Steve Fisher, Geoffrey P. Lewis, Kenneth A Linberg, Edward Barawid and Mark V. Verardo
1. Introduction.
What is retinal detachment?
The retina is firmly attached to the apical surface of the retinal pigmented epithelium, or RPE (see earlier retinal anatomy sections). When the retina is separated from its normal position apposed to the RPE surface, it is said to be “detached.” This detachment creates a pathological, fluid-filled space between the neural retina and the retinal pigmented epithelium. It also creates a greater distance between the photoreceptors and their sole blood supply, the choroidal circulation.
Clinical retinal detachments occur as different types
There are three recognized types of retinal detachment in clinical practice:1) Rhegmatogenous , the most common type. In this form the retina experiences a physical tear through the retinal layers and the torn retina peals away from the retinal pigmented epithelium by the movement of fluid into the space between the two. 2) Tractional , in which some force (usually contracting cells or vitreal “strands”) acts on the surface of the retina to pull it away from the retinal pigmented epithelium. 3) Exudative , in which fluid accumulates between the neural retina and the retinal pigmented epithelium pushing the two apart; the retinal tissue is not torn.
Retinal detachment can cause permanent visual loss or permanent reduction of visual function, especially if the macula is involved, in which case it is considered a medical emergency in the United States. For more information on the types and causes of retinal detachment see the National Eye Institute’s website at: http://www.nei.nih.gov/health/retinaldetach/index.asp#2
Cellular remodeling in the retina
Many specific neural circuits have been identified in the retina (Dowling, 1970; Kolb & Famiglieti, 1976; Linberg et al., 2001b; Kolb et al., 2001) and only recently has evidence been found that these circuits change (remodel) in an adult mammal, usually in response to injury and disease. Prior to this the retina was considered a “hard-wired” part of the central nervous system by most scientists. A change in circuitry may mean a change in vision, so understanding these changes and ways to prevent them or repair them is important.
Some examples of retinal remodeling
The few earlier descriptions of cellular remodeling in vertebrate retina came from studies of fish retinas (Wagner, 1975; Wagner & Ali; 1977; Wagner, 1980) where specific synaptic connections between photoreceptors and 2 order neurons structurally changed with the daily lighting cycle. In 1984, Peichl and Bolz described structural remodeling of retinal neurons in mammals in response to severe retinal degeneration induced by a neuro-toxin, kainic acid. It was nearly a decade later that reports of cellular remodeling in mammalian retina in response to injury or disease began to appear with some regularity (Chu, Humphrey & Constable, 1993, Li et al., 1995; Lewis, Linberg & Fisher, 1998, Fariss et al., 2000). Even total photoreceptor cell loss had not been regarded as causing significant changes to the inner retina until that time.
Photoreceptor cell death differs among models
Many recent descriptions of structural remodeling in mammalian retina are from studies in humans or rodent species in which massive photoreceptor cell death is induced by light damage or genetic mutations (Marc et al., 2003). Retinal detachment provides information that complements those data because in most species there is not massive photoreceptor cell death after detachment. Another important distinction is that the earliest and most obvious damage induced by detachment, outer segment degeneration, is reversible by reattaching the retina. Retinal reattachment surgery probably induces its own remodeling of retinal circuits as recovery occurs but this has been less explored at the present time.
Retinal detachment and reattachment as experimental systems
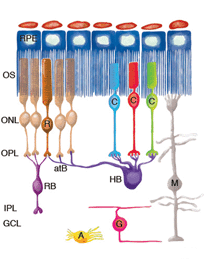
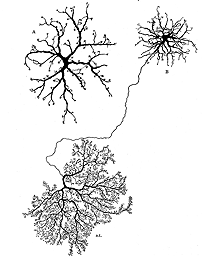
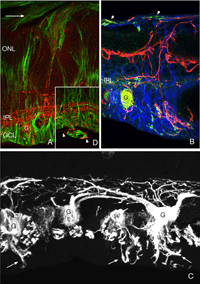
Figure 1 illustrates cell types observed to remodel after detachment, these include: RPE, Muller cells, photoreceptors, rod bipolar cells, horizontal cells, ganglion cells, and astrocytes. It seems likely that remodeling will be identified in other cell types as well.
Outer segment degeneration, photoreceptor cell death, Muller cell hypertrophy, and changes in the RPE apical surface were all recognized as events induced by detachment in early studies (Kroll and Machemer, 1968; Erickson et al., 1983; Anderson et al., 1983). More recently studies have demonstrated photoreceptor cell death occurs by apoptosis in both animals and humans. Currently there is no method for replacing lost photoreceptors. Other events may be reversed by reattachment, often incompletely and usually slowly –over a time-course that can vary from days to years.
Why remodeling is difficult to discover
The huge numbers of neurons and glial cells involved in the retina, the vast range of neuronal cell architectures, the small size of neuronal cell bodies relative to other cells, and the small size of the neuronal processes that intertwine to make up the plexiform layers of the retina, make discovery of subtle changes in these cells difficult. Historically, it was the Golgi impregnation method that provided the breakthrough allowing for a detailed description of individual neurons and their morphologic diversity (Ramon y Cajal, 1892). A similar reliable method that would allow us to observe changes in the branching of individual neurons would be ideal for studying remodeling. Unfortunately, the Golgi method is unreliable and quixotic, and therefore does not provide a method for the systematic study of events such a neuronal remodeling. What will undoubtedly emerge as technology evolves will be the invaluable tools for observing structural remodeling of retinal neurons in living tissue.
Making use of new technology to describe remodeling events
Immunocytochemistry and other techniques that allow for the labeling of individual cells or populations of cells coupled with advances in image technology such as laser scanning confocal microscopy, have provided us with new and powerful tools for describing remodeling events in the retina in recent years.Rod photoreceptor, rod bipolar, and horizontal cell remodeling (Lewis et al., 1998) was the first to be studied in detail with this technology. In fact, results with antibody labeling confirmed speculation based on EM data published in 1983: “In addition to the effects of retinal detachment in the outer retina, we strongly suspect that the inner nuclear layer, inner plexiform layer, ganglion cell layer and, perhaps, more central areas of the visual system may be affected as well, ” (Erickson et al., 1983). Remodeling has now been firmly established in the inner retina, and leads one to believe more strongly that central changes, for example ganglion cell axonal arborization and synaptic contacts will be eventually identified as well. Technologies involving other forms of imaging, including the imaging of living cells in retinal wholemounts or tissue slices, and techniques such as dye injection into single cells will undoubtedly contribute greatly to this rapidly growing knowledge base.
2. Levels of Remodeling.
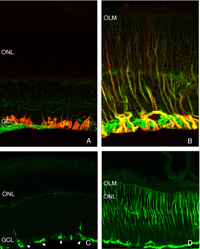
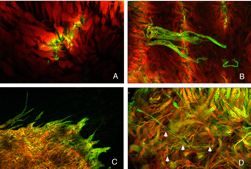
Remodeling can involve whole populations of cells, for instance, all RPE cells remodel their apical processes (Figs. 2A-C, asterisk) and all photoreceptors undergo outer segment degeneration after detachment, or it can involve some subset of cells within a population. The apical surface of every RPE cell must remodel in response to both detachment and reattachment as the complex apical processes are transformed into microvilli after detachment and then regenerated after reattachment. Interestingly, the remodeling after reattachment does not appear to be consistent from cell to cell (Figs. 2D-E). Thus, regeneration of the apical surface is not a perfect recapitulation of development. All Muller cells in the zone of detachment upregulate intermediate filament proteins, and all probably undergo some structural remodeling, but only some show extreme changes where they actually grow out of the retina, creeping into the subretinal space or onto the vitreal surface. Both of these conditions result in serious sight-threatening ophthalmic complications. Defining what stimulates this subpopulation to undergo such growth is medically important. Only a subpopulation of ganglion cells appears to remodel in response to detachment (Coblentz et al., 2003), and identifying which types may lead to a better understanding of some of the visual disturbances that occur after successful reattachment surgery.
Retinal deafferentation
Deafferentation in the CNS refers to removing the sensory input from some motor pathway. Although there is no “motor” component to the retina’s output to the brain, The massive loss of photoreceptors (usually approaching 100%) that occurs in some diseases such as retinitis pigmentosa (or in animal models of this disease) has been described as “deafferentation.” (see Marc et al., 2003). Remodeling in these systems and in such conditions as light damage in albino rats has been described as largely in response to this “deafferentation.” The changes that we are focused on here are those that occur within hours or days of detachment, because it is these changes that may be successfully manipulated by therapeutic intervention and thus lead to better visual recovery. These are not responses to massive death of photoreceptors although they may represent responses to the death of individual photoreceptors and therefore partial deafferentation of individual cells or neural circuits. Remodeling is also associated with the recovery phase after retinal reattachment, during a time when photoreceptor cell death does not occur.
Basic information relevant to understanding the responses to retinal detachment
Although the retina is a developmental outgrowth of the brain, it does have several features unique to its specialized functions. Photoreceptors have the highest metabolic rate of any cells in the body and yet there are no blood vessels among them (their presence presumably would blur the visual image)–they are nourished almost solely by the capillaries of the choroid which lie on the opposite side of the RPE (see Fig. 1; Linsenmeier & Padnick-Silver, 2000). Traditional astrocytes are not scattered throughout the retina as they are in the brain and spinal cord but reside only among the ganglion cells and their axons. The retina has a large population of highly differentiated, polarized radial glia, or Muller cells that may assume many of the functions of astrocytes in the brain and spinal cord, but are at the same time distinct from them. Ganglion cell axons in most species are not myelinated until after they enter the optic nerve, and thus, the retina does not have a population of oligodendrocytes, the myelin-producing glial cells. The retina does have a resident population of microglial (scavenger) cells but these appear to be restricted to the inner and outer plexiform layers in the healthy eye.
Animal models of detachment and reattachment
The choice of which species to use has been driven by many issues including: 1) How closely the retina structurally resembles that of humans. 2) Knowledge of retinal circuitry, physiology, or biochemistry in the species. 3) The reliability with which controlled detachments and reattachments can be created. 4) The specific goals of the study.
Different species react differently: finding the best model
Different species do react differently to detachment and not all of these accurately reflect what little we know about the reaction of the human retina. The rabbit retina (with about the same rod/cone ratio as the feline retina) exhibits very rapid and complete degeneration of much of the neural retina (Berglin et al., 1997; Faude et al., 2002), thus it is not a good model for longer-term events. The ground squirrel has a retina dominated by cones (Long & Fisher, 1983; Kryger et al., 1998), and thus is a potential model for the reactivity of the human macula (except that ground squirrel cones do not structurally resemble macular cones). The ground squirrel retina, however, shows a rapid and eventually complete degeneration of the photoreceptor layer, but almost no RPE or glial reactivity or neuronal remodeling (Linberg et al., 2002a; Sakai et al., 2001). Unlike the rabbit, it does not show inner retinal degeneration. We have used all of these in various studies, but the species that seems to most closely model what is known about events in human detachments is the common domestic cat. The retina of many primate species are foveated, but their use is often prohibitive for a variety of ethical and financial reasons.
Developing new model systems
Because of the availability of genetic information and the ability to readily do genetic manipulations, developing a reliable method for producing large, controlled detachments in the mouse eye has become a high priority in recent years (e.g., Nour et al., 2003; Yang et al., 2004). Current data from that model show many of the same reactions found in the feline model, although there may be less Muller cell reactivity. The height of a detachment, that is the distance separating the detached neural retina from the RPE, is probably an important parameter in human detachments and high detachments are harder to produce in mice because of the small size of the eye and the fact that the lens fills much of the vitreous cavity. Reattachments using the same procedures as in humans can be done in larger species, whether they will be possible in a mouse eye remains to be seen. Simply allowing the retina to settle instead of actively reattaching it provides one method, but with less precise control over the time of reattachment. Another promising area is the use of cold-blooded animals (e.g. frogs, fish, salamanders) in similar experiments because their retinas are relatively easy to maintain in culture, and because many of them have very large photoreceptor cells which are amenable for high-resolution imaging and other types of single-cell analyses. Currently most work is, however, being done with mammalian species.
The feline model
The feline retina, like the peripheral human retina, is rod-dominated. It is a species with a robust intraretinal circulation (as in all species, it is excluded from the photoreceptors). The cat retina has also been the subject of decades worth of anatomical and physiological studies. The feline eye is large, allowing for easy surgical access, and for the production of defined detachments and simple reattachments using the same procedures as in human patients.
Detachments are created by slowly infusing fluid into the interface between the RPE and neural retina through a tapered glass pipette with a tip diameter of about 100 µm (Lewis et al., 1999). It has been argued (Aaberg, 1999) that this does not model a rhegmatogenous detachment because there is no large tear (referred to as a “retinal break”) through the retinal tissue. However, our observations of tissue from human rhegmatogenous detachments and reattachments tend to validate the feline model as producing the same cellular responses (Sethi et al., 2004).
3. The Details of Cellular Remodeling after Detachment and Reattachment
Figure 1 shows the cell types that have been identified to date as undergoing remodeling after detachment and reattachment. We have only preliminary evidence for the structural remodeling of amacrine cells, although this has been described in late-stage human retinitis pigmentosa (Fariss et al., 2000). Little is known about changes in retinal astrocytes except that they proliferate after detachment. Retinal microglia respond robustly to detachment by assuming macrophage-like characteristics (Thanos et al., 1996; Lewis et al., 2005 in press). They will not be discussed here although they undoubtedly play an important role in the overall “injury response” of the retinal tissue.
Retinal pigmented epithelium
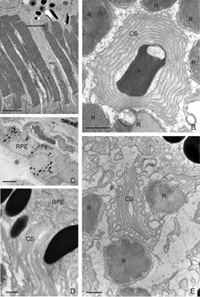
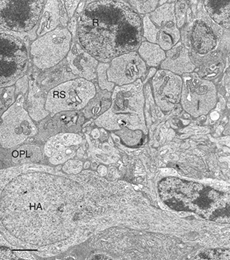
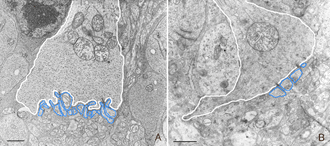
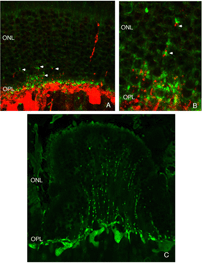
The morphology of the RPE changes stereotypically after detachment in all species studied. In species like cat, dog, rabbit, and human there are vast differences between the apical processes that interact with rods and cones (Anderson & Fisher, 1979; Steinberg et al., 1977; Steinberg & Wood, 1974; Fisher & Steinberg, 1982), with the “cone sheath” forming a unique structure consisting of a highly complex, multi-layered array of sheets of apical processes (Figs. 2A, B). Regardless of their structure in the normal eye, these apical processes all disappear after detachment, and are replaced very quickly, probably within hours, by a fringe of simple microvillus-like processes (Immel, Negi & Marmor, 1986; Fig. 2C, asterisk). The RPE has the remarkable ability to re-form these elaborate apical processes after reattachment. In the case of the feline retina, this also means regenerating the highly complex cone sheaths. However, a month after reattachment these cone sheaths still do not appear “normal.” The presence of slightly truncated, often thickened or misaligned cone sheaths is almost always a clear indicator that the retina was detached at some earlier time (compare the structures labeled “CS” in Figs. 2A, B, D, E). The fact that the cone sheaths re-differentiate only in association with cone outer segments indicates some form of signaling mechanism retained in the adult retina that allows for the RPE to know when its apical surface is opposite a cone.
The RPE proliferates in response to detachment and this can result in a complete remodeling of the geometry of this layer. Newly proliferated RPE cells can migrate into the subretinal space where they assume complex geometric arrangements: single cells, long strands, or multiple layers with reversed apical-basal polarity (Anderson et al., 1981). If the basal surface faces the neural retina, photoreceptor outer segment regeneration does not occur after reattachment (Anderson et al., 1986). It is not known if photoreceptor function is compromised when outer segments regenerate in the presence of multiple layers of RPE.
Photoreceptor deconstruction, outer segment degeneration & cell death
In the feline model, and probably in the human retina, detachment sends all of the photoreceptors, both rods and cones within the zone of detachment, along a pathway of structural changes that we have termed “deconstruction” (Mervin et al., 1999). The outer and inner segment response appears to be the same in rods and cones, but the synaptic terminal responses differ. In both feline and human retinae it is clear that many cells survive detachment for very long periods of time. Deconstructive changes may occur as a mechanism to assure the cells’ survival under adverse environmental conditions, i.e. a means of saving metabolic energy. It is not clear why in some species (ground squirrel, rabbit) detachment leads to deconstruction and cell death of virtually all photoreceptors while in other species the majority survive for long periods of time.
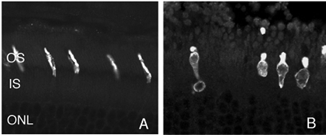
After detachment, outer segment material is lost until only a few disks remain along with the connecting cilium (Anderson et al., 1983). The complex mechanism of disc morphogenesis is not lost in these cells (Steinberg, Fisher & Anderson, 1980) because once reattached, they retain their ability to reconstruct an outer segment even though that reconstruction may not be perfect. Remaining rod photoreceptor outer segments, even if composed of only a few discs (Figs. 3A-C) are still positive for their respective opsins (Fig. 3; Fig. 4; Lewis et al., 1991; Fariss et al., 1997) as well as other proteins.
Death by apoptosis
Most photoreceptor cell death after detachment is by apoptosis (programmed cell death). Based on our results in the feline retina, there is an early period, around the first 3 days after detachment when about 20% of the photoreceptors die by apoptosis (Cook et al., 1995; Lewis et al., 2002), but retinas detached for 450 days can retain at least 50% of their photoreceptors (Erickson et al., 1983). Observations of human detachments also show that apoptosis is a part of their response to detachment, but as in the feline model, large portions of the photoreceptor population also survive (Chang et al., 1995). There is no information about the survivability of foveal photoreceptors after detachment. Cell death in the inner retina in response to detachment is very rarely observed in the feline model, although sporadic cell death may be important it is also difficult to document.
Internal reorganization of photoreceptor inner segments
After detachment, electron microscopy as well as immunocytochemical labeling for mitochondrial components shows a decrease in the number of mitochondria in the inner segments as well as a much less distinctive compartmentalization of all organelles (Anderson et al., 1983; Erickson et al., 1983; Mervin et al., 1999). Maintaining the highly compartmentalized, and polarized structure of a photoreceptor must be metabolically costly. Having the cells assume a much simpler organization may assure their survival under environmentally challenging conditions. In the case of detachment that challenge would include hypoxia and probably hypoglycemia that is created by physically moving the retina away from its choroidal blood supply (Mervin et al., 1999; Lewis et al., 1999; Linsenmeier & Padnick-Silver, 2000). This hypothesis is supported by studies in which providing increased environmental oxygen lessens photoreceptor deconstruction and cell death after detachment (Mervin et al., 1999; Sakai et al., 2001; Lewis et al., 2004).
A comparison of rod and cone responses
The outer and inner segment of rods and cones undergo similar structural changes but a prominent response of rods is the withdrawal of their axon (Figs. 5A,B) and a reconfiguration of the single synaptic invagination with its 3-5 postsynaptic processes (Boycott & Kolb, 1973; Kolb, 1974). This response is not observed in cones.
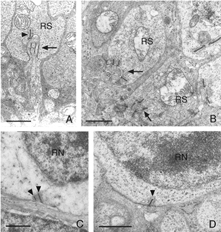
This disruption can be appreciated by immunocytochemical labeling with antibodies to presynaptic vesicle proteins, such as synaptophysin (Fig. 5) or VAMP (synaptobrevin). After detachment, labeled terminals appear near the rod nuclei scattered throughout the outer nuclear layer (Fig. 5B). Terminal withdrawal and reorganization begins within a day. The electron micrograph in Figure 6 shows the highly compact organization of the rod terminals in the outer plexiform layer in a normal feline retina. After detachment, the deep synaptic invagination in each becomes more shallow, (arrows, Figs. 7A, B), and is eventually lost altogether (Figs. 7C, D). There are still membrane specializations associated with the “postsynaptic” processes adjacent to the base of the retracted terminals (Figs. 7C, D). (Fig. 7B). Those terminals that do not retract may also change with a “looser” organization of postsynaptic processes (Fig. 7B).
Withdrawn rod terminals show a sparse population of synaptic vesicles and synaptic ribbons that vary more in size, configuration and location than expected. While there are one or two long, arc-shaped synaptic ribbons in normal feline rod spherules (Boycott & Kolb, 1973; Migdale et al., 2003), the presence of 1-3 shortened ribbons is common in the retracted terminals (arrowheads Fig. 7A, C, D).
Cone pedicles do not withdraw from the OPL. However, the pedicle itself undergoes unpredictable changes in shape. The axons of some become tortuous instead of streaming straight across the outer nuclear layer from their cell body (Figs. 8A-C). In all cases however, these terminals appear to lose the 9-14 synaptic invaginations (Boycott & Kolb, 1973) (Figs. 9A, B), giving their base a flattened appearance (Fig. 8C) (also see Erickson et al., 1983). Presynaptic ribbons change in both rods and conse
Presynaptic ribbons appear to grow shorter, and some may disappear (Figs. 9A,B). This ribbon response is dramatic when observed by immunocytochemical labeling using antibodies specific to them (Schmitz et al., 2000). Ribbons in rod terminals appear as “clumps” (compare the red colored synaptic ribbons in Fig. 10B and C to those in Fig. 10D). Within the cone pedicles the characteristic array of ribbons (arrowheads, Fig. 10C) is no longer detected in the outer plexiform layer (Fig. 10D). By electron microscopy, very short ribbons remain within the affected cone terminals, and some post-synaptic processes are still recognizable (Fig. 9).
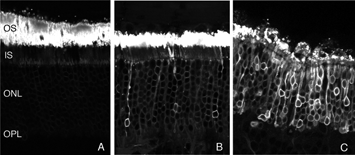
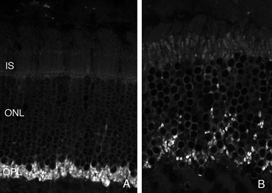
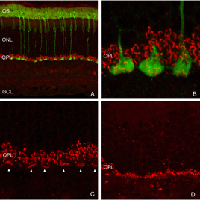
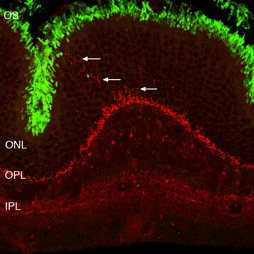
Following the remodeling of rod terminals has been made relatively simple because of their outlining by displaced rhodopsin. Documenting longer-term changes in cone synapses is complicated by the fact that the expression of proteins in the cones rapidly decreases to levels that are below detection (Rex et al., 2002a; Linberg et al., 2001a) making them difficult to visualize by immunocytochemistry. (Figs. 8 A, B, C,D). In figure 11 there are long expanses of the retina in which only the rod outer segments (green) are labeled by the anti-PDEg_and there are no labeled cones.
The population of cones is heterogeneous
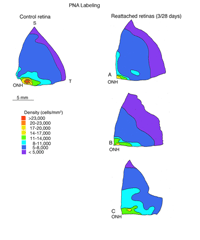
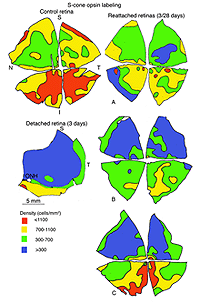
The lectin, peanut agglutinin (PNA), labels the extracellular matrix domain (matrix sheath) around cone photoreceptors (Johnson et al., 1986) and can be used to define the total population of cone cells. Figure 12A is a density map of PNA labeled cone sheaths in the superior temporal quadrant of a control (normal) feline retina (also see, Steinberg et al., 1973).
There are specific antibodies that recognize the different spectral classes of cone photoreceptors (Wang et al., 1992; Szel et al., 1985; 1988). The feline retina contains mid-wavelength sensitive (M) and short-wavelength sensitive (S) cones, and the latter are not distributed evenly over the retinal topography (Fig. 13A). Whereas the total number of cones peaks in the area centralis, the density of S-cones is highest in the inferior retina but even there they comprise only about 20% of the cone population (Linberg et al., 2001a).
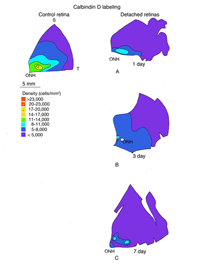
The expression of opsin genes reliably defines distinct subpopulations of photoreceptors. The expression of other proteins by these cells is less consistent. An antibody to the calcium binding protein calbindin D labels entire cone cells in many species, including humans and felines. The density of calbindin D positive cells matches very closely the density of PNA labeled cone matrix sheathes in the peripheral retina, but there is significant disparity in the area centralis where the antibody fails to label a majority of cones (Fig. 14A). This phenomenon also occurs in monkey and human retina where foveal cones do not label with anti-calbindin D (Rohrenbeck et al, 1989; Pasteels et al., 1990; Haley et al., 1995).
4. Protein expression in cone photoreceptors after detachment: analyzing the surviving cone photoreceptor array
What follows is a fairly detailed description of changes in the cone population after detachment and reattachment as summarized in Figures 12 and 13. For those who want to skip the details: cones appear to be lost from the retina after detachment because they 1) lose their outer segments and 2) lose the expression of specific proteins. Using the return of cone proteins as an indicator of cone recovery after reattachment shows that at 28 days after reattachment the cone mosaic is not the same as in normal retinas. Both types of cones recover, although the recovery of the S-cones may not be as complete as that of the M cones. The retinal cone mosaic is disturbed by detachment and inconsistencies in its recovery may account for visual defects after reattachment especially if the fovea is involved. There is nothing known about the recovery of the cone mosaics within the fovea.
As rod outer segments degenerate rod opsin labeling begins to increase in the plasma membrane of the cells until the whole cell is outlined (see Fig. 3; Lewis et al., 1991; Fariss et al., 1997; Rex et al. 2002a; Linberg et al., 2002a). Thus antibodies to rod opsin can be used as markers for the presence of rod cells (and the redistribution of labeling is also a remarkable indicator of stress or injury to them). While antibodies to the cone opsins begin to show a similar redistribution, (Fig. 4) after only 24h of detachment many cones fail to label with these antibodies (see Rex et al., 2002a). A similar phenomenon occurs in retinas of humans with late stage genetic degeneration (John et al., 2000).
Thus, markers for cones in the normal retina are not reliable for estimating the cone population that survive detachment (Linberg et al., 2001a). Indeed, if the lack of labeled cones after detachment was an accurate reflection of cone survival, then the effects of detachment on the cone population would be devastating (Linberg et al. 2001a). Further confounding the use of markers for cones is the fact that the response is not consistent from marker to marker nor even from one retinal region to another.
In the central region of a control retina, anti-calbindin D labels about 19,700 photoreceptors/mm2, and the antibody to S-cone opsin about 1,100/mm2. After 24 h of detachment these numbers drop to 9,000/mm2 (46%of control values) and 700/mm2 (63% of control values) respectively. By 28 days of detachment there were no cells labeled with the anti-calbindin D, but 200/mm2 (18% of control values) labeled with the anti-S-opsin (Linberg et al., 2001a and see Figs. 13B, 14B-D). In detached retinas there are large areas in which no labeling appears at all with the cone markers (see the example for PDEg in Figs. 10 and 11), as if all of the cones in the region were gone. These dramatic changes are reflected in the density maps for both S-opsin and anti-calbindin D labeling (Figs. 13, 14). In the latter, the central area is nearly unrecognizable by 1 day of detachment, and completely undefined after 3-days. Similarly, the number of S-cones drops from between 700-1100/mm2 in the control retina to less than 300 in a 3-day detachment. Because of the relatively large bins used to create these maps, they do not show the substantial islands in which there were no labeled cones. The wide variations in numbers of labeled cones gives the retinal wholemounts a “patchiness” that is not observed in control retinas where the transitions in cone density are smoothly graded.
We approached the question of cone survival in another way: by examining the recovery of cone markers after reattachment (Linberg et al., 2002b). We chose 3 days of detachment because there is already a significant drop in the cone population labeled with peanut agglutinin (PNA), anti-cone-opsin, or anti-calbindin D at that time. The superior retina in the right eye of three animals was detached for 3 days and then reattached for 28. The retinas were harvested and labeled with PNA (Fig. 12, PNA = all cones, only the superior temporal quadrant is illustrated) and the antibody to S-opsin (Fig. 13). Comparisons can be made to the maps for total cones (calbindin D labeling, control and 3 days, Figs. 14A, C; PNA labeling, control retina, Fig. 12A) and S-cones (S-cone opsin labeling, control and 3 days, Figs. 13 A, B). There is a recovery of both the PNA and S-cone population after reattachment; indeed a central-to-peripheral gradient is apparent in the PNA labeling pattern, although the density remains depressed. None of the animals recovered densities greater than 17,000-20,000 cones/ mm2 (Figs. 12B-D). Similarly, the S-cone population recovers, but recovery is not complete (Figs. 13C-E). The high density area of S-cones in the far periphery of the superior-temporal quadrant is not recovered at 28 days of reattachment, and in all three animals, recovery in the central retina was in the range of 300-700 cells/mm2, compared to the 700-1100 cells/ mm2 observed in the control retina (the detachment did not extend into the yellow/red colored area in the central area of figure 13E).
Because these numbers are generated as counts from small sampling areas, and the contour lines on the maps drawn by eye, it is difficult to choose an average value for cone density within the reattached retina, but using estimates of cone density sampled over a fairly broad region, results from two animals with reattachments show a recovery of 40-60% of PNA labeling in the area centralis and approaching 100% recovery in the periphery.
S-cone recovery seems more variable, ranging between zero and 40% with no pattern readily discernable across the retina. Whereas PNA labeled cone matrix sheaths were remarkably evenly distributed across the retina and showed a central to peripheral decline, S-opsin labeling showed large swatches within the reattached retinal map with no visible labeling. These data demonstrate quantitatively that the absence of marker molecules in the detached retinas does not indicate the absence of cone photoreceptors because cells recovered across the entire retina, in some cases to numbers comparable to those in the control eyes. It is unknown of course, if there would be more recovery of these proteins over a longer reattachment time.
Structurally, the S-cone outer segments in the 28-day reattached retina are not equivalent to those in the normal eye. They are often only punctate “dots,” a fraction of their normal size. The S-cone population has been reported to be more sensitive to damage in human retinas with detachments (Nork et al., 1995) with all S-cones either lost or showing signs of “irreversible damage,” within a few days of detachment. Our data from feline retina may support the concept that S cones are more fragile, more susceptible to cell death, and slower to recover than the M-cones, however, it does not support the conclusion that all of the S-cones are irreversibly damaged.
Combined electrophysiological (ERG) and immunocytochemical studies in the cone-dominant ground squirrel retina did not show any particular difference in recovery of signals from S- and M/L-cones in that species (Jacobs et al., 2003).
What may be the most remarkable conclusion from this and other studies, is that cones appear to stop expressing specific proteins when the cells are under some type of physiological stress, and then, at least in the case of reattachment, to begin re-expressing these proteins as part of a recovery process. In their studies of human retinal tissue, Nork et al. (1995) reported that carbonic anhydrase reactivity became an unreliable marker for M-cones after detachment, indicating that it may join the list of severely down-regulated proteins, and its loss may not be an indicator of irreversible damage.
Whether or not foveal cones show similar responses is an unanswered question. Peripheral cones are structurally different from foveal cones in the primate retina (Anderson et al., 1978; Anderson & Fisher, 1979) so their reaction to injury and their capacity for recovery may differ as well.
A differential loss of M/L- and S-cones, or a differential recovery of these cells in humans may explain some of the color vision deficiencies reported in reattachment patients, and could underlie losses in visual acuity when the fovea is involved.
5. Remodeling of photoreceptors after reattachment
The fact that photoreceptor outer segments re-grow after reattachment has been recognized since 1968 (Machemer, 1968; Kroll and Machemer, 1969a,b). This remarkable ability is mechanistically explained by the fact that photoreceptors constantly add new outer segment material as part of the outer segment renewal process (Young, 1967). Thus they are poised to re-build their outer segment as soon as favorable conditions allow.
Studies of detached retina in fact show that radiolabeled proteins continue to be transported into the truncated outer segments of rods that degenerate after detachment (Lewis et al., 1991), although studies of rod-opsin, peripherin/rds (Fariss, et al. 1997) and ROM-1 (Lewis, G.P. & Fisher, S. K., unpublished observations) distribution suggest that protein targeting and trafficking is altered when the outer segment degenerates in the rod cells.
There is some evidence from experimental data that the presence of opsin in the plasma membrane may make photoreceptors more vulnerable to apoptotic cell death (Zhang & Townes-Anderson, 2002).
The recovering rods appear structurally more like those in a normal retina than do cones which are often distorted in shape. Recovering primate cones also show altered outer segment structure when observed by electron microscopy (Guerin et al., 1989). It is now recognized that vision may continue to recover over years in reattachment patients (Liem et al., 1994; Ross, 2002) and this may reflect a slow recovery of cone outer segments in the fovea. The recovery of cone vision may be further complicated by the complex optical nature of cones and their alignment with respect to the pupil (see discussion in Rodieck, 1998; the Stiles-Crawford effect, Stiles & Crawford, 1933; reviewed by Enoch, 1963 and in Rodieck, 1998), and their structurally specialized interface with the apical RPE .
The Recovery of Photoreceptor Synaptic Terminals
The opposite pole of rod cells must also recover. Some recovery of the withdrawn rod terminals occurs because after a month of reattachment, the outer border of the outer plexiform layer is again composed of a relatively compact layer of rod synaptic terminals. This is in contrast to the highly disrupted layer occurring in the detached retina (compare Fig. 5B to 15A). There have been no detailed structural, molecular, or physiological analyses of these regenerated synapses.
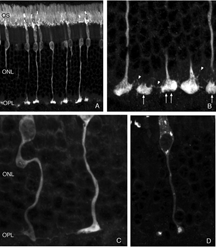
Some rod axons overgrow
Because some rods continue to express opsin in their plasma membrane after reattachment, we were able to identify rod axons that extend well beyond the outer plexiform layer and into the inner retina, terminating at different levels in the INL and IPL (arrowheads, Figs. 15B-D). The endings of these “overgrown” axons also label with antibodies to the proteins associated generally with synaptic vesicles, synaptophysin and VAMP (not shown), but they do not express the ribbon specific protein, ribeye.
A population of rods also “overshoot” their synaptic target layer during early retinal development providing much the same picture as we observed in the reattached retina (Johnson et al., 1999), in human retinas with advanced inherited retinal degeneration (Fariss et al., 2000) and in those with a history of complex detachments and reattachments (Sethi et al., 2004). It is curious that they do not seem to occur in the detached feline retinas, only appearing after reattachment. The ectopic terminals have fine filopodia-like extensions (Fig. 15C,D, arrowheads). Whether or not the overgrown axons remain in place, or are eventually retracted, or even if the cells bearing them eventually die is unknown for both development and reattachment.
Remodeling of cone terminals after reattachment
To date, there is little data on the “redifferentiation” or remodeling of the extraordinarily complex synapses of the cone terminals after reattachment. Cone pedicles with synaptic invaginations do re-appear in retinas detached for 3 days and reattached for 28. In general, the re-differentiation of photoreceptor synapses, along with profiles for the expression of specific pre- and post-synaptic molecules after reattachment is unexplored territory, but one that seems critical to fully understand the events related to visual recovery.
6. Remodeling of second and third order neurons
Rod bipolar cells
Rod bipolar cells innervate the rod spherules. Each rod spherule is usually presynaptic to two different rod bipolar cells, and each rod bipolar cell contacts between 16 and 20 rod spherules (Freed et al., 1987). The rod bipolar dendrites penetrate deeply into the invagination of the rod spherule to terminate opposite one of the two (on average) synaptic ribbons (Figs. 6, 7A; Boycott & Kolb, 1973).
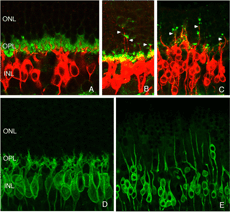
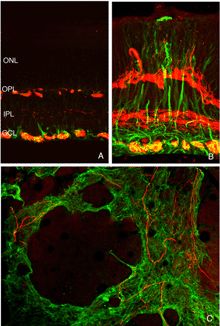
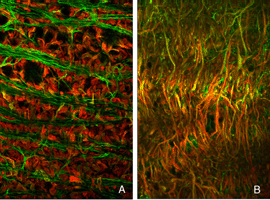
The general relationship between rod bipolar cells and rod spherules can be observed by confocal imaging using antibodies (Negishi et al., 1988; Wassle et al., 1991) to label each cell type (Fig. 16A, green = anti-synaptophysin, synaptic terminals, red = anti-PKC, rod bipolar cells). In the normal feline retina the compact layer of rod spherules stands out (Figs. 5A, 16A, green). Rod bipolar dendrites do not extend beyond them into the outer nuclear layer.
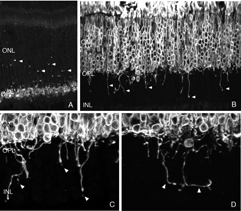
Rod bipolar dendrites remodel as rod terminals withdraw
As the layer of rod terminals becomes disrupted after detachment, there is an emergence of fine, tapered dendritic processes that reach from the rod bipolar cells into the outer nuclear layer, usually ending adjacent to withdrawn rod synaptic terminals (arrowheads, Fig. 16B). Such remodeled dendritic branches are readily apparent within 3 days of a detachment and their number increases with detachment time.
There probably is also “pruning” of dendritic branches on these cells. In control retinas the dendrites appear as fine, “wispy” outgrowths extending from the cell body into the outer plexiform layer (OPL, Fig. 16D). In detached retina these are not nearly as prominent, leading to the impression that some dendrites have withdrawn while others have grown in length (Fig. 16E). Pruning of dendrites appears to be the major response of bipolar cells in other forms of retinal degeneration (Marc et al., 2003), but in those cases the response appears late in the diseases.
Horizontal cells
The feline retina has two morphologically distinct horizontal cells (Fig 17); one with stout tapering dendrites and no axon (A-type) and the other with somewhat finer, highly branched dendrites and a long thin axon that forms an elaborate axon terminal (B-type; Dowling et al., 1966; Fisher & Boycott, 1974). Electron microscopy demonstrated that the dendrites of both the A- and B-type cells innervate the cone terminals, while only the branches of the axon terminal innervate rod spherules (Kolb, 1974). The thin B cell axon does not communicate electrically with cell body (Nelson et al., 1975).
Antibody labeling signatures define horizontal cell subtypes
Neurofilaments are plentiful in the A-type cell, and while not completely absent, they are sparse in the B-type cell. This is reflected in the heavy labeling of the A-type cell by antibodies to the 70 and 200 Kd subunits of the neurofilament protein complex. Antibodies to the calcium binding proteins calbindin D and calretinin also label horizontal cells. The use of these 3 antibodies allowed us to differentiate the 2 subtypes in feline retina.
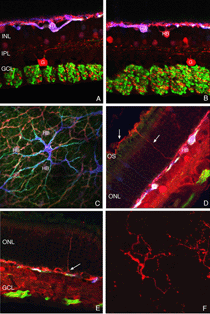
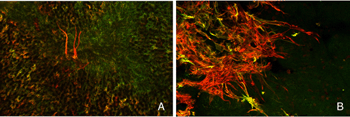
We initially observed fairly thick, beaded calbindin D-positive processes in the outer nuclear layer after detachment (red process, left of Fig. 18A, green processes Fig. 18C). These outgrowths label with the antibody to neurofilament protein. Indeed, there is a large increase in the intensity of horizontal cell labeling with this antibody after detachment (red, OPL, Figs. 19A, B), most likely indicating an upregulation of protein expression in the remodeled cells, (Linberg et al., 2004).
Using any of these three antibodies, we can actually detect two types of horizontal cell outgrowths; those that terminate adjacent to retracted rod spherules (arrowheads, Figs. 18A, B), i.e. “directed” and those that do not, i.e. “undirected” (examples in Figs. 18A, 19B, 20D, 20E). Whether the strikingly dramatic “undirected” outgrowths serve some functional or survival role for the horizontal cells or are merely vestiges of an injury response is an unknown. Amazingly, in detachments of 3 days or longer, these outgrowths often extend beyond the outer limiting membrane and into the subretinal space. This always occurs in conjunction with Muller cell processes (green, Fig. 19B, C). Once in the subretinal space the horizontal cell outgrowths (red, Fig. 19D) were never observed growing on the exposed photoreceptor cells themselves, but always embedded within a meshwork of Muller cell processes (green, Fig. 19D), where they could run for long distances.
Horizontal cells extend both ascending and descending neuritis
Some horizontal cell outgrowths are fairly thick processes descending from the horizontal cells into the inner retina, although these were observed with much less regularity than those ascending into the outer nuclear layer. The descending processes were also observed by Marc et al. (1998; 2003) in the detached feline retina and in other forms of retinal degeneration. Indeed, they seem to be the most commonly encountered form of horizontal cell remodeling in cases of extreme photoreceptor loss (Marc et al., 2003). The “undirected” ascending processes in both sectioned material and whole mounts appear in two structural types; one composed of thin, often beaded cylindrical processes and the other as flattened ribbon-like processes. These can both occur singly and in clusters (Fig. 18C). Within the subretinal space the long extended processes, all appear thin, cylindrical and often beaded (Fig. 19D). But, when thicker, fleshier processes occur there, they usually branch profusely, giving rise to complex assemblies reminiscent of sparse versions of axon terminals of the B-type cell within the outer plexiform layer ( Fig. 20F).
The origin of the horizontal cell outgrowths
By labeling with a combination of antibodies to neurofilament protein, calbindin D, and calretinin and assigning the output colors as green, blue, and red, respectively we determined that the characteristically shaped A-type cells are blue/white in color (i.e. heavily labeled with all 3 antibodies; HA in Figs. 20A,B,C), while cells with the appropriate shape and location to be the B-type are red or near red in color (due to the near lack of labeling with the anti-neurofilament antibody; HB in Figs. 20B,C). The outgrowths into the ONL, including the long processes that reach the subretinal space all bear an immunochemical labeling “signature” of the B-type cell (arrows Figs. 20D, E; 20F). When an outgrowth that gives rise to a branched process in the subretinal space was traced back through the outer nuclear layer, it was found to arise from a complex plexus with an organization characteristic of the axon terminal and with the B-type cell immunochemical signature (arrows, Fig. 20D, E). Thus, the evidence points to these processes as arising from the axon terminal of the B-type horizontal cell.
What drives the outgrowth of rod bipolar and horizontal cell neurites?
Although growth in response to the release (or lack) of some factor from the rods is one possibility, another mechanism is suggested from studies of cultured neurons in which mechanical tension can elicit neurite outgrowth (Lamoureux et al., 2002). If the rod bipolar cell dendrites and the horizontal cell axon terminal endings remain mechanically connected to retracting rod spherules, tension generated by the retracting rod spherules may initiate a growth response from the post-synaptic neurons. Whether or not the same mechanism accounts for the generation of directed and undirected outgrowths is unanswered.
7. Remodeling of Ganglion Cells
A subset of ganglion cell remodels vigorously in response to detachment. Ganglion cells are the retinal neurons farthest removed from the site of the detachment. It is important, however, to remember that ganglion cells are surrounded by Muller cell processes-and the apical end of the Muller cells are in direct physical contact with the actual site of the injury (the subretinal space). Muller cells also react to detachment very quickly (Geller et al., 2001), and thus could easily drive ganglion cell reactivity. Additionally, ganglion cell responsiveness could be dependent on the trans-neuronal changes originating with changes at the rod and cone synapses.
GAP 43 expression, neurofilament protein expression, and ganglion cell remodeling
Growth associated protein (GAP 43) expression is generally associated with axonal growth cones and synaptogenesis (Skene, 1989; Benowitz and Perrone-Bizzozero, 1991a,b; Strittmatter et al., 1992) and it is generally down-regulated significantly after the developmental period associated with synaptogenesis (Benowitz et al., 1988; Skene, 1989; Gispen et al., 1991; Meberg and Routtenberg, 1991; Benowitz and Perrone-Bizzozero, 1991a,b; Kruger et al., 1993; Kapfhammer et al., 1994; Benowitz and Perrone-Bizzozero, 1991b). Mice in which GAP 43 has been genetically removed show a tangling of axons in the optic nerve (Strittmatter et al., 1995) while mice over-expressing GAP 43 show an enhanced sprouting of axon terminals in both central and peripheral neurons (Aigner et al., 1995).
GAP 43 expression in the retina appears as the ganglion cells migrate into their specific layer and begin the process of axon outgrowth. It remains in adults only as stratified labeling in the inner plexiform layer (De la Monte et al., 1989; Benowitz & Perrone-Bizzozero, 1991b; Reh et al., 1993; Kapfhammer et al, 1994; McIntosh and Blazynski, 1991). This is the pattern observed in the adult feline retina (Coblentz et al., 2003). Immunoblot analysis also reveals that there is some GAP 43 protein expressed in normal adult feline retina (Coblentz et al., 2003).
Immunoblot, immunocytochemical, and real-time PCR data all shows a rise in GAP 43 expression beginning a day after a retinal detachment and continuing to increase at the last experimental timepoint at 28 days. Immunohistochemistry data reveals a population of ganglion cell bodies that are heavily labeled in the seven-day postdetachment animals (red processes, Fig. 21A; Coblentz et al., 2003). The GAP 43-positive ganglion cells also show a major increase in labeling with the anti-neurofilament protein antibody. In the normal retina, this antibody labels only a few processes in the inner plexiform layer and ganglion cell axons (red, IPL and GCL labeling, Fig. 19A; Coblentz,et al., 2003). After detachment it heavily labels processes in the IPL and cell bodies in the ganglion cell layer ( red, IPL, GCL labeling Fig. 19B, Fig. 21B, C). This labeling co-localizes with the increased anti-neurofilament labeling.
Ganglion cell morphology after detachment
After detachment the GAP 43/neurofilament-positive ganglion cells exhibit an unusual morphology, unlike any reported in the literature for feline (or other mammalian retinae) ganglion cells, with numerous small, spikey processes extending from their cell body toward the nerve fiber layer (Fig. 21C; Coblentz et al., 2003). As detachment duration increases, there is an astonishing increase in GAP 43/neurofilament-positive neurites that extend from the labeled ganglion cells and course completely across the neural retina and into the subretinal space (Fig. 21B, C, arrows). These processes are generally of a uniform caliber without obvious branching.
Remodeling of ganglion cells is “extreme”
Thus, the picture that emerges for remodeled ganglion cells is very much like that of the remodeled horizontal cell axon terminals, inasmuch as both produce a large number of “undirected” processes that can grow for long distances through the retina and into the subretinal space. The neurites from ganglion cells also tend to appear adjacent to the processes of reactive Muller cells either within the retina or in the subretinal space. Interestingly, neuronal processes have been identified in subretinal membranes removed surgically from human eyes (Lewis & Fisher, unpublished observations).
Which ganglion cell type(s)remodel?
Size measurements from retinal wholemounts were used to produce a frequency distribution for the somal area of the GAP 43 labeled ganglion cells. The data show a large peak at 600 µm2, but also a small population of cells clustering around 3000 µm2 (Coblentz et al., 2003), suggesting that it is the alpha ganglion cell types (Kolb et al., 1981; Boycott & Wassle, 1974) that remodel.
Ganglion cell remodeling after reattachment.
Retinal reattachment appears to elicit the extensive growth of ganglion cell neurites into the vitreous where they associate with epiretinal membranes produced by Muller cells (arrows, Fig. 21C, arrowheads Fig. 21D). Thus, the response in the vitreous is similar to the response in the subretinal space; neurite outgrowths associate with reactive Muller cell processes, but the vitreal growth is initiated only after reattachment, probably because reattachment also initiates the growth of Muller cells into the vitreous cavity (Lewis et al., 2003). Data from pathology specimens of human vitreal membranes also show the presence of anti-neurofilament labeled processes (Sethi et al., 2004).
8. Glial cell remodeling
Muller cells
Muller cells undergo extensive hypertrophy after detachment. They also show nuclear migration, cell division and growth into the subretinal space. All of these are obvious by simple histological observation (Machemer, 1968; Erickson et al., 1983; Anderson et al., 1983; Anderson et al., 1986).
The intermediate filament cytoskeleton and remodeling
The structural hypertrophy of these cells correlates with increased expression of intermediate filament proteins, glial fibrillary acidic protein (GFAP) and vimentin (Lewis et al., 1989; Lewis et al., 1995). Electron microscopy also shows a large increase in the number of intermediate filaments in the cytoplasm of these reactive cells (Fig. 22; Erickson et al., 1987).
Astrocytes in the brain and spinal cord react similarly, especially with respect to an upregulation of GFAP (Kerns & Hinsman, 1973; Eng and DeArmond, 1981; 1989).
The Muller cell response is distinct and dramatic
What makes the Muller cell GFAP response so dramatic is the fact that these filamentous proteins are almost exclusively localized to the endfoot region in a normal retina (red/yellow labeling, Fig. 23A), and in some species such as rats and mice, barely detectable by current immunocytochemical technology (Figs. 23C, Bignami and Dahl, 1979; Eisenfeld et al., 1984; Sarthy and Ripps, 2001). On injury the cytoskeletal remodeling in these cells correlates closely with changes in morphology. Indeed the correlation is so close that they are generally assumed to be functionally linked events. In support of this argument is the fact that in the ground squirrel retina Muller cells do not hypertrophy in response to detachment, and their intermediate filament cytoskeleton remains unchanged (Linberg et al., 2002a). Following brain injury astrocytic scar formation is impaired in vim-/-/GFAP-/- mice, but not in mice lacking only one of the two intermediate filament genes (Pekny et al., 1999). Whether a similar principle applies to Muller cells remains to be determined.
The Muller cell endfoot as the origin of the intermediate filament response
Within a day of a detachment intermediate filament proteins within the endfoot become more dense in number, often forming whorl-like or wavy bundles (red/yellow labeling, Fig. 23B; Erickson et al., 1987). They appear to grow from this distal mass, extending both into branches of the endfoot, which increase in size and in number with detachment time, and apically towards the cell body and then into the outer retina (Fig. 23; 24, Lewis et al., 1995; 2003).
Subretinal growth of Muller cells after detachment
As intermediate filaments expand into the apical cytoplasm of the activated Muller cells, some of them begin to grow, expanding into the subretinal space where they can elaborate into multilayered clinical “membranes” (Figs. 25A,B).
GFAP and vimentin: Details of their responses in the Muller cells Subretinal space
GFAP and vimentin can copolymerize to form intermediate filaments, but we have observed in double-labeling studies that there is a differential expression of the two in feline Muller cells. In the normal retina, vimentin predominates. The endfeet label most heavily with this antibody and labeling extends farther into the neural retina than it does for GFAP. In the main trunk of the reactive cells, GFAP seems to predominate. As the apical microvilli begin to grow into the subretinal space, vimentin labeling predominates (Figs. 25A, red tapering forked processes). This imbalance in expression continues as the processes expand into the subretinal space (Fig. 25B; Lewis & Fisher, 2003). The “leading edge” of the growing processes always shows a predominance of anti-vimentin labeling, with GFAP expression becoming increasingly intense at the point of exit from the neural retina. These processes or “membranes” essentially form glial scars in the subretinal space. Their presence does not appear to prevent physical reattachment of the retina, but it does inhibit the regeneration of outer segments (Anderson et al., 1986).
Reattachment stimulates growth of Muller cells
Vitreous space
A novel endfoot response occurs in the feline retina in response to reattachment. In this case the branched endfoot processes that remained closely adherent to the vitreal surface of the retina begin growing into the vitreous chamber. It is these “branchlets” of the endfoot that expand into the vitreous to form epiretinal membranes. The initial outgrowths appear as fine, flat “ribbons” of cytoplasm extending away from the neural retina into the vitreous cavity (Figs. 26A, B).
These delicate, flattened processes have a very different morphology from the fine, tapered, and branched processes that expand into the subretinal space. In these endfoot outgrowths, GFAP expression dominates over that of vimentin (Fig. 26A,B). While vimentin expression “leads the way” in the expanding Muller cell processes within the subretinal space, it is GFAP that holds this position in the endfoot processes that grow into the vitreous (Figs. 26C,D). The growth of these cellular processes into the vitreous can have significant consequences on vision because they can become contractile (Ryan, 1985) and cause wrinkling and eventual re-detachment of the neural retina; a serious sight-threatening event if the fovea is involved.
Astrocytes
There is little known about the reactive capacity of these cells in the retina. They proliferate in response to detachment (Fisher et al., 1991; Geller et al., 1995) and observations of optic nerve fiber layer in retinal wholemounts show that their regular array and stellate shape are lost as the endfeet of the Muller cells expand on the retinal surface (Lewis & Fisher, 2003).
9. Retinal Remodeling after Detachment and Reattachment: an overview
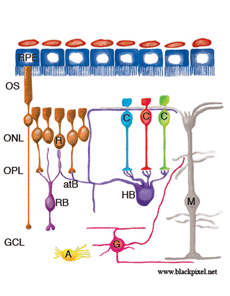
Fig. 27. A drawing summarizing the remodeling events in the feline retina as a result of detachment
Studies of detached (and reattached) retina have shown us that the mammalian retina has remarkable remodeling capabilities. Figure 27 shows in summary form the remodeling of the neural retina described here.
ROS (R) and COS (C) are greatly shortened and separated from the apical surface of the pigmented epithelium (RPE). Many RS are withdrawn from the OPL. After reattachment some rod axons grow into the inner retina (the cell on the left). CP remain in place, although they undergo significant structural remodeling. The dendrites of RB cells grow into the ONL where they terminate adjacent to withdrawn RS. The axon terminals of HB remodel extensively, with some processes terminating next to withdrawn RS, while others grow wildly into the outer retina, and into the subretinal space adjacent to reactive Muller cells. HB axon terminals can also grow into the inner retina, although this reaction appears less frequently. A subpopulation of ganglion cells (G) extend short, spikey, neurites from their base after, and can also grow processes into the outer retina where they behave much like the neurites of horizontal cells. In the reattached retinas, ganglion cell processes can also grow into epiretinal membranes formed by Muller cell growth into the vitreous. Muller cells (M) are highly reactive to detachment and grow in the subretinal space to form membranes or glial scars on the exposed photoreceptor OS. After detachment their endfeet expand but remain within the ILM. After reattachment their specialized endfeet can grow into the vitreous to form epiretinal membranes as part of the disease, proliferative vitreoretinopathy. Astrocytes (A) proliferate and often appear in epiretinal membranes but their responses to detachment have not been characterized in detail.
Muller cell changes may occur in response to photoreceptor cell deconstruction and/or cell death or they may arise independently. While it would seem logical that the remodeling changes in second order neurons would be signaled from photoreceptor changes, and remodeling of third order neurons signaled by changes in bipolar cells, this has not been proven experimentally.
10. Future challenges
Data from the detachment model, as well as from a variety of other studies now suggests that retinal neurons remain capable of significant structural remodeling in adult mammals. This in turn may provide increased optimism for a variety of therapies for blinding diseases in which photoreceptor degeneration is the primary cause of visual loss. Although preventing photoreceptor cell death is the optimum therapy in these diseases, alternatives to this daunting challenge include technology for replacing photoreceptors, whether it is by way of cellular transplantation (Aramant & Seiler, 2004) or the use of progenitor cells (Fischer & Reh, 2001; 2003; Tropepe et al., 2003). This success, however, would be hollow if the second-order neurons did not retain sufficient plasticity to form functional connections with the new photoreceptors. It may seem far-fetched at the present that such connections would form circuitry for functional vision. However, the degree of remodeling we have observed may be an indicator that the inner retina has more of a capacity for remodeling itself than previously imagined. Studies of patients with foveal reattachments indicating that visual recovery may occur over years, not weeks could conceivably represent the re-formation of appropriately functional retinal circuits, or even a remodeling of the RPE/photoreceptor interface to properly align the foveal cones. In the case of detachment, preventing both photoreceptor and Muller cell reactivity may also be key to treating the injury and preventing threats to sight through diseases such as subretinal fibrosis, and proliferative vitreoretinopathy. Adjuncts to therapy do not seem so distant in these cases, since treatment with something as simple as elevated oxygen concentration appear to help attain these goals in animal models (Mervin et al., 1999; Lewis et al., 1999; Sakai et al., 2001; Lewis et al., 2004).
11. References
Aaberg TM. Does hyperoxygenation limit retina degeneration after retinal detachment? Am J Ophthalmol. 1999;128:231. [PubMed]
Aigner L, Arber S, Kapfhammer JP, Laux T, Schneider C, Botteri F, Brenner HR, Caroni P. Overexpression of the neural growth-associated protein GAP-43 induces nerve sprouting in the adult nervous system of transgenic mice. Cell. 1995;83:269–278. [PubMed]
Anderson DH, Fisher SK, Steinberg RH. Mammalian cones: disc shedding, phagocytosis, and renewal. Invest Ophthalmol Vis Sci. 1978;17:117–133. [PubMed]
Anderson DH, Fisher SK. The relationship of primate foveal cones to the pigment epithelium. J Ultrastruct Res. 1979;67:23–32. [PubMed]
Anderson DH, Stern WH, Fisher SK, Erickson PA, Borgula GA. The onset of pigment epithelial proliferation following retinal detachment. Invest Ophthalmol Vis Sci. 1981;21:10–16. [PubMed]
Anderson DH, Stern WH, Fisher SK, Erickson PA, Borgula GA. Retinal detachment in the cat: the pigment epithelial-photoreceptor interface. Invest Ophthalmol Vis Sci. 1983;24:906–926. [PubMed]
Anderson DH, Gurin CJ, Erickson PA, Stern WH, Fisher SK. Morphological recovery in the reattached retina. Invest Ophthalmol Vis Sci. 1986;27:168–183.[PubMed]
Aramant RB, Seiler MJ. Progress in retinal sheet transplantation. Prog Retin Eye Res. 2004;23:475–494. [PubMed]
Benowitz LI, Apostolides PJ, Perrone-Bizzozero N, Finkelstein SP, Zwiers H. Anatomical distribution of the growth-associated protein GAP-43/B-50 in the adult rat brain. J Neurosci. 1988;8:339–352. [PubMed]
Benowitz LI, Perrone-Bizzozero NI. The expression of GAP-43 in relation to neuronal growth and plasticity: when, where, how, and why? Prog Brain Res.1991;89:69–87. [PubMed]
Benowitz LI, Perrone-Bizzozero NI. The relationship of GAP-43 to the development and plasticity of synaptic connections. Ann N Y Acad Sci. 1991;627:58–74.[PubMed]
Berglin L, Algvere PV, Seregard S. Photoreceptor decay over time and apoptosis in experimental retinal detachment. Graefes Arch Clin Exp Ophthalmol.1997;235:306–312. [PubMed]
Bignami A, Dahl D. The radial glia of Muller in the rat retina and their response to injury. An immunofluorescence study with antibodies to the glial fibrillary acidic (GFA) protein. Exp Eye Res. 1979;28:63–69. [PubMed]
Boycott BB, Kolb H. The connections between bipolar cells and photoreceptors in the retina of the domestic cat. J Comp Neurol. 1973;148:91–114. [PubMed]
Boycott B, Wassle H. Parallel processing in the mammalian retina: the Proctor Lecture. Invest Ophthalmol Vis Sci. 1999;40:1313–1327. [PubMed]
Cajal S. Ramon y La r tine des vert br s. Cellule. 1892;9:119–225.
Chang CJ, Lai WW, Edward DP, Tso MO. Apoptotic photoreceptor cell death after traumatic retinal detachment in humans. Arch Ophthalmol. 1995;113:880–886.[PubMed]
Chu Y, Humphrey MF, Constable IJ. Horizontal cells of the normal and dystrophic rat retina: a wholemount study using immunolabeling for the 28 kDa calcium binding protein. Exp Eye Res. 1993;57:141–148. [PubMed]
Coblentz FE, Radeke MJ, Lewis GP, Fisher SK. Evidence that ganglion cells react to retinal detachment. Exp Eye Res. 2003;76:333–342. [PubMed]
Cook B, Lewis GP, Fisher SK, Adler R. Apoptotic photoreceptor degeneration in experimental retinal detachment. Invest Ophthalmol Vis Sci. 1995;36:990–996.[PubMed]
De la Monte SM, Federoff HJ, Ng SC, Grabczyk E, Fishman MC. GAP-43 gene expression during development: persistence in a distinctive set of neurons in the mature central nervous system. Brain Res Dev Brain Res. 1989;46:161–168. [PubMed]
Dowling JE, Brown JE, Major D. Synapses of horizontal cells in rabbit and cat retinas. Science. 1966;153:1639–1641. [PubMed]
Dowling JE. Organization of vertebrate retinas. Invest Ophthalmol. 1970;9:655–680. [PubMed]
Eisenfeld AJ, Bunt-Milam AH, Sarthy PV. Muller cell expression of glial fibrillary acidic protein after genetic and experimental photoreceptor degeneration in the rat retina. Invest Ophthalmol Vis Sci. 1984;25:1321–1328. [PubMed]
Eng LF, DeArmond SJ. 1981. Glial fibrillary acidic (GFA) protein immunocytochemistry in development and neuropathology. In: Eleventh Interantional Congress of Anatomy, Part A; Glial and Neuronal Cell Biology. Acosta Vidreo, E. & Fedoroff, A, (eds.). Liss, N.Y. pp 65-79.
Enoch JJ. Optical properties of ther etinal receptors. J Opt Soc Am. 1963;53:71–85.
Erickson PA, Fisher SK, Anderson DH, Stern WH, Borgula GA. Retinal detachment in the cat: the outer nuclear and outer plexiform layers. Invest Ophthalmol Vis Sci. 1983;24:927–942. [PubMed]
Erickson PA, Fisher SK, Gurin CJ, Anderson DH, Kaska DD. Glial fibrillary acidic protein increases in Muller cells after retinal detachment. Exp Eye Res.1987;44:37–48. [PubMed]
Fariss RN, Molday RS, Fisher SK, Matsumoto B. Evidence from normal and degenerating photoreceptors that two outer segment integral membrane proteins have separate transport pathways. J Comp Neurol. 1997;387:148–156. [PubMed]
Fariss RN, Li ZY, Milam AH. Abnormalities in rod photoreceptors, amacrine cells, and horizontal cells in humans with retinitis pigmentosa. Am J Ophthalmol.2000;129:215–223. [PubMed]
Faude F, Francke M, Makarov F, Schuck J, Gartner U, Reichelt W, Wiedemann P, Wolburg H, Reichenbach A. Experimental retinal detachment causes widespread and multilayered degeneration in rabbit retina. J Neurocytol. 2001;30:379–390. [PubMed]
Fischer AJ, Reh TA. Muller glia are a potential source of neural regeneration in the postnatal chicken retina. Nat Neurosci. 2001;4:247–252. [PubMed]
Fischer AJ, Reh TA. Potential of Muller cells to become neurogenic regeneration retinal progenitor cells. Glia. 2003;43:70–76. [PubMed]
Fisher SK, Boycott BB. Synaptic connexions of the horizontal cells in the outer plexiform layer of the retina of the cat and the rabbit. Proc R Soc Lond B Biol Sci.1974;186:317–331. [PubMed]
Fisher SK, Steinberg RH. Origin and organization of pigment epithelial apical projections to cones in cat retina. J Comp Neurol. 1982;206:131–145. [PubMed]
Fisher SK, Erickson PA, Lewis GP, Anderson DH. Intraretinal proliferation induced by retinal detachment. Invest Ophthalmol Vis Sci. 1991;32:1739–1748.[PubMed]
Fisher SK, Anderson DH. Cellular effects of detachment on the neural retina and the retinal pigment epithelium. In: Ryan SJ, Wilkinson CP, editors. Retina, Surgical retina.Vol. 3. 3rd ed. St. Louis (MO): Mosby; 2001.
Freed MA, Bsmith RG, Sterling P. Rod bipolar array in the cat retina: pattern of input from rods and GABA-accumulating amacrine cells. J Comp Neurol.1987;266:445–455. [PubMed]
Geller SF, Lewis GP, Anderson DH, Fisher SK. Use of the MIB-1 antibody for detecting proliferating cells in the retina. Invest Ophthalmol Vis Sci. 1995;36:737–744. [PubMed]
Geller SF, Lewis GP, Fisher SK. FGFR1, signaling, and AP-1 expression following retinal detachment: reactive Muller and RPE cells. Invest Ophthalmol Vis Sci.2001;42:1363–1369. [PubMed]
Gispen WH, Nielander HB, De Graan PN, Oestreicher AB, Schrama LH, Schotman P. Role of the growth-associated protein B-50/GAP-43 in neuronal plasticity.Mol Neurobiol. 1991;5:61–85. [PubMed]
Guerin CJ, Anderson DH, Fariss RN, Fisher SK. Retinal reattachment of the primate macula: photoreceptor recovery after short-term detachment. Invest Ophthalmol Vis Sci. 1989;30:1708–1725. [PubMed]
Haley TL, Pochet R, Baizer L, Burton MD, Crabb JW, Parmentier M, Polans AS. Calbindin D-28k immunoreactivity of human cone cells varies with retinal position.Vis Neurosci. 1995;12:301–307. [PubMed]
Immel J, Negi A, Marmor MF. Acute changes in RPE apical morphology after retinal detachment in rabbit. An SEM study. Invest Ophthalmol Vis Sci.1986;27:1770–1776. [PubMed]
Jacobs GH, Calderone JB, Sakai T, Lewis GP, Fisher SK.In: Mollon JD, Pokorny J, Knoblauch K, editors. Normal & defective colour vision. Effects of retinal detachment on S and M cone function in an animal model. Oxford (UK): Oxford University Press; 2003. p. 381-388.
John SK, Smith JE, Aguirre GD, Milam AH. Loss of cone molecular markers in rhodopsin-mutant human retinas with retinitis pigmentosa. Mol Vis. 2000;6:204–215.[PubMed]
Johnson LV, Hageman GS, Blanks JC. Interphotoreceptor matrix domains ensheath vertebrate cone photoreceptor cells. Invest Ophthalmol Vis Sci.1986;27:129–135. [PubMed]
Johnson PT, Williams RR, Cusato K, Reese BE. Rods and cones project to the inner plexiform layer during development. J Comp Neurol. 1999;414:1–12.[PubMed]
Kapfhammer JP, Christ F, Schwab ME. The expression of GAP-43 and synaptophysin in the developing rat retina. Brain Res Dev Brain Res. 1994;80:251–260.[PubMed]
Kerns JM, Hinsman EJ. Neuroglial response to sciatic neurectomy. II. Electron microscopy. J Comp Neurol. 1973;151:255–280. [PubMed]
Kolb H. The connections between horizontal cells and photoreceptors in the retina of the cat: electron microscopy of Golgi preparations. J Comp Neurol.1974;155:1–14. [PubMed]
Kolb H, Famiglietti EV. Rod and cone pathways in the retina of the cat. Invest Ophthalmol. 1976;15:935–946.
Kolb H, Nelson R, Mariani A. Amacrine cells, bipolar cells and ganglion cells of the cat retina: a Golgi study. Vision Res. 1981;21:1081–1114. [PubMed]
Kolb H, Nelson R, Ahnelt P, Cuenca N. Cellular organization of the vertebrate retina. In: Kolb H, Ripps H, Wu S, editors. Prog. Brain Res. 131. Concepts and challenges in retinal biology. A tribute to John E. Dowling. Amsterdam: Elsevier; 2001. p. 3-26.
Kroll AJ, Machemer R. Experimental retinal detachment in the owl monkey. III. Electron microscopy of retina and pigment epithelium. Am J Ophthalmol.1968;66:410–427. [PubMed]
Kroll AJ, Machemer R. Experimental retinal detachment in the owl monkey. V. Electron microscopy of reattached retina. Am J Ophthalmol. 1969;67:117–130.[PubMed]
Kroll AJ, Machemer R. Experimental retinal detachment and reattachment in the rhesus monkey. Am J Ophthalmol. 1969;68:58–77. [PubMed]
Kruger L, Bendotti C, Rivolta R, Samanin R. Distribution of GAP-43 mRNA in the adult rat brain. J Comp Neurol. 1993;333:417–434. [PubMed]
Kryger Z, Galli-Resta L, Jacobs GH, Reese BE. The topography of rod and cone photoreceptors in the retina of the ground squirrel. Vis Neurosci. 1998;15:685–691. [PubMed]
Lamoureux P, Ruthel G, Buxbaum RE, Heidemann SR. Mechanical tension can specify axonal fate in hippocampal neurons. J Cell Biol. 2002;159:499–508.[PubMed]
Lewis GP, Erickson PA, Guerin CJ, Anderson DH, Fisher SK. Changes in the expression of specific Muller cell proteins during long-term retinal detachment. Exp Eye Res. 1989;49:93–111. [PubMed]
Lewis GP, Erickson PA, Anderson DH, Fisher SK. Opsin distribution and protein incorporation in photoreceptors after experimental retinal detachment. Exp Eye Res. 1991;53:629–640. [PubMed]
Lewis GP, Matsumoto B, Fisher SK. Changes in the organization of cytoskeletal proteins during retinal degeneration induced by retinal detachment. Invest Ophthalmol Vis Sci. 1995;36:2404–2416. [PubMed]
Lewis GP, Linberg KA, Fisher SK. Neurite outgrowth from bipolar and horizontal cells following experimental retinal detachment. Invest Ophthalmol Vis Sci.1998;39:424–434. [PubMed]
Lewis GP, Linberg KA, Geller SF, Guerin CJ, Fisher SK. Effects of the neurotrophin BDNF in an experimental model of retinal detachment. Invest Ophthalmol Vis Sci. 1999;40:1530–1544. [PubMed]
Lewis GP, Sethi CS, Charteris DG, Leitner WP, Linberg KA, Fisher SK. The ability of rapid retinal reattachment to stop or reverse the cellular and molecular events initiated by detachment. Invest Ophthalmol Vis Sci. 2002;43:2412–2420. [PubMed]
Lewis GP, Sethi CS, Linberg KA, Charteris DG, Fisher SK. Experimental retinal reattachment. A new perspective. Mol Neurobiol. 2003;28:159–175. [PubMed]
Lewis GP, Fisher SK. 2003. Upregulation of GFAP in response to retinal injury: Its potential role in glial remodeling and a comparison to vimentin expression. In: K.W. Jeon, editor. Int. Rev. Cytol. A Survey of Cell Biology. Vol. 230. San Diego (CA): Elsevier Academic Press; 2003. p. 263-290.
Lewis GP, Talaga KC, Linberg KA, Avery RL, Fisher SK. The efficacy of delayed oxygen therapy in the treatment of experimental retinal detachment. Am J Ophthalmol. 2004;137:1085–1095. [PubMed]
Lewis GP, Sethi CS, Carter KM, Charteris DG, Fisher SK. Microglial cell activation following retinal detachment: a comparison between species. Mol Vis. 2005[PubMed]
Li ZY, Kljavin IJ, Milam AH. Rod photoreceptor neurite sprouting in retinitis pigmentosa. J Neurosci. 1995;15:5429–5438. [PubMed]
Liem AT, Keunen JE, van Meel GJ, van Norren D. Serial foveal densitometry and visual function after retinal detachment surgery with macular involvement.Ophthalmology. 1994;101:1945–1952. [PubMed]
Linberg K, Cuenca N, Ahnelt P, Fisher S, Kolb H. (2001). Comparative anatomy of major retinal pathways in the eyes of nocturnal and diurnal mammals. In: Kolb H, Ripps H, Wu S, editors. Concepts and challenges in retinal biology. A tribute to John E. Dowling. Prog Brain Res. 131: 27-52.
Linberg KA, Lewis GP, Shaaw C, Rex TS, Fisher SK. Distribution of S- and M-cones in normal and experimentally detached cat retina. J Comp Neurol.2001;430:343–356. [PubMed]
Linberg KA, Sakai T, Lewis GP, Fisher SK. Experimental retinal detachment in the cone-dominant ground squirrel retina: morphology and basic immunocytochemistry. Vis Neurosci. 2002;19:603–619. [PubMed]
Linberg K.A., Lewis G.P., Barawid E.L., Sakai T., Fisher S.K. A quantitative study of cone matrix sheath and S-cone recovery in reattached retina. Invest Ophthalmol Vis Sci. 2002;43:ARVO E-Abstract 4536.
Linberg K.A., Lewis G.P., Carter K.M., Fisher S.K. Immunocytochemical evidence that B-type horizontal cell axon terminals remodel in response to retinal detachment in the cat. Invest Ophthalmol Vis Sci. 2004;45:ARVO E-Abstract 4604.
Linsenmeier RA, Padnick-Silver L. Metabolic dependence of photoreceptors on the choroid in the normal and detached retina. Invest Ophthalmol Vis Sci.2000;41:3117–3123. [PubMed]
Long KO, Fisher SK. The distributions of photoreceptors and ganglion cells in the California ground squirrel Spermophilus beecheyi. J Comp Neurol.1983;221:329–340. [PubMed]
Machemer R. Experimental retinal detachment in the owl monkey. IV. The reattached retina. Am J Ophthalmol. 1968;66:1075–1091. [PubMed]
Marc RE, Murry RF, Fisher SK, Linberg KA, Lewis GP. Amino acid signatures in the detached cat retina. Invest Ophthalmol Vis Sci. 1998;39:1694–1702.[PubMed]
Marc RE, Jones BW, Watt CB, Strettoi E. Neural remodeling in retinal degeneration. Prog Retin Eye Res. 2003;22:607–655. [PubMed]
McIntosh H, Blazynski C. GAP-43-like immunoreactivity in the adult retina of several species. Brain Res. 1991;554:321–324. [PubMed]
Meberg PJ, Routtenberg A. Selective expression of protein F1/(GAP-43) mRNA in pyramidal but not granule cells of the hippocampus. Neuroscience.1991;45:721–733. [PubMed]
Mervin K, Valter K, Maslim J, Lewis GP, Fisher SK, Stone J. Limiting the death and deconstruction during retinal detachment: the value of oxygen supplementation. Am J Ophthalmol. 1999;128:155–164. [PubMed]
Migdale K, Herr S, Klug K, Ahmad K, Linberg K, Sterling P, Schein S. Two ribbon synaptic units in rod photoreceptors of macaque, human, and cat. J Comp Neurol. 2003;455:100–112. [PubMed]
Negishi K, Kato S, Teranishi T. Dopamine cells and rod bipolar cells contain protein kinase C-like immunoreactivity in some vertebrate retinas. Neurosci Lett.1988;94:247–252. [PubMed]
Nelson R, von Lutzow A, Kolb H, Gouras P. Horizontal cells in cat retina with independent dendritic systems. Science. 1975;189:137–139. [PubMed]
Nork TM, Millecchia LL, Stickland BD, Linberg JV, Chao G. Selective loss of blue cones and rods in human retinal detachment. Arch Ophthalmol. 1995;113:1066–1073. [PubMed]
Nour M, Quiambao AB, Peterson WM, Al-Ubaidi MR, Naash MI. P2Y2 receptor agonist INS37217 enhances functional recovery after eetachment caused by subretinal injection in normal and rds mice. Invest Ophthalmol Vis Sci. 2003;44:4505–4514. [PubMed]
Pasteels B, Rogers J, Blachier F, Pochet R. Calbindin and calretinin localization in retina from different species. Vis Neurosci. 1990;5:1–16. [PubMed]
Peichl L, Bolz J. Kainic acid induces sprouting of retinal neurons. Science. 1984;223:503–504. [PubMed]
Pekny M, Johansson CB, Eliasson C, Stakeberg J, Wallen A, Perlmann T, Lendahl U, Betsholtz C, Berthold C-H, Frisen J. Abnormal reaction to central nervous system injury in mice lacking glial fibrillary acidic protein and vimentin. J Cell Biol. 1999;145:503–514. [PubMed]
Reh TA, Tetzlaff W, Ertlmaier A, Zwiers H. Developmental study of the expression of B50/GAP-43 in rat retina. J Neurobiol. 1993;24:949–958. [PubMed]
Rex TS, Fariss RN, Lewis GP, Linberg KA, Sokal I, Fisher SK. A survey of molecular expression by photoreceptors after experimental retinal detachment. Invest Ophthalmol Vis Sci. 2002;43:1234–1247. [PubMed]
Rodieck RW. In: The first steps of seeing. Chapter 4. Sunderland (MA): Sinauer Assoc, Inc.; 1998. p. 70 -87.
Rohrenbeck J, Wassle H, Boycott BB. Horizontal cells in the monkey retina: immunocytochemical staining with antibodies against calcium binding proteins. Eur J Neurosci. 1989;1:407–420. [PubMed]
Ross WH. Visual recovery after macula-off retinal detachment. Eye. 2002;16:440–446. [PubMed]
Ryan SJ. The pathophysiology of proliferative vitreoretinopathy in its mangagement. Am J Ophthalmol. 1985;100:188–193. [PubMed]
Sakai T, Lewis GP, Linberg KA, Fisher SK. The ability of hyperoxia to limit the effects of experimental detachment in cone dominated retina. Invest Ophthalmol Vis Sci. 2001;42:3264–3273. [PubMed]
Sarthy V, Ripps H. The retinal Muller cell. In: Blakemore C, editor. Perspectives in vision research. New York: Plenum Press; 2001.
Schmitz F, Konigstorfer A, Sudhof TC. RIBEYE, a component of synaptic ribbons: a protein’s journey through evolution provides insight into synaptic ribbon function. Neuron. 2000;28:857–872. [PubMed]
Sethi CS, Lewis GP, Fisher SK, Leitner WP, Mann DL, Luthert PJ, Charteris DG. Glial remodeling and neural plasticity in human retinal detachment with proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci. 2005;46:329–342. [PubMed]
Skene JH. Axonal growth-associated proteins. Annu Rev Neurosci. 1989;12:127–156. [PubMed]
Steinberg RH, Reid M, Lacy PL. The distribution of rods and cones in the retina of the cat (Felis domesticus). J Comp Neurol. 1973;148:229–248. [PubMed]
Steinberg RH, Wood I. Pigment epithelial cell ensheathment of cone outer segments in the retina of the domestic cat. Proc R Soc Lond Biol. 1974;187:461–478.[PubMed]
Steinberg RH, Wood I, Hogan MJ. Pigment epithelial ensheathment and phagocytosis of extrafoveal cones in human retina. Philos Trans R Soc Lond Biol.1977;277:459–474. [PubMed]
Steinberg RH, Fisher SK, Anderson DH. Disc morphogenesis in vertebrate photoreceptors. J Comp Neurol. 1980;190:501–518. [PubMed]
Stiles WS, Crawford BH. The luminous efficiency of rays entering the eye pupil at different points. Proc R Soc Lond B Biol Sci. 1933;112:428–450.
Strittmatter SM, Vartanian T, Fishman MC. GAP-43 as a plasticity protein in neuronal form and repair. J Neurobiol. 1992;23:507–520. [PubMed]
Strittmatter SM, Fankhauser C, Huang PL, Mashimo H, Fishman MC. Neuronal path finding is abnormal in mice lacking the neuronal growth cone protein GAP-43.Cell. 1995;80:445–452. [PubMed]
Szel A, Takacs L, Monostori E, Vigh-Teichmann I, Rohlich P. Heterogeneity of chicken photoreceptors as defined by hybridoma supernatants. An immunocytochemical study. Cell Tissue Res. 1985;240:735–741. [PubMed]
Szel A, Diamantstein T, Rohlich P. Identification of the blue-sensitive cones in the mammalian retina by anti-visual pigment antibody. J Comp Neurol.1988;273:593–602. [PubMed]
Thanos S, Moore S, Hong Y. Retinal microglia. Prog Retin Eye Res. 1996;15:331–361.
Tropepe V, Coles BL, Chiasson BJ, Horsford DJ, Elia AJ, McInnes RR, van der Kooy D. Retinal stem cells in the adult mammalian eye. Science. 2000;287:2032–2036. [PubMed]
Wagner H-J. Quantitative changes of synaptic ribbons in the cone pedicles of Nannacara: light dependent or governed by a circadian rhythm? In: Ali MA, editor. Vision in fishes: new approaches in research. 1974 NATO Advanced Study Institute. New York: Plenum Press; 1975. p. 679-686.
Wagner H-J, Ali MA. Cone synaptic ribbons and retinomotor changes in the brook trout, Salvelinus fontinalis (Salmonidae, Teleostei), under various experimental conditions. Can J Zool. 1977;55:1684–1691. [PubMed]
Wagner H-J. Light-dependent plasticity of the morphology of horizontal cell terminals in cone pedicles of fish retinas. J Neurocytol. 1980;9:573–590. [PubMed]
Wang Y, Macke JP, Merbs SL, Zack DJ, Klaunberg B, Bennett J, Gearhart J, Nathans J. A locus control regtion adjacent to the human read and green visual pigment genes. Neuron. 1992;9:429–440. [PubMed]
Wassle H, Yamashita M, Greferath U, Grunert U, Muller F. The rod bipolar cell of the mammalian retina. Vis Neurosci. 1991;7:99–112. [PubMed]
Yang L, Bula D, Arroyo JG, Chen DF. Preventing retinal detachment-associated photoreceptor cell loss in Bax-deficient mice. Invest Ophthalmol Vis Sci.2004;45:648–654. [PubMed]
Young RW. The renewal of photoreceptor cell outer segments. J Cell Biol. 1967;33:61–72. [PubMed]
Zhang N, Townes-Anderson E. Regulation of structural plasticity by different channel types. J Neurosci. 2002;22:7065–7079. [PubMed]