Josh Morgan and Rachel Wong
1. Introduction.
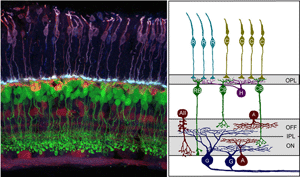
Synaptic connections of the vertebrate retina are organized into distinct laminae (Figure 1). In the outer retina, photoreceptors contact horizontal cells and bipolar cells within a single lamina, the outer plexiform layer (OPL). Within the inner retina, synapses between retinal ganglion cells and their presynaptic partners, the amacrine and bipolar interneurons are localized to the inner plexiform layer (IPL). Connections between cells that are depolarized upon an increase in illumination (ON) occupy approximately the inner half of the IPL, whereas connectivity of cells hyperpolarized (OFF) by light is confined to the outer half of the IPL. What factors control the precision by which each cell type form synaptic connections within the IPL and OPL are not completely understood. However, structural and functional studies have significantly improved our understanding of the mechanisms underlying retinal circuit development, aided in part by new technological advances.
2. Sequence of circuit assembly in the vertebrate retina.
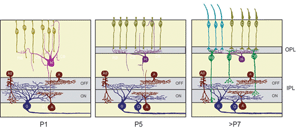
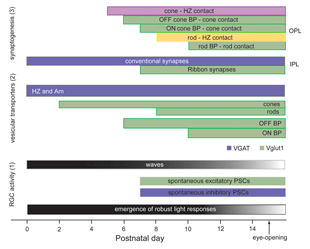
Ultrastructural studies (Olney, 1968; Fisher, 1979; Blanks et al., 1974) suggest that synaptogenesis between the major neuronal classes of the vertebrate retina occurs in three major phases (Figure 2; illustrated for the mouse retina). Retinal ganglion cells and amacrine cells are the first cell classes to differentiate and form the earliest functional circuits in the IPL of the developing retina. Shortly after, horizontal cells and photoreceptors differentiate and contact each other in the outer retina, forming the OPL. The vertical networks in the inner and outer retina are later interconnected when bipolar cells are born and connections with ganglion cells are established. This sequential pattern of retinal circuit development is common across species, although the separation in time between inner and outer retinal circuit development varies, ranging from a few hours in animals such as the zebrafish to many days and weeks in mammals. The emergence of the IPL and OPL plexuses can be observed in the zebrafish embryo by in vivo time-lapse confocal imaging as visualized in Movie M1 below.
3. Structural assembly.
New approaches
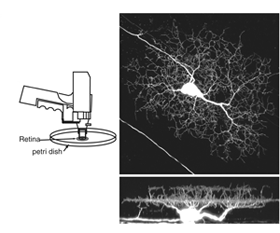
The morphology of the pre- and postsynaptic arbors of retinal neurons have been documented from Golgi staining many decades ago (Cajal, 1960). These studies provided a wealth of information, not only aiding classification of distinct morphological cell types in the mature retina, but also enabling visualization of the structure of their immature counterparts. More recently, fluorescent markers have been used to specifically label retinal cell classes and their subtypes. Cells can be targeted by intracellular dye filling methods using glass micropipettes (Vaney, 1984; Sakaguchi, 1989). This method leads to rapid labeling of the cell and its processes, within seconds to minutes. Intracellular dye filling has been used to visualize developing retinal neurons, and remains a popular technique to determine their morphology at different ages.
Since its discovery, green fluorescent protein (GFP) has been used extensively as a live-cell marker. Its resistance to bleaching, compared to most fluorescent dyes, together with its lack of toxicity, make GFP ideal for studying how retinal neurons alter their structure in tissue explants, or in vivo. GFP and its spectral variants can be expressed in retinal neurons either by transient transfection or in stable transgenic lines. To randomly label retinal neurons in explant cultures, a gene-gun can be used to deliver plasmids encoding GFP (Wong et al., 2000; Figure 3). Other color fluorescent proteins (FPs: yellow, cyan, red) can also be expressed this way. Electroporation can be used to specifically target and label individual cells in vitro and in vivo (Haas et al., 2002). This technique involves intraocular DNA injection before high voltage pulses are applied across the eye (Figure 4; see Matsuda and Cepko, 2004).
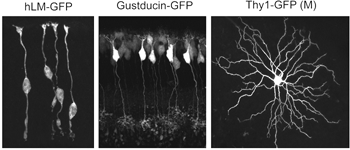
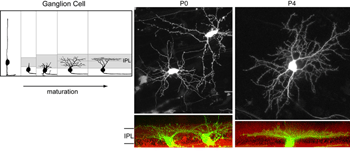
Alternatively, as cell-type specific promoters become increasingly available, labeling of retinal neurons in the developing and adult retina can be achieved by generating transgenic animals. For example, GFP expression is driven in cone photoreceptors in mice shortly after birth using the human L and M cone promoter (Figure 5; Fei and Hughes, 2001). Also, a subset of bipolar cells expresses GFP in the gustducin-GFP mouse (Figure 5; Huang et al., 2003) and retinal ganglion cells are found to express fluorescent proteins under the Thy-1 promoter (Figure 5; Feng et al., 2000).
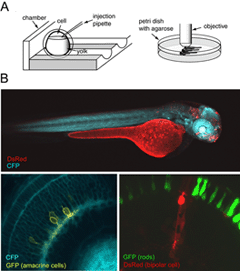
Zebrafish in which retinal neurons express fluorescent proteins have also been generated (Figure 6). The major advantage for generating fluorescent protein expressing transgenic lines is that structural development of the various retinal neuronal cell types can be followed in live tissue, or in the intact animal using time-lapse microscopy. The zebrafish, in particular, is highly suited for in vivo imaging because of its relatively rapid development and transparency during embryonic development. Details of how to image fluorescently labeled retinal neurons in zebrafish embryos is provided by Lohmann et al. (2005a).
4. Development of pre- and postsynaptic processes.
There are at least two features of the structure of retinal neurons that impact their connectivity and therefore the circuits that they form. The first relates to the lateral branching patterns of their axons and dendrites. The lateral organization of the pre- and postsynaptic arbors of retinal neurons determines the spatial coverage of the cell, and possibly the density of input and output that they form. Second, as mentioned earlier, the pre- and postsynaptic arbors of retinal neurons are highly restricted to laminae in the inner and outer retina. This laminar organization in structure reflects the connectivity between specific subsets of cells. Thus, one way to gain a better understanding of how retinal circuits form appropriately, is to determine the mechanisms that regulate the lateral and vertical organization of the axonal and dendritic arbors of retinal neurons.
(a) Retinal ganglion cells
There are many types of retinal ganglion cells, each with characteristic branching patterns, arbor size and amount of overlap with neighbors of the same subtype. Both intrinsic and environmental cues appear to shape the branching patterns of retinal neurons, at least for ganglion cells (reviewed in Sernagor et al., 2001). When retinal ganglion cells are isolated in culture without contact with other cells, they elaborate a dendritic arbor that is complex in branching and resembles cells in vivo. Stereotypic organization of the branching patterns of the various cell types (for example, alpha cells and beta cells in the cat retina) also argue that cell intrinsic mechanisms help define their branching patterns.
However, the shape and size of the ganglion cell dendritic arbor changes when the density of neighboring ganglion cells is altered during development (Figure 7). Laser ablation or axon section results in a local lesion depleted of retinal ganglion cells (Eysel et al., 1985). This manipulation results in cells at the edge of the lesion projecting their dendrites into the ganglion cell-free region. Experimental manipulation of eye size that causes an increase in the spacing between cells is paralleled by an increase in their arbor size (Troilo et al., 1996). However, for certain subtypes of ganglion cells in the mouse retina, a reduction in ganglion cell density does not affect the size and patterning of their dendritic arbors (Lin et al., 2004). These differences raise the possibility that the balance between cell-intrinsic and cell-extrinsic cues in shaping the lateral organization of ganglion cell dendritic arbors may vary from one cellular subtype to another, or perhaps even across species.
If cell-cell interactions influence the branching and territorial size of ganglion cell dendritic arbors, what might be the signals? One way to address this is to better understand the dynamic behavior of ganglion cell dendrites during the period when their branching patterns and territories are established. Time-lapse imaging experiments of developing chick and mouse retinal ganglion cells reveal that dendritic branches are both added and eliminated over time, before the adult branching patterns are attained. Dynamic alterations to the dendritic territory upon branch addition and loss can be appreciated in the following movie M3.
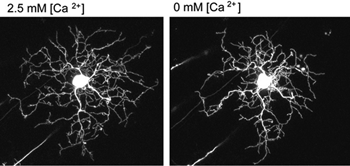
What cellular mechanisms might neurotransmission evoke to regulate dendritic branching in retinal ganglion cells? Live-imaging studies of embryonic chick retinas reveal that ganglion cell dendritic stability is calcium-dependent. In low extracellular calcium, the dendrites of retinal ganglion cells retract (Figure 8). Dendritic retraction can be locally controlled by calcium induced calcium release (CICR) from intracellular stores (Lohmann et al., 2002). Spontaneous CICR is observed in small dendritic segments during calcium imaging of immature retinal ganglion cells. The pathways CICR activates to stabilize the dendritic cytoskeleton remains to be determined. One possibility is that transmission from presynaptic terminals causes local CICR that then stabilize the contacted dendritic segments (Lohmann et al., 2002). Future time-lapse imaging experiments are needed to directly address whether nascent synapses stabilize dendrites of retinal ganglion cells upon contact, as has been demonstrated in zebrafish tectal neurons (Niell et al., 2004).
What factors shape dendritic stratification of retinal ganglion cells during development? In mammals, retinal ganglion cells initially project dendrites throughout the depth of the IPL (Figure 9). Stratification occurs progressively and becomes precise by maturity (Figure 9). Some insight into what mechanisms underlie ganglion cell dendritic stratification was gained by early studies where intraocular injections of 2-amino-4-phosphonobutyrate, APB (a mGluR6 receptor agonist) prevented the emergence of stratified alpha and beta ganglion cell dendritic arbors in cat (Bodnarenko and Chalupa, 1993; Bodnarenko et al., 1995) and ferret (Bodnarenko et al., 1999) retina. Thus, neurotransmission may be involved in sculpting the final arrangement of ganglion cell dendrites. This is supported by pharmacological studies in turtles and chick whereby blockade of cholinergic or glutamatergic transmission, respectively, resulted in ganglion cells with relatively smaller, and less branched arbors (Wong et al., 2000; Sernagor and Mehta, 2001).
(b) Amacrine cells
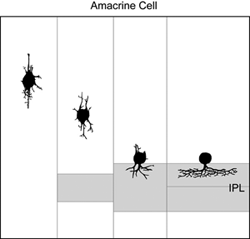
How immature amacrine cells elaborate their processes after neuronal differentiation has been described by Golgi (Prada et al., 1987) and electron microscope (Hinds and Hinds, 1978; 1983) studies a few decades ago. From observations of retinas fixed at different ages, it is thought that cells in the differentiating inner nuclear layer that are multipolar in shape are immature amacrine cells. Such cells extend neurites in many different directions prior to reaching the border of the INL and IPL. Thereafter, amacrine cells elaborate processes within the IPL to form their arbors (Figure 10).
Time-lapse studies of amacrine cells labeled by expression of fluorescent protein in stable transgenic zebrafish lines have provided some insight into the lamination of amacrine cell neurites (Kay et al., 2004). In a transgenic line in which subpopulations of ON and OFF amacrine cells express GFP, it is possible to visualize the emergence of a plexus of processes from these amacrine cells (see movie M1). Initially, it appears that GFP-positive amacrine cell processes contributing to the nascent IPL are diffusely arranged, as no sublamination is observed. However, over time, two distinct bands of bright fluorescence resolve, corresponding in depth to sublamina a (OFF) and sublamina b (ON)of the IPL. In order to determine how individual amacrine cells target their appropriate sublamina during development, it will be necessary to visualize and follow the development of individual amacrine cells over time in vivo. This is because it is impossible to differentiate between closely apposed, but segregated, arbors in the IPL from arbors that are intermingled in depth.
It is evident that even after amacrine cell neurites have attained their correct sublamination, their arbors still undergo significant reoroganization in the lateral dimension prior to achieving their mature form. Starburst amacrine cells in the mammalian retina show a distinctive radial morphology early in development, but their detailed branching pattern alters with maturation. Like retinal ganglion cells, the morphological changes primarily involve the loss of small protrusions, and the emergence of bouton-like structures at the distal branches (Wong and Collin, 1989) that in the mature cell, are sites of presynaptic transmitter release (See chapters on amacrines and neuroactive substances).
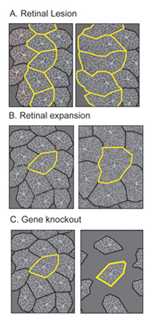
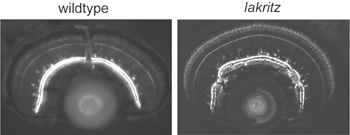
What factors influence the development of amacrine cell neurites? First, it is evident that retinal ganglion cells, although a major postsynaptic target of amacrine cells, are not required for lamination of amacrine cell arbors. Optic nerve section in rodents that leads to ganglion cell death, does not alter the stratification of starburst amacrine cells (reviewed in Chalupa and Gunhan, 2004). In mutant animals in which ganglion cells do not differentiate (Math5 knockout mice (Brown et al., 2001; Wang et al., 2001a) and the zebrafish lakritz mutant), amacrine cell lamination also occurs (Figure 11). Since bipolar cells differentiate only after amacrine cell lamination has taken place, it is unlikely that these neurons influence the initial stratification of amacrine cells. It is possible that interactions between amacrine cells that are specific to each subtype result in the formation of their separate laminae. Alternatively, lamination cues may arise from Muller glial cells or their precursors. Such cues may be diffusible, creating a gradient of permissive or repulsive factors with retinal depth, or are contact-mediated. Because amacrine cells express neurotransmitters even before their cell bodies reach the INL/IPL border, it also remains plausible that communication between these cells via secretion of transmitters influences their arborization. Mutant animals in which specific forms of neurotransmission are absent would help address this possibility.
(c) Bipolar cells
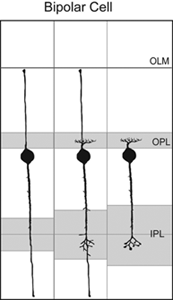
Axo- and dendrogenesis of bipolar cells are not well understood because of the lack of markers for these cells early in development. However, Golgi studies of the chick retina suggest that the axonal and dendritic arbors of developing bipolar cells elaborate from vertical processes terminating in the inner and outer limiting membranes (Quesada et al., 1981; see Figure 12). The use of cell-specific markers to identify bipolar cells would further test this model of bipolar cell development.
Fig. 12. Proposed sequence of development of bipolar cell axons and dendrites from Golgi studies
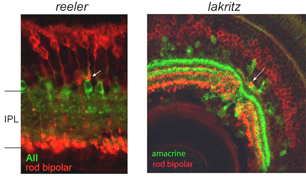
Stratification of the axonal arbors of bipolar cells may be influenced by interactions with amacrine cells. AII amacrine cells, which are the postsynaptic targets of rod bipolar cells, have a bistratified arbor, each with stereotypic morphology. The outer arbor (OFF sublamina) comprises lobular appendages whereas the inner arbor (ON) is finely branched (see chapter on AII amacrine cells). AII amacrine cells express Disabled1, an adaptor protein involved in the reelin pathway (Rice et al., 2001). In the reeler mouse in which reelin is absent, the distribution of lobular and non-lobular appendages of the AII amacrine cells are imprecise. Likewise, some rod bipolar cells are found to have axonal stratification defects (Figure 13). Also in the lakritz mutant, bipolar axonal terminals are similarly perturbed in their organization in local regions of disrupted amacrine cell lamination (Figure 13). ON and OFF bipolar cell lamination persists in the absence of ganglion cells (Gunhan-Agar et al., 2000; Wang et al., 2001a; Brown et al., 2001). Together, these observations raise the possibility that amacrine cells, rather than ganglion cells, provide lamination cues for bipolar axon terminals.
(d) Horizontal cells
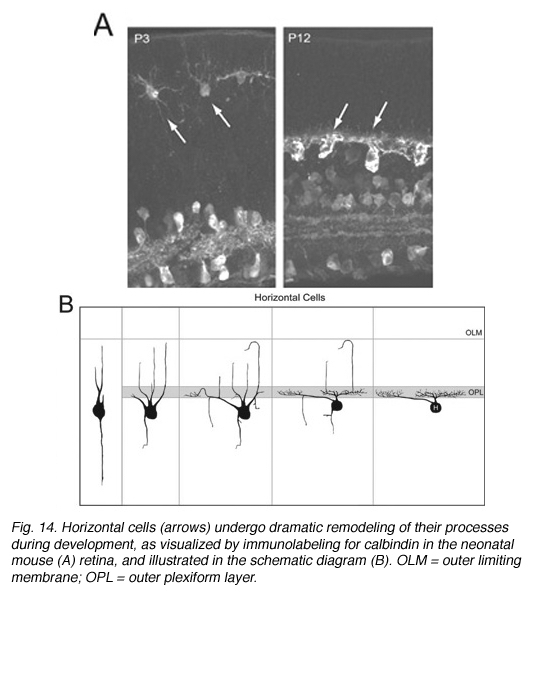
Early Golgi studies and immunolabeling for GAD67 (Schnitzer and Rusoff, 1984), one of the two synthetic enzymes for GABA, suggest that horizontal cell processes undergo dramatic progression from a radial to lateral organization during development (Figure 14). Horizontal cells achieve this lateral organization in the absence of cone photoreceptors (Reese et al., 2005). However, abnormal lamination patterns in horizontal cells are observed in the Rb mouse during development in regions where rods are absent (Donovan and Dyer, 2004).
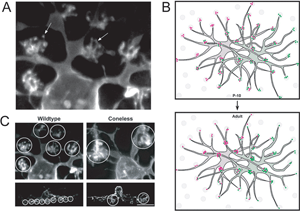
In the mature retina, horizontal cells do not contact cones uniformly within their dendritic fields. The number of dendritic terminals (examples of terminals, Figure 15A) that contact cones decreases as a function of distance from the cell body (Figure 15B). Interestingly, in developing horizontal cells, the number of terminals does not vary greatly with distance from the cell body. Because the overlap of dendritic fields of neighboring horizontal cells is relatively unchanged after birth in the mouse, horizontal cells appear to lose peripheral terminals but gain central terminals with maturation (Figure 15B). The terminal branching pattern of horizontal cell dendrites is altered in the coneless mouse (see Reese et al., 2005; Figure 15C). This suggests that contact with cones is either important in the initial organization, or in the maintenance, of these structures.
(e) Photoreceptors
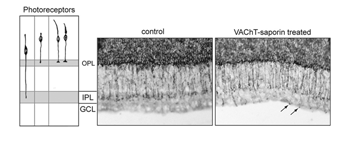
Structural changes in the morphology and axonal targeting of photoreceptors have been assessed using immunolabeling methods (schematized in Figure 16A). In a variety of species, these studies reveal that the terminals of a large number of rods (80% of the population) and some cones project beyond the forming outer plexiform layer, reaching the IPL (Johnson et al., 1999; reviewed by Reese, 2004; see Figure 16). With maturation, all photoreceptor terminals become restricted to the OPL. The transient projection of photoreceptor terminals to the IPL is not perturbed when retinal ganglion cells are ablated. However, pharmacological deletion of amacrine cells, for example with VAChT-saporin, results in these photoreceptor terminals ending at various depths of the IPL, and even extending into the GCL. This implicates the amacrine cells as targets of the immature photoreceptor terminals (see Johnson et al., 2001; Figure 16).
5. Functional assembly
New approaches
Physiological properties of developing retinal neurons have often been assessed at the single cell level using patch clamp, intracellular and extracellular recording methods. Simultaneous recording from large populations of developing retinal neurons became possible upon the development of multielectrode arrays (Meister et al., 1991) and advances in optical imaging methods (reviewed in Morgan et al., 2005). Both approaches have been used to assess activity patterns of retinal networks during development.
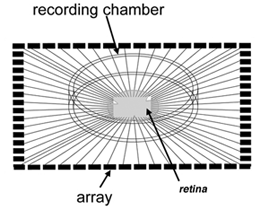
Multielectrode recording Planar electrode arrays with 60 or more extracellular electrodes permit simultaneous recordings from tens of ganglion cells (Figure 17; see Movie below). However, because they are extracellular recordings, it is not straightforward to determine which cells within the sampled area are associated with spike trains detected at each electrode site. These arrays are commercially available from several sources – shown below is an example of an array from Multichannel systems used for recording spikes from the developing retina (Tian and Copenhagen, 2001; Demas et al., 2003).
Calcium imaging
Calcium imaging approaches have been used to assess retinal neuronal activity at the single cell level and to simultaneously monitor intracellular calcium levels in populations of cells (See chapter on retinal development by Marla Feller). The use of calcium indicator dyes is widely documented (see Lohmann et al., 2005b for detailed description of methodology). Both membrane-permeant forms of indicators as well as genetically encoded indicators are available and have been used successfully. Shown in Movie M6 is an example of spontaneous activity in an embryonic chick retina showing elevations in intracellular calcium levels as there is increase in fluorescence intensity. The calcium levels elevate across the array in a wave formation. Ballistic delivery of calcium indicators can be used to actually visualize the processes of individual cells (Lohmann et al., 2002).
6. Development of synaptic connectivity
Functional drive onto retinal ganglion cells is first observed shortly after conventional synapses are present in the IPL, as assessed by electron microscopy. During this period of development, spontaneous excitatory postsynaptic currents (EPSCs) are detected in retinal ganglion cells and amacrine cells. These excitatory currents are mediated by acetylcholine, GABA or glycine (Sernagor et al., 2001), transmitters used by amacrine cells. As in other parts of the CNS, GABA is depolarizing to retinal ganglion cells prior to the appearance of glutamatergic drive. Spontaneous glutamatergic drive is first observed in the mouse retina around P7, when bipolar terminals are clearly present in the IPL (Johnson et al., 2003). Whether photoreceptors transiently projecting to the IPL provide glutamatergic drive to inner retinal neurons is not known, although it is suggested by the presence of synaptophysin positive terminals (Reese, 2004).
Figure 18 summarizes the time-line of key events associated with circuit development in the developing mouse retina.
7. Spontaneous activity
Prior to vision, the inner retina generates a pattern of activity that is unique to the developing retina. Neighboring retinal ganglion cells exhibit rhythmic bursting activity that is correlated by propagating waves (reviewed in Wong, 1999; see Marla Feller’s chapter). These waves are visualized by calcium imaging and are also detected using multielectrode arrays (see Movie M5). Retinal waves disappear shortly after eye-opening (Figure 19). The properties and significance of these retinal waves are discussed in the chapter by Marla Feller. Although there is increasing evidence for a role of waves in the refinement of connectivity between the retina (McLaughlin et al., 2003) and its central targets, their role in shaping intra-retinal circuits although possible, remains to be determined.
8. Light responses
Emergence of ON versus OFF responses
Immunolabeling studies show that VGlut1, a vesicular glutamate transporter, first appears in the axonal terminals of OFF bipolar cells, before being present in ON bipolar cells (Sherry et al., 2003). This would suggest that the OFF pathway may become functional somewhat earlier than the ON pathway. However, immature amacrine cells in the developing rabbit retina show a predominantly ON response to light (Dacheux and Miller, 1981b). Thus, to further ascertain whether ON and OFF transmission matures at different rates, it is necessary to record spontaneous glutamatergic currents and visual responses from identified ON and OFF retinal ganglion cells during development.
At the stage when ferret retinal ganglion cells are still diffusely unstratified, they are found to respond to both increments and decrements in light intensity (Wang et al., 2001b). In mouse, multielectrode recordings show that a higher proportion of units sampled have ON-OFF responses during development, compared to the adult (Tian and Copenhagen, 2003). Thus, it appears that significant refinement of ON and OFF bipolar axonal projections to the retinal ganglion cells occurs during maturation of the mammalian retina. One set of projections becomes dominant and the other eliminated. In addition to preventing dendritic stratification of retinal ganglion cells, intraocular injections of APB also results in a higher incidence of ON-OFF cells in the cat retina (Bodnarenko and Chalupa, 1993). Because APB suppresses spontaneous activity of both ON and OFF ganglion cell subtypes during development, how chronic treatment with this glutamate analog might affect the maturation of ON and OFF connectivity is not yet known.
Emergence of receptive fields
In vivo recordings from cat retina show that center-surround organization of receptive fields is apparent as soon as light responses are detected (Tootle, 1993). Unlike the mature retina, however, receptive field center sizes appear larger than dendritic field size during development (Tootle, 1993). In contrast to ganglion cells, the surrounds of rabbit bipolar cells appear after receptive field centers are clearly present (Dacheux and Miller, 1981a).
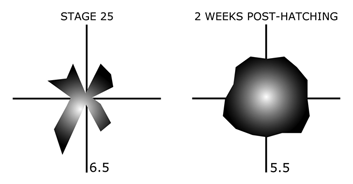
Specialized receptive field properties, such as direction-selectivity, can be detected prior to eye-opening in the rabbit retina (Masland, 1977). Recordings from the developing turtle retina, however, suggest that ganglion cells initially have “anisotropic” receptive fields, with more “isotropic” fields emerging only with maturation (Sernagor and Grzywacz, 1995; Figure 20).
Influence of light in shaping receptive field properties
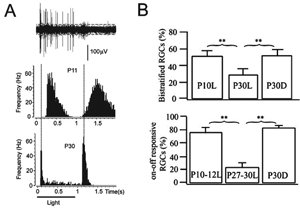
Multielectrode recordings in the mouse retina suggest that light stimulation is necessary to facilitate the age-related loss of ON-OFF ganglion cells with maturation (Tian and Copenhagen, 2003). Dark-rearing mice until about 3 weeks after birth leads to an increase in the fraction of bistratified, ON-OFF ganglion cells in the retina (Figure 21). This observation would suggest that interactions between bipolar cells and the retinal ganglion cells, or amacrine cells, act to fine-tune the extent of convergence of the vertical pathways onto an individual ganglion cell. This study also underscores the ability of retinal circuits to undergo reorganization, physiologically and structurally, after eye-opening.
Experiments in the turtle retina also implicate light stimulation in prolonging the period of spontaneous wave activity. This in turn affects receptive field maturation (Sernagor and Grzywacz, 1996). Dark-rearing causes an expansion of receptive field size; however, this expansion is prevented when cholinergic-driven spontaneous activity is blocked.
9. Summary
Much remains to be determined as to how retinal circuits are assembled precisely. Because the retina comprises a large diversity of cell components and numerous circuits, determining the cellular and molecular mechanisms regulating its wiring is a challenging task. Nevertheless, as retinal subcircuits with defined visual properties become identified (such as the direction-selective circuit), elucidating the developmental mechanisms regulating their assembly should become more tractable. Combined electrophysiological and anatomical techniques are necessary, and with rapid advancement in both approaches, it is likely that significant progress will be made in the near future.
10. References
Blanks JC, Adinolfi AM, Lolley RN. Synaptogenesis in the photoreceptor terminal of the mouse retina. J Comp Neurol. 1974;156:81–93. [PubMed]
Bodnarenko SR, Chalupa LM. Stratification of ON and OFF ganglion cell dendrites depends on glutamate-mediated afferent activity in the developing retina. Nature. 1993;364:144–146. [PubMed]
Bodnarenko SR, Jeyarasasingam G, Chalupa LM. Development and regulation of dendritic stratification in retinal ganglion cells by glutamate-mediated afferent activity. J Neurosci. 1995;15:7037–7045. [PubMed]
Bodnarenko SR, Yeung G, Thomas L, McCarthy M. The development of retinal ganglion cell dendritic stratification in ferrets. Neuroreport. 1999;10:2955–2959. [PubMed]
Brown NL, Patel S, Brzezinski J, Glaser T. Math5 is required for retinal ganglion cell and optic nerve formation. Development. 2001;128:2497–2508.[PubMed] [Free Full text in PMC]
Cajal RY. Studies on vertebrate neurogenesis . Springfield (IL): Thomas; 1960.
Chalupa LM, Gunhan E. Development of On and Off retinal pathways and retinogeniculate projections. Prog Retin Eye Res. 2004;23:31–51. [PubMed]
Dacheux RF, Miller RF. An intracellular electrophysiological study of the ontogeny of functional synapses in the rabbit retina. I. Receptors, horizontal, and bipolar cells. J Comp Neurol. 1981;198:307–326. [PubMed]
Dacheux RF, Miller RF. An intracellular electrophysiological study of the ontogeny of functional synapses in the rabbit retina. II. Amacrine cells. J Comp Neurol. 1981;198:327–334. [PubMed]
Demas J, Eglen SJ, Wong RO. Developmental loss of synchronous spontaneous activity in the mouse retina is independent of visual experience. J Neurosci. 2003;23:2851–2860. [PubMed]
Donovan SL, Dyer MA. Developmental defects in Rb-deficient retinae. Vision Res. 2004;44:3323–3333. [PubMed]
Eysel UT, Peichl L, Wassle H. Dendritic plasticity in the early postnatal feline retina: quantitative characteristics and sensitive period. J Comp Neurol.1985;242:134–145. [PubMed]
Fadool JM. Development of a rod photoreceptor mosaic revealed in transgenic zebrafish. Dev Biol. 2003;258:277–290. [PubMed]
Fei Y, Hughes TE. Transgenic expression of the jellyfish green fluorescent protein in the cone photoreceptors of the mouse. Vis Neurosci. 2001;18:615–623. [PubMed]
Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, Nerbonne JM, Lichtman JW, Sanes JR. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28:41–51. [PubMed]
Fisher LJ. Development of synaptic arrays in the inner plexiform layer of neonatal mouse retina. J Comp Neurol. 1979;187:359–372. [PubMed]
Gunhan-Agar E, Kahn D, Chalupa LM. Segregation of on and off bipolar cell axonal arbors in the absence of retinal ganglion cells. J Neurosci.2000;20:306–314. [PubMed]
Haas K, Jensen K, Sin WC, Foa L, Cline HT. Targeted electroporation in Xenopus tadpoles in vivo–from single cells to the entire brain. Differentiation.2002;70:148–154. [PubMed]
Hinds JW, Hinds PL. Early development of amacrine cells in the mouse retina: an electron microscopic, serial section analysis. J Comp Neurol.1978;179:277–300. [PubMed]
Hinds JW, Hinds PL. Development of retinal amacrine cells in the mouse embryo: evidence for two modes of formation. J Comp Neurol. 1983;213:1–23.[PubMed]
Huang L, Max M, Margolskee RF, Su H, Masland RH, Euler T. G protein subunit G gamma 13 is coexpressed with G-alphao, G-beta3, and G-beta4 in retinal ON bipolar cells. J Comp Neurol. 2003;455:1–10. [PubMed]
Johnson PT, Williams RR, Cusato K, Reese BE. Rods and cones project to the inner plexiform layer during development. J Comp Neurol. 1999;414:1–12.[PubMed]
Johnson PT, Raven MA, Reese BE. Disruption of transient photoreceptor targeting within the inner plexiform layer following early ablation of cholinergic amacrine cells in the ferret. Vis Neurosci. 2001;18:741–751. [PubMed]
Johnson J, Tian N, Caywood MS, Reimer RJ, Edwards RH, Copenhagen DR. Vesicular neurotransmitter transporter expression in developing postnatal rodent retina: GABA and glycine precede glutamate. J Neurosci. 2003;23:518–529. [PubMed]
Kay JN, Roeser T, Mumm JS, Godinho L, Mrejeru A, Wong RO, Baier H. Transient requirement for ganglion cells during assembly of retinal synaptic layers.Development. 2004;131:1331–1342. [PubMed]
Lin B, Wang SW, Masland RH. Retinal ganglion cell type, size, and spacing can be specified independent of homotypic dendritic contacts. Neuron.2004;43:475–485. [PubMed]
Lohmann C, Myhr KL, Wong RO. Transmitter-evoked local calcium release stabilizes developing dendrites. Nature. 2002;418:177–181. [PubMed]
Lohmann C, Mumm JS, Morgan J, Godinho L, Schroeter E, Stacy R, Wong WT, Oakley D, Wong ROL. Imaging the developing retina. In: Rafael Y, Konnerth A, editors. Imaging in neuroscience and development. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 2005. p. 159-170.
Lohmann C, Demas J, Morgan JL, Wong ROL. A practical guide: calcium imaging of the retina. In: Rafael Y, Konnerth A. Imaging in neuroscience and development. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 2005. p. 283-288.
Masland RH. Maturation of function in the developing rabbit retina. J Comp Neurol. 1977;175:275–286. [PubMed]
Matsuda T, Cepko CL. Electroporation and RNA interference in the rodent retina in vivo and in vitro. Proc Natl Acad Sci U S A. 2004;101:16–22. [PubMed] [Free Full text in PMC]
McLaughlin T, Torborg CL, Feller MB, O’Leary DD. Retinotopic map refinement requires spontaneous retinal waves during a brief critical period of development. Neuron. 2003;40:1147–1160. [PubMed]
Meister M, Wong RO, Baylor DA, Shatz CJ. Synchronous bursts of action potentials in ganglion cells of the developing mammalian retina. Science.1991;252:939–943. [PubMed]
Morgan J, Huckfeldt R, Wong RO. Imaging techniques in retinal research. Exp Eye Res. 2005;80:297–306. [PubMed]
Mumm JS, Godinho L, Morgan JL, Oakley DM, Schroeter EH, Wong RO. Laminar circuit formation in the vertebrate retina. Prog Brain Res. 2005;147:155–169. [PubMed]
Niell CM, Meyer MP, Smith SJ. In vivo imaging of synapse formation on a growing dendritic arbor. Nat Neurosci. 2004;7:254–260. [PubMed]
Olney JW. An electron microscopic study of synapse formation, receptor outer segment development, and other aspects of developing mouse retina. Invest Ophthalmol. 1968;7:250–268. [PubMed]
Prada C, Puelles L, Genis-Galvez JM, Ramirez G. Two modes of free migration of amacrine cell neuroblasts in the chick retina. Anat Embryol (Berl).1987;175:281–287. [PubMed]
Quesada A, Prada F, Armengol JA, Genis-Galvez JM. Early morphological differentiation of the bipolar neurons in the chick retina. A Golgi analysis. Anat Histol Embryol. 1981;10:328–341. [PubMed]
Reese BE. Developmental plasticity of photoreceptors. Prog Brain Res. 2004;144:3–19. [PubMed]
Reese BE, Raven MA, Stagg SB. Afferents and homotypic neighbors regulate horizontal cell morphology, connectivity, and retinal coverage. J Neurosci.2005;25:2167–2175. [PubMed]
Rice DS, Nusinowitz S, Azimi AM, Martinez A, Soriano E, Curran T. The reelin pathway modulates the structure and function of retinal synaptic circuitry.Neuron. 2001;31:929–941. [PubMed]
Sakaguchi DS. The development of retinal ganglion cells deprived of their targets. Dev Biol. 1989;134:103–111. [PubMed]
Schnitzer J, Rusoff AC. Horizontal cells of the mouse retina contain glutamic acid decarboxylase-like immunoreactivity during early developmental stages. J Neurosci. 1984;4:2948–2955. [PubMed]
Sernagor E, Grzywacz NM. Emergence of complex receptive field properties of ganglion cells in the developing turtle retina. J Neurophysiol.1995;73:1355–1364. [PubMed]
Sernagor E, Grzywacz NM. Influence of spontaneous activity and visual experience on developing retinal receptive fields. Curr Biol. 1996;6:1503–1508.[PubMed]
Sernagor E, Eglen SJ, Wong RO. Development of retinal ganglion cell structure and function. Prog Retin Eye Res. 2001;20:139–174. [PubMed]
Sernagor E, Mehta V. The role of early neural activity in the maturation of turtle retinal function. J Anat. 2001;199:375–383. [PubMed] [Free Full text in PMC]
Sherry DM, Wang MM, Bates J, Frishman LJ. Expression of vesicular glutamate transporter 1 in the mouse retina reveals temporal ordering in development of rod vs. cone and ON vs. OFF circuits. J Comp Neurol. 2003;465:480–498. [PubMed]
Tian N, Copenhagen DR. Visual deprivation alters development of synaptic function in inner retina after eye opening. Neuron. 2001;32:439–449.[PubMed]
Tian N, Copenhagen DR. Visual stimulation is required for refinement of ON and OFF pathways in postnatal retina. Neuron. 2003;39:85–96. [PubMed]
Tootle JS. Early postnatal development of visual function in ganglion cells of the cat retina. J Neurophysiol. 1993;69:1645–1660. [PubMed]
Troilo D, Xiong M, Crowley JC, Finlay BL. Factors controlling the dendritic arborization of retinal ganglion cells. Vis Neurosci. 1996;13:721–733. [PubMed]
Vaney DI. ‘Coronate’ amacrine cells in the rabbit retina have the ‘starburst’ dendritic morphology. Proc R Soc Lond B Biol Sci. 1984;220:501–508.[PubMed]
Wang GY, Liets LC, Chalupa LM. Unique functional properties of on and off pathways in the developing mammalian retina. J Neurosci. 2001;21:4310–4317. [PubMed]
Wang SW, Kim BS, Ding K, Wang H, Sun D, Johnson RL, Klein WH, Gan L. Requirement for math5 in the development of retinal ganglion cells. Genes Dev. 2001;15:24–29. [PubMed] [Free Full text in PMC]
Wong ROL, Collin SP. Dendritic maturation of displaced putative cholinergic amacrine cells in the rabbit retina. J Comp Neurol. 1989;287:164–178.[PubMed]
Wong ROL. Retinal waves and visual system development. Annu Rev Neurosci. 1999;22:29–47. [PubMed]
Wong WT, Faulkner-Jones B, Sanes JR, Wong ROL. Rapid dendritic remodeling in the developing retina: dependence on neurotransmission and reciprocal regulation by Rac and Rho. J Neurosci. 2000;20:5024–5036. [PubMed]
Last Updated: July 30, 2018.
The author
Rachel Wong obtained her PhD from the Australian National University, Canberra, Australia, in Visual Neuroscience under the mentorship of Abbie Hughes. She then worked in the laboratory of Carla Shatz at Stanford University, focusing on early activity patterns of the retina together with Markus Meister and Denis Baylor. Subsequently, Rachel moved back to Australia to join the Vision group in the Vision, Touch and Hearing Research Centre, University of Queensland. From 1994-2010, she joined the Department of Anatomy and Neurobiology at Washington University School of Medicine, St. Louis. Now she is Professor of Neurobiology in the Department of Biological Structure, University of Washington, Seattle. Research interests concern how neural circuits are developed in the vertebrate visual system using mice and chick and zebrafish retinas. They use confocal and multiphoton imaging, electron microscopy and transgenic methods along with electrophysiology to investigate the retina in development and in diseased retinas. Presently Rachel and Phil Horner’s lab are exploring neuron circuit disassembly in a mouse model of glaucoma. Contact Rachel at wongr2@uw.edu
The author
Josh Morgan received his B.A at New College of the University of South Florida with an area of concentration in neurobiology. He is currently a graduate student in Rachel Wong’s lab the Anatomy and Neurobiology Dept. of Washington University in St. Louis . He expects to spend his life imaging neural circuit development and has focused his graduate work on studying the development of bipolar cells, retinal ganglion cells and the connections between the two.