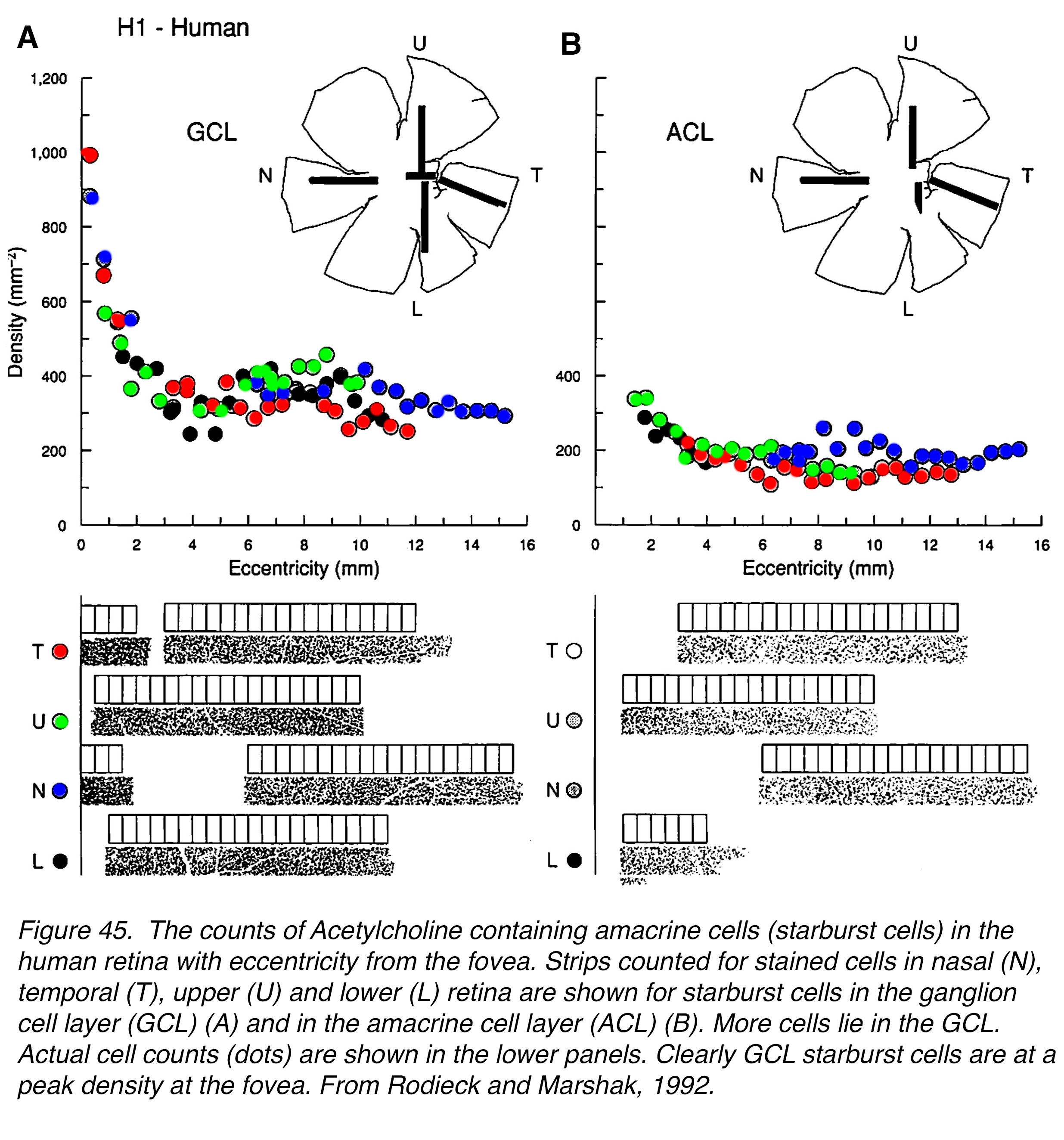 Figure 45. The counts of acetylcholine containing amacrine cells (starburst cells) in the human retina with eccentricity from the fovea. Strips counted for stained cells in nasal (N), temporal (T), upper (U) and lower (L) retina are shown for starburst cells in the ganglion cell layer (GCL) (A), and in the amacrine cell layer (ACL) (B). More cells lie in the GCL. Actual cell counts (dots) are shown in the lower panels. Clearly GCL starburst cells are at a peak density at the fovea. From Rodieck and Marshak, 1992 (82).
Figure 45. The counts of acetylcholine containing amacrine cells (starburst cells) in the human retina with eccentricity from the fovea. Strips counted for stained cells in nasal (N), temporal (T), upper (U) and lower (L) retina are shown for starburst cells in the ganglion cell layer (GCL) (A), and in the amacrine cell layer (ACL) (B). More cells lie in the GCL. Actual cell counts (dots) are shown in the lower panels. Clearly GCL starburst cells are at a peak density at the fovea. From Rodieck and Marshak, 1992 (82).
Amacrine cells with small dendritic fields are well characterized and classified into different types in human retina (79). As mentioned above all these different small field cells are glycinergic. Although they haven’t been so demonstrated in human retina, they have in mouse retina by immunostaining in the thy1-GFP-O mouse retina (88, 89) (Figure 46). These cells are clearly recognizable across species and are seen in human and monkey retina too. We are presuming and have evidence on some of these small field cells that they are most frequent in and near the fovea.
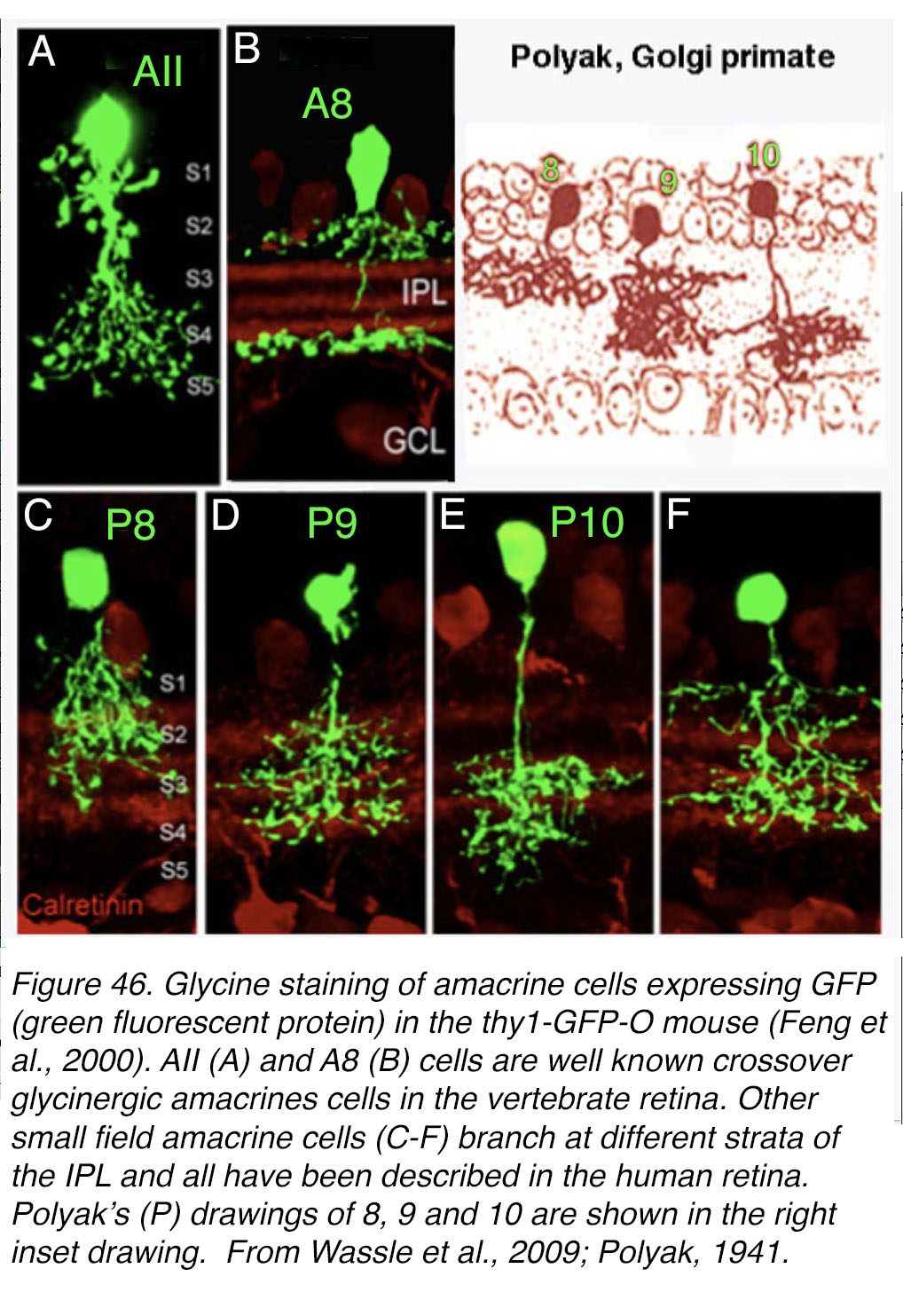 Figure 46. Glycine staining of amacrine cells expressing GFP (green fluorescent protein) in the thy1-GFP-O mouse (88). AII (A) and A8 (B) cells are well known crossover glycinergic amacrine cells in mammalian retina. Other small field amacrine cells (C-F) branch at different strata of the IPL, and all have been described in the human retina. Polyak’s (#8, #9, #10) drawings are shown in the right inset drawing and compared to counterparts in mouse retina (C-E, P8, P9, P10). From Wässle et al., 2009 (89); Polyak, 1941 (90).
Figure 46. Glycine staining of amacrine cells expressing GFP (green fluorescent protein) in the thy1-GFP-O mouse (88). AII (A) and A8 (B) cells are well known crossover glycinergic amacrine cells in mammalian retina. Other small field amacrine cells (C-F) branch at different strata of the IPL, and all have been described in the human retina. Polyak’s (#8, #9, #10) drawings are shown in the right inset drawing and compared to counterparts in mouse retina (C-E, P8, P9, P10). From Wässle et al., 2009 (89); Polyak, 1941 (90).
Kolb and coauthors Golgi study of small field amacrine cells (79) was done on whole mount preparations so a vertical section view of some of these small field amacrines has to come from Polyak’s book (90). See Webvision chapter on Roles of Amacrine Cells to see more details of Polyak’s Golgi staining of amacrine cells. In Figure 46 he shows a small-field amacrine that stratifies in the top strata (number P8), another type in the middle strata (number P9) and third type in the lower strata (number P10) of the IPL neuropil. These are quite possibly the same as the glycinergic amacrine cells seen in mouse retina (89) (Figure 46 C, D and E). Cells A and B and F (Figure 46) were not described by Polyak but one or more were described by more recent authors (79, 84, 85, 91-94). See more details in the Webvision chapter on Roles of Amacrine Cells.
A great many small field amacrine cells are stained with calbindin from the foveal rim into the foveal slope, as we have seen in Figure 24. Figure 47 (a) shows calbindin immunoreative (CB-IR) cells close to the fovea and in the foveal pit (Figure 47; a, c; green and orange cells). The orange amacrine cells co-label mixed calbindin and calretinin (CR) immunoreactivity. Some calbindin cells do not co-label with calretinin (green cells) and some calretinin cells do not co-label with calbindin (red cells). Thus, there are at least 3 different types of small field cell in the fovea. Probably they are equivalent to cells A, E and F in Figure 46. In addition, there are 2 cell types in Figure 47, in the monkey fovea, viz. one that colocalizes parvalbumin (PV) and calretinin (CR) (white arrows, Figure 47, b) and one solely PV-IR (green cells; Fig. 47, b). We know that A in Figure 46 is the well-known AII amacrine cell that is a cross over bistratified cell well documented to receive rod bipolar cell input and transmit rod signals to ON cone bipolar cells via gap junctions and to OFF cone bipolar cells and OFF ganglion cells via glycinergic synapses (84, 95-97) (see Webvision chapter on AII Amacrine Cells).
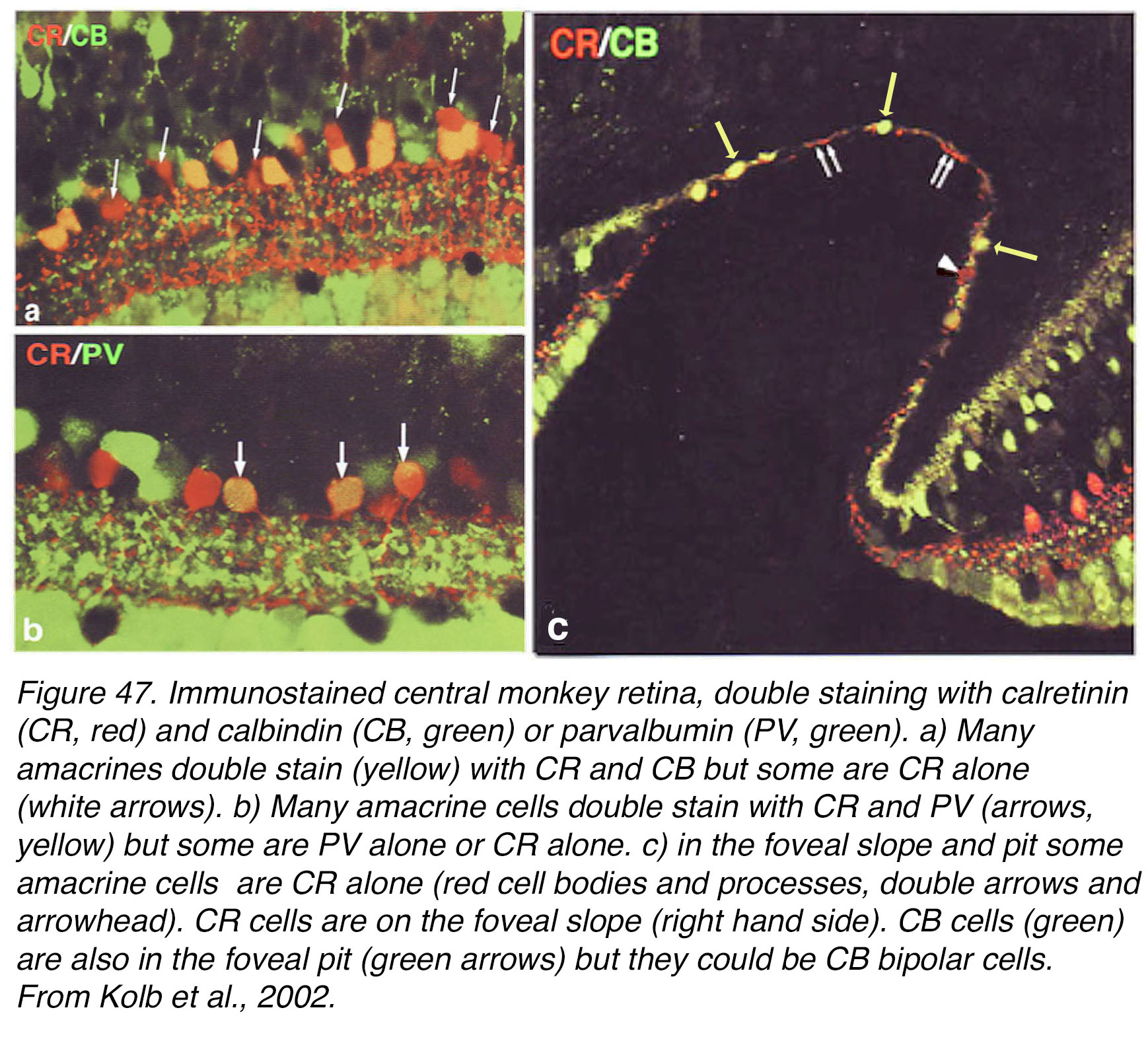 Figure 47. Immunostained central monkey retina, double staining with calretinin (CR, red) and calbindin (CB, green) or parvalbumin (PV, green). (a) Many amacrines double stain (yellow) with CR and CB (AII cells) but some are CR alone (white arrows). (b) Many amacrine cells double stain with CR and PV (arrows, yellow) but some are PV alone or CR alone. (c) In the foveal slope and pit, some amacrine cells are CR alone (red cell bodies and processes, double arrows and arrowhead). CR cells are on the foveal slope (right hand side). CB cells (green) are also in the foveal pit (green arrows), but they could be CB bipolar cells. From Kolb et al., 2002 (85).
Figure 47. Immunostained central monkey retina, double staining with calretinin (CR, red) and calbindin (CB, green) or parvalbumin (PV, green). (a) Many amacrines double stain (yellow) with CR and CB (AII cells) but some are CR alone (white arrows). (b) Many amacrine cells double stain with CR and PV (arrows, yellow) but some are PV alone or CR alone. (c) In the foveal slope and pit, some amacrine cells are CR alone (red cell bodies and processes, double arrows and arrowhead). CR cells are on the foveal slope (right hand side). CB cells (green) are also in the foveal pit (green arrows), but they could be CB bipolar cells. From Kolb et al., 2002 (85).
AII cells would not be expected to occur right in the rod-free fovea of the human retina but only to occur as the rods and rod bipolar cells appear on the foveal slope. AII amacrines are well known to be calbindin-IR, calretinin-IR and glycine-IR (85-87). AII amacrine cells reach their peak density of 10,500 cells/mm2 at 1 mm from the foveal center. The cells of Figure 47 (c) right in the foveal pit and immediate foveal slope are CR –IR but not CR/CB-IR and therefore we suggest they are not the AII amacrine cells (Figure 47, arrow head points to a cell body, and small double arrows point to many CR dendrites) (85). The same conclusion can be drawn from a recent immunostained human retina shown in Figure 48.
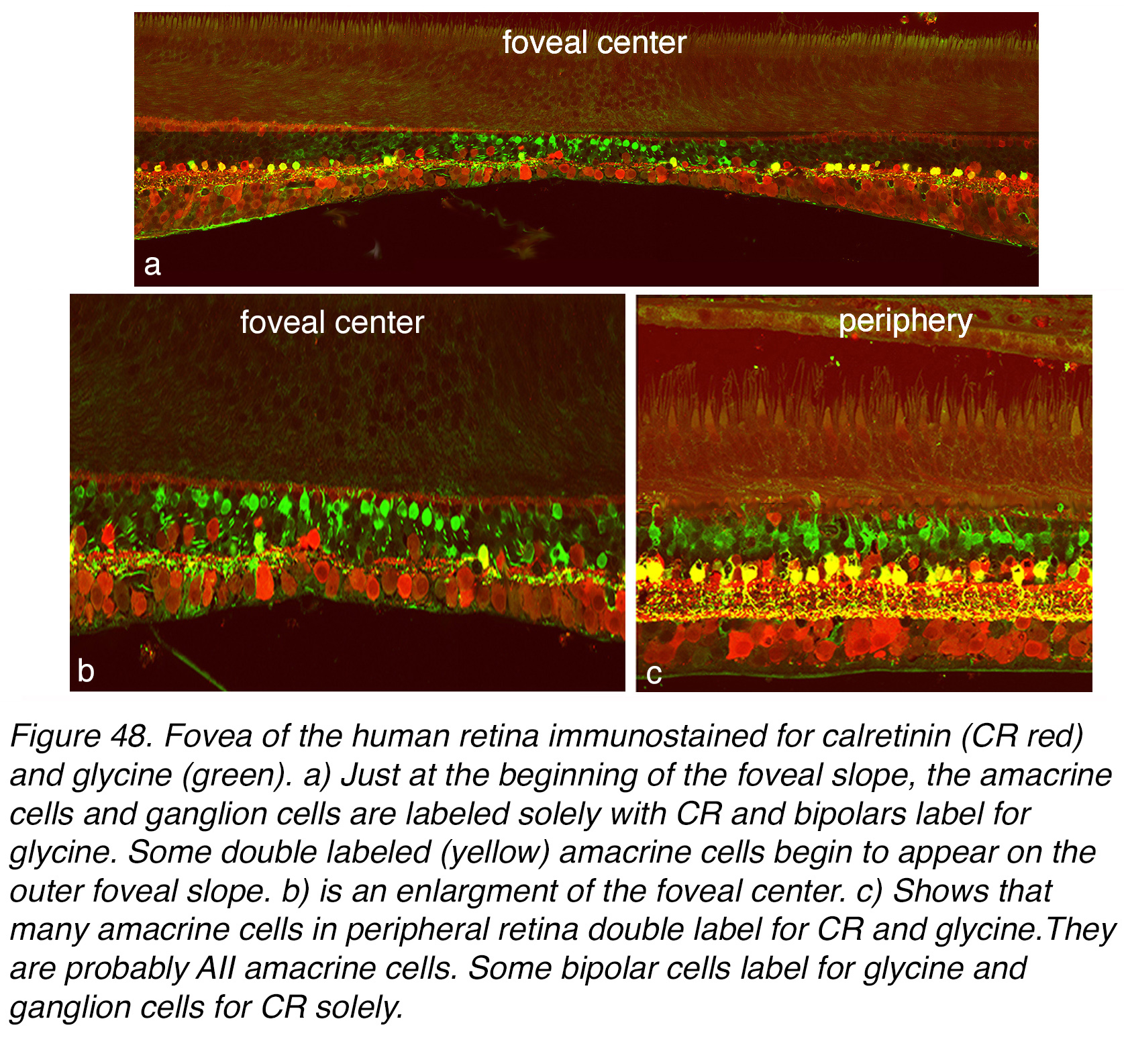 Figure 48. Fovea of the human retina immunostained for calretinin (CR red) and glycine (green). (a) Just at the beginning of the foveal slope, the amacrine cells and ganglion cells are labeled solely with CR, while bipolar cells label for glycine. Some double labeled (yellow) amacrine cells begin to appear on the outer foveal slope. (b) is an enlargement of the foveal center. (c) Shows that many amacrine cells in peripheral retina double label for CR and glycine (yellow). They are probably AII amacrine cells. Some bipolar cells label for glycine, while ganglion cells label for CR solely.
Figure 48. Fovea of the human retina immunostained for calretinin (CR red) and glycine (green). (a) Just at the beginning of the foveal slope, the amacrine cells and ganglion cells are labeled solely with CR, while bipolar cells label for glycine. Some double labeled (yellow) amacrine cells begin to appear on the outer foveal slope. (b) is an enlargement of the foveal center. (c) Shows that many amacrine cells in peripheral retina double label for CR and glycine (yellow). They are probably AII amacrine cells. Some bipolar cells label for glycine, while ganglion cells label for CR solely.
The foveal retina (Figures 48, a-b) is compared to peripheral retina (Figure 48, c) in this double stained section for glycine (green cells) and CR (red cells). CR-IR amacrine cells are in the foveal pit area but are not double stained AII cells. AII cells are seen on the foveal slope (Figure 48, a-b) as double stained cells (yellow cells). They are very common in the peripheral retina (Figure 48, c, yellow cells). In all areas of retina there are still some pure CR-IR amacrine cells that are not AII cells (Figure 48, c, red cell bodies). These CR-IR amacrines do not appear to stain for glycine (probably for some technical staining reasons) in our human material. Green cells in the foveal pit and periphery that immunostain for glycine are mostly cone bipolar cells in Figure 48 a-c.
Our detailed EM study (85) showed that the CR-IR amacrine cells of the foveal slope closest to the foveal pit occurred internal to the first rod spherule and rod bipolar (Figure 49, a, arrow 4, 350 µm from the foveal pit). Cells at position arrows 3 and 1, 2 were amacrine cells that occurred at the same position that the first rod bipolar cells entered the IPL neuropil. They were a mixed group of CA-IR amacrine cells, many with AII characteristics i.e. input from rod bipolar axons and gap junctions with diffuse ON center bipolar cell types. In the foveal region we and others have never seen invaginating midget cone bipolar cells getting gap junction contacts from an amacrine cell (44, 46, 63, 69). Thus, we conclude that AII amacrine cells have nothing to do with the midget system in the foveal retina at least. We also conclude a different small field amacrine cell from AII cells, that is CR-IR, occurs in central, rod-free fovea. Recent studies by Lee and coauthors (86) and Strettoi et al. (98) come to a different conclusion.
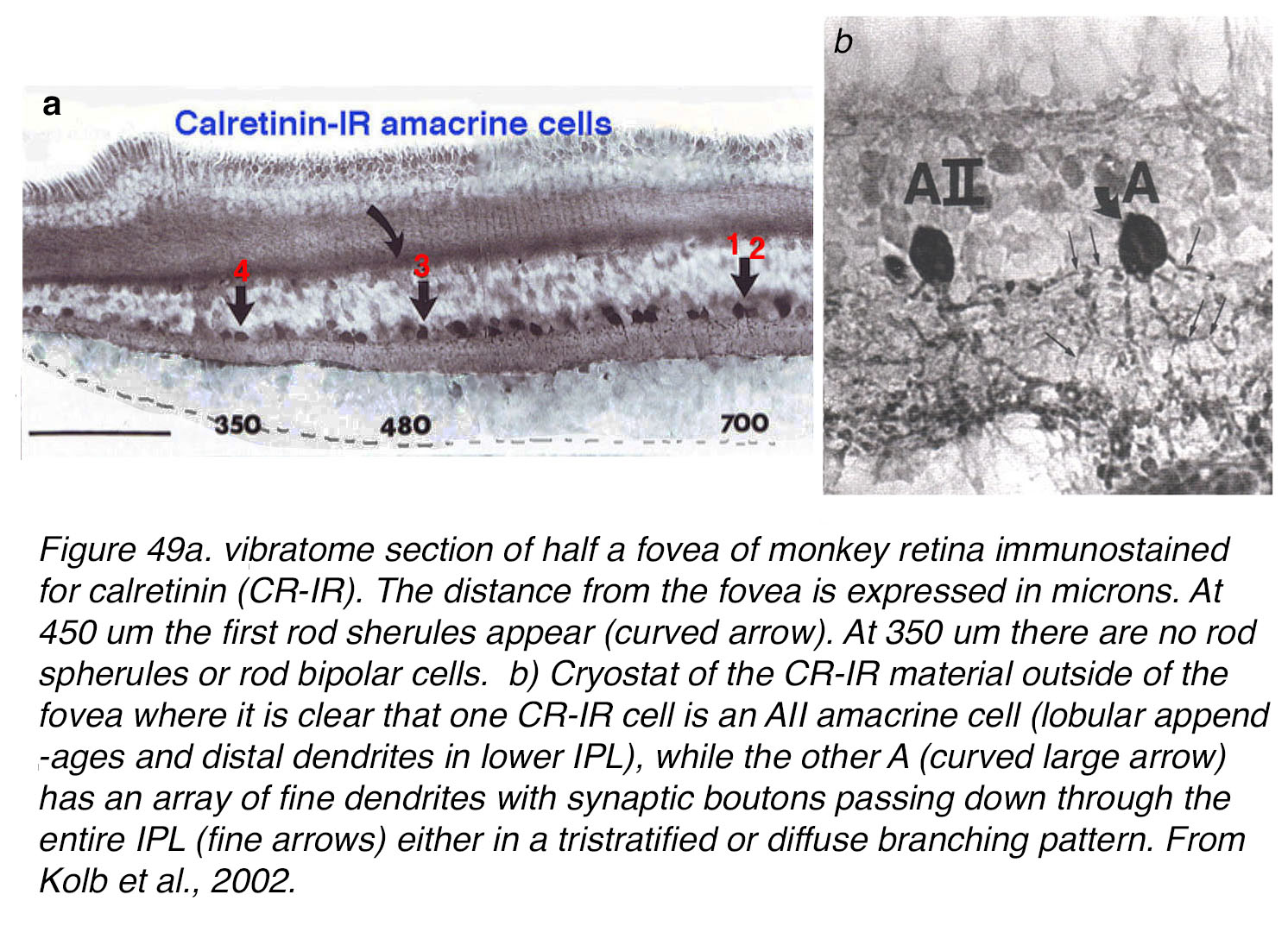 Figure 49. (a) Vibratome section of half a fovea of monkey retina immunostained for calretinin (CR-IR). The distance from the fovea is expressed in µm. At 480 µm the first rod spherules appear (curved arrow). At 350 µm there are no rod spherules or rod bipolar cells. (b) Cryostat of the CR-IR material outside of the fovea where it is clear that one CR-IR cell is an AII amacrine cell (lobular appendages and distal dendrites in lower IPL), while the other amacrine (A, curved large arrow) has an array of fine dendrites with synaptic boutons passing down through the entire IPL (fine arrow) either in a tristratified or diffuse branching pattern. From Kolb et al., 2002 (85).
Figure 49. (a) Vibratome section of half a fovea of monkey retina immunostained for calretinin (CR-IR). The distance from the fovea is expressed in µm. At 480 µm the first rod spherules appear (curved arrow). At 350 µm there are no rod spherules or rod bipolar cells. (b) Cryostat of the CR-IR material outside of the fovea where it is clear that one CR-IR cell is an AII amacrine cell (lobular appendages and distal dendrites in lower IPL), while the other amacrine (A, curved large arrow) has an array of fine dendrites with synaptic boutons passing down through the entire IPL (fine arrow) either in a tristratified or diffuse branching pattern. From Kolb et al., 2002 (85).
The question is what type of amacrine cell is the CR-IR cell of our foveal study (Figure 49, cell 4 at 350 µm from the foveal center). Light microscopy suggest this CR-IR cell has a tristratified or diffuse dendritic tree with fine dendrites throughout the IPL (Figure 49 b, A curved arrow). The electron microscope study of the cells at 350 µm from the foveal center indicated that the CR-IR processes were receiving ribbon input from OFF midget bipolar cells and fed reciprocal synapses to the same bipolar terminal (Figure 50, a-b). The CR-IR amacrine received input from a bipolar cell’s axon terminal in the lower IPL neuropil, therefore from an ON center bipolar (Figure 50, c). This was likely an ON midget bipolar but an ON diffuse bipolar axon terminal could not be ruled out (Figure 50, d), because this study is looking at neuropil with many stained processes from multiple stained cells. The CR-IR amacrines also made clear synapses upon a large ganglion cell process that was almost certainly an ON-center midget ganglion cell (Figure 50, c and d). In the fovea the CR-IR amacrine cell seems to be driven by OFF and ON midget bipolar cells and make reciprocal synapses upon the OFF bipolar type at least, and crossover inhibitory synapses upon ON midget ganglion cells (Figure 50 a, b, c, d, and summarized in e).
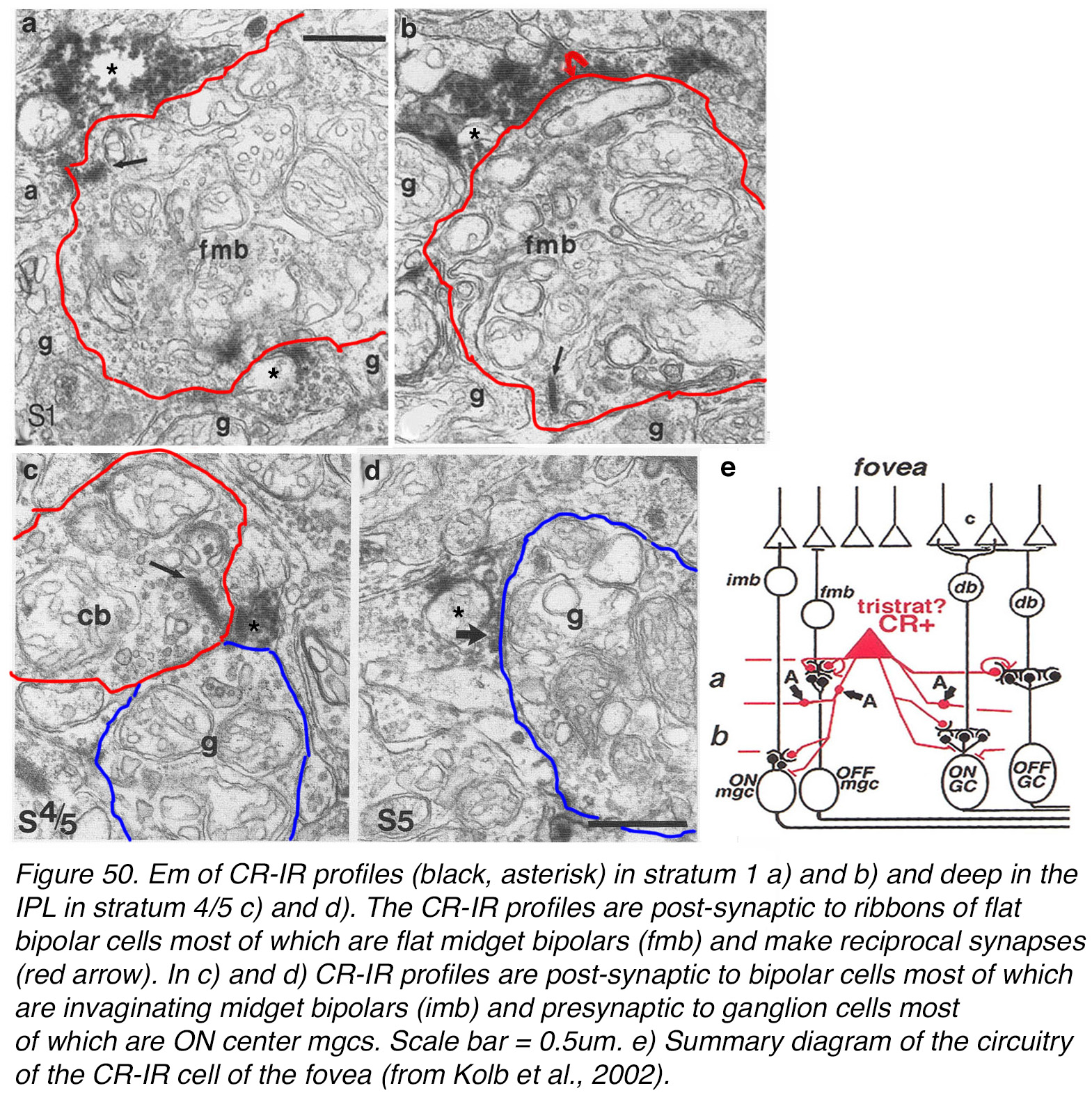 Figure 50. EM of CR-IR profiles (black, asterisk) in stratum 1 (a, b) and deep in the IPL in stratum 4/5 (c, d). The CR-IR profiles are postsynaptic to ribbons of flat bipolar cells, most of which are flat midget bipolar cells (fmb) and make reciprocal synapses (red arrow). In (c, d) CR-IR profiles are post-synaptic to bipolar cells, most of which are invaginating midget bipolar cells (imb) and presynaptic to ganglion cells, most of which are ON center midget ganglion cells (mgcs). Scale bar = 0.5 µm. (e) Summary diagram of the circuitry of the CR-IR amacrine cell of the fovea. From Kolb et al., 2002 (85).
Figure 50. EM of CR-IR profiles (black, asterisk) in stratum 1 (a, b) and deep in the IPL in stratum 4/5 (c, d). The CR-IR profiles are postsynaptic to ribbons of flat bipolar cells, most of which are flat midget bipolar cells (fmb) and make reciprocal synapses (red arrow). In (c, d) CR-IR profiles are post-synaptic to bipolar cells, most of which are invaginating midget bipolar cells (imb) and presynaptic to ganglion cells, most of which are ON center midget ganglion cells (mgcs). Scale bar = 0.5 µm. (e) Summary diagram of the circuitry of the CR-IR amacrine cell of the fovea. From Kolb et al., 2002 (85).
Besides the CR-IR putative small field diffuse amacrine cell, there are at least two other small field, glycinergic amacrine cells that are probably part of the foveal and midget bipolar-midget ganglion cell system of transmission of cone specific pathways. These are the A8 bistratified cell (79, 92) which can be stained with synuclein antibodies (93, 94) and a small field glycinergic amacrine equivalent to Figure 46, E, A3 or knotty type 2 cells, which can be marked with Parvalbumen immunoreactivity (91, 99) (see Webvision chapter on Roles of Amacrine Cells). An additional small field glycinergic cell described in rat, mouse and macaque monkey retina is immunoreactive to the vesicular glutamate transporter 3 (100). This vGluT3 cell is likely found in human retina too. Its dendritic tree lies between the two cholinergic amacrine types’ branching levels, where it receives input from bipolar cells and from other amacrine cells. The axon terminals of OFF diffuse cone bipolar types DB3 and ON diffuse cone bipolar DB5 (see Figure 43) run within the vGluT3 dendritic tree, so it is possible that vGluT3 cells are driven by both ON and OFF light responses and that they transmit this response to (at least) OFF ganglion cells (100, 101). In mouse retina, this amacrine cell selectively targets suppressed-by-contrast ganglion cells (102). It remains to be seen whether a glutamatergic amacrine cell is present in human fovea.
Furthermore there is at least one wide-field CR immunoreactive GABAergic amacrine that has dendrites coursing through the IPL on the foveal slope and even crossing the foveal pit floor (85, 86). This wide field A19 cell, immunostains for GABA, CB and CR (85). There is indirect evidence that the perifoveal blue-cone amacrine, perhaps the human A12, is GABAergic (77, 79).
Other wide-field amacrine cells are certainly present around the fovea at high density, as are all amacrine and ganglion cell types. Figure 51 shows the distribution of dopaminergic amacrine cells immunostained with tyrosine hydroxylase at the human fovea. These cells are at quite high concentration here and have a meshwork of dendrites forming a nest of processes around the foveal rim (Figure 51). The few dopaminergic synaptic beads in the foveal slope and pit (Figure 51, green spots) are probably synapsing upon small field A8 cell types, the CR immunoreactive small, diffuse amacrine. These are the same CR-IR amacrines we immunostain in the foveal center (Figure 48, Figure 49) (85). Dopaminergic amacrine cells synapse on AII amacrine cells in perifovea and peripheral retina (Figure 52) and on the stratum 1 melanopsin ganglion cell (103). Dopamine amacrine cells are also GABAergic and reach their highest concentration at the 4 mm ring around the foveal center where the highest concentration of rods is found (Figure 51) (104).
 Figure 51. Wholemount of the fovea in a human retina that is immunostained for tyrosine hydroxylase. The stained dopaminergic amacrine cells are numerous and have a meshwork of dendrites surrounding the fovea. Some fine dendrites penetrate the foveal slope and surround the foveal pit.
Figure 51. Wholemount of the fovea in a human retina that is immunostained for tyrosine hydroxylase. The stained dopaminergic amacrine cells are numerous and have a meshwork of dendrites surrounding the fovea. Some fine dendrites penetrate the foveal slope and surround the foveal pit.
Figure 52. Wholemount human retina immunostained for tyrosine hydroxylase (Th, green) and Calretinin (CR, red). The Th-IR cells are dopamine cells and exhibit rings of synaptic profiles around the CR-IR cell bodies of AII amacrine cells in this case.
Summary
The diagram in Figure 53 summarizes the different neural types and their circuitry in the human fovea. The central foveal pit is devoid of second and third order neurons because of the close packing of the cone photoreceptors and the pushing out radially of these circuits to drive ganglion cells piled up at the outer edge of the foveal slope and thence passing visual messages to the visual centers in the brain. The cone mosaic in the foveal pit and throughout the rod free zone through the foveal slope is the basis of our visual acuity. We know that the human visual system is capable of acuity of 1 min of arc or 60 cycles/degree of visual angle. One degree of visual angle is thought to cover approximately 280-300 µm of retinal distance. The center of the fovea is about one degree across thus allowing visual discrimination of 1 min of arc i.e. the center to center spacing (3 µm) of the cones of the central mosaic in the foveal pit.
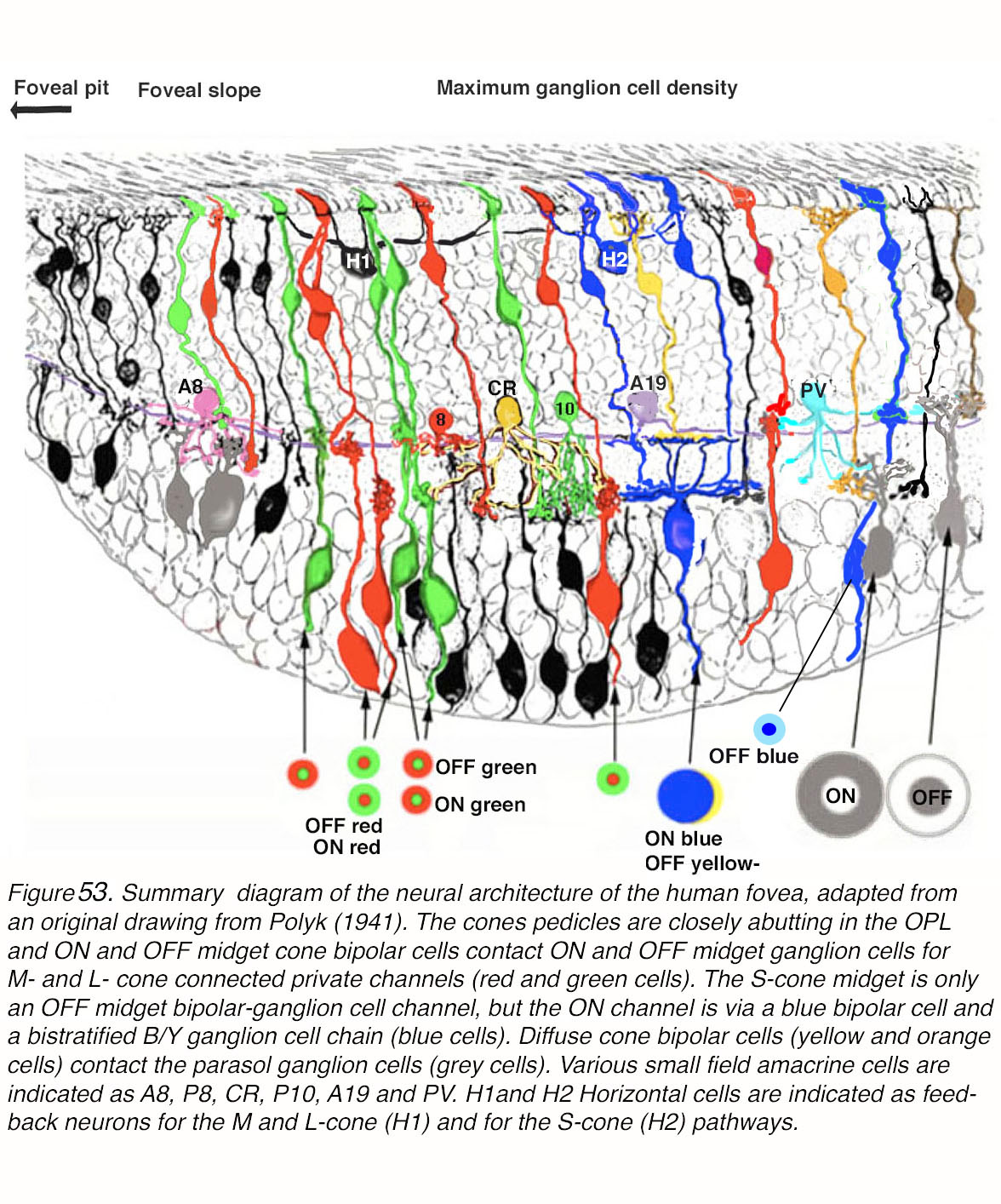 Figure 53. Summary diagram of the neural architecture of the human fovea, adapted from an original drawing of Polyak (1941) (90). The cone pedicles are closely abutting in the foveal OPL where ON and OFF midget cone bipolar cell dendrites contact them. ON and OFF midget bipolar cells contact ON and OFF midget ganglion cells in the inner retina providing M- and L-cone-connected private channels (red and green cells). The S-cone midget system (small blue cells) is only an OFF midget bipolar-ganglion cell channel, but the S-ON channel is via a blue-selective bipolar cell and a bistratified B/Y ganglion cell chain. A diffuse bipolar cell (yellow) provides OFF L/M cone input. Diffuse cone bipolar cells (brown and orange cells) contact the parasol ganglion cells (grey cells). Various small field amacrine cells are indicated as A8, P8, CR, P10, A19 and PV. H1 and H2 horizontal cells are indicated as feedback neurons for the M and L-cone (H1) and for the S-cone (H2) pathways.
Figure 53. Summary diagram of the neural architecture of the human fovea, adapted from an original drawing of Polyak (1941) (90). The cone pedicles are closely abutting in the foveal OPL where ON and OFF midget cone bipolar cell dendrites contact them. ON and OFF midget bipolar cells contact ON and OFF midget ganglion cells in the inner retina providing M- and L-cone-connected private channels (red and green cells). The S-cone midget system (small blue cells) is only an OFF midget bipolar-ganglion cell channel, but the S-ON channel is via a blue-selective bipolar cell and a bistratified B/Y ganglion cell chain. A diffuse bipolar cell (yellow) provides OFF L/M cone input. Diffuse cone bipolar cells (brown and orange cells) contact the parasol ganglion cells (grey cells). Various small field amacrine cells are indicated as A8, P8, CR, P10, A19 and PV. H1 and H2 horizontal cells are indicated as feedback neurons for the M and L-cone (H1) and for the S-cone (H2) pathways.
The central fovea with no superficial cell bodies and neural circuitry allows light to directly stimulate cones and give us this maximal visual acuity, but the circuitry underlying the transmission of this acuity to the brain must occur at the rim of the fovea. The rim of the fovea is where the ganglion cells are piled 6 to 7 deep (Figure 53) and is the foveal area containing all the intervening cells and connectivity to drive those ganglion cells. The predominant circuitry is concerned with the private cone to midget bipolar and midget ganglion cells. Every cone drives two midget bipolar cells and two midget ganglion cells so that the message from a single cone is provided to the brain as a contrast between lighter signals (ON pathways) or darker signals (OFF pathways) (Figure 53). The sharpening of this contrast message is provided by horizontal-cell feedback circuits and, in some pathways by amacrine circuitry. The human retina with 3 spectral types of cone means that many of these midget pathways will carry a concentric color opponent message (Figure 53). For example, a red cone will transmit to a midget bipolar cell and a midget ganglion cell a red ON center, green OFF surround or a red OFF center and a green ON surround. The same occurs for the green cone message to midget bipolar cells and midget ganglion cells (Figure 53, red and green neurons). In these red and green color pathways, the H1 horizontal cell is thought to play a role via feedback mechanisms to give the midget bipolar and midget ganglion cells the concentric opponent message (Figure 53). A current model of color vision channels in the human fovea, however, suggests that the red and green cone midget systems are not channels for color hue, but are primarily chromatic edge and visual acuity channels (70).
Signals from blue cones in the foveal slope have different pathways, in the case of ON center blue signals, to a non midget small bistratified ganglion cell (Figure 53) via the lower tier of dendrites. The upper tier of dendrites receives red and green cone OFF input from a diffuse cone bipolar type (Figure 53, yellow/orange cells). An OFF-center blue midget bipolar cell is known to be present in the fovea and connect to a blue-OFF midget ganglion cell (Figure 53). Another OFF blue message is sent to giant melanopsin ganglion cells that are present in the foveal ring area, but the circuitry driving these is uncertain and possibly involves an intermediate amacrine cell (not drawn in Figure 53). The H2 horizontal cells are thought to be feedback neurons of the blue cone system providing a concentric color-opponent organization of blue cones (35), nonetheless there is no concentric opponent visual message for the bistratified blue-driven ganglion cell types (Figure 53, blue and yellow opponent fields are coextensive), unlike the red and green cone midget ganglion cells.
Apart from the midget system that dominates the fovea, there is a presumed non-color channel (i.e. luminosity channel) in the fovea via the diffuse bipolar cells that get input from several cones of different spectral type and carry that non-color-specific message about light to a large field ganglion cell type known as a parasol ganglion cell (Figure 53, grey cells with receptive field structure in grey). Parasols also come in ON center and OFF center varieties, and are thought to have a center–surround organization concerning light and dark although they may have an imbalance of the strength of spectral mechanisms in center and surround (51, 105). These cells respond strongly to a sequence of slits consecutively displaced along a fixed direction, but much more poorly to a sequence of randomly positioned slits. This is evidence of tuning for motion (58).
Amacrine cells of the fovea are mostly small field and glycinergic. The larger field amacrines are present but more typically surround the fovea in a ring of processes, with little or no penetration into the foveal center (Figure 53). Thus, the small field glycinergic amacrines are important in some sort of interplay with the midget bipolar cell – midget ganglion cell channels (Figure 53, A8, CR and PV cells). We have anatomical descriptions of their synaptology but only the A8, P8 (called A2 in cat retina) (106) and PV cells have been recorded from. All have cone driven responses. Some are OFF center (A2, A8), and some are ON center (PV cells). All have been hypothesized to have a role in the generation of antagonistic surrounds of ganglion cells, but this remains to be proven. Foveal midget ganglion cells appear to receive little direct synaptic inhibition (107).
In sum, the fovea is a small but vital area of the central retina, unique in neural circuits, and molecular expression (108). The foveal cones themselves have evolved a sustained response characteristic, suited to standing contrast, that differs from peripheral cones (107). Foveal midget ganglion cells show virtually no synaptic inhibition, as compared to peripheral midgets (107). The fovea appears to have evolved in development from a central mound of cells as seen, for instance, in cat area centralis, to a virtually neuron-free pit, to concentrate cone photoreceptors into a dense array to be maximally sensitive to the smallest stimuli, and further, displace all ensuing circuitry into an exquisitely organized small surrounding rim of real estate in the retina foveal slope. If this small area is damaged by looking at the sun, by inherited cone atrophies or degenerated by age related macular degeneration (AMD), or even underdeveloped, as happens in albino people (see Webvision chapter on albinism), our eyesight becomes very poor.
Acknowledgements
P.A., N.C., I.O.-L. and R.F.N. thank H.K. for initiating and organizing this project, in part arising from long and fruitful collaboration among our laboratories. P.A thanks collaborators at the Medical University of Vienna and elsewhere, in particular Christian Schubert, Martin Glösmann and Dietmar Pum. Eduardo Fernandez obtained part of the human donor tissue. P.A work was supported by grants from the Austrian Science Fund (FWF)and EC-grant QLK6-CT-2001-00279. N.C. work was supported by the Spanish government, MINECO-FEDER-BFU2015-67139-R, RETICS-FEDER-RD16/0008/0016 and FPU14/03166. Generalitat Valenciana-FEDER, IDIFEDER/2017/064 and Prometeo-2016/158. R.F.N. work was supported by the Intramural Research Program of the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda MD.
About the authors
Helga Kolb, Ph.D. is the originator and chief editor of Webvision. She was born in England and educated with degrees from Bristol University. Helga has been in eye research since 1961, at first doing electrophysiology at the Institute of Ophthalmology, Moorfields Eye Hospital, and then emigrating to the USA. At the Wilmer Institute, Baltimore, in John Dowling’s lab, along with Brian Boycott she continued work in anatomy of the retina concentrating on understanding the neurons and wiring of the monkey retina. Later at the NEI, and the NINDS, Bethesda, she collaborated with Peter Gouras and Ralph Nelson on joint physiology and electron microscopy of retinal neurons to understand the connections of cell types involved in light, dark and color pathways in cat and monkey retinas. On moving to Utah, Helga continued collaborative projects with Ralph Nelson and started new collaborations with various postdocs in her lab from Europe and China mainly. Thus, Peter Ahnelt and Nicolas Cuenca aided and abetted studies of the monkey and human retina, before going on to their stellar careers in Austria and Spain respectively. All of us have been fascinated with the organization of the human fovea and this chapter represents our collaboration over the years.
 Ralph F. Nelson, Ph.D. received his B.A. in Biophysics from Amherst College and his Ph. D. in Biophysics from Johns Hopkins University. At Johns Hopkins, under the preceptorship of John Dowling, he studied conductance mechanisms responsible for light responses of retinal bipolar cells. In his postdoctoral studies at the National Institutes of Health in Dr. Peter Gouras’ lab, he formed a close collaboration with Helga Kolb in pioneering a type of study in which the light responses of retinal neurons were tightly interpreted in terms of synaptic connectivity. This group was the first to show that separation of bipolar/ganglion cell connections into two layers within the IPL underlaid the ON-center and OFF-center responses of the retina and whole visual system. Ralph Nelson’s laboratory is the Neural circuitry Unit of the Basic Neurosciences Program at the National Institute of Neurological Diseases and Stroke, NIH, Bethesda. His recent studies are on the neural circuitry for image processing in zebrafish retina.
Ralph F. Nelson, Ph.D. received his B.A. in Biophysics from Amherst College and his Ph. D. in Biophysics from Johns Hopkins University. At Johns Hopkins, under the preceptorship of John Dowling, he studied conductance mechanisms responsible for light responses of retinal bipolar cells. In his postdoctoral studies at the National Institutes of Health in Dr. Peter Gouras’ lab, he formed a close collaboration with Helga Kolb in pioneering a type of study in which the light responses of retinal neurons were tightly interpreted in terms of synaptic connectivity. This group was the first to show that separation of bipolar/ganglion cell connections into two layers within the IPL underlaid the ON-center and OFF-center responses of the retina and whole visual system. Ralph Nelson’s laboratory is the Neural circuitry Unit of the Basic Neurosciences Program at the National Institute of Neurological Diseases and Stroke, NIH, Bethesda. His recent studies are on the neural circuitry for image processing in zebrafish retina.
 Dr. Peter Ahnelt, Ph.D. was born in Bernstein, Austria. He received his Ph.D. in Biology at the University of Viennain 1980. His foremost interest was the identification of color specific cone photoreceptors in ground squirrel retinas at the Dept. of General and Comparative Physiology, Medical University of Vienna (MUW). In 1984/85 he worked as a post-doc in Helga Kolb’s lab at the University of Utah, identifying two spectral subtypes of human cone photoreceptors based on morphological and topographic criteria. Further studies at the U of U, revealed patterns of color-specific connections between human cone and horizontal cell subtypes. As associate professor at the MUW, in collaboration with Jan Nora Hokoc, Eduardo Fernandez, and Martin Glösmann he worked on understanding the various evolutionary and ecological trends underlying mammalian/primate photoreceptor mosaic organization. When retinal OCT imaging was advancing in labs such as Wolfgang Drexler’s, Peter made correlations of OCT signal profiles with retinal sublayers. More recently, Peter is interested in the role of Fractalkine in microglial activation in a retinitis pigmentosa mouse model.
Dr. Peter Ahnelt, Ph.D. was born in Bernstein, Austria. He received his Ph.D. in Biology at the University of Viennain 1980. His foremost interest was the identification of color specific cone photoreceptors in ground squirrel retinas at the Dept. of General and Comparative Physiology, Medical University of Vienna (MUW). In 1984/85 he worked as a post-doc in Helga Kolb’s lab at the University of Utah, identifying two spectral subtypes of human cone photoreceptors based on morphological and topographic criteria. Further studies at the U of U, revealed patterns of color-specific connections between human cone and horizontal cell subtypes. As associate professor at the MUW, in collaboration with Jan Nora Hokoc, Eduardo Fernandez, and Martin Glösmann he worked on understanding the various evolutionary and ecological trends underlying mammalian/primate photoreceptor mosaic organization. When retinal OCT imaging was advancing in labs such as Wolfgang Drexler’s, Peter made correlations of OCT signal profiles with retinal sublayers. More recently, Peter is interested in the role of Fractalkine in microglial activation in a retinitis pigmentosa mouse model.
 Isabel Ortuño-Lizarán, Ph.D. is from Elda, Alicante, Spain and studied for a Biotechnology degree in the Polytechnic University of Valencia, Spain. After, she received a master’s degree in Neurosciences at the University of Valencia, she recently finished a doctorate degree (2019) at the University of Alicante, under the supervision of Dr. Nicolás Cuenca. Her research has been mainly focused on studying the human retina and foveal organization in Cuenca’s lab. She has also been working on understanding the visual impairment in Parkinson’s disease using human retinas and examining the melanopsin ganglion cell density and degeneration rates with the disease progress
Isabel Ortuño-Lizarán, Ph.D. is from Elda, Alicante, Spain and studied for a Biotechnology degree in the Polytechnic University of Valencia, Spain. After, she received a master’s degree in Neurosciences at the University of Valencia, she recently finished a doctorate degree (2019) at the University of Alicante, under the supervision of Dr. Nicolás Cuenca. Her research has been mainly focused on studying the human retina and foveal organization in Cuenca’s lab. She has also been working on understanding the visual impairment in Parkinson’s disease using human retinas and examining the melanopsin ganglion cell density and degeneration rates with the disease progress
 Nicolás Cuenca, Ph.D. was born in Elda, Alicante, Spain. He has a B.Sc. Degree in Biological Sciences from the University of Valencia, Spain and a Ph.D. Degree from the University of Alicante, Spain. He carried out postdoctoral studies at the Department of Physiology, University of Utah (USA) in Helga Kolb’s laboratory. Currently, Nicolas is Full Professor in the Department of Physiology, Genetics and Microbiology at the University of Alicante. His research is dedicated to the understanding the functional organization of the mammalian retina. He has extensive expertise in the field of retinal connectivity, morphology and functional correlations, and is an expert in confocal microscopy of immunostained retinas. Nicolas has won many awards throughout Europe for his photography of immunostained retinas. Presently he is studying the disruption of the retinal architecture including blood vessels and glial cells in inherited retinal degenerations. He is concentrated on therapeutic treatments using animal models for retinitis pigmentosa, glaucoma and macular degeneration. His team is aiming to slow down the progression of neurodegenerative diseases, through the use of cell transplant, antiapoptotics, gene therapy and antioxidant drugs. All are welcome to visit his imaging website at www.retinalmicroscopy.com.
Nicolás Cuenca, Ph.D. was born in Elda, Alicante, Spain. He has a B.Sc. Degree in Biological Sciences from the University of Valencia, Spain and a Ph.D. Degree from the University of Alicante, Spain. He carried out postdoctoral studies at the Department of Physiology, University of Utah (USA) in Helga Kolb’s laboratory. Currently, Nicolas is Full Professor in the Department of Physiology, Genetics and Microbiology at the University of Alicante. His research is dedicated to the understanding the functional organization of the mammalian retina. He has extensive expertise in the field of retinal connectivity, morphology and functional correlations, and is an expert in confocal microscopy of immunostained retinas. Nicolas has won many awards throughout Europe for his photography of immunostained retinas. Presently he is studying the disruption of the retinal architecture including blood vessels and glial cells in inherited retinal degenerations. He is concentrated on therapeutic treatments using animal models for retinitis pigmentosa, glaucoma and macular degeneration. His team is aiming to slow down the progression of neurodegenerative diseases, through the use of cell transplant, antiapoptotics, gene therapy and antioxidant drugs. All are welcome to visit his imaging website at www.retinalmicroscopy.com.
References
References
1. O’Brien J.J., Chen X., MacLeish P.R., O’Brien J., Massey S.C. Photoreceptor coupling mediated by connexin36 in the primate retina. Journal of Neuroscience. 2012;32(13):4675–4687. [PMC free article] [PubMed]
2. Hendrickson, A., D. Possin, L. Vajzovic, and C.A. Toth, Histologic development of the human fovea from midgestation to maturity. American journal of ophthalmology. 2012; 154(5):767-778. e2. [PMC free article] [PubMed]
3. Hendrickson A.E., Yuodelis C. The morphological development of the human fovea. Ophthalmology. 1984;91(6):603–612. [PubMed]
4. Bringmann A., Syrbe S., Görner K., Kacza J., Francke M., Wiedemann P., Reichenbach A. The primate fovea: Structure, function and development. Progress in retinal and eye research. 2018;66:49–84. [PubMed]
5. Curcio C.A., Sloan K.R. Packing geometry of human cone photoreceptors: variation with eccentricity and evidence for local anisotropy. Visual neuroscience. 1992;9(2):169–180. [PubMed]
6. Pum D., Ahnelt P.K., Grasl M. Iso-orientation areas in the foveal cone mosaic. Visual neuroscience. 1990;5(6):511–523. [PubMed]
7. Ahnelt, P., C. Schubert, and E. Anger, Macular Photoreceptor Organization. Modular substructuring–a consistent feature along all foveal cone elements, in The Macula Diagnosis, Treatment and Future Trends. 2004, Springer: Vienna.
8. Curcio C.A., Packer O., Kalina R.E. A whole mount method for sequential analysis of photoreceptor and ganglion cell topography in a single retina. Vision research. 1987;27(1):9–15. [PubMed]
9. Schein S.J. Anatomy of macaque fovea and spatial densities of neurons in foveal representation. Journal of Comparative Neurology. 1988;269(4):479–505. [PubMed]
10. Curcio C.A., Allen K.A. Topography of ganglion cells in human retina. Journal of comparative Neurology. 1990;300(1):5–25. [PubMed]
11.Ahnelt P.K., Kolb H., Pflug R. Identification of a subtype of cone photoreceptor, likely to be blue sensitive, in the human retina. Journal of Comparative Neurology. 1987;255(1):18–34. [PubMed]
12. Peichl L., Behrmann G., Kröger R.H. For whales and seals the ocean is not blue: a visual pigment loss in marine mammals. European Journal of Neuroscience. 2001;13(8):1520–1528. [PubMed]
13. Nathans J., Piantanida T.P., Eddy R.L., Shows T.B., Hogness D.S. Molecular genetics of inherited variation in human color vision. Science. 1986;232(4747):203–210. [PubMed]
14. Kolb, H. and L. Lipitz, The anatomical basis for colour vision in the vertebrate retina, Volume 6: The perception of colour, in Vision and Visual Dysfunction, P. Gouras, Editor. 1991, Macmillan Press Ltd,: London. p. 128-145.
15. Marc R.E., Sperling H.G. Chromatic organization of primate cones. Science. 1977;196(4288):454–456. [PubMed]
16. Williams D.R., MacLeod D.I., Hayhoe M.M. Punctate sensitivity of the blue-sensitive mechanism. Vision research. 1981;21(9):1357–1375. [PubMed]
17. Cicerone C.M., Nerger J.L. The relative numbers of long-wavelength-sensitive to middle-wavelength-sensitive cones in the human fovea centralis. Vision research. 1989;29(1):115–128. [PubMed]
18. Mollon J., Bowmaker J. The spatial arrangement of cones in the primate fovea. Nature. 1992;360(6405):677. [PubMed]
19. Roorda A., Williams D.R. The arrangement of the three cone classes in the living human eye. Nature. 1999;397(6719):520. [PubMed]
20. Roorda A., Metha A.B., Lennie P., Williams D.R. Packing arrangement of the three cone classes in primate retina. Vision research. 2001;41(10-11):1291–1306. [PubMed]
21. Hofer H., Singer B., Williams D.R. Different sensations from cones with the same photopigment. Journal of Vision. 2005;5(5):5–5. [PubMed]
22. Schmidt B.P., Boehm A.E., Foote K.G., Roorda A. The spectral identity of foveal cones is preserved in hue perception. Journal of vision. 2018;18(11):19–19. [PMC free article] [PubMed]
23. Drasdo N., Fowler C. Non-linear projection of the retinal image in a wide-angle schematic eye. The British journal of ophthalmology. 1974;58(8):709. [PMC free article] [PubMed]
24. Ahnelt P., Pflug R. Telodendrial contacts between foveolar cone pedicles in the human retina. Experientia. 1986;42(3):298–300. [PubMed]
25. Xiao M., Hendrickson A. Spatial and temporal expression of short, long/medium, or both opsins in human fetal cones. Journal of Comparative Neurology. 2000;425(4):545–559. [PubMed]
26. Hendrickson A., Zhang C. Development of cone photoreceptors and their synapses in the human and monkey fovea. Journal of Comparative Neurology. 2019;527(1):38–51. [PMC free article] [PubMed]
27. Cornish E.E., Hendrickson A.E., Provis J.M. Distribution of short-wavelength-sensitive cones in human fetal and postnatal retina: early development of spatial order and density profiles. Vision research. 2004;44(17):2019–2026. [PubMed]
28. Kolb H., Fernandez E., Schouten J., Ahnelt P., Linberg K.A., Fisher S.K. Are there three types of horizontal cell in the human retina? Journal of Comparative Neurology. 1994;343(3):370–386. [PubMed]
29. Ahnelt P., Kolb H. Horizontal cells and cone photoreceptors in primate retina: a Golgi‐light microscopic study of spectral connectivity. Journal of Comparative Neurology. 1994;343(3):387–405. [PubMed]
30. Ahnelt P., Kolb H. Horizontal cells and cone photoreceptors in human retina: A Golgi‐electron microscopic study of spectral connectivity. Journal of Comparative Neurology. 1994;343(3):406–427. [PubMed]
31. Ahnelt, P.K. and H. Kolb, Short-wavelength-sensitive cones: morphology and color-specific connections, in Colour Vision Deficiencies XII. 1995, Springer. p. 285-297.
32. Drinnenberg, A., F. Franke, R.K. Morikawa, J. Jüttner, D. Hillier, P. Hantz, A. Hierlemann, R.A. da Silveira, and B. Roska, How diverse retinal functions arise from feedback at the first visual synapse. Neuron. 2018; 99(1):117-134. e11. [PMC free article] [PubMed]
33. Verweij J., Hornstein E.P., Schnapf J.L. Surround antagonism in macaque cone photoreceptors. Journal of Neuroscience. 2003;23(32):10249–10257. [PMC free article] [PubMed]
34. Dacey D.M., Crook J.D., Packer O.S. Distinct synaptic mechanisms create parallel S-ON and S-OFF color opponent pathways in the primate retina. Visual Neuroscience. 2014;31(2):139–151. [PMC free article] [PubMed]
35. Packer O.S., Verweij J., Li P.H., Schnapf J.L., Dacey D.M. Blue-yellow opponency in primate S cone photoreceptors. Journal of Neuroscience. 2010;30(2):568–572. [PMC free article] [PubMed]
36. Cajal, S.R.y., The structure of the retina. 1933/1972, Translated by S. Thorpe and M. Glickstein: Charles C. Thomas Publisher.
37. Kolb H. Organization of the outer plexiform layer of the primate retina: electron microscopy of Golgi-impregnated cells. Philosophical Transactions of the Royal Society of London. B, Biological Sciences. 1970;258(823):261–283. [PubMed]
38. Kolb H., Boycott B., Dowling J. A second type of midget bipolar cell in the primate retina. Philos Trans R Soc Lond B Biol Sci. 1969;255:177–184.
39. Stell W.K. Correlation of retinal cytoarchitecture and ultrastructure in Golgi preparations. The Anatomical Record. 1965;153(4):389–397. [PubMed]
40. Boycott B.B., Dowling J.E. Organization of the primate retina: light microscopy, with an appendix: a second type of midget bipolar cell in the primate retina. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 1969;255(799):109–184.
41. Ritchey E.R., Bongini R.E., Code K.A., Zelinka C., Petersen-Jones S., Fischer A.J. The pattern of expression of guanine nucleotide-binding protein β3 in the retina is conserved across vertebrate species. Neuroscience. 2010;169(3):1376–1391. [PMC free article] [PubMed]
42. Milam A.H., Dacey D.M., Dizhoor A.M. Recoverin immunoreactivity in mammalian cone bipolar cells. Visual neuroscience. 1993;10(1):1–12. [PubMed]
43. Zang J., Neuhauss S.C. The Binding Properties and Physiological Functions of Recoverin. Frontiers in molecular neuroscience. 2018:11. [PMC free article] [PubMed]
44. Kolb H., Dekorver L. Midget ganglion cells of the parafovea of the human retina: a study by electron microscopy and serial section reconstructions. Journal of Comparative Neurology. 1991;303(4):617–636. [PubMed]
45. Marshak D.W., Yamada E.S., Bordt A.S., Perryman W.C. Synaptic input to an ON parasol ganglion cell in the macaque retina: a serial section analysis. Visual neuroscience. 2002;19(3):299–305. [PMC free article] [PubMed]
46. Kolb H., Marshak D. The midget pathways of the primate retina. Documenta Ophthalmologica. 2003;106(1):67–81. [PubMed]
47. Jusuf P.R., Martin P.R., Grünert U. Synaptic connectivity in the midget‐parvocellular pathway of primate central retina. Journal of Comparative Neurology. 2006;494(2):260–274. [PubMed]
48. Nelson R., Famiglietti E. Jr, Kolb H. Intracellular staining reveals different levels of stratification for on-and off-center ganglion cells in cat retina. Journal of Neurophysiology. 1978;41(2):472–483. [PubMed]
49. Nelson R., Kolb H. ON and OFF pathways in the vertebrate retina and visual system. The visual neurosciences. 2004;1:260–278.
50. Field G.D., Gauthier J.L., Sher A., Greschner M., Machado T.A., Jepson L.H., Shlens J., Gunning D.E., Mathieson K., Dabrowski W. Functional connectivity in the retina at the resolution of photoreceptors. Nature. 2010;467(7316):673. [PMC free article] [PubMed]
51. Kling A., Field G., Brainard D., Chichilnisky E. Probing Computation in the Primate Visual System at Single-Cone Resolution. Annual review of neuroscience. 2019:42. [PMC free article] [PubMed]
52. Telkes I., Lee S.C., Jusuf P.R., Grünert U. The midget‐parvocellular pathway of marmoset retina: A quantitative light microscopic study. Journal of Comparative Neurology. 2008;510(5):539–549. [PubMed]
53. Gouras P. Identification of cone mechanisms in monkey ganglion cells. The Journal of physiology. 1968;199(3):533–547. [PMC free article] [PubMed]
54. Gouras P., Zrenner E. Color coding in primate retina. Vision Research. 1981;21(11):1591–1598. [PubMed]
55. Dacey D.M., Packer O.S. Colour coding in the primate retina: diverse cell types and cone-specific circuitry. Current opinion in neurobiology. 2003;13(4):421–427. [PubMed]
56. Dacey D., Packer O.S., Diller L., Brainard D., Peterson B., Lee B. Center surround receptive field structure of cone bipolar cells in primate retina. Vision research. 2000;40(14):1801–1811. [PubMed]
57. Wool L.E., Crook J.D., Troy J.B., Packer O.S., Zaidi Q., Dacey D.M. Nonselective wiring accounts for red-green opponency in midget ganglion cells of the primate retina. Journal of Neuroscience. 2018;38(6):1520–1540. [PMC free article] [PubMed]
58. Manookin, M.B., S.S. Patterson, and C.M. Linehan, Neural mechanisms mediating motion sensitivity in parasol ganglion cells of the primate retina. Neuron. 2018; 97(6):1327-1340. e4. [PMC free article] [PubMed]
59. Slaughter M.M., Miller R.F. 2-amino-4-phosphonobutyric acid: a new pharmacological tool for retina research. Science. 1981;211(4478):182–5. [PubMed]
60. McGregor J.E., Yin L., Yang Q., Godat T., Huynh K.T., Zhang J., Williams D.R., Merigan W.H. Functional architecture of the foveola revealed in the living primate. PloS one. 2018;13(11):e0207102 [PMC free article] [PubMed]
61. Mariani A.P. Bipolar cells in monkey retina selective for the cones likely to be blue-sensitive. Nature. 1984;308(5955):184. [PubMed]
62. Kouyama N., Marshak D. Bipolar cells specific for blue cones in the macaque retina. Journal of Neuroscience. 1992;12(4):1233–1252. [PMC free article] [PubMed]
63. Calkins D.J., Tsukamoto Y., Sterling P. Microcircuitry and mosaic of a blue–yellow ganglion cell in the primate retina. Journal of Neuroscience. 1998;18(9):3373–3385. [PMC free article] [PubMed]
64. Dacey D.M., Lee B.B. The’blue-on’opponent pathway in primate retina originates from a distinct bistratified ganglion cell type. Nature. 1994;367(6465):731. [PubMed]
65. Mullen K.T. The contrast sensitivity of human colour vision to red‐green and blue‐yellow chromatic gratings. The Journal of physiology. 1985;359(1):381–400. [PMC free article] [PubMed]
66. Sekiguchi N., Williams D.R., Brainard D.H. Efficiency in detection of isoluminant and isochromatic interference fringes. JOSA A. 1993;10(10):2118–2133. [PubMed]
67. Stromeyer C. III, Kranda K., Sternheim C. Selective chromatic adaptation at different spatial frequencies. Vision research. 1978;18(4):427–437. [PubMed]
68. Curcio C.A., Allen K.A., Sloan K.R., Lerea C.L., Hurley J.B., Klock I.B., Milam A.H. Distribution and morphology of human cone photoreceptors stained with anti‐blue opsin. Journal of Comparative Neurology. 1991;312(4):610–624. [PubMed]
69. Klug K., Herr S., Ngo I.T., Sterling P., Schein S. Macaque retina contains an S-cone OFF midget pathway. Journal of Neuroscience. 2003;23(30):9881–9887. [PMC free article] [PubMed]
70. Patterson S.S., Kuchenbecker J.A., Anderson J.R., Bordt A.S., Marshak D.W., Neitz M., Neitz J. An S-cone circuit for edge detection in the primate retina. Scientific Reports. 2019;9(1):11913. [PMC free article] [PubMed]
71. Wool L.E., Packer O.S., Zaidi Q., Dacey D.M. Connectomic identification and three-dimensional color tuning of S-OFF midget ganglion cells in the primate retina. Journal of Neuroscience. 2019;39(40):7893–7909. [PMC free article] [PubMed]
72. Wiesel T.N., Hubel D.H. Spatial and chromatic interactions in the lateral geniculate body of the rhesus monkey. Journal of neurophysiology. 1966;29(6):1115–1156. [PubMed]
73. Dacey D.M., Liao H.-W., Peterson B.B., Robinson F.R., Smith V.C., Pokorny J., Yau K.-W., Gamlin P.D. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature. 2005;433(7027):749. [PubMed]
74. Mure L.S., Vinberg F., Hanneken A., Panda S. Functional diversity of human intrinsically photosensitive retinal ganglion cells. Science. 2019;366(6470):1251–1255. [PMC free article] [PubMed]
75. Hannibal J., Christiansen A.T., Heegaard S., Fahrenkrug J., Kiilgaard J.F. Melanopsin expressing human retinal ganglion cells: Subtypes, distribution, and intraretinal connectivity. Journal of Comparative Neurology. 2017;525(8):1934–1961. [PubMed]
76. Miyagishima K.J., Gruenert U., Li W. Processing of S-cone signals in the inner plexiform layer of the mammalian retina. Visual neuroscience. 2014;31(2):153–163. [PubMed]
77. Patterson S.S., Kuchenbecker J.A., Anderson J.R., Neitz M., Neitz J. A Color Vision Circuit for Non-Image-Forming Vision in the Primate Retina. Current Biology. 2020 [PMC free article] [PubMed]
78. Mariani A.P. Giant bistratified bipolar cells in monkey retina. The Anatomical Record. 1983;206(2):215–220.
79. Kolb H., Linberg K.A., Fisher S.K. Neurons of the human retina: a Golgi study. Journal of comparative neurology. 1992;318(2):147–187. [PubMed]
80. Jusuf P.R., Lee S.C., Hannibal J., Grünert U. Characterization and synaptic connectivity of melanopsin‐containing ganglion cells in the primate retina. European Journal of Neuroscience. 2007;26(10):2906–2921. [PubMed]
81. Mariani A.P. Amacrine cells of the rhesus monkey retina. Journal of comparative neurology. 1990;301(3):382–400. [PubMed]
82. Rodieck R., Marshak D. Spatial density and distribution of choline acetyltransferase immunoreactive cells in human, macaque, and baboon retinas. Journal of Comparative Neurology. 1992;321(1):46–64. [PubMed]
83. Marshak D.W. Peptidergic neurons of the macaque monkey retina. Neuroscience Research Supplements. 1989;10:S117–S130. [PubMed]
84. Kolb H., Famigilietti E. Rod and cone pathways in the inner plexiform layer of cat retina. Science. 1974;186(4158):47–49. [PubMed]
85. Kolb H., Zhang L., Dekorver L., Cuenca N. A new look at calretinin‐immunoreactive amacrine cell types in the monkey retina. Journal of Comparative Neurology. 2002;453(2):168–184. [PubMed]
86. Lee S.C., Weltzien F., Madigan M.C., Martin P.R., Grünert U. Identification of AII amacrine, displaced amacrine, and bistratified ganglion cell types in human retina with antibodies against calretinin. Journal of Comparative Neurology. 2016;524(1):39–53. [PubMed]
87. Wässle H., Grüunert U., Chun M.N., Boycott B.B. The rod pathway of the macaque monkey retina: identification of AII‐amacrine cells with antibodies against calretinin. Journal of Comparative Neurology. 1995;361(3):537–551. [PubMed]
88. Feng G., Mellor R.H., Bernstein M., Keller-Peck C., Nguyen Q.T., Wallace M., Nerbonne J.M., Lichtman J.W., Sanes J.R. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28(1):41–51. [PubMed]
89. Wässle H., Heinze L., Ivanova E., Majumdar S., Weiss J., Harvey R.J., Haverkamp S. Glycinergic transmission in the Mammalian retina. Frontiers in molecular neuroscience. 2009;2:6. [PMC free article] [PubMed]
90. Polyak, S.L., The retina. 1941, Chicago: Chicago University Press.
91. Klump K.E., Zhang A.-J., Wu S.M., Marshak D.W. Parvalbumin-immunoreactive amacrine cells of macaque retina. Visual neuroscience. 2009;26(3):287–296. [PMC free article] [PubMed]
92. Kolb H., Nelson R. Hyperpolarizing, small‐field, amacrine cells in cone pathways of cat retina. Journal of Comparative Neurology. 1996;371(3):415–436. [PubMed]
93. Lee S.C., Meyer A., Schubert T., Hüser L., Dedek K., Haverkamp S. Morphology and connectivity of the small bistratified A8 amacrine cell in the mouse retina. Journal of Comparative Neurology. 2015;523(10):1529–1547. [PMC free article] [PubMed]
94. Neumann S., Haverkamp S. Characterization of small‐field bistratified amacrine cells in macaque retina labeled by antibodies against synaptotagmin‐2. Journal of Comparative Neurology. 2013;521(3):709–724. [PubMed]
95. Bloomfield S.A., Xin D., Osborne T. Light-induced modulation of coupling between AII amacrine cells in the rabbit retina. Visual neuroscience. 1997;14(3):565–576. [PubMed]
96. Dacheux R.F., Raviola E. The rod pathway in the rabbit retina: a depolarizing bipolar and amacrine cell. Journal of Neuroscience. 1986;6(2):331–345. [PMC free article] [PubMed]
97. Nelson R. AII amacrine cells quicken time course of rod signals in the cat retina. Journal of Neurophysiology. 1982;47(5):928–947. [PubMed]
98. Strettoi E., Masri R.A., Grünert U. AII amacrine cells in the primate fovea contribute to photopic vision. Scientific reports. 2018;8(1):16429. [PMC free article] [PubMed]
99. Bordt A.S., Hoshi H., Yamada E.S., Perryman‐Stout W.C., Marshak D.W. Synaptic input to OFF parasol ganglion cells in macaque retina. Journal of Comparative Neurology. 2006;498(1):46–57. [PMC free article] [PubMed]
100. Haverkamp S., Wässle H. Characterization of an amacrine cell type of the mammalian retina immunoreactive for vesicular glutamate transporter 3. Journal of comparative neurology. 2004;468(2):251–263. [PubMed]
101. Johnson J., Sherry D.M., Liu X., Fremeau R.T. Jr, Seal R.P., Edwards R.H., Copenhagen D.R. Vesicular glutamate transporter 3 expression identifies glutamatergic amacrine cells in the rodent retina. Journal of comparative neurology. 2004;477(4):386–398. [PMC free article] [PubMed]
102. Lee S., Zhang Y., Chen M., Zhou Z.J. Segregated glycine-glutamate co-transmission from vGluT3 amacrine cells to contrast-suppressed and contrast-enhanced retinal circuits. Neuron. 2016;90(1):27–34. [PMC free article] [PubMed]
103. Liao H.W., Ren X., Peterson B.B., Marshak D.W., Yau K.W., Gamlin P.D., Dacey D.M. Melanopsin‐expressing ganglion cells on macaque and human retinas form two morphologically distinct populations. Journal of Comparative Neurology. 2016;524(14):2845–2872. [PMC free article] [PubMed]
104. Mariani A.P., Kolb H., Nelson R. Dopamine-containing amacrine cells of rhesus monkey retina parallel rods in spatial distribution. Brain research. 1984;322(1):1–7. [PubMed]
105. De Monasterio F., Gouras P. Functional properties of ganglion cells of the rhesus monkey retina. The Journal of physiology. 1975;251(1):167–195. [PMC free article] [PubMed]
106. Kolb H., Nelson R., Mariani A. Amacrine cells, bipolar cells and ganglion cells of the cat retina: a Golgi study. Vision Res. 1981;21(7):1081–1114. [PubMed]
107. Sinha, R., M. Hoon, J. Baudin, H. Okawa, R.O. Wong, and F. Rieke, Cellular and circuit mechanisms shaping the perceptual properties of the primate fovea. Cell. 2017; 168(3):413-426. e12. [PMC free article] [PubMed]
108. Peng, Y.-R., K. Shekhar, W. Yan, D. Herrmann, A. Sappington, G.S. Bryman, T. Van Zyl, M.T.H. Do, A. Regev, and J.R. Sanes, Molecular classification and comparative taxonomics of foveal and peripheral cells in primate retina. Cell. 2019; 176(5):1222-1237. e22. [PMC free article] [PubMed]
109. Saari J.C., Bunt-Milam A.H., Bredberg D.L., Garwin G.G. Properties and immunocytochemical localization of three retinoid-binding proteins from bovine retina. Vision research. 1984;24(11):1595–1603. [PubMed]
110. Cuenca N., Ortuño-Lizarán I., Pinilla I. Cellular characterization of OCT and outer retinal bands using specific immunohistochemistry markers and clinical implications. Ophthalmology. 2018;125(3):407–422. [PubMed]
111. Curcio C.A., Provis J., Wong R., Neitz M. Obituary: Anita Hendrickson, PhD. The Journal of comparative neurology. 2019;527(1):11–12.
112. Ahnelt P.K. The photoreceptor mosaic. Eye. 1998;12(3b):531–540. [PubMed]
113. Ahnelt P.K., Pflug R. Telodendrial contacts between foveolar cone pedicles in the human retina. Experientia. 1986;42(3):298–300. [PubMed]
114. Nasir‐Ahmad S., Lee S.C., Martin P.R., Grünert U. Melanopsin‐expressing ganglion cells in human retina: Morphology, distribution, and synaptic connections. Journal of Comparative Neurology. 2019;527(1):312–327. [PubMed]