Helga Kolb
A certain degree of integration of the visual message goes on at the first synapse in the retina, in the outer plexiform layer. Here cone pedicles and rod spherules are synaptic upon various bipolar cell and horizontal cell types. In addition, as mentioned in the previous section, cone pedicles pass electrical messages between each other and between rod spherules so that a small amount of rod and cone signal mixing occurs at this layer (Kolb, 1977; Nelson, 1977).
Two important synaptic interactions that occur at the outer plexiform layer are:
- the splitting of the visual signal into two separate channels of information flow, one for detecting objects lighter than background and one for detecting objects darker that background
- the instillation of pathways to create simultaneous contrast of visual objects.
The first pathways are known as the basis of successive contrast or ON and OFF pathways respectively while the second puts light and dark boundaries in simultaneous contrast and forms a receptive field structure with a center contrasted to an inhibitory surround. We shall see how these two necessary visual information processes are created by synaptic interactions at the outer plexiform layer in this chapter.
1. Techniques that have been used to understand neural pathways in the retina.
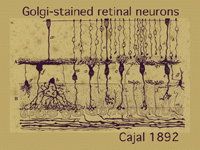
The morphologies of individual neurons that make up the retina and contribute processes for synaptic interaction in the plexiform layers have been described over the years from using various anatomical techniques. Principal amongst these is a specific neural stain named after a famous early Italian neuroanatomist, Camillo Golgi (1885), who lived at the end of the last century. This staining method was used most extensively and with extraordinary success by the great Spanish anatomist Ramon y Cajal (1892).
It is, in fact, the monumental studies of Ramon y Cajal that form the basis of neuroanatomy for the vertebrate nervous system in general and the retina in particular. One of Cajal’s drawings of nerve cells in the retina stained by Golgi techniques is in Figure 2.
In this one figure from Cajal’s works we see photoreceptors, bipolar cells, horizontal cells and some amacrine cells types. Cajal pointed out that photoreceptors-bipolar cells-ganglion cells were involved in passing rod or cone information through the vertical pathways and that horizontal and amacrine cells were involved in lateral interactions. He introduced the idea that synaptic connections were set up between specific cell types in the plexiform layers by virtue of nerve cells’ dendrites and axons costratifying precisely to ensure that the correct presynaptic cell talked to the correct postsynaptic cell.
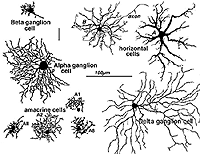
Stephen Polyak, was the man most responsible for applying the Golgi stain to monkeys and chimpanzees, authoring landmark books concerning the organization of the primate retina and visual system in 1941. More contemporaneously, we have used the Golgi method in research on cat, monkey and human retinas (Boycott and Dowling, 1969; Kolb, 1970; Mariani, 1984a,b; Kolb, et al., 1981, 1992). Some of the cells we have described since Cajal and Polyak are shown in Figure 3.
Fig. 3. Golgi-stained neurons of cat retina
It becomes immediately obvious that our drawings of Golgi stained cells differ from those of Cajal, in that we have drawn complete cells from a surface or wholemount view looking into the retina mounted flat. Being able to prepare wholemounts of retinas was a great advance for our understanding of the morphologies of nerve cells in the retina for it allowed us to see complete dendritic tree spreads of stained cell, which were often truncated in the sectioned retinas that Cajal and Polyak studied. Thus we have been able to add significant numbers of new cell types to the original descriptions. Also, with the advent of electron microscopy, histochemical and immunocytochemical staining and electrophysiological single cell recording and staining, we can now direct these techniques at elucidating neural circuits in the retina in a way not available to our predecessors. All the descriptions of cells and circuits that follow in this chapter come from experiments over the years using a combination of these techniques but always with the morphological data from Golgi staining as a basis.
2. Bipolar cells.
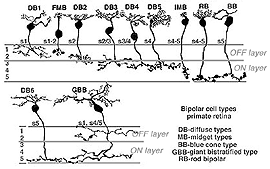
In human retina eleven different bipolar cell types are revealed by Golgi staining (Boycott and Wassle, 1991; Kolb et al., 1992; Mariani, 1984, 1985). Ten are for cones and one type is for rods (Fig. 4).
Fig. 4. Bipolar cell types in human retina
Human retina like most mammalian retinas is rod dominated outside of the fovea. Therefore rod bipolar cells form the numerically superior part of the bipolar population in human retinas.
The rod bipolar is typically a stout bipolar with a cell body situated middle to high in the inner nuclear layer and producing a tuft of dendrites entering the OPL and reaching up to different levels between cone pedicles to reach the stacked rod spherules (Fig. 5).
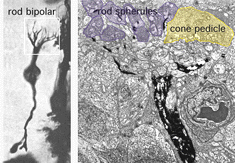 Fig. 5. Rod bipolar cell at low magnification |
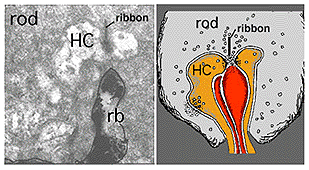 Fig. 6. Rod bipolar dendritic terminal |
The rod bipolar dendritic terminals end one to a rod spherule as the central invaginating dendrite (Fig. 6)) (Kolb, 1970). In central retina rod bipolar dendritic trees are small (15 µm across) and 15-20 rods are contacted. In peripheral retina the dendritic tree is 30 µm across and contacts 40-50 rods.
Ten different types of cone bipolar are present in human retina. Seven of them are concerned with converging information from many cones. They are known as diffuse cone bipolar types (DBs). Three cone bipolar types are concerned only with one cone in a one-to one relationship. These are known as midget bipolars and blue cone specific types (Fig. 4, FMB, IMB and BB).
Some of the diffuse cone bipolars are very wide field (giant) in dendritic spread (70-100 µm) and connect with as many as 15-20 cones (Mariani, 1984a). Little is known concerning the wide-field cells and and their role in retinal processing. These cells are badly in need of further exploration. Commonly the smaller diffuse bipolar cells collect information from 5-7 cones in central retina, and 12-14 cones in peripheral retina. The midget bipolar cells contact single cones but there are two different varieties of them per cone. Thus, cones of the fovea have output to two midget bipolar cells and of course, also some output to the diffuse bipolar cells. The two types of midget bipolar differ in their contact with the cone pedicle.
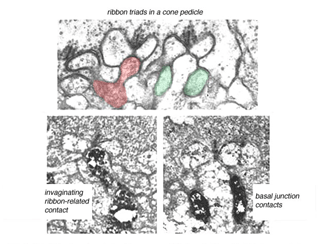
Fig. 7. Midget bipolar cell contacts
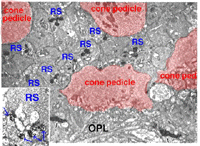
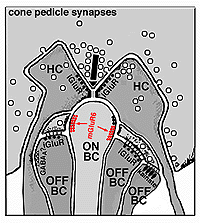
The invaginating midget bipolar type (IMB) connects with the cone pedicle as central invaginating dendrites (red profiles) at ribbon synapses in the cone pedicles (Fig. 7) (Kolb, 1970). Flat midget bipolar cells (FMB) contact the cone pedicle by means of semi-invaginating, wide-cleft basal junctions (green profiles, Fig. 7). Often FMB dendrites make two contacts with the cone pedicle on either side of the central invaginating dendrite from the other midget bipolar cell (Kolb, 1970).
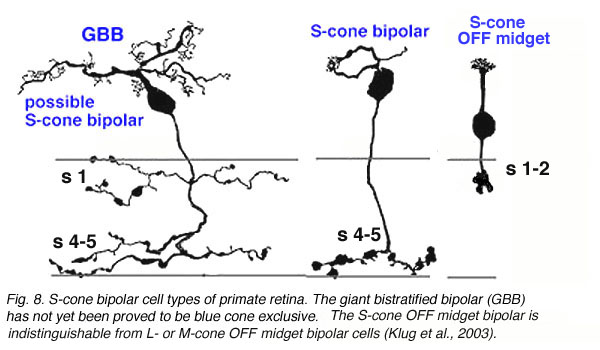
A cone bipolar cell that is thought to be specific for the short wavelength cones or blue cones (S-cones) has been described in monkey (Mariani, 1984b; Kouyama and Marshak, 1990) and in human retina (Kolb et al., 1992). This blue S-cone bipolar (Fig. 8, s-cone bipolar) typically contacts one cone heavily, by several dendrites converging on that particular cone pedicle as central elements at the ribbons: so it is essentially another type of midget bipolar cell, but it differs from regular IMBs and FMBs, in having also two or more wispy dendrites contacting either another cone pedicle or ending blindly in the OPL (see later chapter on S-cone pathways). There is also a giant bistratified cone bipolar cell type in primate retina (Fig. 8, GBB) that has been proposed to be involved in the blue cone system because its axonal branching level in the IPL corresponds exactly to the dendritic branching levels of the bistratified blue/yellow ganglion cell of the S-cone pathways through the retina (see later chapter on S-cone pathways) (Kolb et al., 1997).
Fig. 8. S-cone bipolar cell types of primate retina
The remaining diffuse bipolar types in primate retinas (Fig. 4) are analogous to cone bipolar cells of other mammalian retinas in contacting clusters of cone pedicles as mentioned above. Like the midget bipolar cells, though, these diffuse types also differ in their types of synaptic contacts with cone pedicles, being either flat contacting types (making basal junctions with cone pedicles) or invaginating and ribbon contacting types or even being types that mix these types of contacts (Hopkins and Boycott, 1995) (see also Kolb and Nelson, 1995, for a review on photoreceptor to bipolar contacts in the vertebrate retina).
3. Horizontal cells.
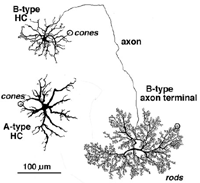
Most mammalian retinas have two types of horizontal cell as the laterally interconnecting neurons in the outer plexiform layer. The cat has been studied extensively as a model mammalian retina and its two types, known as A-type and B-types, are illustrated in Figure 9 (Kolb, 1974). The A- and B-type HCs are very similar in appearance in the rabbit retina too. Other species may have more than two types of horizontal cell and often these other types are concerned with color coding (see chapter on Horizontal cells by Perlman et al., webvision). Moreover, in all species studied, the different horizontal cell types are connected homotypically by gap junctions, so they form a large interconnected cell network across the whole outer plexiform layer.
The A-type HC is a large sturdy cell with radiating dendrites covering a dendritic field of 150-250 µm (depending on area of retina) (all neurons of the retina are small in dendritic expanse in the central retina and increase in dendritic tree size with eccentricity from the central area). The B-type HC in the mammalian retina differs from the A-type in having a smaller bushier (in general) dendritic tree (75-150 µm diameter) and bearing an axon that travels 300 µm or more before ending in a huge expansive axon terminal tree. The dendrites of both A-type and B-type HCs end in cone pedicles while the axon terminals of the B-type HCs end in rod spherules (see above) (Kolb, 1974).
Fig. 9. Horizontal cells in cat retina
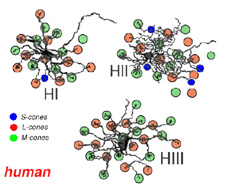
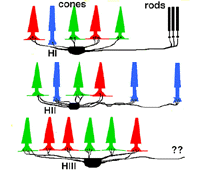
Primates, although obviously mammals, have been thought of as somewhat different in their horizontal cell make-up compared with cats. Originally there was only thought to be one HC type, called an HI, looking like a miniature version of the cat B-type HC (Polyak, 1941). In 1980, we described a second type of HC in the rhesus monkey retina and called it the HII type (Kolb et al., 1980). Most recently we have been able to distinguish a third type, HIII type, of horizontal cell in the human retina (Kolb et al., 1994). Light micrographs and drawings of Golgi stained examples of these three types are illustrated in Figures 10 and 11.
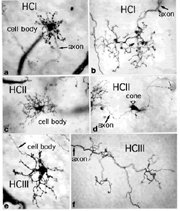 Fig. 10. Light micrograph of human horizontal cells |
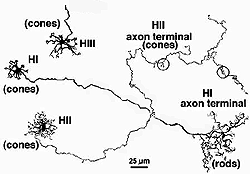 Fig. 11. Drawings of monkey horizontal cells |
HI is the classic horizontal cell of primate retina ( Polyak, 1941). It is a small-field cell (15 µm diameter dendritic tree in the fovea, 80-100 µm in the periphery) with stout dendrites giving rise to distinct clusters of round or donut-shaped terminals contacting cones as lateral elements of the ribbon synapses (Fig. 12).
Fig. 12. HI horizontal cell of primate retina
In peripheral retina the HI cells have much bigger dendritic trees and their radiating dendrites contact as many as 18 cones. The HI cell has a single thick axon that passes laterally in the outer plexiform layer to terminate more than 1mm away in a thickened axon terminal stalk bearing a fan-shaped profusion of lollipop-like terminals. HI axon terminals end in rod spherules as lateral elements of the ribbon synapses (Kolb, 1970) (Fig. 13).
Fig. 13. HI horizontal cell terminals
HIII cells are similar in appearance to HI cells, but are everywhere in the retina 1/3 bigger in dendritic tree size and typically, particularly in peripheral retina, asymmetrical in shape (one or two dendrites are much longer than others). The clusters of terminals contact cones in the same manner as the HI cell terminals and because of their bigger field size contact more cone pedicles (9-12 in foveal retina, 20-25 in peripheral retina). The axon of the HIII has not been conclusively followed to a terminal yet so we know nothing of the nature of the photoreceptor type they contact, although we suspect it is a mixture of rods and cones.
HII cells are more spidery and intricate in dendritic field characteristics than either of the other types (Kolb et al., 1980). Their terminals are not clearly seen as clusters approaching cone pedicles but they are known to end in cone pedicles (Kolb, 1980; Ahnelt and Kolb 1994a,b). HII cells also bear an axon, but this is quite different from that of the other two horizontal cell types. It is short (100-200 µm) curled instead of straight and has contacts to cone pedicles by means of small whispy terminals (Fig. 11).
Recent findings from electron microscopic studies of Golgi-stained horizontal cells of the human retina show that there is some colour specific wiring going on for the three cell types (Fig. 14) (Ahnelt and Kolb, 1994a,b).
Fig. 14. Three cell types of horizontal cells in human retina
Thus HIs contact medium and long wavelength cones primarily but with a small number of contacts to any short wavelength cones in the dendritic field (Fig. 14). HII cells contact short wavelength cones, directing major dendrites to these cones in their dendritic fields where they occur (Fig. 14), and contacting with lesser numbers of terminals other types of non-short wavelength cone. The HII cells axon contacts short wavelength cones only. HIII cells have large dendritic terminals in medium and long wavelength cones, seemingly avoiding short wavelength cones in their dendritic tree (Fig. 14) (Ahnelt and Kolb, 1994b). Thus a wiring diagram can be made (Fig. 15) that summarizes our present understanding of the spectral connections of the three HC cell types of the primate retina.
Fig. 15. Summary of the spectral connections of the three HC types of the primate retina
4. ON and OFF center pathways and center surround organization of the retina are initiated at the photoreceptor to bipolar and horizontal cell contacts in the outer plexiform layer.
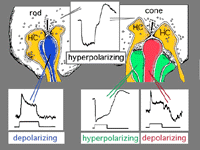
We know that photoreceptor neurotransmitter (which is glutamate, see Dowling, 1987 and Massey, 1990, and glutamate chapter in webvision for reviews) is released in the dark in the vertebrate retina (Trifonov, 1968). Thus the photoreceptor, whether it be rod or cone is in a depolarized state in the dark. On light stimulation the photoreceptor responds with a hyperpolarization, transmitter release ceases but the postsynaptic bipolar cells respond with either hyperpolarization or depolarization of their membranes.The hyperpolarizing type of bipolar cell is called an OFF-center cell while the depolarizing bipolar cell is called an ON-center cell (Werblin and Dowling, 1969; Werblin, 1991).
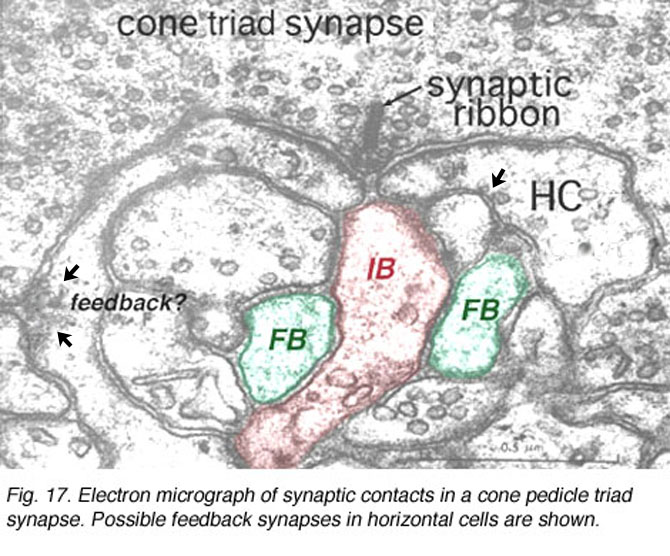
The origin of these two important ON-center and OFF-center channels, is the types of synaptic contacts these bipolars make with cone pedicles or rod spherules. Thus, the type of bipolar cell making invaginating contacts (several cone bipolar types, amongst them the IMB type and the rod bipolar type), responds to light with an inverted sign compared with the photoreceptor (Fig. 16). They give depolarizing responses to light and are thought to be stimulated via metabotropic glutamate receptors, specifically mGluR6, and signal via a G protein cascade (Fig. 17)(Jahr, 1999; Dhingra et al. 2001) (see chapters on rod and cone pathways). These mGluR6 receptors are APB sensitive (Slaughter and Miller, 1981).
On the other hand, the type of bipolar cell that makes contact with the photoreceptor at a basal junction, responds to light just like the photoreceptor by hyperpolarizing (Fig. 16). The hyperpolarizing types are driven via ionotropic (iGluR) AMPA-kainate glutamate channels in their synapses with photoreceptors (Fig. 17)(Slaughter and Miller, 1983). Hyperpolarizing (basal junction contacting) bipolar cells are the start of OFF-center channels and the depolarizing (invaginating, ribbon-related) bipolar types are the start of ON-center channels through the whole retina and visual system. For, as we shall see in a later chapter, at the level of information transfer between cone bipolar cells and ganglion cells in the inner plexiform layer, only excitatory channels are present. Therefore, the status of the signal transmitted by the ganglion cell to the brain is essentially determined by the nature of the cone bipolar contacting it which in turn is determined by its photoreceptor synapse.
CLICK HERE to see a movie of the intracellular recordings of a cone and bipolar cells (mp4 movie)
In submammalian species intracellular recordings from the cones have indicated that they receive a feed-back inhibitory message from horizontal cells (Baylor et al., 1971). In fish retinas there is morphological evidence that the horizontal cell lateral elements at the triad synapse produce spinules at which feed-back signalling could occur to the cone (Djamgoz and Kolb, 1993, for a review). Unfortunately, in mammalian cones there is ongoing arguments about this feed-back synapse.
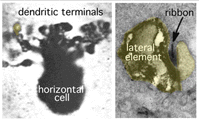
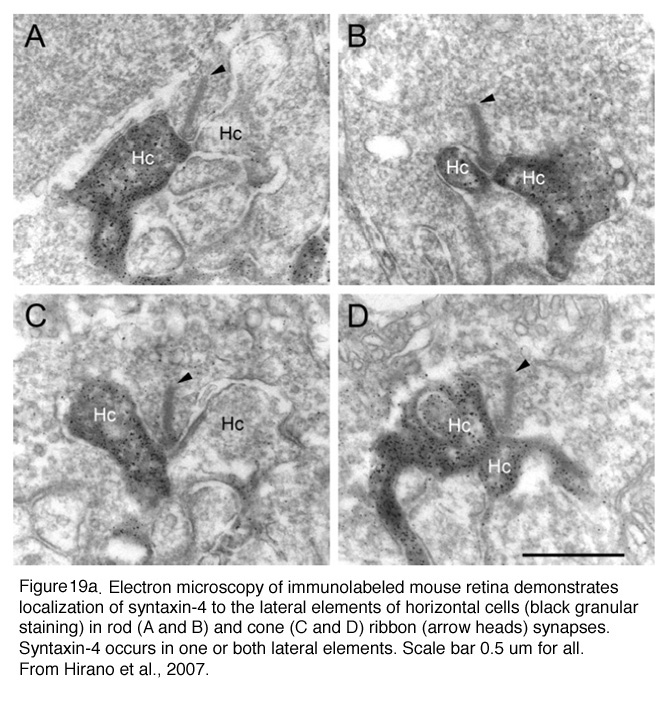
Horizontal cell lateral elements, i.e. horizontal cell dendrites, sometimes are seen to have vesicles within them directed at the cone membrane in electron microscope images (Fig, 18, unpublished image in primate retina). At this site syntaxin-4, a synaptic vesicle anchoring protein has been seen (Fig. 19) in a recent study in mouse retina by Hirano and coauthors (2007). It suggests a feedback synapse is here from horizontal cell to cone or rod photoreceptor. Because this evidence for a real synapse is still equivocal there are other theories of horizontal cell feedback. The two current ones are: 1) pH changes in the cleft modulates photoreceptor calcium currents (Davenport et al., 2008) and 2) current flow into horizontal cells occurs through hemigap junctions in horizontal cell dendrites causing a highly localized ephaptic change in the membrane potential at the cone pedicle (Kamermans and Fahrenfort, 2004). It is possible that these different findings may ultimately be reconciled within a single common mechanism (e.g., by incorporating pH sensitivity of hemigap junctions). Alternatively, it is possible that multiple mechanisms may contribute to negative feedback from horizontal cells to cones (personal communication by Wallace Thoreson). See also chapter on Horizontal cells and S-potentials by Perlman, Kolb and Nelson, webvision, for more details of these feedback pathways.
We know that photoreceptor neurotransmitter is released in the dark in the vertebrate retina. Thus the photoreceptor, whether it be rod or cone is in a depolarized state in the dark. On light stimulation the photoreceptor reacts with a hyperpolarization, transmitter release ceases but the postsynaptic bipolar cells respond with either hyperpolarization or depolarization of their membranes. The horizontal cells respond too, with a hyperpolarization. They then sum information from the horizontal cell network connected over a wide spatial area of the outer plexiform layer via the gap junctions between them. Thus, this large horizontal cell response feeds back somehow to both the photoreceptor and the bipolar cell. This local circuit provides the bipolar cell with a center-surround organization (Werblin and Dowling, 1969).
5. References.
Ahnelt P, Kolb H. Horizontal cells and cone photoreceptors in primate retina: a Golgi-light microscope study of spectral connectivity. J Comp Neurol.1994;343:387–405. [PubMed]
Ahnelt P, Kolb H. Horizontal cells and cone photoreceptors in human retina: a Golgi-electron microscopic study of spectral connectivity. J Comp Neurol.1994;343:406–427. [PubMed]
Baylor DA, Fuortes MGF, O’Bryan PM. Receptive fields of cones in the retina of the turtle. J Physiol. 1971;214:265–294. [PubMed] [Free Full text in PMC]
Boycott BB, Dowling JE. Organization of the primate retina: light microscopy. Phil Trans R Soc B. 1969;255:109–184.
Boycott BB, Wassle H. Morphological classification of bipolar cells of the primate retina. Eur J Neurosci. 1991;3:1069–1088. [PubMed]
Cajal SR. The Structure of the Retina. 1892 In: Thorpe SA, Glickstein M, translators. Springfield (IL): Thomas, 1972.
Dhingra A, Lyubarsky A, Jiang M, Pugh EN, Jr, Birnbaumer L, Sterling P, Vardi N. The light response of ON bipolar neurons requires Gαo. J Neurosci.2000;20:9053–9058. [PubMed]
Djamgoz MBA, Kolb H. Ultrastructural and functional connectivity of intracellularly stained neurones in the vertebrate retina: correlative analyses. Microscopy Res Techniques. 1993;24:43–66.
Dowling JE. The retina: an approachable part of the brain Cambridge (MA): The Belknap Press, Harvard University Press; 1987.
Golgi C. Sulla fina anatomie degli organi centrali del sistemi nervosa 1885; Calderini.
Hopkins JM, Boycott BB. Synapses between cones and diffuse bipolar cells of a primate retina. J Neurocytol. 1995;24:680–694. [PubMed]
Kolb H. Organization of the outer plexiform layer of the primate retina: electron microscopy of Golgi-impregnated cells. Phil Trans R Soc B. 1970;258:261–283.
Kolb H. The connexions between horizontal cells and photoreceptors in the retina of the cat: electron microscopy of Golgi-preparations. J Comp Neurol.1974;155:1–14. [PubMed]
Kolb H. The organization of the outer plexiform layer in the retina of the cat: electron microscopic observations. J Neurocytol. 1977;6:131–153. [PubMed]
Kolb H, Mariani A, Gallego A. A second type of horizontal cell in the monkey retina. J Comp Neurol. 1980;189:31–44. [PubMed]
Kolb H, Nelson R, Mariani A. Amacrine cells, bipolar cells and ganglion cells of the cat retina: a Golgi study. Vision Res. 1981;21:1081–1114. [PubMed]
Kolb H, Linberg KA, Fisher SK. Neurons of the human retina: a Golgi study. J Comp Neurol. 1992;31:147–187.
Kolb H, Fernandez E, Schouten J, Ahnelt P, Linberg KA, Fisher SK. Are there three types of horizontal cell in the human retina? J Comp Neurol. 1994;343:370–386. [PubMed]
Kolb H, Nelson R. The organization of photoreceptor to bipolar synapses in the outer plexiform layer. In: Djamgoz MBA, Archer SN, Vallerga S, editors. Neurobiology and clinical aspects of the outer retina. London: Chapman and Hall; 1995. p. 273–296.
Kolb H, Goede P, Roberts S, McDermott R, Gouras P. Uniqueness of the S-cone pedicle in the human retina and consequences for color processing. J Comp Neurol. 1997;386:443–460. [PubMed]
Kouyama N, Marshak DW. Bipolar cells specific for blue cones in the macaque retina. J Neurosci. 1992;12:1233–1252. [PubMed]
Mariani AP. A diffuse, invaginating cone bipolar cell in primate retina. J Comp Neurol. 1981;197:661–671. [PubMed]
Mariani AP. Giant bistratified bipolar cells in monkey retina. Anat Rec. 1983;206:215–220.
Mariani AP. Bipolar cells in monkey retina selective for the cones likely to be blue-sensitive. Nature. 1984;308:184–186. [PubMed]
Mariani AP. Multiaxonal horizontal cells in the retina of the tree shrew, Tupaia glis. J Comp Neurol. 1985;233:553–563. [PubMed]
Massey SC. Cell types using glutamate as a neurotransmitter in the vertebrate retina. Prog Retinal Res. 1990;9:399–425.
Nawy S, Jahr CE. Suppression by glutamate of cGMP activated conductance in retinal bipolar cells. Nature. 1990;346:269–271. [PubMed]
Nelson R. Cat cones have rod input: a comparison of the response properties of cones and horizontal cell bodies in the retina of the cat. J Comp Neurol.1977;172:109–136. [PubMed]
Polyak SL. The Retina Chicago: The University of Chicago Press; 1941.
Slaughter MM, Miller RF. 2-Amino-4-phosphonobutyric acid: a new pharmacological tool for retina research. Science. 1981;211:182–184. [PubMed]
Slaughter MM, Miller RF. An excitatory amino acid antagonist blocks cone input to sign-conserving second-order retinal neurons. Science. 1983;219:1230–1232.[PubMed]
Trifonov YA. Study of synaptic transmission between the photoreceptor and the horizontal cell using electrical stimulation of the retina. Biofizika. 1968;10:673–680.
Werblin FS, Dowling JE. Organization of the retina of the mudpuppy, Necturus maculosus. II. Intracellular recording. J Neurophysiol. 1969;32:339–355. [PubMed]
Werblin F. Synaptic connections, receptive fields, and patterns of activity in the tiger salamander retina. Invest Ophthalmol Vis Sci. 1991;32:459–483. [PubMed]
Last Updated: October 8, 2011.