Helga Kolb
1. General characteristics.
Todays research on the retina focuses a great deal of attention on neurotransmission between the neurons of the retina. Varous techniques using autoradiography, immunocytochemistry and molecular biology are being used to mark neurons for neurochemicals, their synthesizing enzymes, calcium binding proteins and receptors and transporters of these neurochemicals. Cells immunostained with antibodies to the various neurotransmitter candidates and neuropeptides, first discovered in nonhuman retinas, are present in human retina too.
2. The neurotransmitter of neurons of the vertical pathways through the retina is glutamate.
Glutamate is the neurotransmitter of the neurons of the vertical pathways through the retina. All photoreceptor types, rods and cones, use the excitatory amino acid glutamate to transmit signals to the next order neuron in the chain (See chapter on glutamate and Massey, 1990, for review). There was originally some evidence for the closely related amino acid, aspartate, being present in rods but the later sophisticated techniques of demonstrating amino acid signatures in retinal neurons cannot confirm aspartate as a retinal neurotransmitter at all (Marc et al., 1995). Uptake, release and action of glutamate and agonists upon second-order neurons in slice preparations or isolated cells in tissue culture have also all confirmed glutamate to be the neurotransmitter acting at the first synapse in the retina (see Lasater, 1992 for review). The action of the photoreceptor neurotransmitter upon the second-order neurons is through two different types of sensory channels though. The one type of postsynaptic receptor type is the metabotropic glutamate channel (mGluR6) that involves a second messenger cascade and cyclic GMP for activation of the channel (in the ON-center bipolar cell) whereas the other is an ionotropic channel via at least two types of AMPA receptors and Na ions (OFF-center bipolar and horizontal cells) (Slaughter and Miller, 1981, 1983a, b; Nawy and Jahr, 1990). Photoreceptors in most vertebrates including human, have a content of D2 dopamine receptors somewhere upon their surface (Witkovsky and Dearry, 1991).
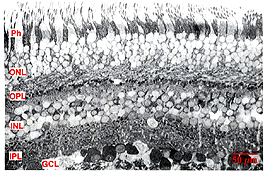
Fig. 1. Distribution of Glutamate immunoreactivity in human retina
Immunocytochemical staining of retina give particularly spectacular images of the glutamatergic neurons. The excitatory retinal neurons of the vertical pathways through the retina are all glutamatergic (see chapter on glutamate). Thus, photoreceptors, bipolar cells and all the ganglion cells appear to label strongly with antibodies to glutamate in all retinas studied including human (Crooks and Kolb, 1992) (Fig. 1).
Bipolar cells have receptor channels that are either of the metabotropic type, mGluR6 (APB sensitive) or ionotropic type (AMPA) at their dendrites in the OPL, while their axonal ending in the IPL have channels and receptors for GABA (A, B and C types), D1 dopamine and glycine because, of course, all kinds of amacrine cells are presynaptic at these sites in the IPL neuropil. Ganglion cells are as diverse in receptor sensors as the bipolar cells with the addition of receptors to acetylcholine (Keyser et al., 1993), and the first appearance in the retina of NMDA glutamate receptors that are typical in the brain (Massey and Miller, 1988; Yazejian and Fain et al., 1992).
Amacrine cells
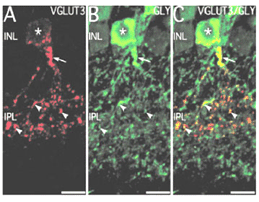
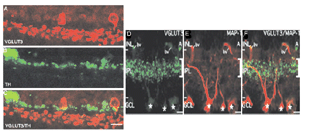
Recently an amacrine cell type has also been shown to contain the glutamate transporter, vesicular glutamate transporter 3, shortened to VGLUT3 (Johnson et al, 2004; Haverkamp and Wassle, 2004). VGLUTs are used to concentrate cystolic glutamate into the synaptic vesicles. Typically in the retina the VGLUT1 isoform is expressed in photoreceptors and bipolar cells and the VGLUT2 in ganglion cells. The amacrine cell type that can be immunostained to the antibody to VGLUT3 is found to be a small-field amacrine cell with varicose processes that are restricted in branching to the OFF laminae (S1 and S2) of the IPL (Fig. 2A). It is also immunoreactive to the transmitter glycine as seen in Figures 2B and C, but not reactive for GABA, dopamine (Fig. 3A, B and C) or acetylcholine. Figures 3A, B, and C show that the dopamine cell and its dendrites run above the branches of the VGLUT3 cell and indeed appear to be able to make synaptic contact with the cell bodies of the latter cell type, in the way we know dopamine cell processes do (see below and amacrine cell chapter). The VGLUT3 amacrines appear to be in a good position to interact with OFF center ganglion cell, stained for MAP-1, dendrites as shown in Figures 3D, E and F.
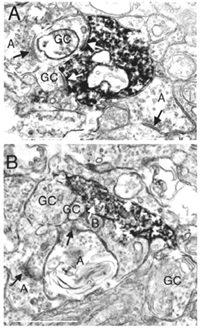
Electron microscopy of VGLUT3 dendrites in the IPL, indicate that they are postsynaptic to OFF cone bipolar cell axons (Fig. 4B) and presynaptic to OFF ganglion cell dendrites (Fig.4A).
Fig. 4 EM of VGLUT3 immunostained processes in the IPL of the rat retina
Recent evidence from the metabolic mapping technique of Marc and Jones (2004) indicates that a dopaminergic amacrine cell type also has a content of glutamate. However, from the above evidence on VGLUT3 immunostaining it is clear that the dopaminergic type 1 cell does not use that particular vesicular glutamate transporter.
3. Gamma aminobutyric acid.
The classical inhibitory neurotransmitter gamma aminobutyric acid (GABA) occurs in many different varieties of amacrine cells, and in one or more classes of horizontal cell in most vertebrate retinas (Marc et al., 1995) There is still some controversy over whether GABA is contained within horizontal cells in monkey and human retina.
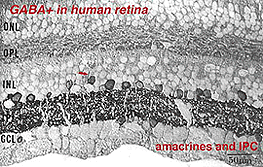
Fig. 5a. Expression pattern of GABA immunoreactivity in human retina
In this figure (Fig. 5a) taken from human peripheral retina, it can be seen that there is heavy staining with antibodies to GABA in the inner plexiform layer (three heavier bands can be discerned) and in about half of the amacrine cell bodies in the lower row of amacrine cells in the inner nuclear layer. Some displaced amacrines and interplexiform cells are also revealed with GABA immunocytochemistry (Crooks and Kolb, 1992). However, the horizontal cells are not stained at all in peripheral retina although they are in foveal retina apparently (Cuenca, personal communication).
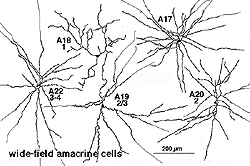
A valuable identification of individual cell types that contain GABA has come from autoradiography on Golgi stained cell types (Pourcho and Goebel, 1983) and more recently from double immunostaining techniques (Marc, 1994). The amacrine cell that contain GABA are all of the medium to large-field types. Thus we now know that A10, A13, A17, A19, A20, A22 and the interplexiform cell accumulate GABA and probably use it as their primary neurotransmitter (Fig. 5b). Most of the large field GABAergic amacrine cells also colocalize with another neurotransmitter. Thus the GABAergic A17 cell colocalizes serotonin, the acetylcholine (starburst amacrine) colocalizes GABA (Vaney and Young, 1988). Neuropetides (see later) are also commonly colocalized with GABA i.e. substance P in A22 is almost certainly the secondary transmitter to GABA as the primary. GABAergic amacrine cells and IPCs act upon bipolar, amacrine and ganglion cell processes or cell bodies in the neuropils of the retina via two common varieties of GABA receptors (a and c types). It appears that the rod A17 type, or the type that makes reciprocal synapse with the rod bipolar axon terminal, uses a GABAA receptor (Chavez et al., 2010) (and see Nelson and Connaughton chapter on bipolar cells in webvision). The rod bipolar terminals in mammals are particularly sensitive at GABAc receptors at non reciprocal GABAergic synapses (Lukasiewicz and Wong, 1997; Chavez et al., 2010; Eggers and Lukasiewicz, 2011).
Immunocytochemical techniques are commonly used on wholemounts of retina to reveal the neurotransmitter content of amacrine cells (Cuenca et al., 2003; Cuenca and Kolb, 1989). The advantage of the wholemount staining technique is that all the neurons using the particular neurotransmitter are stained so one can understand their topographical organization into mosaics across the entire retina. Unfortunately, where many different amacrine types are stained, as in the case of GABA, the various morphological types have been difficult to sort one from another. Some immunocytochemistry for the common neurotransmitter candidates has been performed on the human retina (Davanger, 1992; Crooks and Kolb, 1992) but most have been done in the cat, rabbit and mouse retinas and so far the findings are the same in general. There is a remarkable consistency of cell types staining with GABA across species boundaries, in mammals at least. The next challenge is to determine the identity of the GABA receptors associated with particular morphological or physiological subtypes of amacrine cell, pre and postsynaptic to bipolar, amacrine and ganglion cell types in the retina. Grunert and co-workers are making some strides in this area (Grunert et al., 1993; Macri et al., 2000; Sassoe-Pognetto and Fritschy, 2000: Sassoe-Pognetto et al., 1995).
4. Glycine.
The other classic inhibitory neurotransmitter glycine, accounts for most of the small-field types of amarine cell. All amacrine cells in the vertebrate retina can be accounted for by the two inhibitory neurotransmitters GABA and glycine (Marc et al., 1995). In addition one or more types of bipolar cell are also thought to contain glycine in mammalian retinas including monkey and human.
In Figure 6 it can be seen that the immunostaining for glycine is just as strong in the inner plexiform layer as GABA staining. About the same number of amacrine cells are revealed. However there is an addition of some small bipolar cell bodies in the inner nuclear layer and the occasional large cell body of a ganglion cell type in the ganglion cell layer (Crooks and Kolb, 1992).
Fig. 6. Expression pattern of Glycine immunoreactivity in human retina
Pourcho and Goebel (1985) showed quite clearly that tritiated glycine accumulated in Golgi stained AII, A4, and A8 cells in cat retina. More recently as many as 10 different morphological types of small field amacrine cells have been demonstrated by immunocytochemistry and GFP (green fluorescent protein) staining in mouse retina (Menger et al., 1998; Waessle et al., 2009). The A8 cell was the most strongly glycinergic of the small field amacrine cells according to Pourcho and Goebel, with the rod amacrine AII cell being also very clearly glycinergic. Figure 7 shows the commonest glycinergic amacrine cells present in the mouse retina (Waessle et al., 2009). These cells are presumably also so in the cat and primate retinas.
Glycine receptors are found on all the neurons that are postsynaptic to these glycinergic (all small-field) amacrine cells. Thus receptors are found on certain bipolar cell axons, and on many amacrine and ganglion cell dendrites. Again like for the GABA receptors, linking receptor type to morphological type of postsynaptic cell is still a hot topic for research in the retina (Grunert and Wassle, 1993; Waessle et al., 2009).
5. Dopamine is present in amacrine cells in the mammalian retina.
The neuromodulator dopamine is found in one or more types of amacrine cell in the mammalian retina. The most robustly stained dopaminergic cell after immunocytochemistry to the rate limiting enzyme of dopamine synthesis, tyrosine hydroxylase (Toh), is recognized as an A18 cell of the Golgi descriptions (Kolb et al., 1981, Kolb et al., 1992; Tauchi et al.,1990).
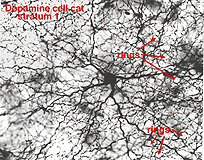 Fig. 8. Wholemount view of a type 1 TOH+ amacrine cell |
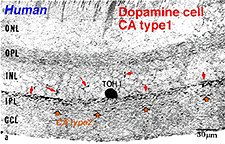 Fig. 9a. Vertical section of a type 1 TOH+ amacrine cell |
It is very characteristic in wholemount appearance, with a large cell body and a dense plexus of dendrites in stratum S1 of the inner plexiform layer (Fig. 8). Holes or “rings” in the plexus of criss-crossing stained dendrites are sites of amacrine cell bodies or large amacrine dendrites (see chapter on amacrine cells) on which the processes of the Toh stained plexus synapse. Some of the processe of TOH cells appear to be axon-like. The Type 1 dopamine cell (A18) is known to synapse upon the AII rod amacrine cell and possibly also upon A8 and A17 cells (Pourcho, 1982; Kolb et al., 1991; Wässle et al., 1987; Casini et al., 1995).
Type 1 dopamine cells provide ascending processes to the outer plexiform layer (Fig. 9a, small red arrows) which are known to synapse on the GABAergic interplexiform cell (see previous chapter on feed-back loops). And its main plexus in S1 sends a few processes to the S3 level of the IPL (Tauchi et al., 1990; Kolb et al., 1991). Glutamate (Marc, personal communication) and dopamine colocalize to the A18 cell type (Type 1 CA cell) and serotonin has also been found to co-exist in the same amacrine cells in cat retina (Wässle and Chun, 1988). The dopamine Type 1 cell exhibits glutamate, GABA and histamine H1 receptors (Kolb et al., 1991; Zhang et al., 2008; Frazao et al., 2011). It is postsynaptic to cone ON-bipolars on its rare branches to stratum 3 of the IPL (Fig.9a, large orange arrows) (Tauchi et al., 1990; Contini et al., 2010) and postsynaptic to GABAergic amacrine cells and probably melanopsin ganglion M1 cells and histaminergic centrifugal fibers (see chapters on amacrine cells and melanopsin ganglion cells).
A second type of dopamine amacrine cell has also been described in primate retinas (Mariani and Hokoc, 1988; Crooks and Kolb, 1992), in rabbit retinas (Tauchi et al., 1990) and mouse retinas (Zhang et al., 2004; Zhang et al., 2007). The Type 2 CA cell has dendrites stratifying in stratum 3 of the IPL. It does not immunostain for TOH alone but can be revealed in transgenetic mouse retina labeled with green fluorescent protein (GFP) (Contini et al., 2010) (Fig 9b). In vertical views of GFP stained mouse Type 2 CA cells have a clear plexus of dendrites in S3 of the IPL (Fig. 9b, green profiles), a distinctly different dendritic plexus than that of the CA1 type (Fig. 9b, blue profiles under the amacrine cells).
Both D1 and D2 receptor types have been found on neurons of the inner and outer retina in many vertebrates. It is thought that both D1 and D2 receptors are found between cells that are coupled by gap junctions, because of dopamine’s known action in cyclic AMP regulation of gap junction channels (Lasater and Dowling, 1985). Thus D1 receptors have been demonstrated in association with horizontal cells of the OPL and amacrine and ganglion cells cells of the inner retina (Djamgoz and Wagner, 1992). D2 receptors are also found in the inner retina but mostly are associated with photoreceptors in the outer nuclear layer, outer limiting membrane and retinal pigment epithelium (Djamgoz and Wagner, 1992).
D1 and D2 receptors are robust on ganglion cell bodies, despite the fact that the dopamine amacrine cell is not known to make direct synapses upon ganglion cells. However several ganglion cell types are connected to amacrine cells via gap junctions and the dopamine effect is probably via diffusion of dopamine from DA cells to the site partners of electrical coupling acting through both D1 and D2 receptors (Mills et al., 2007; Hu et al., 2010).
The best known effect of dopamine on gap junctions is that upon AII to AII amacrine cell gap junctionss, and AII to cone bipolar gap junctions (see chapter on AII amacrine cells) (Mills and Massey, 1995; Bloomfield and Volgyi, 2009). Dopamine particularly affects the former homologous gap junctions while the gas transmitter, nitric oxide, affects the heterologous gap junction involving the ON cone bipolar gap junction (Mills and Massey, 1995).
6. Acetylcholine.
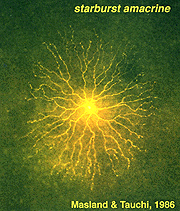
The classic fast excitatory neurotransmitter of the peripheral nervous system, acetylcholine (ACh), is found in a mirror symmetric pair of amacrine cells in the vertebrate retina. In the rabbit such cells have been named starburst cell (Famiglieti, 1983; Masland and Tauchi, 1986). One of the mirror pair occurs in the amacrine cell layer with dendrites in sublamina a (OFF sublamina of the IPL). The other of the pair has its cell body displaced to the ganglion cell layer and its dendrites stratify in sublamina b (ON sublamina of the IPL).
These ACh containing amacrine cells are common to almost all vertebrate retinas and have been described morphologically in human retina too (Hutchins and Hollyfield, 1987; Kolb et al., 1992) (see previous chapter on amacrine cells). ACh starburst amacrine cells co-localize GABA (Vaney and Young, 1988). Both muscarinic and nicotinic receptors have been demonstrated in the mammalian retina, particularly associated with transient phasic ganglion cells (Y cells) (Keyser et al., 1989; Hughes, 1991) and directionally selective ganglion cells (Grzywacz et al., 1998; Strang et al., 2007). Apparently starburst amacrine cells are excitatory with ACh release early in development of the retina and this release is necessary for development of retinal waves. Later in development the starburst cells use inhibitory GABA release to influence directional selectivity in the DS ganglion cells (Zheng et al., 2004; Masland, 2005).
Fig. 10. ACh containing amacrine cell stained with Lucifer yellow
7. Serotonin.
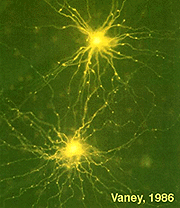
There are two types of serotonin-accumulating amacrine cell in the rabbit retina (Vaney, 1986). One of these is almost certainly the A17 cell or the reciprocal amacrine cell of the rod system in the rabbit (see previous chapters) (Fig. 11). However, in cat retina, a completely different amacrine cell type stains with antibodies against serotonin. One cell type in cat is similar to the wide-field cell A20, while the other may be the A18 or dopamine cell (Wässle and Chun, 1988). Even where serotonin is strongly demonstrated in these amacrine cells in rabbit retina, it is not thought to be the neurotransmitter.
Serotonin co-exists with GABA, in the A17 cell and the latter is thought to be the releasable transmitter (Ehinger and Dowling, 1987; Vaney, 1990). A few cold-blooded vertebrates have a bistratified amacrine cell and a bipolar cell type that immunostain for serotonin (Schütte and Weiler, 1987; Hurd and Eldred, 1993).
8. Adenosine may be a retinal neurotransmitter.
The purine nucleotide, adenosine may be a neurotransmitter or neuromodulator in the mammalian retina. Autoradiography and immunocytochemistry for adenosine has revealed cell bodies in the amacrine and ganglion cell layers (Blazynski and Perez, 1991). Probably most of these cells are amacrine cells but some in the ganglion cell layer may be true ganglion cells. In human retina additional cells that could be bipolar or horizontal cell label too. K+ and light evoked release of adenosine can be measured in rabbit and chick retinas. And the vertical pathway neurotransmitter glutamate can induce adenosine release from [3H]-adenosine preloaded rabbit retina. Additionally some effects of adenosine on the ERG generated in the retina and on the activity of ganglion cell terminals in the superior colliculus have been recorded and the information points to the strong likelihood that adenosine does play a neurotransmitter role in the vertebrate retina (See review by Blazynski and Perez, 1991). Adenosine colocalizes with GABA, acetylcholine and serotonin in various retinas. Much more research is needed in this area.
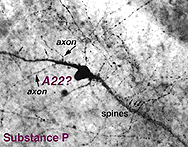
9. Substance P occurs in an amacrine type and a ganglion cell type.
Substance P (SP) is a neuropeptide belonging to the tachykinin family that include neurokinin A, neuropeptide K and neurokinin B, as well. Substance P is thought to be a neurotransmitter or neuromodulator in the vertebrate retina (See Kolb et al., 1995, for a review).
SP-IR amacrine cells appear to be of a single type in the human retina (Fig. 12, above). They are large-field cells with large cell bodies (16 um diameter) lying in normal or displaced positions on either side of the inner plexiform layer (IPL).
Fig. 12. Substance P containing amacrine cell in human retina
Their sturdy, spiny and appendage-bearing dendrites stratify in stratum 3 (S3) of the IPL, where many overlapping fine dendrites intermingle to form a plexus of stained processes. Either cell bodies or primary dendrites emit “axon-like” process which divides typically into two long, fine processes that run in opposite directions for hundreds of microns in S5 and S3 before disappearing as distinct entities in the stained plexus in S3. Long fine dendrites also pass from the dendritic plexus to run in S5 and down to the nerve fibre layer to end as large varicosities at blood vessel walls. In addition fine processes are emitted from the dendritic plexus that run in S1, and some pass up to the outer plexiform layer (OPL) to run therein for short distances. The SP-IR amacrine cell has many similarities to thorny type 2 amacrine cells described in Golgi studies. SP amacrine cells co-localize GABA as a neurotransmitter (Pourcho and Goebel, 1988).
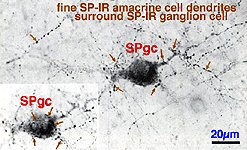
Fig. 13. Substance P containing ganglion cell in human retina
In addition to the SP-IR amacrine cells, a ganglion cell type is immunostained with SP (Fig. 13). Its 20- 22 um cell body gives rise to a radiate, sparsely-branched, wide-spreading dendritic tree running in S3. Its dendrites and cell body become enveloped by the more intensely SP-IR processes and boutons from the SP-IR amacrine cell type (Fig. 13, fine arrows). The SP-IR ganglion cell type most resembles G21 of a Golgi study. A ganglion cell type in rabbit retina has definitely been proved to contain substance P. Such ganglion cells almost certainly colocalize a more standard neurotransmitter like glutamate too (Brecha et al., 1987).
10. Other neuropeptides.
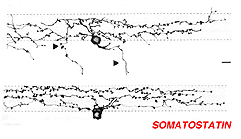
Immunostaining with antibodies against somatostatin has revealed a small population of neurons in the ganglion cell layer in the rabbit retina (Sagar, 1987). They are distributed only in the inferior retina and in the far peripheral circumference of the retina. However, in the monkey and human retinas these amacrines are more uniformly distributed. The somatostatin-IR amacrines have long fibers that distribute across the entire retina running in three plexuses in the middle and outer and inner strata of the inner plexiform layer (Fig. 14, from Marshak, 1989). In the human and monkey most of the somatostatin-IR cells have their cell bodies in the ganglion cell layer (Fig. 14) and they emit fibers or axon-like processes that can be measured running 20 mm across the entire retina (Sagar and Marshall, 1988). Because these axon like processes stay within the retina, not passing to the optic nerve, the somatostatin-immunoreactive cells are likened to the associational neurons of Cajal (1892).
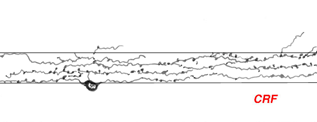
Corticotropin releasing factor (CRF) is contained within a population of wide-field tristratified amacrines with long axon like processes, very similar in morphology to those containing somatostatin (Fig. 15) (Marshak, 1989). Colocalization of the two peptides has not been attempted yet but it seems probably that they are one and the same cell type.
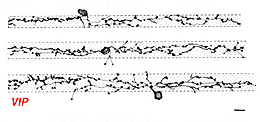
Vasoactive Intestinal peptide (VIP) immunostains a population of amacrine cells that can be normally place in the INL or displaced to the ganglion cell layer (Fig. 16). They appear to have a medium size dendritic field and dendrites that branch diffusely through the middle strata of the IPL. They may be equivalent to the A12 type of Mariani (1990).
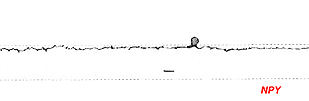
Amacrine cells that immunostain for neuropeptide Y are quite regularly stained in the primate and rodent retina. These amacrine cells have a characteristic sparsely branched wide-field dendritic tree with dendrites running in stratum 1 of the IPL (Fig. 17) (Marshak, 1989). Recent research whereby NPY amacrine cells can be selectively ablated indicates that these cells have a role in low spatial frequency tuning of certain large ganglion cell types (Sinclair et al., 2004). Both ON and OFF center ganglion cell types are said to be equally affected by loss of NPY amacrine cells, which is a strange finding considering the OFF stratum branching of these particuar amacrine cells. Presumably another chain of amacrine cells is involved in spatial tuning of ganglion in addition to the NPY type.
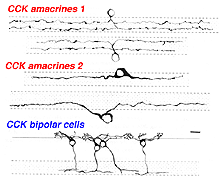
Using antibodies to the glycine extended form of cholecystokinin, Marshak and colleagues were able to demonstrate the presence of two different morphological types of amacrine cell and a single morphological type of bipolar cell in the monkey retina (Marshak, 1989; Kouyama and Marshak. 1992). The amacrines come as pairs that are either bistratified in the strata 2 and 4 and a larger bodied monstratified pair, again branching in either stratum 2 or 4 (Fig. 18). The blue cone specific bipolar cell is well characterized by CCK immunostaining and its distribution, contact with blue cones specfically and its probably ON center physiology is now well known (Kouyama and Marshak, 1992; Kolb et al., 1997) (See chapter on S-cone pathways).
11. NADPH-diaphorase staining and the possibility that there are nitric oxide containing neurons in the retina.
Nitric oxide is formed in many nerve cells of the peripheral and central nervous system. It is known to play a role in second messenger cascades by activating guanylyl cyclase and elevating cyclic GMP levels. Since many neurotransmitters and transduction events utilize cyclic GMP in the retina (notable phototransduction in the photoreceptors and activation of metabotropic glutamate receptors) the idea that nitric oxide might play a key role in retinal neurotransmission has been forwarded. Nitric oxide synthase the enzyme required for synthesis of nitric oxide requires NADPH as a cofactor in the cell. Thus the simple histochemical technique to reveal NADPH in a cell by the reduction of tetrazolium salts has proved to be a good marker of neurons that contain NADPH-diaphorase and hence possibly also nitric oxide synthase. An antibody has been made against the synthase but staining and identification of morphological cell types that contain this enzyme have been unsatisfactory to date for the retina. Thus the NADPH-diaphorase histochemical technique is still more reliable.
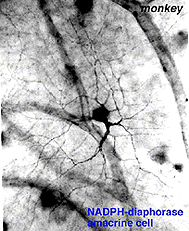 Fig. 19. NADPH-diaphorase histochemical staining of an amacrine cell in monkey retina |
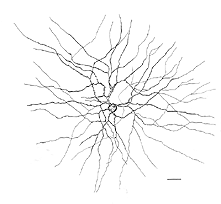 Fig. 20. Drawing from wholemount retina stained with NADPH-diaphorase of the commonly stained amacrine cell |
In monkey retina, three types of amacrine cell and one type of ganglion cell appear clearly stained with NADPH-diaphorase. The commonest type is the one shown above (Figs. 19 and 20) and occurs at a maximum density of 280 cell/mm2 at 1 mm from the fovea (Cuenca, personal communication). It has a large cell body that lies either in the amacrine cell layer or can be displaced to the ganglion cell layer. The dendrites radiate out from the cell body like the spokes of a wheel, contain, many fine spines, and lie on one plane in stratum 3 of the IPL. Fine axon like processes arise from the main dendritic tree that is about 300 um in diameter to run for mm in all directions. The cell is probably the equivalent of a spiny amacrine described by Mariani (1990) and a cell type stained by Dacey as axon bearing (1989).
12. Amacrine cell populations and mosaics arrangements are revealed by neurotransmitter immunocytochemistry.
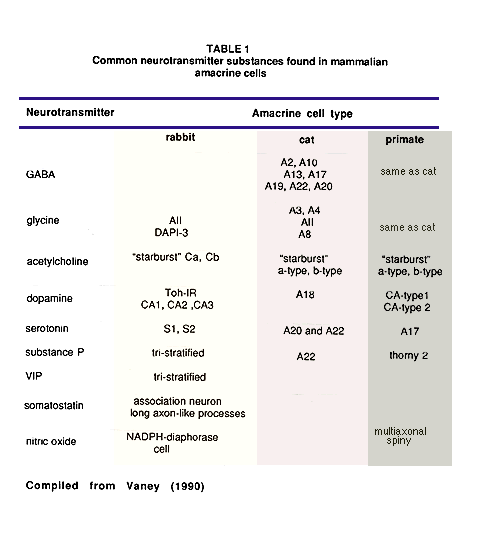
The summary table below lists the amacrine cell types that have now become associated with particular neurotransmitter substances in the mammalian retina. The list is compiled primarily from data of cat and rabbit retinas. However, some clearly recognizable amacrine cell types of the monkey and human retina have been included. The list is as yet incomplete concerning an exact classification of each cell type by neurotransmitter signature because of the difficulty of agreeing on correspondence between different authors classification schemes and making cross species comparisons. The review article by Vaney (1990) is the most comprehensive treatment on the subject of the differences between cat and rabbit retina in this regard.
Immunocytochemical staining of amacrine cells, when done on wholemounts of retina, can reveal every cell of the population that are immunoreactive to the antibody used. Thus we are increasingly acquiring population maps and distribution maps of all the different types of amacrine cells according to neurotransmitter content. Most amacrine types are arranged in regular mosaics and the individual cells have certain overlap characteristics that can be calculated from nearest neighbor statistics. The cat and monkey amacrine mosaics peak in cell density with closest packing of their smallest dendritic trees in the fovea or area centralis. Then from center to periphery the neurons distribute evenly in concentric rings of decreasing density and increasing dendritic field sizes. The rabbit amacrine mosaics peak along the horizontally-organized visual streak and fall off therefrom linearly into superior and inferior retina (Vaney, 1990). Some of the more sparsely distributed neurotransmitter types, have unique distributions. For example, the somatostatin-immunoreactive associational neurons in rabbit retina are located almost exclusively in inferior retina.
The commonest amacrine cell type of the cat and rabbit retina is the glycinergic AII amacrine (estimated 512,000 total in cat retina) followed by the serotonin-accumulating amacrines (between 170,000 and 230,000 cells in rabbit), cholinergic cells (approximately 130,000 in rabbit retina) and substance P-containing cell types (39,000 cells in cat retina). Dopaminergic amacrine cells are some of the lowest density populations of cells (3-5,000 in cat; 6-8,000 in rabbit). Somatostatin occurs in a small population of only 1000 cells in rabbit retina.
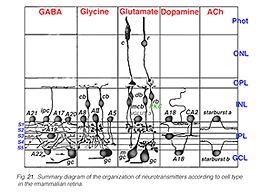
Fig. 21. Summary diagram of the organization of neurotransmitters in the retina
13. References.
Blazynski C, Perez, MTR. Neuroregulatory functions of adenosine in the retina. Prog ret Res. 1991; 11: 293-332.
Brecha NC, Johnson D, Bolz J, Sharma S, Parnavelas JG, Lieberman AR. Substance P immunoreactive retinal ganglion cells and their central axon terminals in the rabbit. Nature. 1987;327:155–158. [PubMed]
Cajal RS. The structure of the retina. (Thorpe SA, Glickstein M, Trans) Springfield, IL: Thomas; 1892, 1972.
Casini G, Rickman DW, Brecha NC. AII amacrine cell population in the rabbit retina: Identification by parvalbumin immunoreactivity. J Comp Neurol.1995;356:132142.
Chavez AE, Grimes WN, Diamond JS (2010) Mechanisms underlying lateral GABAergic feedback onto rod bipolar cells in rat retina. J Neurosci. 30, 2330-9. [PubMed]
Crooks J, Kolb H. Localization of GABA, glycine, glutamate and tyrosine hydroxylase in the human retina. J Comp Neurol. 1992;315:287–302. [PubMed]
Dacey DM. Axon-bearing amacrine cells of the Macaque monkey retina. J Comp Neurol. 1989;284:275–293. [PubMed]
Davanger S, Ottersen OP, Storm-Mathisen J. Glutamate, GABA and glycine in the human retina: An immunocytochemical investigation. J Comp Neurol.1991;311:483–494. [PubMed]
Eggers, ED and Lukasiewicz, PD (2011) Multiple pathways of inhibition shape bipolar cell responses in the retina. Vis Neurosci. 28, 95-102. [PubMed]
Ehinger B, Dowling JE. Retinal neurocircuitry and transmission. In”Handbook of chemical neuroanatomy, vol. 5 Integrated systems of the CNS, part 1 (A Bjorklund, T Hokfelt and LW Swanson, eds) Elsevier Science Publishers BV; 1987, pp. 389-446.
Famiglietti EV. ‘Starburst’ amacrine cells and cholinergic neurons: mirror-symmetric ON and OFF amacrine cells of rabbit retina. Brain Res. 1983;261:138–144. [PubMed]
Grünert U, Wässle H. Immunocytochemical localization of glycine receptors in the mammalian retina. J Comp Neurol. 1993;335:523–537. [PubMed]
Haverkamp S, Wassle H. Characterization of an amacrine cell type of the mammalian retina immunoreactive for vesicular glutamate transporter 3. J Comp Neurol. 2004;468:251–263. [PubMed]
Hughes TE, Grunert U, Karten HJ. GABAA receptors in the retina of the cat: an immunohistochemical study of wholemounts, sections, and dissociated cells. Vis Neurosci. 1991;6:229–238. [PubMed]
Hurd LB, Eldred WD. Synaptic microcircuitry of bipolar and amacrine cells with serotonin-like immunoreactivity in the retina of the turtle Pseudemys scripta elegans. Vis Neurosci. 1993;10:455–472. [PubMed]
Hutchins JB, Hollyfield JG. Cholinergic neurons in the human retina. Exp Eye Res. 1987;44:363–376. [PubMed]
Johnson J, Sherry DM, Liu X, Fremeau RT, Seal RP, Edwards RH, Copenhagen DR. Vesicular glutamate transporter 3 expression identifies glutamatergic amacine cells in the rodent retina. J Comp Neurol. 2004;477:386–398. [PubMed]
Keyser KT, Hughes TE, Whiting PJ, Lindstrom JM, Karten HJ. Cholinoceptive neurons in the retina of the chick: an immunohistochemical study of the nicotinic acetylcholine receptors. Vis Neurosci. 1988;1:349–366. [PubMed]
Keyser KT, Britto LRG, Schoepfer R, Whiting P, Cooper J, Conroy W, Brozozowska-Prechtl A, Karten HJ, Linstrom J. Three subtypes of alpha-bungarotoxin-sensitive nicotinic acetylcholine receptors are expressed in chick retina. J Neurosci. 1993;13:442–454. [PubMed]
Kolb H, Nelson R, Mariani A. Amacrine cells, bipolar cells and ganglion cells of the cat retina: A Golgi study. Vision Res. 1981;21:1081–1114. [PubMed]
Kolb H, Cuenca N, DeKorver L. Postembedding immunocytochemistry for GABA and glycine reveals the synaptic relationships of the dopaminergic amacrine cell of the cat retina. J Comp Neurol. 1991;310:267–284. [PubMed]
Kolb H, Linberg KA, Fisher SK. The neurons of the human retina: a Golgi study. J Comp Neurol. 1992;318:147–187. [PubMed]
Kolb H, Fernandez E, Ammermüller J, Cuenca N. Substance P: A neurotransmitter of amacrine and ganglion cells in the vertebrate retina. Histol Histopathol. 1995;10:947–968. [PubMed]
Kolb H, Goede P, Roberts S, McDermott R, Gouras P. The uniqueness of the S-cone pedicle in the human retina and consequences for color processing. J Comp Neurol. 1997;386:443–460. [PubMed]
Kouyama N, Marshak DW. Bipolar cells specific for blue cones in the macaque retina. J Neurosci. 1992;12:1233–1252. [PubMed]
Lasater EM, Dowling JE. Dopamine decreases conductance of the electrical junctions between cultured retinal horizontal cells. Proc Natl Acad Sci USA.1985;82:3025–3029. [PubMed]
Lasater EM, Lam DMK. Membrane properties of distal retinal neurons. Prog Ret Res. 1992;11:215–246.
Lukasiewicz PD, Wong ROL. GABAC receptors on ferret retinal bipolar cells: a diversity of subtypes in mammals? Vis Neurosci. 1997;14:989–994.[PubMed]
Marc RE, Murry RF, Basinger SF. Pattern recognition of amino acid signatures in retinal neurons. J Neurosci. 1995;15(7 Pt 2):5106–29. [PubMed]
Marc RE, Jones BW. Molecular phenotyping of retinal ganglion cells. J Neurosci. 2002;22(2):413–27. [PubMed]
Mariani AP, Hokoc JM. Two types of tyrosine hydroxylase-immunoreactive amacrine cells in the rhesus monkey. J Comp Neurol. 1988;276:81–91.[PubMed]
Mariani AP. Amacrine cells of the rhesus monkey retina. J Comp Neurol. 1990;301:382–400. [PubMed]
Marshak DW. Peptidergic neurons of the Macaque monkey retina. Neurosci Res. (Suppl., 10) 1989; S117-130.
Masland RH, Tauchi M. The cholinergic amacrine cell. TINS. 1986;9:218–223.
Massey SC, Miller RF. Glutamate receptors of ganglion cells in the rabbit retina: Evidence for glutamate as a bipolar cell transmitter. J Physiol (Lond).1988;405:635–655. [PubMed]
Massey SC. Cell types using glutamate as a neurotransmitter in the vertebrate retina. Prog Ret Res. 1990;9:399–425.
Menger N, Pow DV. Glycinergic amacrine cells of the rat retina. J Comp Neurol. 1998;401:34–46. [PubMed]
Nawy S, Jahr CE. Supression by glutamate of cGMP activated conductance in retinal bipolar cells. Nature. 1990;346:269–271. [PubMed]
Pourcho RG. Dopaminergic amacrine cells in the cat retina. Brain Res. 1982;252:101–109. [PubMed]
Pourcho RG, Goebel DJ. Neuronal subpopulations in cat retina which accumulate the GABA agonist (3H)muscimol: a combined Golgi and autoradiographic study. J Comp Neurol. 1983;219:25–35. [PubMed]
Pourcho RG, Goebel DJ. A combined Golgi and autoradiographic study of 3(H)glycine-accumulating amacrine cells in the cat retina. J Comp Neurol.1985;233:473–480. [PubMed]
Pourcho RG, Goebel DJ. Colocalization of substance P and GABA in amacrine cells of the cat retina. Brain Res. 1988;447:164–168. [PubMed]
Sagar SM. Somatostatin-like immunoreactive material in the rabbi retina: immunohistochemical staining using monoclonal antibodies. J Comp Neurol.1987;266:291–299. [PubMed]
Sagar SM, Marshall PE. Somatostatin-like immunoreactive material in associational ganglion cells of human retina. Neurosci. 1988;27:507–516.
Schütte M, Weiler R. Morphometric analysis of serotonergic bipolar cells in the retina and its implication for retinal image processing. J Comp Neurol.1987;260:619–626. [PubMed]
Sinclair JR, Jacobs AL, Nirenberg S. Selective ablation of a class of amacrine cells alters spatial processing in the retina. J Neurosci. 2004;24:1459–1467.[PubMed]
Slaughter MM, Miller RF. 2-amino-4-phosphonobutyric acid: A new pharma-cological tool for retina research. Science. 1981;211:182–184. [PubMed]
Slaughter MM, Miller RF. An excitatory amino acid antagonist blocks cone input to sign-conserving second-order retinal neurons. Science.1983a;219:1230–1232. [PubMed]
Slaughter MM, Miller RF. Bipolar cells in the mudpuppy retina use an excitatory amino acid neurotransmitter. Nature. 1983b;303:537–538. [PubMed]
Vaney DI. Morphological identification of serotonin-accumulating neurons in the living retina. Science. 1986;233:444–446. [PubMed]
Vaney DI. The mosaic of amacrine cells in the mammalian retina. Prog Ret Res. 1990;9:49–100.
Wässle H, Voight T, Patel B. Morphological and immunocytochemical identification of indoleamine-accumulating neurons in the cat retina. J Neurosci.1987;7:1574–1585. [PubMed]
Wässle H, Chun MH. Dopaminergic and indoleamine-accumulating amacrine cells express GABA-like immunoreactivity in cat retina. J Neurosci.1988;8:3383–3394. [PubMed]
Waessle H, Heinze L, Ivanova E, Majumdar S, Weiss J, Harvey RJ, Haverkamp S. (2009). Glycinergic transmission in the mammalian retina. Frontiers in Molecular Neuroscience, www.frontiersin.org.
Witkovsky P, Dearry A. Functional roles of dopamine in the vertebrate retina. Prog Ret Res. 1991;11:247–292.
Yazejian B, Fain GL. Excitatory amino acid receptors on isolated retinal ganglion cells from the goldfish. J Neurophysiol. 1992;67:94–106. [PubMed]
Last Updated: August 19, 2011.