Ning Tian
Introduction
The neuronal information of the visual scene that is processed by the retina is conducted to the brain by a set of separate spatio-temporal synaptic pathways. The morphological basis for the formation of these parallel synaptic pathways is the laminar-specific structure of the retina, in which specific subtypes of retinal neurons form synapses only with highly selective presynaptic and postsynaptic cells (Famiglietti and Kolb, 1976; Nelson et al., 1978; Schiller, 2010).
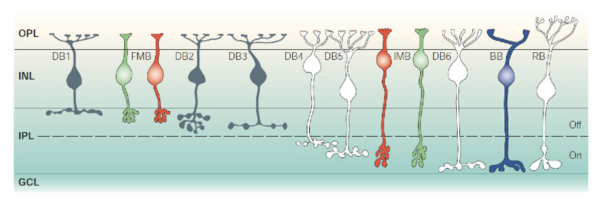
Retinal ganglion cells (RGCs) are the output neurons of the retina. In the retina, RGCs synapse with bipolar and amacrine cells in the inner plexiform layer (IPL) to receive excitatory and inhibitory synaptic inputs respectively. The axons of RGCs travel through the optic nerve to retinorecipient structures in the brain, where they transfer their specific aspects of visual information to the higher centers (Schiller, 2010). Because different subtypes of bipolar cells (Fig 1) (Euler and Wässle, 1995) and amacrine cells (Fig. 2) (MacNeil and Masland, 1998) have their axonal/dendritic terminals in the specific sublaminae of the IPL, it is crucial that dendrites of individual RGCs are also confined to specific strata in order to synapses with them.
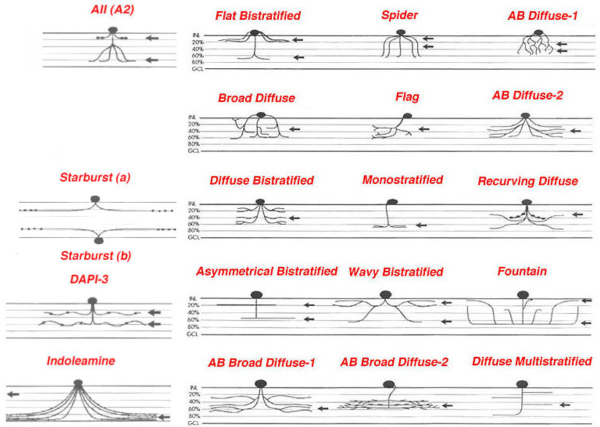 Figure 2: Schematic drawings of some of the amacrine cells of rabbit retina to show each type has a characteristic morphology and stratification of the dendrites to specific strata (1-5) of the inner plexiform layer. All other mammals have similar cells. (Adapted from MacNeil and Masland, 1998).
Figure 2: Schematic drawings of some of the amacrine cells of rabbit retina to show each type has a characteristic morphology and stratification of the dendrites to specific strata (1-5) of the inner plexiform layer. All other mammals have similar cells. (Adapted from MacNeil and Masland, 1998).
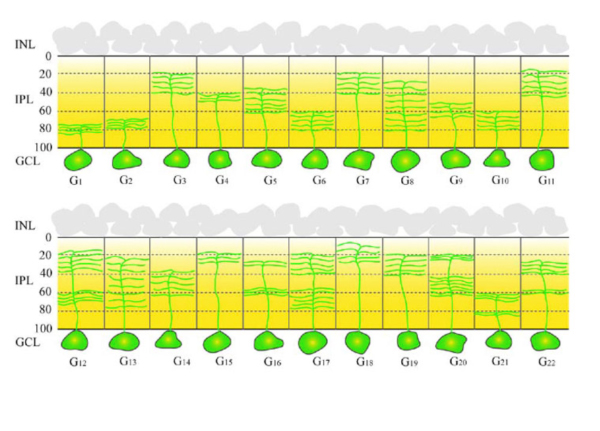
Thus, the synaptic circuitries processing distinct visual features, the so called “parallel pathways” (Coombs and Chalupa, 2008; Famiglietti and Kolb, 1976; Ghosh et al. 2004; Kuffler, 1953; Masland, 2001; Nelson et al., 1978; Wässle, 2004), start in the retina. In most mammals, RGCs can be divided into about 20 morphological subtypes based on their distinctive dendritic structure and synaptic connections (Kolb et al., 1981; 1992; Badea and Nathans, 2004; Berson, 2008; Coombs et al., 2006; Dacey and Packer, 2003; Kong et al., 2005; Rockhill et al., 2002; Sun et al., 2002; Volgyi et al., 2009). The wholemount drawings of mouse RGCs (Fig. 3) illustrate the diversity of morphologies present in mammalian RGCs (Volgi et al., 2009. See also RGCs of human, cat and rabbit retinas in the ganglion cell chapter in Webvision).
Most of these RGCs have specific dendritic distribution in the IPL in adult retina as exemplified by the schematic (Figure 4) showing the branching patterns of mouse RGCs. In most mammals, these lamina-restricted distributions of RGC dendrites and synaptic connections are formed during pre- and post-natal development. The question is how this lamination arises.
Neurogenesis and synaptogenesis of retina
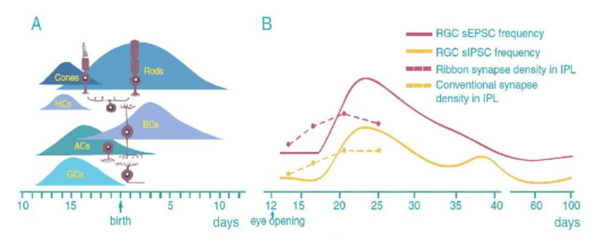
The neurogenesis and synaptogenesis of mammalian retina is an orderly process. Fig 5 shows an overview drawing of the development of mouse retinal neurons. RGCs differentiate first followed by amacrine cells, cones and horizontal cells. Rod photoreceptors differentiate shortly afterward. Bipolar cells are the last neurons to differentiate. Similarly, most retinal neurons differentiate before birth in other mammals (Altshuler et al. 1991; Cepko et al. 1996; Marquardt and Gruss, 2002).
The order of synaptogenesis of retinal neurons is somewhat different from the order of neurogenesis. The synapses of amacrine cells in the IPL appear first. These are followed by the synaptic formation between photoreceptors and horizontal cells in the OPL. The last synaptic element to link photoreceptors in the outer retina and RGCs in the inner retina is the synaptic connection between bipolar cells and RGCs (Fig. 5A) (Stone et al. 1984; Nishimura and Rakic, 1987). In mouse, the density of both ribbons and conventional synapses in the IPL continuously increases after eye opening and reaches the peak level by the age of P21 (Fig. 5B). Functionally, the strength of RGC synaptic inputs measured by the frequency of spontaneous synaptic activity is low before eye opening in mice. After eye opening, a surge of glutamate receptor-mediated spontaneous excitatory postsynaptic currents (sEPSCs) and GABA/glycine receptor-mediated spontaneous inhibitory postsynaptic currents emerges around P25 (Fig. 5B). Amplitudes of RGC light responses in cat and ferret retina are also found to increase after eye opening (Tootle 1993; Wang et al., 2001). In rabbit and rat, the amplitudes of retinal light responses measured by electroretinography continuously increases in the first month after birth and reaches the adult level by the ages of P30 to P40 (Gorfinkel et al., 1988; Wachtmeister 1998).
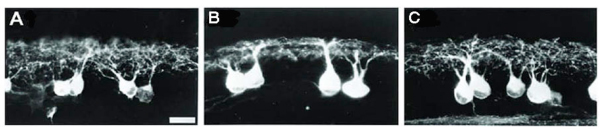
During synaptogenesis, the dendrites of mouse RGCs undergo very active remodeling. More than 30% of dendritic filapodial branches in the mouse are replaced every hour by continuous dendritic growth and elimination (pruning) between P10-13 (see Fig 6 and movie 1). This developmental remodeling of RGC dendrites is thought to play an important role in synaptogenesis and the formation of lamina-restricted dendritic distributions of RGCs.
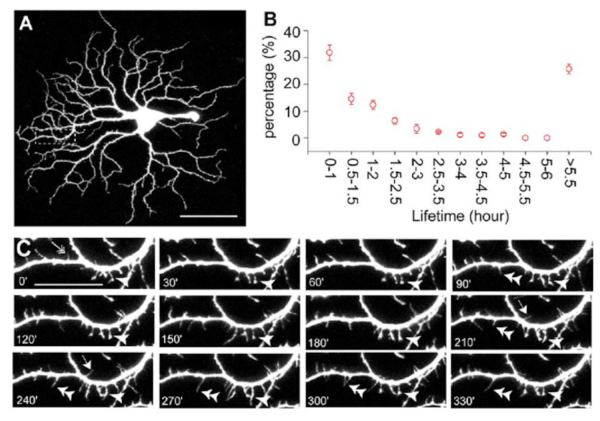 Figure 6: Dendrites of mouse RGCs undergo very active remodeling during synaptogenesis in postnatal development. RGC dendritic motility was examined using time-lapse confocal imaging on retinas of YFP+ mice at P13-14. A: Representative image of an A1 YFP+ RGC of a Thy1-YFP mouse. Scale bar, 60 µm. B: Average lifetime of RGC filopodia of P13-14 mice. Note that more than 30% of the filopodia are replaced every hour. Filopodia that existed at the beginning and disappeared before the end of the recording period and filopodia that emerged during the recording period and persisted until the end of the recording were discarded since the lifetime of these filopodia could not be determined. C: Representative time-lapse images of a segment of the dendrite from the RGC shown in (A) (indicated by the dash-line box) taken at 30 min intervals. Arrow, transient filopodia; arrowhead, static filopodia: double arrowhead, new filopodia; double-headed arrow, filopodia lost over the recording period. Scale bar, 20 µmm. See also Movie (Adapted from Xu et al., 2010).
Figure 6: Dendrites of mouse RGCs undergo very active remodeling during synaptogenesis in postnatal development. RGC dendritic motility was examined using time-lapse confocal imaging on retinas of YFP+ mice at P13-14. A: Representative image of an A1 YFP+ RGC of a Thy1-YFP mouse. Scale bar, 60 µm. B: Average lifetime of RGC filopodia of P13-14 mice. Note that more than 30% of the filopodia are replaced every hour. Filopodia that existed at the beginning and disappeared before the end of the recording period and filopodia that emerged during the recording period and persisted until the end of the recording were discarded since the lifetime of these filopodia could not be determined. C: Representative time-lapse images of a segment of the dendrite from the RGC shown in (A) (indicated by the dash-line box) taken at 30 min intervals. Arrow, transient filopodia; arrowhead, static filopodia: double arrowhead, new filopodia; double-headed arrow, filopodia lost over the recording period. Scale bar, 20 µmm. See also Movie (Adapted from Xu et al., 2010).
Movie 1. Changes in filapodial growth and shape in the course of one hour.
Formation of lamina-restricted dendritic distributions of RGCs
Many studies have shown that the dendritic morphology and synaptic connections of RGCs undergo profound refinement during postnatal development. Early in postnatal development, the dendrites of many RGCs ramify diffusely throughout the IPL of the retina in cats, rats and mice (Fig. 7, A1, A2). With subsequent maturation, RGC dendrites become much more narrowly stratified in the IPL (Fig. 7, A2, B2 and B3) (Bodnarenko and Chalupa, 1993; Bodnarenko et al. 1995, 1999; Coombs et al., 2007; Diao et al. 2004; Kim et al., 2010; Maslim and Stone, 1988; Wang et al. 2001b) at least partially due to a developmental restriction of RGC dendrites (Coombs et al., 2007). Recent studies suggest that different subtypes of RGCs acquire their lamina-restricted dendritic ramification patterns in different ways.
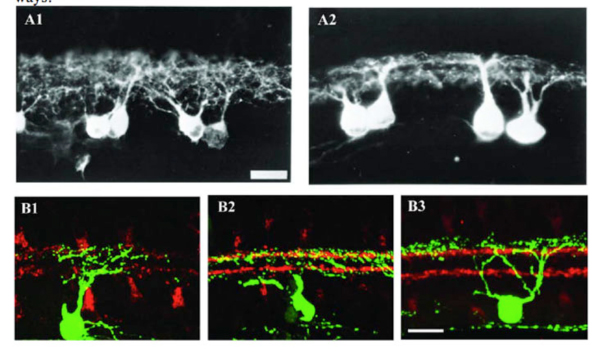 Figure 7: Dendrites of RGCs can reach mature stratified pattern through selective pruning. A1 and A2: Light micrographs of DiI (1,10-dioctadecyl-3,3,30,30-tetramethyl indocarbocyanine perchlorate) labeled RGCs from transverse sections of the central region of a P2 and a P10 cat, respectively. In A1, the RGC dendrites are distributed throughout the total IPL at P2 while, in A2, the dendrites have the distinct appearance of two tiers of dendrites within the IPL and the stratification pattern of ON and OFF layers. Scale bar in A1, 20 um. (Reproduced from Bodnarenko and Chalupa, 1993.) B1, B2 and B3: Retinal sections from P5, P8, and P12-P13 JAM-B mice, respectively. RGCs were labeled with anti-GFP (green) and starburst amacrines with anti-ChAT (red). In B1, the dendrites of JAM-B RGC are distributed throughout the total IPL at P5. At P8 (B2), the JAM-B RGC selectively loses dendrites in the inner IPL. At P12-13 (B3), the dendrites of JAM-B RGC exhibit a lamina-restricted distribution in the outer IPL. Scale bar in B3, 20 um. (Adapted from Kim et al., 2010).
Figure 7: Dendrites of RGCs can reach mature stratified pattern through selective pruning. A1 and A2: Light micrographs of DiI (1,10-dioctadecyl-3,3,30,30-tetramethyl indocarbocyanine perchlorate) labeled RGCs from transverse sections of the central region of a P2 and a P10 cat, respectively. In A1, the RGC dendrites are distributed throughout the total IPL at P2 while, in A2, the dendrites have the distinct appearance of two tiers of dendrites within the IPL and the stratification pattern of ON and OFF layers. Scale bar in A1, 20 um. (Reproduced from Bodnarenko and Chalupa, 1993.) B1, B2 and B3: Retinal sections from P5, P8, and P12-P13 JAM-B mice, respectively. RGCs were labeled with anti-GFP (green) and starburst amacrines with anti-ChAT (red). In B1, the dendrites of JAM-B RGC are distributed throughout the total IPL at P5. At P8 (B2), the JAM-B RGC selectively loses dendrites in the inner IPL. At P12-13 (B3), the dendrites of JAM-B RGC exhibit a lamina-restricted distribution in the outer IPL. Scale bar in B3, 20 um. (Adapted from Kim et al., 2010).
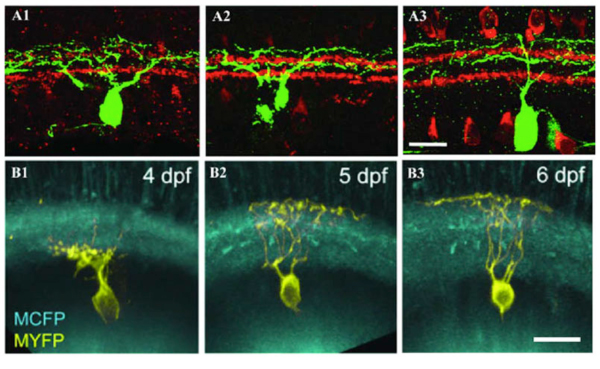
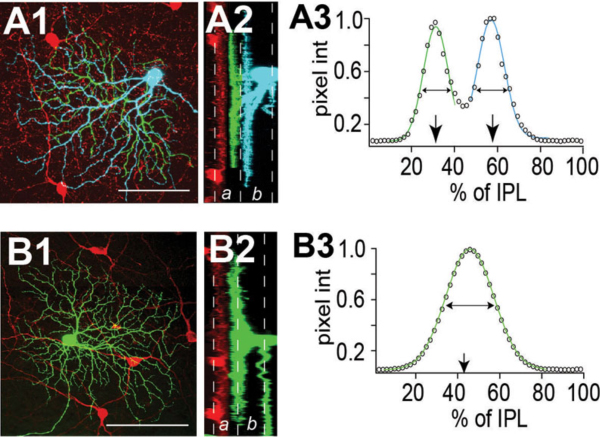
Some RGCs seem to achieve their restricted lamina patterns by direct targeting without significant pruning. In Figure 8 A1-A3, a bistratified RGC has a bistratified dendritic distribution pattern early at P5 (A1) and retains this bistratified pattern into adulthood (A2 and A3) without an initial diffuse distribution pattern. Similarly in Zebrafish some RGCs directly elaborate their dendrites to the middle of the IPL and later became strictly monostratified, occupying a single stratum in the middle of the IPL (Fig. 8 B1, B2 and B3) (Mumm et al., 2006).
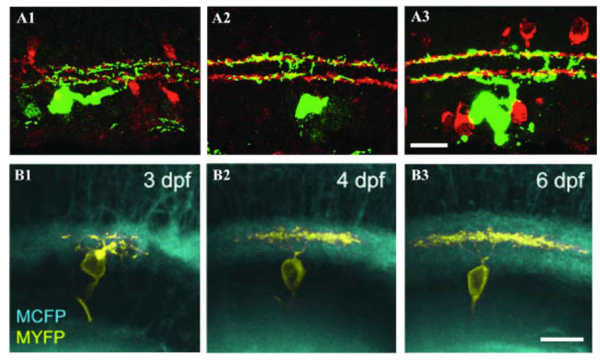 Figure 8: Dendrites of RGCs can reach mature stratified pattern by direct targeting. A1, A2 and A3: Retinal sections from P5, P8, and P12-P13 BD mice, respectively. RGCs were labeled with anti-GFP (green) and starburst amacrines with anti-ChAT (red). The BD RGC forms the distinct bistratified dendritic distribution pattern early during postnatal development at P5 (A1) without an initial diffuse distribution pattern. The bistratified distribution pattern remains throughout the postnatal development to adulthood (A2 and A3). Scale bar in A3, 20 um. (Adapted from Kim et al., 2010). B1, B2 and B3: In vivo time-lapse image of an isolated immature RGC (MYFP) in the Q01 background (MCFP) transgenic zebrafish retina. Time-lapse images taken at 3, 4 and 6 dpf, respectively, show orthogonally oriented image stacks of the entire arbor of the RGC. This RGC directly elaborates its dendrites targeting the inner half of the IPL that later became monostratified, occupying a single stratum in the middle of the IPL. Scale bar in B3, 10 um. (Adapted from Mumm et al., 2006).
Figure 8: Dendrites of RGCs can reach mature stratified pattern by direct targeting. A1, A2 and A3: Retinal sections from P5, P8, and P12-P13 BD mice, respectively. RGCs were labeled with anti-GFP (green) and starburst amacrines with anti-ChAT (red). The BD RGC forms the distinct bistratified dendritic distribution pattern early during postnatal development at P5 (A1) without an initial diffuse distribution pattern. The bistratified distribution pattern remains throughout the postnatal development to adulthood (A2 and A3). Scale bar in A3, 20 um. (Adapted from Kim et al., 2010). B1, B2 and B3: In vivo time-lapse image of an isolated immature RGC (MYFP) in the Q01 background (MCFP) transgenic zebrafish retina. Time-lapse images taken at 3, 4 and 6 dpf, respectively, show orthogonally oriented image stacks of the entire arbor of the RGC. This RGC directly elaborates its dendrites targeting the inner half of the IPL that later became monostratified, occupying a single stratum in the middle of the IPL. Scale bar in B3, 10 um. (Adapted from Mumm et al., 2006).
It is also clear that some RGCs form their lamina-restricted dendritic patterns through both direct targeting and selective dendritic pruning (Fig 9). In Fig. 9, A1-A3, the dendritic trees of a subtype of RGCs are diffusely ramified with many side branches originally and become bistratified to two strata above and below the cholinergic starburst type a cell with significant pruning of their dendritic branches (Kim et al., 2010). Similarly in Figure 9 B1-B3, a Zebrafish RGC starts its dendrites in the inner strata of the IPL and then selectively prunes the dendrites in the inner strata and grows the dendrites in the outer strata of the IPL over time (Mumm et al., 2006).
Regulation of the formation of lamina-restricted dendritic patterns of RGCs
The regulatory mechanisms for the formation of the lamina-restricted dendritic patterns of RGCs are not completely understood. It has been reported that many molecular cues play crucial roles in the formation of laminar-restricted dendritic pattern of some subtypes of RGCs. The immunoglobulin superfamily adhesion molecules, DSCAMs and sidekicks, have been reported to direct laminar-specific axonal and dendritic ramification of bipolar cells and RGCs in chick retina (Yamagata and Sanes, 2008) and RGC neurite arborization and mosaic formation in mouse retina (Fuerst et al. 2008). The transmembrane semaphorin Sema6A and its receptor PlexinA4 (PlexA4) have also been reported to control the stratification of the dendrites of dopaminergic amacrine cells, melanopsin containing RGCs and calbindin-positive cells into ON and OFF sublaminae of the IPL in mouse retina (Matsuoka et al., 2011a). Fig 10A shows that transmembrane semaphorin Sema5A and Sema5B normally constrain dendritic targeting of melanopsin-expressing RGCs to the IPL. In Sema5A-/- and Sema5B-/- mice the RGCs exhibit aberrant dendritic branching in INL, OPL and ONL (Fig. 10B, 10C and 10G).
Several reports have also shown that both spontaneous synaptic activity mediated by glutamate receptor (GluR) before eye opening and light evoked retinal activity after eye opening regulate the normal development of the lamina-restricted dendritic patterns of RGCs. In an early developing vertebrate retina like mouse, RGCs fire periodic bursts of action potentials that are highly correlated and propagate across the RGC layer in a wave-like fashion (Wong 1999). These spontaneous retinal waves are mainly mediated by cholinergic and glutamatergic synaptic transmission (Bansal et al. 2000; Demas et al. 2003; Feller and Blankenship 2008; Feller et al. 1996; Xu et al., 2010; Zhou 2001) (see chapter by Ford and Feller, Webvision). The retinal wave mediated by AChR seems to have little effect on the formation of laminar-restricted dendritic pattern of RGCs. In mice, genetic deletion of β2 subunits of nAChR or the sole synthetic enzyme for acetylcholine, choline acetyltransferase, eliminates the retinal waves mediated by nAChRs and causes an insignificant or non detectable change of the development of the lamina-restricted dendritic ramification of RGCs (Bansal et al. 2000; Stacy et al., 2005).
On the other hand, intraocular injection of APB, an agonist for class III metabotropic GluRs (mGluR6), results in a blockade of glutamate release from ON and rod bipolar cells and causes an arrest of the developmental stratification and segregation of RGC dendrites into ON and OFF synaptic pathways in cats, ferrets and rats (Bodnarenko and Chalupa, 1993; Bodnarenko et al., 1995; 1999; Deplano et al., 2004) (Fig 11).
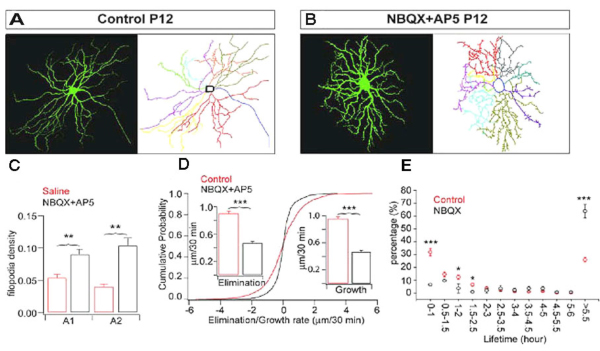
Also, intraocular injection of antagonists for NMDA and AMPA receptors, AP5 and NBQX, increases the density of filopodia by more than 100% after 5 days of treatment in mice (see Fig 12, compare A and B). Xu et al. (2010) showed that pharmacological blockade of GluR-mediated activity reduces the kinetic of RGC dendritic growth and elimination by approximately 50% (Fig. 12D). The disrupted GluR-mediated activity in retina during early postnatal development is associated with profound and permanent defects of RGC dendritic morphology and synaptic function in adults (Xu et al., 2010. Similarly, Lau et al. (1992) showed that blockade of NMDA receptors before eye opening increases the spine density of RGCs in hamsters.
However, genetic blockade of glutamate release from ON bipolar cells eliminates spontaneous and light evoked synaptic inputs to ON RGCs without effect on the spontaneous and light evoked synaptic activity of OFF RGCs and causes no detectable effect on the lamina-restricted dendritic ramification of either ON or OFF RGCs (Kerschensteiner et al., 2009). In addition, genetic deletion of the mGluR6 receptor, which blocks ON bipolar cell light evoked synaptic activity, failed to impair dendritic stratification of mouse RGCs (Tagawa et al., 1999). Therefore, the effect of GluR-mediated synaptic activity on the development of the lamina-restricted dendritic ramification and synaptic connections of RGCs is somewhat controversial and needs to be further investigated. The effect of light evoked synaptic activity on the development of RGC dendritic restriction and synaptic connection seems to vary among subtypes of RGCs and selective to some synaptic features. Morphologically, dark rearing blocks an age-dependent remodeling of dendritic complexity of a class of “aberrant” RGCs in hamster retina (Wingate and Thompson 1994). In mice, light deprivation increases the density of conventional synapses in the IPL (Fisher 1979a). The developmental ramification of RGC dendrites into OFF lamina of the IPL is selectively impaired by light deprivation in RGCs of mouse retina (Xu and Tian, 2007). Functionally, light deprivation blocks the surge of spontaneous synaptic inputs to RGCs and an age-dependent increase of inner retinal light responses measured by ERG oscillatory potentials (Tian and Copenhagen 2001; Vistamehr and Tian, 2004), the segregation of RGC synaptic inputs from ON and OFF synaptic pathways (Xu and Tian, 2007), and the maturation of the size of inhibitory receptive field of RGCs (Di Marco et al., 2009).
However, light deprivation seems preferentially to affect the maturation of dendrites of OFF RGCs, but not ON RGCs. Xu and Tian (Xu and Tian, 2007) quantitatively analyzed the developmental refinement of the dendrites of a random group of RGCs in mouse retina and determined the ramification depth and width of RGC dendrites in the IPL at different postnatal ages (Fig. 13).
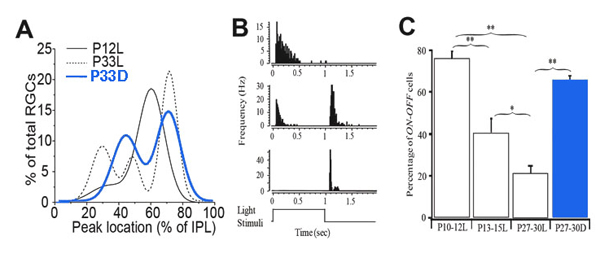
They showed that a large proportion of RGCs have a single layer of narrowly stratified dendritic plexus ramifying near the centre of the IPL before eye opening (P12), where they could synapse with both ON and OFF bipolar cells. After eye opening, a significant portion of RGCs redistribute their dendrites from the centre of the IPL toward the inner and outer borders of the IPL (Fig. 14A). This laminar-specific redistribution of RGC dendrites is associated with an age-dependent decrease of the number of RGCs receiving synaptic inputs from both ON and OFF bipolar cells (Fig. 14C). In dark reared mice, the RGC dendritic redistribution from the centre of the IPL to sublamina a of the IPL is blocked, which results in a significant increase of the number of RGCs ramifying at the center of the IPL, and a decrease of the number of RGCs ramifying only in sublamina a, in comparison with age-matched controls (Fig. 14A). Physiologically, the number of RGCs responding to both the onset and the offset of light stimulation of mice raised in constant darkness from birth to the ages of P27-30 was 4-fold higher than that of age-matched controls raised in cyclic light, but comparable to the percentage of ON-OFF responsive RGCs of P10-12 mice (Tian & Copenhagen, 2003) (Fig. 14C). Similarly, long-term treatment of cat eyes with intraocular injection of APB significantly reduced the number of αRGCs ramifying in the sublamina a and increased the number of multistratified α cells (Deplano et al., 2004).
The possible mechanisms of developmental regulation of RGC dendrites
During developmental refinement, the dendritic arborizations of RGCs undergo dynamic elaboration, maintenance or elimination to attain their lamina-restricted ramification pattern. Although neuronal activity influences this remodeling in many subtypes, the underlying molecular mechanisms have not yet been identified. Several studies suggest that calcium is important to link the neuronal activity with dendritic growth and patterning (Wong and Ghosh, 2002). Thus, it has been reported that synaptic stimulation induces calcium influx through voltage-dependent calcium channels and is sufficient to activate a transcriptional program that regulates dendritic growth (Redmond et al., 2002).
BDNF/TrkB has also been shown to play an essential role in the activity-dependent development of RGC dendrites (Landi et al., 2007). Activation of BDNF promotes the anatomical segregation of the dendrites of ON- and OFF-center RGCs in different sublaminae of the IPL (Liu et al., 2007; Landi et al., 2007), while deletion of TrkB strongly inhibits visual experience-dependent refinement of RGC dendrites (Liu et al., 2007). In addition, the expression of BDNF in the retina is up-regulated by visual stimulation (Pollock et al., 2001; Seki et al., 2003; Mandolesi et al., 2005; Landi et al., 2007). This suggests that light deprivation retards RGC dendritic maturation by reduction of the expression of BDNF. Conversely, over-expression of BDNF precludes the retardation of laminar refinement in dark reared mice (Liu et al., 2007).
Recent studies demonstrated that genes typically associated with the immune system, such as those in the major histocompatibility complex (MHC), are expressed by neurons in various regions of the CNS, including retina, and play important roles in synapse formation and activity-dependent synaptic plasticity (Baudouin et al., 2008; Corriveau et al., 1998; Huh et al., 2000; Ishii et al., 2003; Syken and Shatz, 2003; Syken et al., 2006; Xu et al., 2010). Genetic deletion or mutation of a number of MHC class I genes result in the failure of eye-specific segregation of RGC axon projections to the dosal lateral geniculat nucleus (dLGN) (Huh et al., 2000; Xu et al., 2010). Also, long-term potentiation, long-term depression, learning, memory, and neurogenesis in hippocampus are impaired (Huh et al., 2000; Ziv et al., 2006).
Xu et al. (2010) reported that the key component of MHCI receptor, CD3ζ is specifically expressed by RGCs in mouse retina. Similar to the pharmacological blockade of GluR-mediated activity, genetic mutation of CD3ζ profoundly reduces the kinetics of RGC dendritic growth and pruning and impairs the lamina-specific segregation of RGC dendrites in the IPL. In addition, CD3ζ-/- mice show a selective reduction of GluR-mediated synaptic transmission in RGCs suggesting that CD3ζ-mediated signaling participates in activity-dependent synaptic maturation of RGCs. However, some of the important questions, such as what are the exact molecule mechanisms with which activation of MHC/CD3ζ on neurons affects the maturation of RGC dendrites, and how MHC/CD3ζ-mediated signaling interacts with neurotransmitter-mediated synaptic activity in dendritic maturation, need to be further addressed.
References
Altshuler D, Turner D, Cepko C. 1991. Specification of cell types in the vertebrate retina. In Development of the Visual System (D. Lam and C. Shatz, eds) Boston, MIT Press, pp37-58.
Badea TC, Nathans J. 2004. Quantitative analysis of neuronal morphologies in the mouse retina visualized by using a genetically directed reporter. J Comp Neurol 480:331-351. [PubMed]
Bansal A, Singer JH, Hwang BJ, Xu W, Beaudet A, Feller MB. 2000. Mice lacking specific nicotinic acetylcholine receptor subunits exhibit dramatically altered spontaneous activity patterns and reveal a limited role for retinal waves in forming ON and OFF circuits in the inner retina. J Neurosci 20:7672-81. [PubMed]
Baudouin SJ, Angibaud J, Loussouarn G, Bonnamain V, Matsuura A, Kinebuchi M, Naveilhan P, Boudin H. 2008. The signaling adaptor protein CD3ζ is a negative regulator of dendrite development in young neurons. Mol Biol Cell 19:2444-2456. [PubMed]
Berson DM 2008. Retinal ganglion cell types and their central projections. In: The senses: a comprehensive reference (Basbaum AI, Kaneko A, Shepherd GM and Westheimer G, eds), San Diego: Academic. Vol 1, pp 491-520.
Bodnarenko SR, Chalupa LM. 1993. Stratification of ON and OFF ganglion cell dendrites depends on glutamate-mediated afferent activity in the developing retina. Nature 364:144-6. [PubMed]
Bodnarenko SR, Jeyarasasingam G, Chalupa LM. 1995. Development and regulation of dendritic stratification in retinal ganglion cells by glutamate-mediated afferent activity. J Neurosci 15:7037-45. [PubMed]
Bodnarenko SR, Yeung G, Thomas L, McCarthy M. 1999. The development of retinal ganglion cell dendritic stratification in ferrets. Neuroreport 10:2955-9. [PubMed]
Boycott, B. B. & WŠssle, H. Morphological classification of bipolar cells of the primate retina. Eur. J. Neurosci. 3, 1069-1088 (1991). [PubMed]
Cepko CL, Austin CP, Yang X, Alexiades M, Ezzedine D. 1996. Cell fate determination in the vertebrate retina. Proc Natl Acad Sci USA 93:589-95. [PubMed]
Cohen, E. & Sterling, P. Demonstration of cell types among cone bipolar neurons of the cat retina. Phil. Trans. R. Soc. Lond. B Biol. Sci. 330, 305-321 (1990). [PubMed]
Coombs J, Chalupa LM. 2008. Morphological, functional, and developmental properties of mouse retinal ganglion cells. In Eye, Retina, and Visual System of the Mouse. Chalupa, editor. MIT, pp189-199.
Coombs J, van der List D, Wang GY, Chalupa LM. 2006. Morphological properties of mouse retinal ganglion cells. Neuroscience 140:123-136. [PubMed]
Coombs JL, Van Der List D, Chalupa LM. 2007. Morphological properties of mouse retinal ganglion cells during postnatal development. J Comp Neurol 503:803- 814. [PubMed]
Corriveau RA, Huh GS, Shatz CJ. 1998. Regulation of class I MHC gene expression in the developing and mature CNS by neural activity. Neuron 21:505-520. [PubMed]
Dacey DM, Packer OS. 2003. Colour coding in the primate retina: diverse cell types and conespecific circuitry. Curr Opin Neurobiol 13:421- 427. [PubMed]
Deplano S, Gargini C, Maccarone R, Chalupa LM, Bisti S. 2004. Long-term treatment of the developing retina with the metabotropic glutamate agonist APB induces long-term changes in the stratification of retinal ganglion cell dendrites. Dev Neurosci 26: 396-405. [PubMed]
Demas J, Eglen SJ, Wong RO. 2003. Developmental loss of synchronous spontaneous activity in the mouse retina is independent of visual experience. J Neurosci 23:2851-2860. [PubMed]
Diao L, Sun W, Deng Q, He S. 2004. Development of the mouse retina: Emerging morphological diversity of the ganglion cells. J Neurobiol 61:236-249. [PubMed]
Euler, T. & WŠssle, H. Immunocytochemical identification of cone bipolar cells in the rat retina. J. Comp. Neurol. 361, 461-478 (1995). [PubMed]
Famiglietti EV Jr, Kolb H 1976. Structural basis for ON-and OFF-center responses in retinal ganglion cells. Science 194:193-195. [PubMed]
Feller MB, Blankenship AG. 2008. The function of the retina prior to vision: The phenomenon of retinal waves and retinotopic refinement. In Eye, Retina, and Visual System of the Mouse. Chalupa, editor. MIT, pp343351.
Feller MB, Wellis DP, Stellwagen D, Werblin FS, Shatz CJ. 1996. Requirement for cholinergic synaptic transmission in the propagation of spontaneous retinal waves. Science 272:1182-1187. [PubMed]
Fisher LJ. 1979a. Development of retinal synaptic arrays in the inner plexiform layer of dark reared mice. J Embryol Exp Morph 54:219-27. [PubMed]
Fisher LJ. 1979b. Development of synaptic arrays in the inner plexiform layer of neonatal mouse retina. J Comp Neurol 187:359-372. [PubMed]
Fuerst PG, Koizumi A, Masland RH, Burgess RW. 2008. Neurite arborization and mosaic spacing in the mouse retina require DSCAM. Nature 451:470-4. [PubMed]
Ghosh KK, Bujan S, Haverkamp S, Feigenspan A, Wassle H. 2004. Types of bipolar cells in the mouse retina. J Comp Neurol 469:70-82. [PubMed]
Gorfinkel J, Lachapelle P, Molotchnikoff S. 1988. Maturation of the electroretinogram of the neonatal rabbit. Doc Ophthalmol 69:237-45. [PubMed]
Huh GS, Boulanger LM, Du H, Riquelme PR, Brotz TM, Shatz CJ. 2000. Functional requirement for class I MHC in CNS development and plasticity. Science 290:2155-2159. [PubMed]
Ishii T, Hirota J, Mombaerts P. 2003. Combinatorial coexpression of neural and immune multigene families in mouse vomeronasal sensory neurons. Cur Biol 13:394-400. [PubMed]
Kerschensteiner D, Morgan JL, Parker ED, Lewis RM, Wong RO. 2009. Neurotransmission selectively regulates synapse formation in parallel circuits in vivo. Nature 460:1016-20. [PubMed]
Kim IJ, Zhang Y, Meister M, Sanes JR. 2010. Laminar restriction of retinal ganglion cell dendrites and axons: subtype-specific developmental patterns revealed with transgenic markers. J Neurosci. 30:1452-62. [PubMed]
Kolb, H., Nelson, R. and Mariani, A. 1981 Amacrine cells, bipolar cells and ganglion cells of the cat retina: a Golgi study. Vision Res., 21 : 1081-1114. [PubMed]
Kolb, H., Linberg, K. A. and Fisher, S. K. 1992 The neurons of the human retina: a Golgi study. J. Comp. Neurol. 318 : 147-187. [PubMed]
Kong JH, Fish DR, Rockhill RL, Masland RH. 2005. Diversity of ganglion cells in the mouse retina: unsupervised morphological classification and its limits. J Comp Neurol 489:293-310. [PubMed]
Kuffler SW. 1953. Discharge patterns and functional organization of mammalian retina. J Neurophysiol 16:37- 68. [PubMed]
Landi S, Cenni MC, Maffei L, Berardi N. 2007. Environmental enrichment effects on development of retinal ganglion cell dendritic stratification require retinal BDNF. PLoS ONE 2:e346. [PubMed]
Lau KC, So KF, Tay D. 1992. APV prevents the elimination of transient dendritic spines on a population of retinal ganglion cells. Brain Res 595:171-4. [PubMed]
Liu X, Grishanin RN, Tolwani RJ, Renteria RC, Xu B, Reichardt LF, Copenhagen DR. 2007. Brain-derived neurotrophic factor and TrkB modulate visual experience-dependent refinement of neuronal pathways in retina. J Neurosci 27:7256-7267. [PubMed]
MacNeil, M.A. and Masland, R. H. 1998. Extreme diversity among amacrine cells: Implications for function. Neuron 29: 971-982. [PubMed]
Mandolesi G, Menna E, Harauzov A, von Bartheld CS, Caleo M, Maffei L. 2005. A role for retinal brain-derived neurotrophic factor in ocular dominance plasticity. Curr Biol 15:2119-2124. [PubMed]
Marquardt T, Gruss P. 2002. Generating neuronal diversity in the retina: one for nearly all. Trends Neurosci 25:32-8. [PubMed]
Masland RH. 2001. The fundamental plan of the retina. Nat Neurosci 4:877-86. [PubMed]
Maslim J, Stone J. 1988. Time course of stratification of the dendritic fields of ganglion cells in the retina of the cat. Dev Brain Res 44:87-93. [PubMed]
Matsuoka RL, Nguyen-Ba-Charvet KT, Parray A, Badea TC, ChŽdotal A, Kolodkin AL. 2011a. Transmembrane semaphorin signalling controls laminar stratification in the mammalian retina. Nature 470:259-263. [PubMed]
Matsuoka RL, Chivatakarn O, Badea TC, Samuels IS, Cahill H, Katayama K, Kumar SR, Suto F, ChŽdotal A, Peachey NS, Nathans J, Yoshida Y, Giger RJ, Kolodkin AL. 2011b. Class 5 transmembrane semaphorins control selective Mammalian retinal lamination and function. Neuron, 71:460-73. [PubMed]
Mu X, KleinWH. 2004. A gene regulatory hierarchy for retinal ganglion cell specification and differentiation. Semin Cell Dev Biol 15:115-123. [PubMed]
Mumm JS, Willams PR, Godinho L, Koerber A, Pittman AJ, Roeser T, Chien CB, Bailer H, Wong ROL. 2006. In vivo imaging reveals dendritic targeting of laminated afferents by zebrafish retinal ganglion cells. Neuron 52: 609-621. [PubMed]
Nassi JJ, Callaway EM. 2009. Parallel processing strategies of the primate visual system. Nat Rev Neurosci 10:360-72. [PubMed]
Nelson, R., Famiglietti, E.V. and Kolb, H. 1978 Intracellular staining reveals different levels of stratification for on-center and off-center ganglion cells in the cat retina. J. Neurophysiol., 41 : 472-483. [PubMed]
Nishimura Y, Rakic P. 1987. Synaptogenesis in the primate retina proceeds from the ganglion cells towards the photoreceptors. Neurosci Res Suppl 6:S253-68. [PubMed]
Pollock GS, Vernon E, Forbes ME, Yan Q, Ma YT, Hsieh T, Robichon R, Frost DO, Johnson JE. 2001. Effects of early visual experience and diurnal rhythms on BDNF mRNA and protein levelsin the visual system, hippocampus, and cerebellum. J Neurosci 21:3923-3931. [PubMed]
Redmond L, Kashani AH, Ghosh A. 2002. Calcium regulation of dendritic growth via CaM kinase IV and CREB-mediated transcription. Neuron 34:999-1010. [PubMed]
Rockhill RL, Daly FJ, MacNeil MA, Brown SP, Masland RH. 2002. The diversity of ganglion cells in a mammalian retina. J Neurosci 22:3831-43. [PubMed]
Schiller PH. 2010. Parallel information processing channels created in the retina. Proc Natl Acad Sci U S A; 107(40): 17087-17094. [PubMed]
Seki M, Nawa H, Fukuchi T, Abe H, Takei N. 2003. BDNF is upregulated by postnatal development and visual experience: quantitative and immunohistochemical analyses of BDNF in the rat retina. Invest Ophthalmol Vis Sci 44:3211-3218. [PubMed]
Stacy RC, Demas J, Burgess RW, Sanes JR, Wong RO. 2005. Disruption and recovery of patterned retinal activity in the absence of acetylcholine. J Neurosci 25:9347-9357. [PubMed]
Stone J, Maslim J, Rapaport D. 1984. The development of the topographical organization of the cat’s retina. In: Development of Visual Pathways in Mammals (J. Stone, B. Breher, D.H. Rapaport, eds) New York, Alan R. Liss, pp1-21.
Sun W, Li N, He S. 2002. Large-scale morophological survey of rat retinal ganglion cells. Vis Neurosci 19:483-93. [PubMed]
Syken J, Shatz CJ. 2003. Expression of T cell receptor _ locus in central nervous system neurons. Proc Natl Acad Sci USA 100:13048-13053. [PubMed]
Syken J, GrandPre T, Kanold PO, Shatz C. 2006. PirB restricts ocular-dominance plasticity in visual cortex. Science 313:1795-1800. [PubMed]
Tagawa Y, Sawai H, Ueda Y, Tauchi M, Nakanishi S. 1999. Immunohistological studies of metabotropic glutamate receptor subtype 6-deficient mice show no abnormality of retinal cell organization and ganglion cell maturation. J Neurosci 19:2568-2579. [PubMed]
Tian N, Copenhagen DR. 2001. Visual deprivation alters development of synaptic function in inner retina after eye opening. Neuron 32:439-43. [PubMed]
Tian N, Copenhagen DR. 2003. Visual stimulation is required for refinement of ON and OFF pathways in postnatal retina. Neuron 39:85-96. [PubMed]
Tootle JS. 1993. Early postnatal development of visual function in ganglion cells of the cat retina. J Neurophysiol 69:1645-60. [PubMed]
Vistamehr S, Tian N. 2004. Light deprivation suppresses the light response of inner retina in both young and adult mouse. Vis Neurosci 21:23-37. [PubMed]
Volgyi B, Chheda S, Bloomfield SA. 2009. Tracer coupling patterns of the ganglion cell subtypes in the mouse retina. J Comp Neurol 512:664-687. [PubMed]
Wachtmeister L. 1998. Oscillatory potentials in the retina: what do they reveal. Prog Retin Eye Res 17:485-521. [PubMed]
Wang GY, Liets LC, Chalupa LM. 2001b. Unique functional properties of on and off pathways in the developing mammalian retina. J Neurosci 21:4310-17. [PubMed]
Wässle H. 2004. Parallel processing in the mammalian retina. Nat Rev Neurosci 5:747-57. [PubMed]
Wingate RJT, Thompson ID. 1994. Targeting and activity-related dendritic modification in mammalian retinal ganglion cells. J Neurosci 14:6621-37. [PubMed]
Wong ROL. 1999. Retinal waves and visual system development. Annu Rev Neurosci 22:29-47. [PubMed]
Wong ROL, Ghosh A. 2002. Activity-dependent regulation of dendritic growth and patterning. Nat Rev Neurosci 3:803-12. [PubMed]
Xu HP, Tian N. 2004. Pathway-specific maturation, visual deprivation, and development of retinal pathways. Neuroscientist 10:337-346. [PubMed]
Xu HP, Tian N. 2007. Retinal ganglion cell dendrites undergo a visual activity-dependent redistribution after eye-opening. J Comp Neurol 503:244-259. [PubMed]
Xu HP, Chen H, Ding Q, Xie ZH, Chen L, Diao L, Wang P, Gan L, Crair MC, Tian N. 2010. Immune molecule, CD3ζ is required for the development of neural circuits in retina. Neuron 65:503-515. [PubMed]
Yamagata M, Sanes JR. 2008. Dscam and Sidekick proteins direct lamina-specific synaptic connections in vertebrate retina. Nature 451:465-9. [PubMed]
Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, Cohen H, Kipnis J, Schwartz M. 2006. Immune cells contribute to the maintenance of neurogenesis and spatial leaning abilities in adulthood. Nature Neurosci 9:268-275. [PubMed]
Zhou ZJ. 2001. The function of the cholinergic system in the developing mammalian retina. Progress in Brain Research 131:599-613. [PubMed]
About the author
Dr. Ning Tian was initially trained as a physician in China (Yichang Medical School) and then received his Master Degree in clinical visual physiology from Zhong-sen Ophthalmic Center, Sun Yat-sen University of Medical Sciences, China. He practiced clinical ophthalmology for a while before doing a PhD in Biophysics and Physiology at the State University of New York at Buffalo with Dr. Malcolm Slaughter. Ning then did a postdoc with Dr. David Copenhagen at University of California, San Francisco. After being an Assistant Research Ophthalmologist at the University of California, San Francisco from 1998-2000, he headed a laboratory at Yale University (2000-2009). Ning is presently an Associate Professor of Ophthalmology and Neurobiology in the Moran Eye Center, University of Utah. His research is focused on understanding the cellular and molecular mechanisms that regulate the maturation of retinal ganglion cell synaptic function and dendritic structure. E-mail: Ning.Tian@hsc.utah.edu