Helga Kolb
1. Rods.
Rod photoreceptors and rod-connected nerve cells through the retina are responsible for pathways concerned with night vision and increased sensitivity of our visual system under what is called scotopic conditions (conditions of very little ambient light). Most vertebrates have a preponderance of rod photoreceptors in their retinas and such animals are very good at hunting and movement at night because of their very sensitive scotopic visual systems. Some animals are even completely nocturnal and have lost some of their cone types and their cone system neurons.
Humans, of course, are highly visual vertebrates and function mostly with their cone systems for color and high acuity form vision. However, the rod system is also important for our capacity to move around and function in scotopic conditions. If our rods or rod system neurons become diseased and degenerated we become night blind as happens to unfortunate people who have a disease called retinitis pigmentosa.
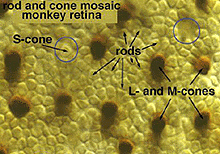
Fig. 1. Light micrograph of the primate photoreceptors
When looking at the mosaic of human photoreceptors it becomes apparent that the human retina is actually rod-dominated numerically (Fig. 1). That is, rod photoreceptors out-number cone photoreceptors by orders of magnitude and the consequent second- and third-order neurons recruited for processing rod-driven vision outnumber the cone pathways neurons everywhere but in the central fovea.
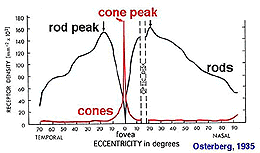
Fig. 2. Densities of cone and rods
Rods peak in density in a ring approximately 5mm (18o) from the center of the fovea (Osterberg, 1935 (human); Mariani et al., 1984, (rhesus monkey)(Fig. 2)). Rods are present in greater numbers than cones from 2 mm from the fovea to the far periphery. As can be seen above the rods always form a nice hexagonal packing around the cones and separate the cones from each other (Fig. 1).
2. Rod bipolar cells.
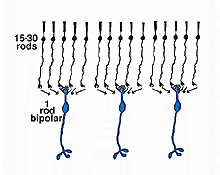
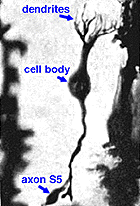
As was pointed out in a previous section, only one morphological type of bipolar cell has been found to make connections with the rod photoreceptors. The rod bipolar collects input from between 15 and 30 rod spherules in the outer plexiform layer (Fig. 3).
Fig. 3. Convergence of rod pathways
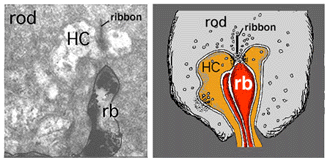
Electron microscope investigation of Golgi-impregnated rod bipolar cells first showed that the rod bipolar dendrite penetrates into the rod spherule to make an invaginating ribbon related type of contact (Fig. 4) (Kolb, 1970). This invaginating contact is known to bear metabotropic glutamate receptors in the bipolar dendrite membrane (Fig. 5). The receptor was proved to be mGluR6 by Numura and coauthors (1994) and Vardi and Morigawa (1997) using immunocytochemistry against the cloned mGluR6 receptor. Since then the mGluR6 receptor is thought to trigger a second messenger cascade involving GalphaO, transducin molecules, to act on voltage gated Ca channels (Nawy, 1999; Dhingra et al, 2000).
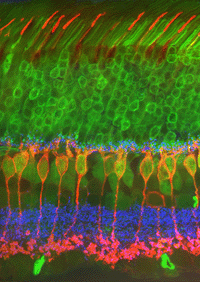
Both Golgi impregnation of single rod bipolar cells (Fig. 6) and immunocytochemical staining of rod bipolar cell populations with protein kinase C (PKC) (Fig. 7) show the characteristic morphology of the rod bipolar cell type in mammalian retina (Kolb et al., 1993; Grünert et al., 1994). The immunocytochemical staining and confocal microscopy is now the most illustrative way of seeing the rod bipolar cells in mammalian retinas (Cuenca personal communication) (Fig. 7). As can be seen in this stunning micrograph (which incidentally won the first place award in science photos at the Spanish Congress of Neuroscience, 2009) rod bipolar cells (red) send dendrites to the rod spherules in the outer plexiform layer (green) to end at synaptic ribbons therein (blue) and axons to the inner plexiform layer where they terminate deep, close to the ganglion cell bodies, as narrow-field, clumpy axon terminals (red).
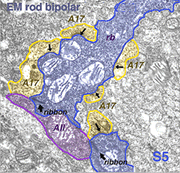
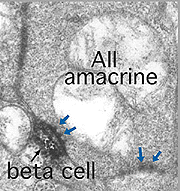
Electron microscopy of the rod bipolar cell axons in the inner plexiform layer shows that they make ribbon synapses only upon amacrine cell profiles (Fig. 8). A ganglion cell dendrite is not seen to be postsynaptic to rod bipolar cell axons (Kolb and Famiglietti, 1974; Kolb, 1979). This allows for both divergence of the rod signal and collection (convergence) of signals from many rods and rod bipolars, by means of these amacrine cells, before synaptic output to ganglion cells. Most commonly the output of the rod bipolar ribbon is to a dyad of amacrine cell processes, one of which is known as AII and the other as A17, a reciprocal amacrine (Figs. 8 and 9). The AII is characterized by making gap junctions with neighboring cone bipolar or other AII profiles (Figs. 8 and 9). The A17 is characterized by always making a return synapse known as a reciprocal synapse to the rod bipolar axon terminal.
3. Rod amacrine cells.
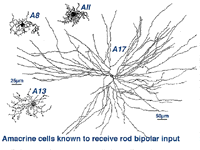
Two amacrine cells are key in the rod pathway circuitry through the mammalian retina (Fig. 10). The one is a small-field, bistratified cell given the name AII in its original description, to compare with the other amacrine common at the rod bipolar ribbon dyad, known then as as AI (Kolb and Famiglietti, 1974). Since then the AII label has stuck to the rod amacrine but the AI label has been dropped in favor of calling the cell the A17 or reciprocal amacrine. The small AII amacrine is probably the commonest amacrine cell in the mammalian retina (512,000 total in cat retina) followed by the A17 cell (between 170,000 and 230,000 cells in rabbit) (Vaney, 1990).
Fig. 10. Rod amacrine cells of human retina
Two other amacrine cells are known to be involved in the rod pathways but to a lesser extend that AII and A17. These cells are A8 and A13 (Fig. 10). But they are more strongly driven by the cone system in that greater numbers of cone bipolar cells have synapses upon them than rod bipolar cells. We shall, therefore, consider them in great detail in the chapter on cone driven pathways through the retina. However one amacrine cell, A18, is definitely involved in the pathways of the rod system by virtue of influencing both AII and A17 amacrine cells. So we will described A18 cells in detail in this chapter.
4. AII amacrine cells.
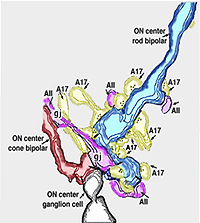
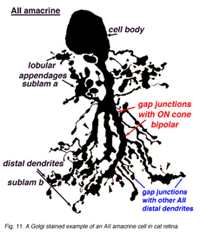
AII amacrines are fascinating, small-field amacrine cells (Fig. 11) that have a seminal role in the rod pathways, and in linking the rod and cone pathways so that the rod signals can also use the cone bipolar pathways to ganglion cells. The narrow-field bistratified rod amacrine cell, AII, is primarily postsynaptic to rod bipolar axon terminals in lower sublamina b of the IPL (30% of its input, Strettoi et al., 1992). Its major output is from its lobular appendages upon ganglion cells that have dendrites only in sublamina a (Figs. 11 and 12)(Famiglietti and Kolb, 1975; Kolb and Nelson, 1993).
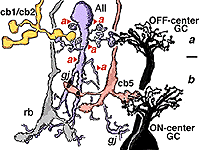
The AII also passes rod-driven information to a cone bipolar cell that makes contact with ganglion cells of sublamina b. It does so through large gap junctions with these cone bipolar axons before they in turn make their ribbon synapses to those ganglion cells (Fig. 12, black spots on AII primary dendrites to pink cb axon). A little OFF-center cone bipolar input is provided to the AII lobular appendages by cb1 and cb2 cone bipolar cells in sublamina a (19% of input, Strettoi et al., 1992) (Fig. 12, yellow cb profiles). AII amacrine cells are also coupled across the retina in a weak electrical syncytium by virtue of their gap junctions between their dendrites in sublamina b (Fig. 12, gj, lower right) (Famiglietti and Kolb, 1975; Nelson, 1982; Vaney, 1994).
Fig. 12. Circuitry for AII amacrine cells
We know from the intracellular recordings done in cat retina that ganglion cells with dendrites in sublamina a respond to light with a hyperpolarizing or OFF-center response and that ganglion cells with dendrites in sublamina b respond with a depolarizing or ON-center response (Nelson et al., 1978).
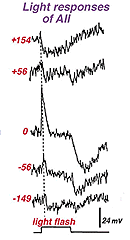
We also know that rod bipolar cells respond to light with a center-depolarizing (ON-center) responses (Dacheux and Raviola, 1986), and by virtue of their deep penetrating axon terminals to sublamina b we expect them to carry rod ON-center signals to the next order neurons i.e. either ganglion cell or amacrine cell of that neuropil. Since rod bipolar cells do not synapses directly with ganglion cells we expect the rod bipolar cell to have output at ribbon synapses instead to center depolarizing (ON-center) amacrine cells. And this is in fact what has been discovered from intracellular recordings in cat and rabbit retinas. AII amacrine cells respond to light with a depolarizing response (ON center response) in their centers (Fig. 13)(Dacheux and Raviola, 1986).
Fig. 13. Intracellular recordings of AII amacrine cells
Thus the center-depolarizing (ON-center) AII cell make a sign-inverting synapse upon the center-hyperpolarizing (OFF-center) type of ganglion cell to contribute rod signals to the center response of the receptive field of that ganglion cell (Fig. 14). (Amacrine cells in general are known to be inhibitory neurons using common inhibitory neurotransmitters of the CNS like glycine and GABA). AII amacrine cells are glycinergic (Pourcho and Goebel, 1985). The AII uses the gap junction with the sublamina b type of cone bipolar (cb5 in Fig. 12) to pass center-depolarizing (excitatory) input to the center-depolarizing ganglion cell with which the cone bipolar has synapses (cb5 to ON-center beta GC, Fig. 12). By this division of function within the small dendritic field of the AII amacrine cell, rod signals can be ensured of reaching both ON- and OFF-center types of ganglion cells.
5. A17 amacrine cells.
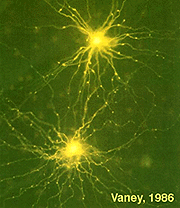
A17 cells are very wide-field diffusely branching amacrine cells types (Fig. 15). Their fine, beaded dendrites pass through the IPL to end running in the lower sublamina b, receiving synapses at intervals from rod bipolar axon terminals upon their beads.
A single A17 amacrine cell receives synaptic input from approximately 1000 rod bipolar cells. Interestingly A17 cells do not appear to make synapses upon other amacrine or ganglion cells, instead they merely interconnect rod bipolar cells by reciprocal synapses. The A17 thus has a completely different receptive field area over which it collects rod signals than its narrow-field partner at the dyad, the AII amacrine cell. Presumably the A17 is an integrating unit that helps set sensitivity levels over a very large area of rod photoreceptors and rod bipolar cells (Nelson and Kolb, 1985).
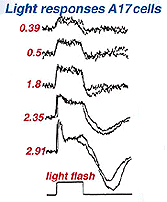
Like the AII amacrine cell A17 cells respond to light flashes with a center depolarizing response (Fig. 16) (Nelson and Kolb, 1985). Its ON-center response reflects the rod generated ON-center message of ints input rod bipolar cell (Dacheux and Raviola, 1986). But unlike the AII, the A17 cell’s output is only known to be reciprocal synapses to the rod bipolar from which it receives input (Nelson and Kolb, 1985).
Fig. 16. Intracellular recordings of AI7 amacrine cells
A17 cells are very sensitive at low light intensities and may play a role in converging and amplifying rod signals from large areas of retina. A17 is known to accumulate serotonin in rabbit retina (Vaney, 1990, Fig. 15) but is thought to be a GABAergic neuron in terms of neurotransmission, in all mammalian retinas (Pourcho and Goebel, 1983).
6. Dopamine containing (A18) cells of the retina.
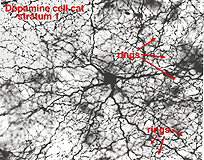
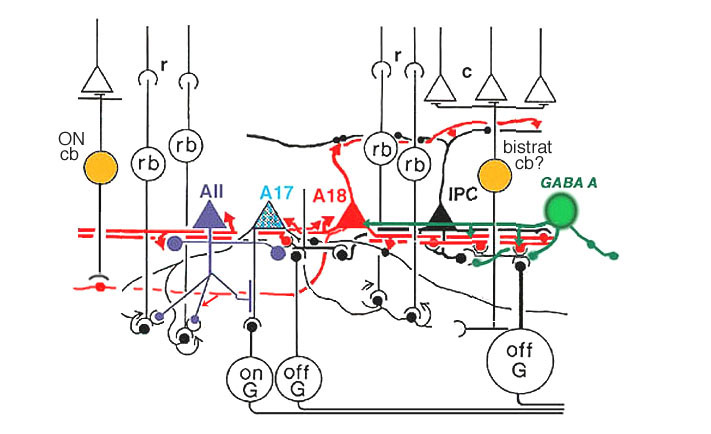
A18 is a wide-field amacrine with extensive dendritic branching in the stratum next to the amacrine cell bodies, i.e. S1 of sublamina a (Fig. 17). The A18 is known to be a dopaminergic amacrine cell and the whole population of these cells in the retina can be revealed by immunostaining to the synthesizing enzyme of dopamine, tyrosine hydroxylase (Fig. 17). A18 has thin dendrites and axon like processes that run for hundreds of microns.
The fine terminals of these processes surround cell bodies and dendrites of amacrine cells AII, A8, A17 and possibly A13 cells (Pourcho, 1982; Kolb et al., 1990; Voigt and Wässle, 1987). The summary diagram in Fig. 18 shows all these connection. The A18 synapses in profusion upon the two major and some of the minor amacrines of the rod pathways (Fig. 18, A13, AII, A17, A8). In addition, the A18 receives many synapses from other amacrine cells and some few synapses from a cone bipolar cell type, as yet not positively identified, but possibly the type known as a giant bistratified cone bipolar cell (Fig. 18) (Hokoc and Mariani, 1987). Recently it has also been discovered that deep axon-like processes of the A18 amacrine passing in S5 of sublamina b make synapses directly upon rod bipolar axon terminals (Fig. 18) (Kolb et al., 1990). A18 has not yet been studied by intracellular recordings, but equivalent dopaminergic amacrine cells in other species are known to be transient-sustained, and center-depolarizing in response type (Ammermüller and Kolb, 1995).
Fig. 18. Wiring diagram of A18 amacrine cells
It is thought that the dopaminergic A18 cell has an effect upon the rod AII amacrine to uncouple the syncytium of AIIs across the retina and to uncouple the AII amacrine from the cone bipolar cell of sublamina b (Hampson et al., 1992; Jensen and Daw, 1986). This could have the affect of increasing receptive field sizes of ganglion cells under scotopic conditions, providing added sensitivity to the rod system messages in the ganglion cells. Dopaminergic amacrine cells are also thought to function in the circadian cycle of the shift from dark to light conditions, and in modulating the adaptational state of the whole retina.
7. The rod pathway is convergent to increase sensitivity.
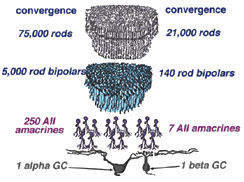
Rod pathways involve huge convergences and divergences of cell contacts. The pathway starts with many rods converging information to the rod bipolar cell in the OPL. Rod responses are carried to the next stage of processing in the IPL where divergence occurs to several different amacrine cell types, the most important of which are the AII and the A17 cells. Rod bipolar cells do not contact ganglion cells directly. Instead they contact amacrine cells which spread out the rod information before converging on ganglion cells. One other cell type is also influential upon the rod pathways. This is the dopaminergic amacrine, A18.
The huge convergence of the rod system in the cat retina has been calculated by Sterling and coauthors (1987, 1988) and Kolb and Nelson (1993). Thus about 1500 rods have input to a single small-field ON-beta ganglion cell via 100 rod bipolars, 5 AII amacrine cells and 4 cone bipolar axons. In the case of a large-field OFF-alpha ganglion cell, 75,000 rods drive 5000 rod bipolars and 250 AII amacrine cells before converging on the ganglion cell (Fig. 19).
Fig. 19. Summary diagram of the convergence in the rod pathway
In terms of divergence of the rod system, we know that a single rod photoreceptor passes information to 2 rod bipolar cells, thence through 5 AII amacrines to 8 cone bipolar cell axons to drive 2 ON-beta cells (Sterling et al., 1987, 1988). So it is, that divergent and then convergent circuitry provides pooling and amplification of the rod signal in very low light levels to allow our visual system to be sensitive to a single quantum of light (Hecht et al., 1942).
8. Summary diagram of the rod system of the mammalian retina.
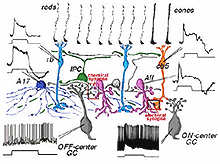
Combining the physiological responses we now understand for the neurons of the rod system in the cat retina, with their anatomical connections, we can construct a summary diagram of the likely pathway for scotopic vision though the human retina (Fig. 20).
Fig. 20. Summary diagram of the neurons and their responses
involved in rod-driven pathways
CLICK HERE to see an animation of the rod circuits
(Quicktime movie)
Signals from hyperpolarizing rod photoreceptors depolarize rod bipolar cells. Rod bipolar cells synapse upon two depolarizing amacrine cells, the wide-field A17 and the small bistratified AII amacrine cells. Rod signals reach OFF-center ganglion cells (a-type beta GC) via chemical synaptic input from the AII amacrine cell lobular appendages (boxed region a). ON-center ganglion cells (b-type beta GC) receive rod signals, via AII amacrine cell electrical synapses at gap junctions to cone bipolar cells (ibc, boxed region b). The latter cone bipolars stimulate the ON-center ganglion cells by excitatory chemical synapses. Dopaminergic amacrine cells (Fig. 20, DA) influence the rod pathways by chemical synapses in the IPL upon AII and A17 amacrine cells and via an internuncial GABA-ergic interplexiform cell (see later section for more details on interplexiform cells), which in turn synapses upon rod bipolar cells in the OPL.
9. References.
Ammermüller J, Kolb H. The organization of the turtle inner retina I. On- and off-center pathways. J Comp Neurol. 1995;358:1–34. [PubMed]
Dacheux RF, Raviola E. The rod pathway in the rabbit: a depolarizing bipolar and amacrine cell. J Neurosci. 1986;6:331–345. [PubMed]
Dhingra A, Lyubarsky A, Jiang M, Pugh EN, Birnbaumer L, Sterling P, Vardi N. The light response of ON bipolar neurons requires Gao. J Neurosci.2000;20:9053–9058. [PubMed]
Famiglietti EV, Kolb H. A bistratified amacrine cell and synaptic circuitry in the inner plexiform layer of the retina. Brain Res. 1975;84:293–300. [PubMed]
Grünert U, Martin PR, Wässle H. Immunocytochemical analysis of bipolar cells in the Macaque monkey retina. J Comp Neurol. 1994;348:607–627. [PubMed]
Hampson ECGM, Vaney DI, Weiler R. Dopaminergic modulation of gap junction permeability between amacrine cells in mammalian retina. J Neurosci.1992;12:4911–4922. [PubMed]
Hecht S, Schlaer S, Pirenne MH. Energy, quanta and vision. J Gen Physiol. 1942;25:819–840. [PubMed]
Hokoc JN, Mariani AP. Tyrosine hydroxylase immunoreactivity in the rhesus monkey retina reveals synapses from bipolar cells to dopaminergic amacrine cells. J Neurosci. 1987;7:2785–2793. [PubMed]
Jensen RJ, Daw NW. Effects of dopamine and its agonists and antagonists on the receptive field properties of ganglion cells in the rabbit retina. Neurosci.1986;17:837–855.
Kolb H. Organization of the outer plexiform layer of the primate retina: electron microscopy of Golgi-impregnated cells. Phil Trans R Soc B (Lond.).1970;258:261–283.
Kolb H, Famiglietti EV. Rod and cone pathways in the inner plexiform layer of the cat retina. Science. 1974;186:47–49. [PubMed]
Kolb H. The inner plexiform layer in the retina of the cat: electron microscopic observations. J Neurocytol. 1979;8:295–329. [PubMed]
Kolb H, Cuenca N, Wang H-H, DeKorver L. The synaptic organization of the dopaminergic amacrine cell in the cat retina. J Neurocytol. 1990;19:343–366.[PubMed]
Kolb H, Nelson R. Off-alpha and off-beta ganglion cells in the cat retina. II. Neural circuitry as revealed by electron microscopy of HRP stains. J Comp Neurol.1993;329:85–110. [PubMed]
Mariani AP, Kolb H, Nelson R. Dopamine-containing amacrine cells of rhesus monkey retina parallel rods in spatial distribution. Brain Res. 1984;322:1–7.[PubMed]
Nawy S. The metabotropic receptor mGluR6 may signal through Go, but not phosphodiesterase, in retinal bipolar cells. J Neurosci. 1999;19:2938–2944.[PubMed]
Nelson R, Famiglietti EV, Kolb H. Intracellular staining reveals different levels of stratification for on-center and off-center ganglion cells in the cat retina. J Neurophysiol. 1978;41:427–483.
Nelson R. AII amacrine cells quicken the time course of rod signals in the cat retina. J Neurophysiol. 1982;47:928–947. [PubMed]
Nelson R, Kolb H. A17: a broad-field amacrine cell of the rod system in the retina of the cat. J Neurophysiol. 1985;54:592–614. [PubMed]
Numura A, Shigemoto R, Nakamura Y, Okomoto N, Mizumo N, Nakanishi S. Developmentally-regulated postsynaptic localization of a metabotropic glutamate receptor in rat rod bipolar cells. Cell. 1994;77:361–369. [PubMed]
Østerberg G. Topography of the layer of rods and cones in the human retina. Acta Ophthal. 1935 suppl. 6:1–103.
Pourcho RG. Dopaminergic amacrine cells in the cat retina. Brain Res. 1982;252:101–109. [PubMed]
Pourcho RG, Goebel DJ. Neuronal subpopulations in cat retina which accumulate the GABA agonist (3H)muscimol: a combined Golgi and autoradiographic study. J Comp Neurol. 1983;219:25–35. [PubMed]
Pourcho RG, Goebel DJ. A combined Golgi and autoradiographic study of 3(H)glycine-accumulating amacrine cells in the cat retina. J Comp Neurol.1985;233:473–480. [PubMed]
Sterling P, Cohen E, Freed M, Smith RG. Microcircuitry of the ON-beta ganglion cell in daylight, twilight, and starlight. Neurosci Res Suppl. 1987;6:S269–S285. [PubMed]
Sterling P, Freed MA, Smith RG. Architecture of rod and cone circuits to the On-beta ganglion cell. J Neurosci. 1988;8:623–642. [PubMed]
Strettoi E, Raviola E, Dacheux RF. Synaptic connections of the narrow-field, bistratified rod amacrine cell (AII) in the rabbit retina. J Comp Neurol.1992;325:152–168. [PubMed]
Vaney DI. The mosaic of amacrine cells in the mammalian retina. Prog Ret Res. 1990;9:49–100.
Vaney DI. Patterns of neuronal coupling in the retina. Prog Ret and Eye Res. 1994;13:301–355.
Vardi N, Morigawa K. ON cone bipolar cells in rat express the metabotropic receptor mGluR6. Vis Neurosci. 1997;14:789–794. [PubMed]
Voigt T, Wässle H. Dopaminergic innervation of AII amacrine cells in mammalian retina. J Neurosci. 1987;7:4115–4128. [PubMed]
Last Updated: July, 2011.