1 Introduction
“Nothing in biology makes sense except in the light of evolution” (1).
Theodosius Dobzhansky’s insight is especially apposite in trying to comprehend the nature of our rod and cone photoreceptors, and the organization of our retina. Unless we understand how these cells and structures arose, through hundreds of millions of years of evolution, we have little prospect of making sense of their morphological and molecular structure, or being able to answer the recurring conundrum ‘Why does the retina do it this way?’. In addition to providing a rationale for the arrangement of our retina, a study of the evolution of our eye and its cones and rods is immensely satisfying, in offering potential answers to questions such as ‘How and when did our eyes originate?’ and ‘Why should we and all other vertebrates possess eyes so different from those of (for example) insects?’.
The apparent lack of transitional forms that have been preserved during the course of vertebrate eye evolution has provided perennial fodder for ‘creationists’. But, as Charles Darwin (2) explained,
“if numerous gradations from a perfect and complex eye to one very imperfect and simple, each grade being useful to its possessor, can be shown to exist … and if any variation or modification in the organ be ever useful to an animal under changing conditions of life, then the difficulty of believing that a perfect and complex eye could be formed by natural selection, though insuperable by our imagination, can hardly be considered real”.
One of the goals of this article is to document evidence for such gradations in the photoreceptors, in the phototransduction cascade, and in the retina, during the course of chordate and vertebrate evolution. A second major aim is to construct a set of ‘scenarios’ for the long sequence of events that contributed; in this regard the term ‘scenario’ is used in its dictionary sense of ‘a postulated sequence or development of events’.
Historically, three main avenues for studying eye evolution have been utilized: examination of eyes in the fossil record, examination of the structure of eyes in extant species, and examination of embryological development. Recently, a number of powerful new avenues have been developed, utilizing molecular evidence; for example, comparative molecular genetics across extant species, as well as the combination of evolutionary and developmental analysis (evo-devo approaches). This article concentrates on the eyes of extant chordates, and examines clues to eye evolution that can be obtained from morphological, embryological and molecular features. In doing so, it builds on the scenarios put forward by Lamb et al (3, 4).
For other recent reviews of various aspects of the evolution of phototransduction and photoreception, see Arendt et al (5), Vopalensky & Kosmik (6), Larhammar et al (7), Shichida & Matsuyama (8), Kusakabe et al (9), Collin et al (10), Fain et al (11), Porter et al (12) and Nilsson (13). For descriptions of the types of eyes that have evolved across the entire animal kingdom (rather than primarily in chordates, as treated here), see the lavishly illustrated book by Schwab (14). For the evolution of vertebrate sensory systems and brains, see Butler (15) and Butler & Hodos (16).
One question that has often been asked is ‘How many times have eyes and photoreceptors evolved independently?’ Answers to this question can vary greatly, depending on one’s concept of ‘independence’. As we shall see below, the common ancestor of cnidaria, protostomes and deuterostomes already possessed the great majority of the components needed for constructing an ocellus and/or a retina; e.g. it already possessed transcription factors, growth factors, opsins, photoreceptor cells (of the ciliary and microvillar forms), pigment cells, and neurons, etc. Using this common set of tools, different events occurred in different lineages, leading to very different eyes. In certain protostomes, a simple ocellus replicated many times to form a compound eye; in our own lineage, an extensive light-sensitive retina formed and a single optical element developed in front of it.
Although the eyes that have resulted are radically different from each other, it turns out that the photoreceptors upon which they are based are remarkably similar to each other, and indeed are derived from a common ancestral type, so that one can now conclusively reject the claim of Salvini-Plawen & Mayr (17) that “Photoreceptors have originated independently in at least 40, but possibly up to 65 or more different phyletic lines”.
As was so aptly pointed out by Jacob (18), it is important to realize that evolution works by ‘tinkering’ with what is already available, and without any overall ‘purpose’ (such as to give rise to vision). In the case of the evolution of phototransduction, photoreceptors, and retina, numerous examples of such tinkering will become apparent in the Sections ahead; see also Goldsmith (19).
2 Origins
By way of background, the following sub-sections briefly summarize in turn: the origin of vertebrates, the origin of the vertebrate-style eye, the origin of opsins, and the origin of photoreceptor cells.
Origin of vertebrates
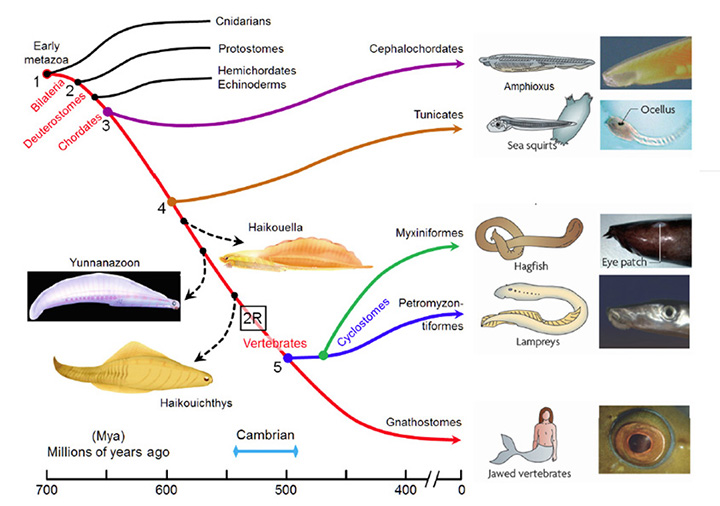
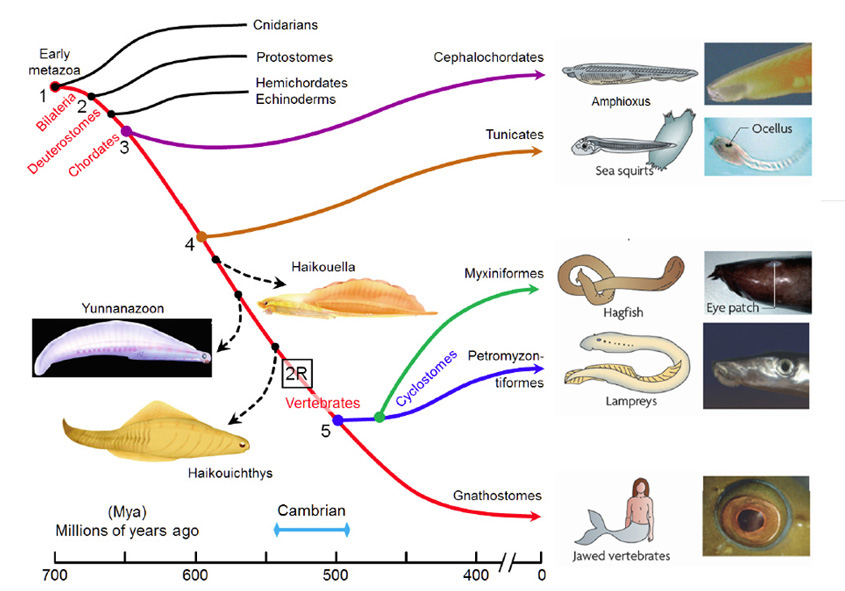
A summary view of the origin of vertebrates is presented in Figure 1, on a timescale extending from 700 to 400 Mya (millions of years ago); this diagram primarily illustrates extant taxa that are relevant to vertebrate evolution, though three extinct taxa of interest are also shown (using dashed lines). Although there is widespread agreement about the sequence of the branchings that occurred, there is less certainty about the timings of the various divergences. Molecular evidence suggests that many of the branchings occurred considerably earlier than is recorded in the fossil record, and the timings shown in Fig. 1 are based on a recent reconciliation of molecular and fossil evidence, as reported by Erwin et al (20). In the following discussion, the branchings will be described from the perspective of our own direct ancestors, shown by the red curve, with various important branchings numbered as #1 to #5.
Around 700 Mya (#1), the early Eumetazoa that were our distant ancestors separated into Cnidaria (e.g. jellyfish, corals, etc.) and our own line of bilaterally symmetric animals (Bilateria). Then, around 665 Mya (#2), the ancestors of the great majority of extant invertebrate species (Protostomes) diverged from our Deuterostome line. A few million years later, the common ancestor of Hemichordates and Echinoderms (e.g. sea urchins) diverged from our ancestors. Shortly afterwards, around 655 Mya (#3), Cephalochordates (e.g. amphioxus) diverged – and from the time of that split (at the latest) our ancestors have been chordates. After perhaps another 50 million years, at ~600 Mya (#4), Tunicates (e.g. sea squirts) diverged, and then a further 100 million years elapsed before the occurrence of the next split from which descendants have survived, when ancestors of the extant jawless vertebrates (Agnathans) diverged from our own lineage of jawed vertebrates (Gnathostomes), around 500 Mya (#5). From the time of that divergence (and possibly from some time before it) our ancestors have been vertebrates.
The preceding interval of ~100 million years, between #4 and #5 (from ~600–500 Mya), was important not only for the evolution of the vertebrate eye, but also more generally as a period of exceptional innovation in the evolution of body plans. Frustratingly, though, two factors complicate analysis of the transitions that occurred through that period. First, none of the numerous intermediate forms that diverged from our own lineage during that 100 million year time-span have survived to the present day. A few important examples of extinct species are indicated in Figure 1, but there is a huge gap between extant species. Secondly, soft tissues are poorly preserved in the fossil record, so that few clues to transitional forms of chordate eyes can be obtained from the extinct species that are known.
Despite these difficulties, there is a remarkable amount that we can surmise about vertebrate eye evolution, (i) from comparative analysis of the eyes of extant animals, (ii) from analysis of embryonic eye development, and (iii) from molecular genetic analysis of photoreceptors and retinas. But before we look in detail at the eyes of living vertebrates, it will help to set the scene if we briefly consider the origin of the vertebrate-style eye, the origin of opsin photopigments, and the origin of photoreceptor cells.
Origin of the vertebrate-style eye
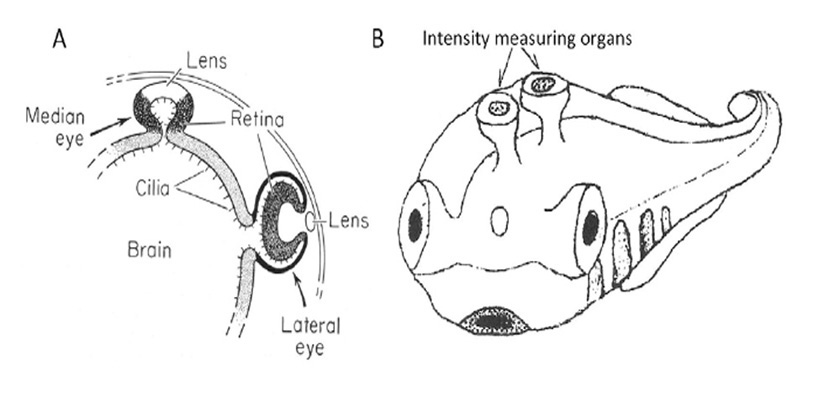
To help answer the question “When did the vertebrate-style eye arise?” one can usefully examine the eyes of extant chordates – jawed vertebrates, cyclostomes, tunicates, and cephalochordates.
Jawed vertebrates. The eyes of all extant jawed vertebrates (e.g. fish, tetrapods, birds) are remarkably similar and appear to be built to a common plan. As a result, we can be certain that the last common ancestor of all extant jawed vertebrates, that lived ~420 Mya, possessed a ‘vertebrate-style eye’. But can we delve even further back into the past?
Lampreys. The ancestors of lampreys diverged from our own ancestors around 500 Mya (Figure 1), yet the lamprey’s camera-style eyes are extremely similar to the eyes of jawed fish and other jawed vertebrates. Thus, the lamprey’s eye has a lens, an iris, and a set of six extraocular muscles that are broadly homologous to those of jawed vertebrates (Section 3). Furthermore, the lamprey retina has a structure closely comparable to that of vertebrates, with the five classes of homologous neurons (photoreceptors, horizontal, bipolar, amacrine and ganglion cells) distributed into three main nuclear layers and two plexiform layers. The southern hemisphere lamprey Geotria australis possesses five morphological classes of retinal photoreceptor cell together with five classes of opsin, each of which is closely related to the retinal opsins of jawed vertebrates (see Section 6 for details); northern hemisphere lamprey species, however, have lost several opsin classes.
In view of the overwhelming parallels between the eyes of lampreys and jawed vertebrates, it seems a near certainty that the last common ancestor of these taxa possessed a camera-type eye, broadly comparable to that of extant lampreys and gnathostomes, and hence that the vertebrate-style eye already existed ~500 Mya. However, one cannot totally reject the possibility that, even though the last common ancestor of lampreys and jawed vertebrates possessed the requisite genes, its eyes might have exhibited a form more rudimentary than a fully-developed camera-style eye, and that both lampreys and gnathostomes perfected the physical manifestation of the eye by some degree of convergent evolution.
Hagfish. The ‘eyes’ of hagfish represent a special case that will be considered in detail in Section 3. Although hagfish are descended from a lamprey-like ancestor, their ‘eyes’ exhibit a much more rudimentary form than the eyes of lampreys, and it will be argued that there are strong grounds for thinking that those features are retained from an earlier stage of eye evolution.
Tunicates and cephalochordates. Of the chordate taxa that diverged prior to the agnathans (lampreys and hagfish), none of those that survive to the present day possess an organ that can properly be described as an eye. The cephalochordate amphioxus possesses several groupings of photoreceptors, and larval tunicates possess a simple ocellus. In both cases ciliary photoreceptors with C-opsins are present, and appear homologous to our own cones and rods. Although their photoreceptors and opsins hold important clues to the evolution of vertebrate photoreceptors, these primitive organisms clearly diverged prior to the evolution of the vertebrate-style camera eye.
Some extinct chordates of interest. Three extinct species of early chordates are indicated schematically in Figure 1 (diagrams from Chen (21)). These are the earliest known chordates to have possessed structures that in extant vertebrates arise from migratory neural crest tissue, and accordingly they have been named ‘crest animals’, or Cristozoa, by Chen and colleagues. These pre-craniate crest fossils have been found only in the Lower Cambrian strata of Yunnan in south-western China; they are around 3 cm in length. Of the three species, Haikouella lanceolata has the most basal form, and Yunnanazoon lividum appears somewhat more developed, while Haikouichthys ercaicunensis is the most developed and appears to be a transitional form to craniates. Each of these fossil species is reported to exhibit paired eyes, with diameters of around 0.3, 0.6 and 0.4 mm respectively, but unfortunately so little detail of these soft tissues is preserved that it is not possible to describe the internal features of the eyes – even, for example, whether they possessed a lens.
Comparison of chordate eye features. Table 1 compares a number of features of the eyes (or eyespots) of the extant chordate species referred to above. There seems little doubt that the last common ancestor we shared with lampreys (~500 Mya, Figure 1, #5) possessed a fully-fledged ‘vertebrate-style camera eye’ while, further back, it seems inconceivable that our last common ancestor with sea squirts (~600 Mya, Figure 1, #4) could have had anything more complicated than a simple eyespot.
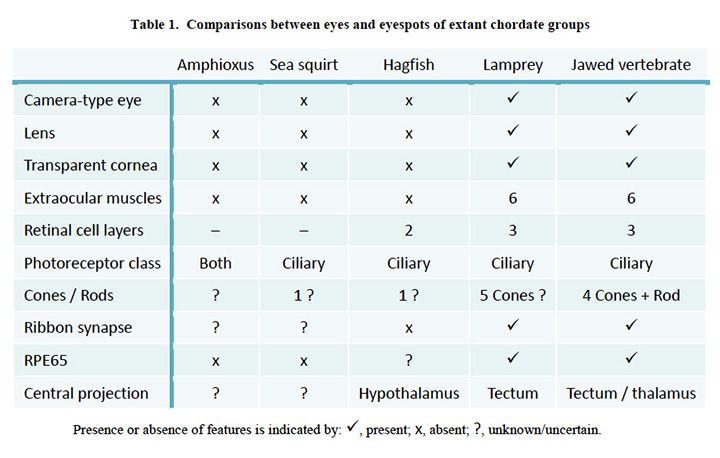 Comparisons between eyes and eyespots of extant chordate groups.
Comparisons between eyes and eyespots of extant chordate groups.
A crucial period for eye evolution in chordates and other phyla. Clearly then, the interval #4 to #5, from 600-500 Mya, was crucial to the evolution of the vertebrate-style eye. Furthermore, it seems plausible that the most profound changes in physical appearance occurred towards the end of that period, during the time of the Cambrian ‘explosion’ in body forms. At roughly the same time, eyes were evolving in a number of other phyla as well, often with radically different physical form (14, 22).
Recent discoveries of exceptionally well preserved fossil eyes from the Early Cambrian (~515 Mya) have shown that some of the earliest arthropods already possessed compound eyes containing many thousands of ommatidia. Paterson et al (23) have reported that Anomalocaris had eyes at least 12 mm in diameter, containing well over 16,000 ommatidia. These animals, which had bodies up to 90 cm long, are acknowledged as free-swimming apex predators, and have now been confirmed to have possessed compound eyes with the potential for high spatial resolution (~1°). Our own ancestors were tardy in developing eyes, and they may have been preyed upon by visually-guided protostomes for tens of millions of years.
Driving force for the evolution of eyes? In analyzing the emergence of sensory systems in the Cambrian, Plotnick et al (24) have proposed that two factors that rendered the acquisition of spatial vision highly valuable were, firstly, the enormous increase in spatial complexity in the landscape that occurred during the Cambrian and, secondly, the need of free-swimming organisms to navigate. Optic-flow information from spatial vision provided at least a partial solution to the latter requirement. On top of these pressures there was of course the need to detect prey and to avoid predators.
Origin of opsins
Opsins, and their major divisions (25) arose very early in metazoan evolution. In this article the term ‘opsin’ will refer only to ‘Type 2 animal opsins’, and not to the ‘Type 1 microbial opsins’ of bacteria or the ‘channelrhodopsins’ of algae, both of which are unrelated and appear to have arisen by convergent evolution. The phylogeny of ciliary opsins will be considered in Sections 5 and 6 for chordates generally, and for the vertebrate retina, but for now the questions are: How did the ancestral opsin originate? and What were the initial stages in its diversification?. In addressing these questions, important clues have been obtained through analysis of a number of cnidarian opsin sequences that have become available since 2007 (12, 26-31).
Animal opsins evolved from within the eponymous ‘Rhodopsin family’ of the ‘GRAFS’ superfamily of G-protein coupled receptors (GPCRs), and it is known that this superfamily originated in an ancient eukaryote that existed prior to the divergence of fungi (32)). Recently, Feuda et al (30)) analyzed the phylogeny of opsins and proposed a scheme for the early origin of opsins. They showed that the closest relatives of the opsins are found in the lineage that includes the vertebrate receptors for melatonin. However, for the corresponding GPCRs in invertebrates the ligand has not yet been identified, and so it is not clear what the ancestral ligand might have been at the time that the opsin lineage diverged.
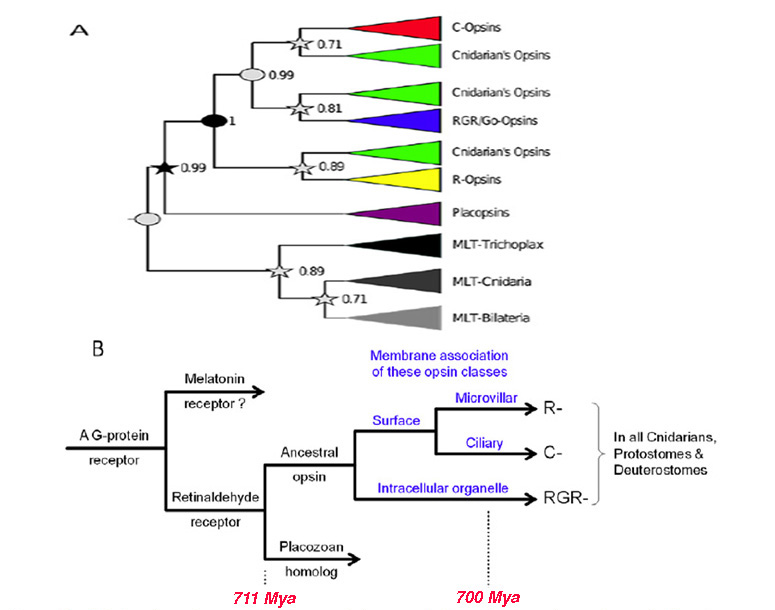
One potential problem with the analysis of Feuda et al (30) is its reliance on the (unproven) existence of R-opsins in cnidaria, but that issue appears to have been resolved by an independent and nearly simultaneous study of opsins from a coral (31), that clearly identified the existence of an R-opsin. The following scenario for the early origin of animal opsins, illustrated in Figure 2B builds on the report of Feuda et al (30), and is presented here as the first in a series of scenarios/hypotheses for the events that gave rise to photoreceptors:
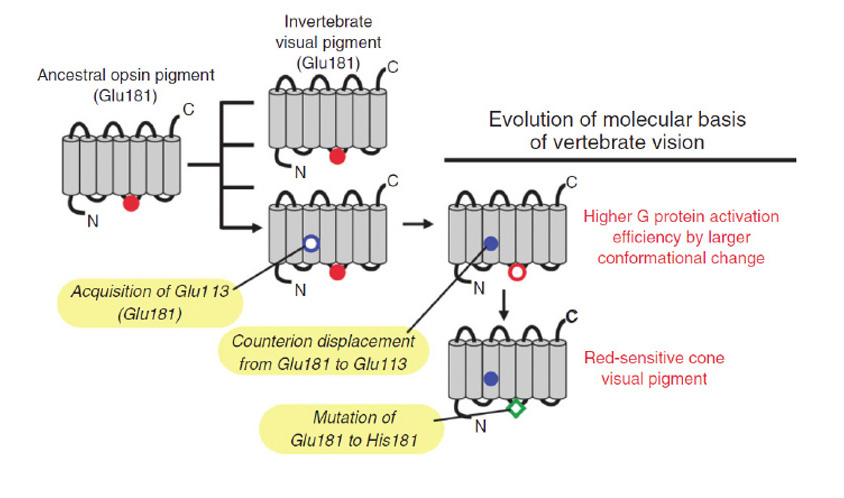
A-1) The forerunner of the first opsin arose through duplication of a GPCR in an ancient metazoan, at a time prior to the divergence of the amoeba-like placozoans.
A-2) That forerunner protein did not possess the retinal-binding lysine (‘K296’) in the seventh transmembrane helix (30); this suggests that retinaldehyde ligand occupied the internal cavity by means of non-covalent binding, as for ligands in conventional GPCRs, and in Figure 2B this pre-opsin is termed a ‘retinaldehyde receptor’. The placozoan Trichoplax has a homolog of opsin (dubbed placopsin by Feuda et al, 2012), that likewise is devoid of the retinal-binding lysine residue.
A-3) Acquisition of an appropriately situated lysine residue within the seventh transmembrane segment of that receptor allowed the retinaldehyde ligand to bind covalently. Initially, the Schiff base bond is likely to have been unprotonated, so that the molecule would have absorbed in the UV. Acquisition of an appropriately located negatively charged residue (e.g. E181) permitted the bond to be protonated, thereby creating the ancestral opsin, and enabling the absorption peak to be shifted into the ‘visible’ spectrum.
A-4) As for most opsins (though not for vertebrate visual opsins), the activated metarhodopsin state of this opsin was thermally stable and could undergo photoreversal to the rhodopsin state. Hence this protein probably did not require a source of 11-cis retinal and could instead utilize all-trans retinal perfectly well.
A-5) Subsequently, two duplications of that earliest opsin occurred, during the relatively short interval between the divergence of placozoa and the divergence of cnidarians from bilaterians. Thus, all of the duplications indicated in Figure 2B took place shortly prior to the first of the numbered branchings shown in Figure 1 (i.e. prior to #1).
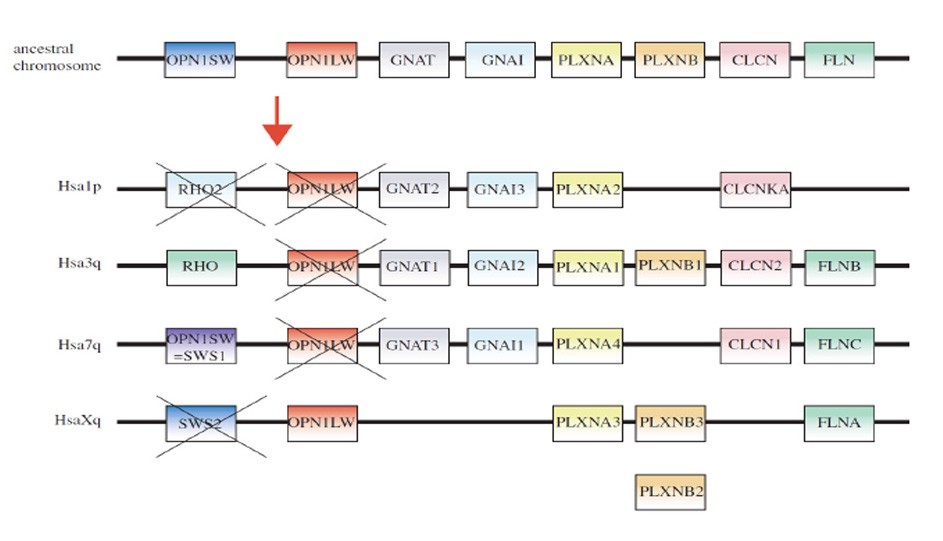
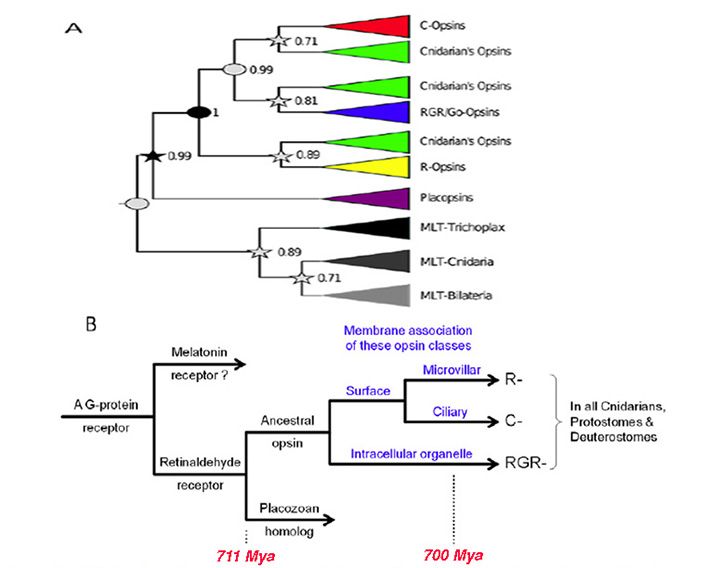 Figure 2. Origin of opsins, and their possible association with membrane type. A, Opsin phylogeny. Cnidarians have orthologs of each bilaterian opsin subfamily; i.e. the C-, R-, and RGR/Go-opsin subfamilies. Numbers indicate support values (Bayesian PPs) for key nodes. From Feuda et al (2012). B, Hypothesized duplications of ancestral opsin and its precursors, and suggested association with membrane type. An ancient GPCR (related to extant vertebrate melatonin receptors) duplicated, and its ligand became retinaldehyde, which bound non-covalently; this is denoted as ‘Retinaldehyde receptor’. After the divergence of the amoeba-like placozoans (~711 Mya), this GPCR evolved a lysine residue in its seventh transmembrane segment and a negatively charged residue (counterion) so that retinaldehyde bound covalently via a protonated Schiff base linkage; this form is denoted ‘Ancestral opsin’. Within a relatively short interval (prior to the divergence of cnidarians, ~700 Mya), this opsin duplicated twice, giving rise to three major families of opsins: C-opsins, R-opsins, and RGR/Go-opsins. It is proposed that these three opsins preferentially associated with ciliary membrane, microvillar membrane, and the membranes of intracellular organelles, respectively. Note that all these events occurred just prior to the starting point of Fig. 1.
Figure 2. Origin of opsins, and their possible association with membrane type. A, Opsin phylogeny. Cnidarians have orthologs of each bilaterian opsin subfamily; i.e. the C-, R-, and RGR/Go-opsin subfamilies. Numbers indicate support values (Bayesian PPs) for key nodes. From Feuda et al (2012). B, Hypothesized duplications of ancestral opsin and its precursors, and suggested association with membrane type. An ancient GPCR (related to extant vertebrate melatonin receptors) duplicated, and its ligand became retinaldehyde, which bound non-covalently; this is denoted as ‘Retinaldehyde receptor’. After the divergence of the amoeba-like placozoans (~711 Mya), this GPCR evolved a lysine residue in its seventh transmembrane segment and a negatively charged residue (counterion) so that retinaldehyde bound covalently via a protonated Schiff base linkage; this form is denoted ‘Ancestral opsin’. Within a relatively short interval (prior to the divergence of cnidarians, ~700 Mya), this opsin duplicated twice, giving rise to three major families of opsins: C-opsins, R-opsins, and RGR/Go-opsins. It is proposed that these three opsins preferentially associated with ciliary membrane, microvillar membrane, and the membranes of intracellular organelles, respectively. Note that all these events occurred just prior to the starting point of Fig. 1.
Hypothesized association between opsin type and membrane type . A contributory factor in the co-evolution of opsin classes and photoreceptor classes may have been a preferential association of the different opsins with different regions of membrane, as indicated in Figure 2B. Accordingly, the hypothetical scenario for the early evolution of opsins is extended as follows:
A-6) The two variants of opsin that emerged after the first duplication event may have trafficked preferentially to the membrane of sub-cellular organelles and to surface membrane. Those variants would have given rise to the RGR- division and the C-/R- division, respectively, of modern opsins.
A-7) Following the duplication event that created the distinction between C- and R-opsins, these two variants trafficked to ciliary and microvillar membrane, respectively. In Figure 2B this duplication is shown as having occurred subsequent to the duplication mentioned in the previous point, but at present one cannot reliably distinguish the order in which this pair of duplication events occurred.
A-8) Subsequently, cells expressing the C- and R-opsin classes became distinct from each other, through a process termed ‘division of labor’ (5, 33), leading to (a) ciliary photoreceptors that possessed C-opsins and (b) microvillar photoreceptors that possessed R-opsins; see next Section. The third variant of opsin, RGR-opsin, tended to be expressed in the membranes of intracellular organelles, possibly as an additional opsin in the first two classes of photoreceptors.
A-9) Later in evolution, further division of labor occurred, so that (for example) RGR-opsin could be expressed in separate cells. This would explain how it is possible, on the one hand, for squid photoreceptors to contain an R-opsin in their microvillar membranes as well as retinochrome (an RGR-opsin) in their intracellular organelles, and, on the other hand, for vertebrate cones and rods to contain only a C-opsin in their outer segments whereas RPE cells contain only RGR-opsin in their endoplasmic reticulum.
Classification and diversity of photoreceptor cell types
A view that pervades quite widely is that photoreceptor cells fall into two varieties: rhabdomeric photoreceptors in the eyes of invertebrates (protostomes) and ciliary photoreceptors in the eyes of chordates. However, neither the rigid association of photoreceptor type with phylum, nor the rigid classification of photoreceptors into two categories, can be supported.
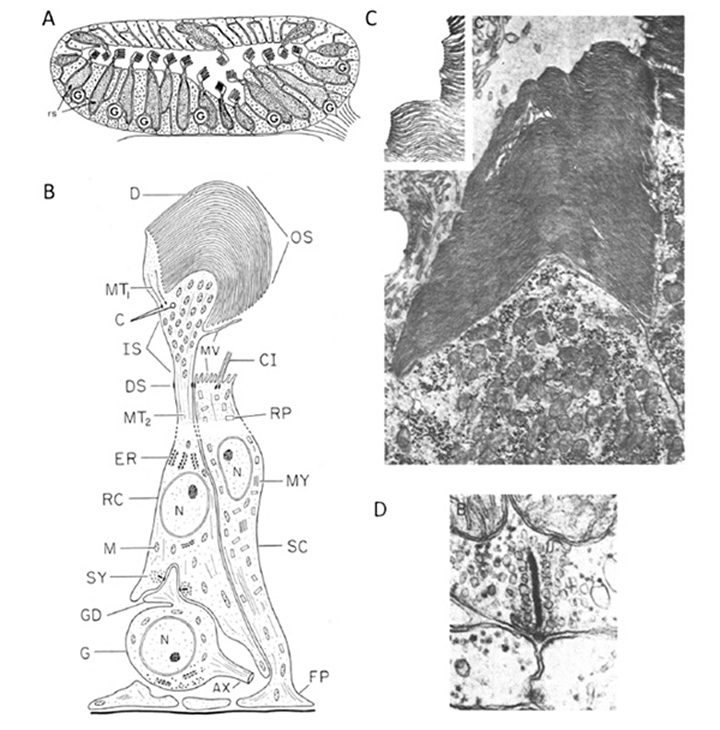
Ciliary and rhabdomeric classification. Based on the manner in which the opsin-containing region is elaborated into an extensive surface area, Eakin and his collaborators discerned two classes of photoreceptor: the ciliary form, exemplified by vertebrate rod and cone photoreceptors, where the membrane expansion forms a modified cilium, radiating from a classic 9+0 non-motile axonemal structure, and the rhabdomeric form, exemplified by insect photoreceptors, where the membrane expansion occurs as microvilli arranged in a highly-ordered manner (33); see for example Figure 3 and Figure 4 below.
Based to a substantial extent on Eakin’s classification, three theories were proposed to account for the evolution of photoreceptor types. Eakin himself proposed what became known as a diphyletic model of photoreceptor evolution, wherein the rhabdomeric line of photoreceptors arose in protostomes, as a variant of the ancestral line of ciliary photoreceptors (34). Eakin acknowledged a number of exceptions to his model (such as deuterostomes with microvillar photoreceptors), but he proposed that those exceptions arose via independent evolution.
In a variant of Eakin’s model, Vanfleteren & Coomans (35) interpreted rhabdomeric photoreceptors to represent a modified form of the ancestral ciliary photoreceptors, in which the elaboration of the microvillar membranes is induced to occur by a ciliary structure that often degenerates subsequently; other authors termed this a monophyletic model of photoreceptor evolution. In contra-distinction to both these models, Salvini-Plawen & Mayr (17) proposed that photoreceptors had evolved independently on at least 40 occasions, in what they termed a polyphyletic origin of photoreceptors. With the benefit of hindsight, one can see that there are aspects of both Eakin’s and Vanfleteren & Coomans’ models that had merit.
Diversity of photoreceptor types. The diversity of photoreceptor types across the animal kingdom is truly remarkable, to the extent that in perhaps the majority of organisms photoreceptor morphology fails to fall neatly into the two categories of ciliary and rhabdomeric. For an overview of the range of light-sensitive structures that occur in different organisms, the interested reader is referred to the comprehensive summary of photoreceptor morphology provided by Vanfleteren (36). Numerous kinds of membrane elaboration occur, quite apart from the conventional ‘ciliary’ and ‘rhabdomeric’ structures.
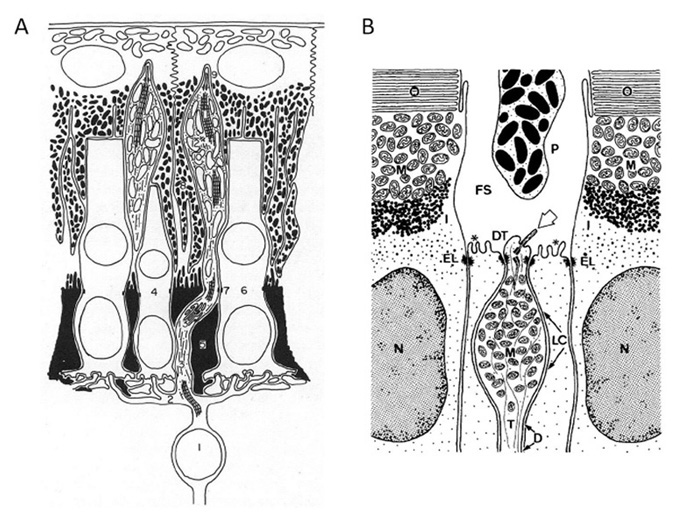
Furthermore both ciliary and microvillar forms of membrane frequently co-occur within a single type of photoreceptor, as sketched in Figure 3 (36). For example, microvillar photoreceptors often exhibit ciliary structures such as centrioles during development (Figure 3A), though in most cases in protostomes these cilia are transient.
Importantly, such co-occurrence is also seen in deuterostome (and even vertebrate) photoreceptors. In the microvillar photoreceptors of amphioxus, the cilia persist; thus, Hesse cells bear a single 9+0 cilium, while Joseph cells bear two (37). And vertebrate ciliary photoreceptors typically possess microvilli that emanate from the distal region of the inner segment and that appear closely associated with the ciliary outer segment (Figure 3B3); however, these microvilli do not exhibit the organization typical of rhabdomeric photoreceptors, at right angles to the incident light, and instead they are arranged longitudinally. A third example amongst deuterostomes is found in hemichordates, where the cerebral eyes in the larvae of an acorn worm contain photoreceptor cells that possess both a well-developed cilium and numerous microvilli closely packed and at right-angles to the axis (38).
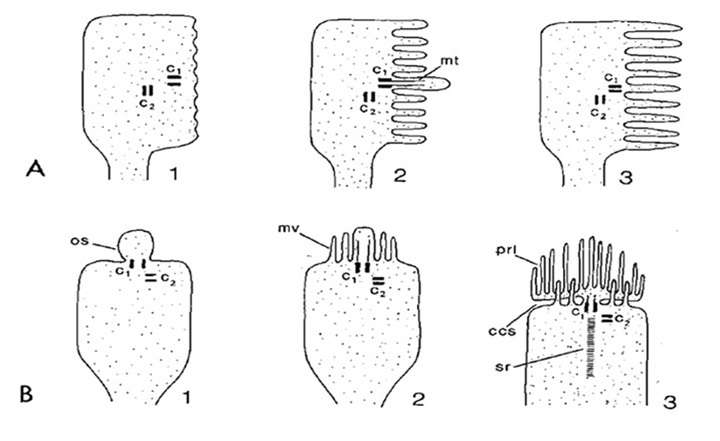 Figure 3. Co-occurrence of ciliary and microvillar structures within photoreceptors. Sketches of three steps in the development of a rhabdomeric photoreceptor (A) and a ciliary photoreceptor (B). A, Arthropod rhabdomeric photoreceptor. B, Tunicate ciliary photoreceptor. In both cases paired centrioles (c1, c2 are seen. In rhabdomeric photoreceptors, the ciliary apparatus may disappear during subsequent development. In the chordate photoreceptor, an outer segment bud appears first, then microvilli develop, and subsequently the ciliary membrane (of the outer segment) expands. Note that this diagram was designed to illustrate Vanfleteren & Coomans’ model of ciliary induction of photoreceptor membrane, but is here presented simply to sketch the concept of co-occurrence of structures. c1, c2, distal and proximal centrioles; ccs, circumciliary space; mt, microtubules; mv, microvilli; os, outer segment; prl, photoreceptive lamellae; sr, striated rootlet. From Vanfleteren (1982).
Figure 3. Co-occurrence of ciliary and microvillar structures within photoreceptors. Sketches of three steps in the development of a rhabdomeric photoreceptor (A) and a ciliary photoreceptor (B). A, Arthropod rhabdomeric photoreceptor. B, Tunicate ciliary photoreceptor. In both cases paired centrioles (c1, c2 are seen. In rhabdomeric photoreceptors, the ciliary apparatus may disappear during subsequent development. In the chordate photoreceptor, an outer segment bud appears first, then microvilli develop, and subsequently the ciliary membrane (of the outer segment) expands. Note that this diagram was designed to illustrate Vanfleteren & Coomans’ model of ciliary induction of photoreceptor membrane, but is here presented simply to sketch the concept of co-occurrence of structures. c1, c2, distal and proximal centrioles; ccs, circumciliary space; mt, microtubules; mv, microvilli; os, outer segment; prl, photoreceptive lamellae; sr, striated rootlet. From Vanfleteren (1982).
A final illuminating example comes from a marine gastropod, Aporrhais pespelecani. In the larval eye, the photoreceptors are ciliary, but at metamorphosis the ciliary photoreceptors additionally develop microvilli, thereby undergoing conversion into mixed ciliary-plus-microvillar photoreceptors in the adult (39); unfortunately, the nature of the opsin and transduction cascade in these photoreceptors is not known.
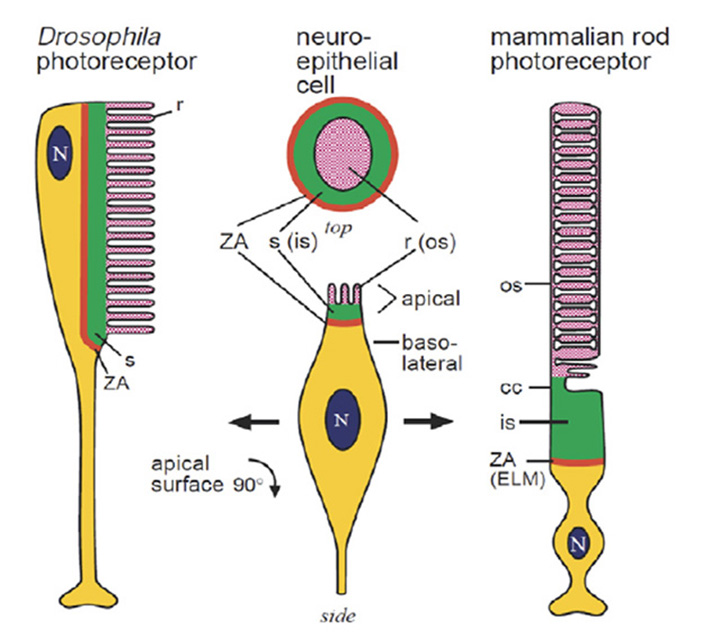
Even the extreme cases are homologous. In light of the sheer diversity in photoreceptor morphologies, one can view the classical cases of the fly photoreceptor and the vertebrate rod cell as representing extrema in a vast gradation of photoreceptor structural types. But even though the morphological disparity between these two cell types is large, the homology between them (Figure 4) is quite remarkable, as noted by Ready & Tepass (40). Both cells develop from a simple columnar epithelium. Both retain a zonula adherens (ZA) region that delineates basal from apical membrane, and that links neighboring cells; in the vertebrate retina, these intercellular contacts form the outer limiting membrane. In developing photoreceptors of both types, two sub-domains develop in the membrane apical to the ZA. The first is a Crumbs-rich supporting domain that in Drosophila forms the fly stalk, and that in vertebrate photoreceptors forms the inner segment. The second more apical sub-domain expands massively to form the light-sensitive membrane, in Drosophila as microvilli, and in vertebrate photoreceptors as the ciliary sacs or discs.
Scenario for the origin of photoreceptor cells
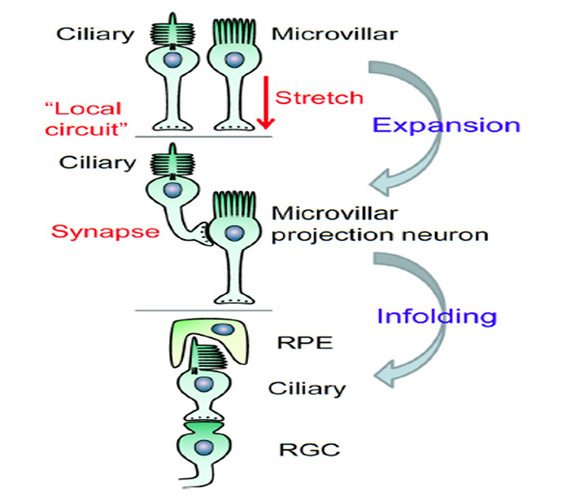
By drawing together threads from the concepts above, the following scenario is proposed for the origin of the main two morphological variants of photoreceptor, namely ciliary and microvillar:
B-1) The ciliary variant represents the ancestral class of photoreceptor. That ancestral photoreceptor expressed the ancestral opsin in its ciliary membrane and it also exhibited microvilli extending from its soma in the vicinity of the cilium.
B-2) In deuterostomes and cnidarians, mechanisms evolved for the massive elaboration of the ciliary region of cell membrane.
B-3) After R-opsins diverged from C-opsins (see above), they tended to traffic to microvillar membranes.
B-4) In bilaterians, mechanisms evolved for the elaboration of those microvillar membranes, leading to the principal distinction between ‘ciliary’ and ‘microvillar’ photoreceptors.
B-5) In certain arthropods and mollusks the elaboration of microvilli culminated in the formation of highly-organized rhabdomeres, and hence the evolution of a number of cases of truly ‘rhabdomeric’ photoreceptors.
B-6) Many different mechanisms for elaboration of the opsin-containing membrane have arisen, that have led to the evolution of many different varieties of membrane specialization, and hence to the formation of a vast number of photoreceptor morphologies in different organisms.
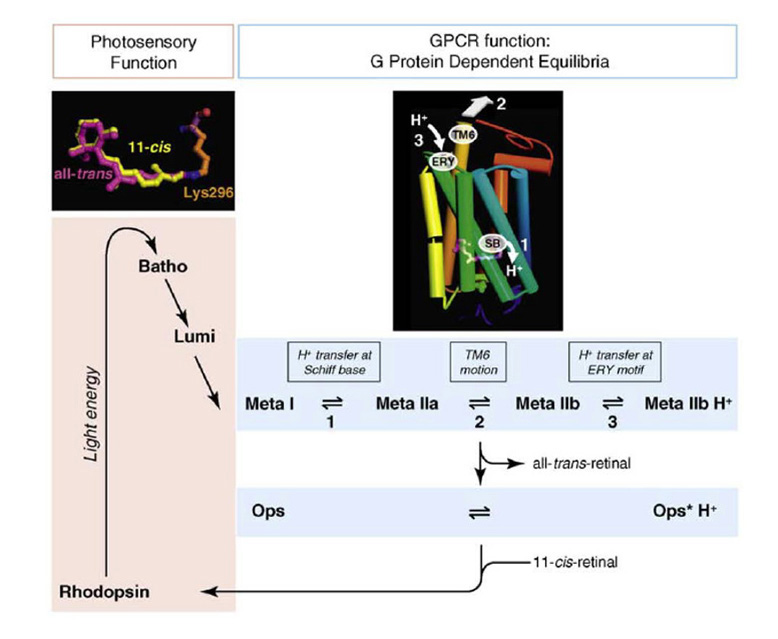
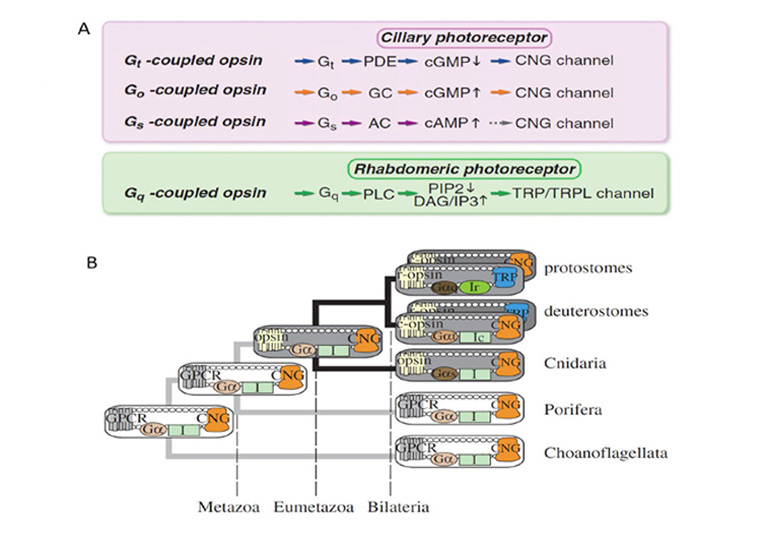
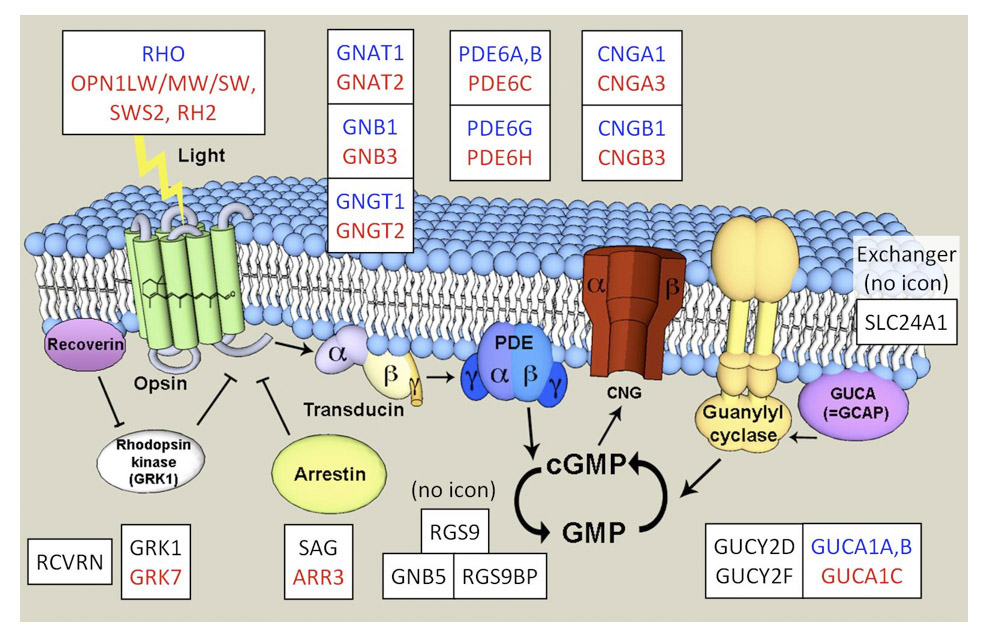
Association between opsin class and transduction cascade. There is a strong association between the class of opsin and the nature of the G-protein transduction cascade in the photoreceptor. In vertebrates, C-opsins typically activate a member of the Gi family (which includes Gt and Ggust), leading to modulation of cyclic nucleotide levels and to altered opening of CNGCs (cyclic nucleotide gated channels) and thereby to generation of the electrical response. R-opsins instead activate a Gq, which uses a PLC (phospholipase C) as the effector protein, with TRP/C channels usually generating the electrical activity. Plachetzki et al (27) provide evidence that the ancestral opsin is likely to have employed CNGCs to mediate its electrical response, and that the linkage of R-opsins to TRP/C channels is likely to have arisen subsequently. The molecular genetic evidence for the co-evolution of opsins and their transduction cascades will be expanded upon in Section 8.
3 Ciliary photoreceptors in the eyes of extant chordates
In order to understand vertebrate eye evolution, one of the primary challenges is to explain the sequence of events by which a handful of photoreceptors and a pigment cell in an ancient chordate ancestor could have evolved into an exquisite retina, by the time that vertebrates appeared. (Closely related issues, that will not be treated here, concern the co-evolution of the requisite optical and motor apparatus, and of appropriate brain areas.) In considering the evolution of the vertebrate retina, we will now survey the light-sensitive organs of living chordate species, beginning with the simplest ocelli and progressing to the vertebrate retina. For each example of chordate light-sensitive organ, we will briefly examine the general features of the organ, and then concentrate on its ciliary photoreceptors.
In Section 7 we will consider why it was ciliary photoreceptors rather than microvillar photoreceptors that triumphed in chordate and vertebrate eyes. Then in later sections we will examine the embryological development of the mammalian retina, as well as molecular clues to eye evolution.
Cephalochordate light-sensitive organs (amphioxus)
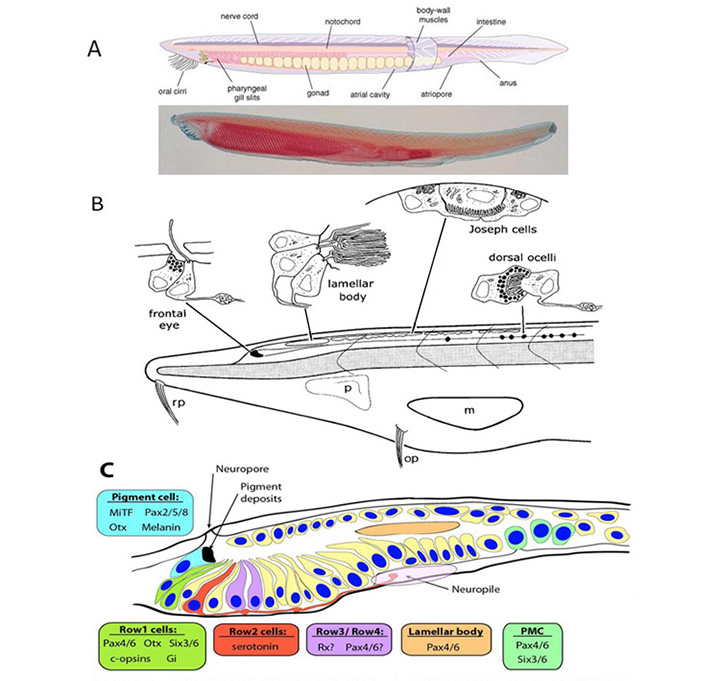
Lacalli (41, 42) has described four distinct light-sensing regions in the cephalochordate, amphioxus (Figure 5). Each of these regions lies along the neural tube, in a fairly dorsal position, on the midline (i.e. unpaired), and none has any kind of lens or other imaging apparatus. Two of these organs contain ciliary cells (usually presumed to be photoreceptors) whereas the other two contain microvillar photoreceptors. Although these latter cells have in the past often been referred to as ‘rhabdomeric’ cells, they do not actually display the highly-organized structure of genuine rhabdomeres and it is preferable to refer to them as ‘microvillar’, as used by Gomez et al (43) and co-workers.
Ciliary photoreceptors. The ‘frontal eye’ is a tiny rostral region containing a few ciliary cells that has been proposed to be the homolog of the vertebrate lateral eyes. The ‘lamellar body’, not far behind the frontal eye, also contains ciliary cells, which in this case exhibit very extensive lamellar membranes; this organ has been proposed as the homolog of the vertebrate pineal. Although the lamellar body is present as a distinct organ in larvae, the cells appear to disperse in the adult. To date, neither of these types of ciliary cell (frontal eye or lamellar body) has actually been shown to be light-responsive.
Microvillar photoreceptors. A little more caudally, a set of ‘Joseph cells’ is found, each being a microvillar photoreceptor, and further caudally a chain of ‘dorsal ocelli’ or ‘organs of Hesse’ are found, that each contain a microvillar photoreceptor partly enveloped by a pigment cell. Recently, it has been established that phototransduction in these cells is closely homologous to that in rhabdomeric photoreceptors of protostomes, utilizing the R-opsin melanopsin (44) to trigger a classical ‘rhabdomeric’ transduction cascade that involves Gq and PLC (45, 46), and that presumably is closely similar to the cascade in the intrinsically photosensitive retinal ganglion cells (ipRGCs) of the vertebrate retina.
Molecular markers. Very recently, Vopalensky et al (47) identified a number of molecular markers expressed in cells of the frontal eye of amphioxus (Figure 5C), and they concluded that this organ indeed appears homologous to the lateral eyes of vertebrates. In particular, the simple ciliary cells in the first row of the frontal eye appear homologous to cone and rod photoreceptors, in co-expressing C-opsins along with the transcription factors Pax4/6, Otx and Six3/6; furthermore, the presence of the inhibitory Gi-type G-protein alpha subunit is suggestive of the vertebrate-style OFF response to light. Cells in the second row might conceivably be homologous to retinal ganglion cells; they project axons to the neuropil, and they contain serotonin, though so far there is insufficient information to decide on their possible homology to ganglion cells. Finally, the pigmented cells appear homologous to vertebrate RPE cells, in terms of melanin content, location adjacent to the photoreceptors, and regulatory signature (of Mitf, Otx, and Pax2/5/8).
The previously proposed homology of the lamellar body to the vertebrate pineal organ is not supported, because of the demonstrated absence of both Otx and Rx – as well as by the inability to detect an opsin. Thus, although the cells of the lamellar body clearly exhibit a ciliary lamellate ultrastructure, there is as yet no evidence that they are photoreceptors, and the organ appears not to be homologous to the vertebrate eyes or pineal.
Electrophysiology. The Joseph cells and dorsal ocellar cells of amphioxus have recently been studied using electrophysiological techniques, and unequivocally identified as microvillar photoreceptors (43), but the ciliary cells of the frontal eye and lamellar body have not yet been studied in this way.
Tunicate ocellus (larval Ciona and Aplidium)
Ocellus. The closest extant sister group to vertebrates comprises the tunicates, including sea squirts such as Ciona intestinalis and Aplidium constellatum. The sessile adult form of the sea squirt has not been reported to possess any kind of discrete eyespot, though scattered opsin-expressing cells apparently occur. However, the tadpole-like larval stage (Fig. 6A) has a simple photosensory organ, termed an ocellus (Figure 6D), that contains a handful of ciliary photoreceptors surrounded by a single large pigment-containing cell. It has been suggested that this ocellus is the remnant of paired ocelli in an ancestor (48). The larva does little, beyond swimming to the bottom, embedding its head on a rock, dissolving its nervous system, and transforming itself into a squirt.
Photoreceptor cells. The ciliary photoreceptors of two species of sea squirt are illustrated in Figures 6B-E (from (49, 50)). A total of a dozen or so photoreceptor cells (about seven are sectioned in Figure 6B) protrude through the single pigment cell, with their outer segments lying beneath the lens formed by the three lens vesicle (LV) cells. A schematized Ciona photoreceptor is shown in Figure 6C. A large somatic region gives rise at its base to an axonal process (AX), while from its upper end a process penetrates the pigmented cell and gives rise to the axoneme (A), from which expands the outer segment (OS) with its lamellae (L). The electron micrographs from the closely-related ascidian, Aplidium, were obtained transversely (Figure 6D) and longitudinally (Figure 6E) with respect to the axis of the outer segment, and show the rather ‘petal-like’ concentric arrangement of lamellae. Both the studies above reported microvilli interdigitating with the lamellae, though Eakin & Kuda (50) suggested that these originated from the pigment cell (Figure 6C) whereas Barnes (49) reported that they originated from the inner segment of the photoreceptor (see Figure 6E).
Electrophysiology. Electrical responses have been recorded from ascidian ciliary photoreceptors in only one study, on Aplidium constellatum (51), where it was reported that it “proved exceedingly difficult to record electrical activity from these preparations, possibly because the retinal cells, which lie close to the surface of the small animal, are often damaged by the removal of the thick tunic”. The rare penetrations gave resting potentials of -5 to -20 mV, with hyperpolarizing light responses of small amplitude (2 to 7 mV) that were accompanied by a decrease in membrane conductance. These responses are qualitatively similar to those of vertebrate retinal photoreceptors (with the small amplitude probably explained by damage), though there is insufficient data to allow a proper comparison to be made.
Hagfish eye (Eptatretus species)
There is now strong evidence that hagfish are descendants of a lamprey-like ancestor and that many of their morphological features have ‘degenerated’ from a more complex form. That interpretation is certainly accepted here but, notwithstanding this, the viewpoint that will be advanced below is that the hagfish eye provides a window into an early transitional form in the evolution of the vertebrate-style eye, as suggested previously by Lamb et al (3).
Although controversy has long surrounded the phylogenic position of hagfish, there is now powerful evidence that hagfish form a clade with lampreys (52), as indicated in Figure 1. Hagfish have the simplest body plan of all vertebrates (Figure 7A, B). They inhabit the oceans around most continents, often at great depths (in many cases 200 m or more) where they scavenge fallen carcasses; recently they have been found also to be predatory (53).
Behaviorally, hagfish appear blind. However, in captivity, exposure to the onset of bright light leads, after a long delay (of the order of 10 s or more), to the onset of swimming and sometimes attempted burrowing. Upon removal of the animal’s eyes, Newth & Ross (54) reported that the delay was unchanged, though Kobayashi (55) instead reported that it nearly doubled from around 10 s to 20 s. In either case, the eyes of the hagfish appear to mediate little in the way of rapid photosensitive behavior.
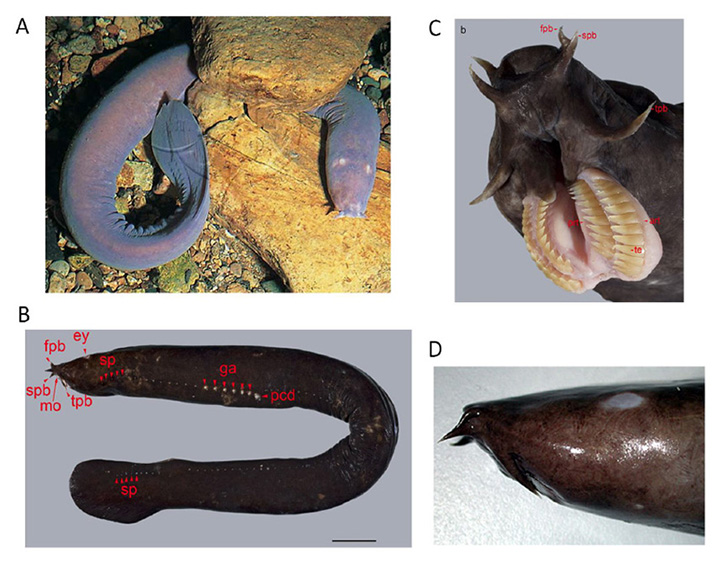 Figure 7. New Zealand Hagfish (Eptatretus cirrhatus) A, Living hagfish. From Image Quest Marine. B, Slime pores (sp) and gill apertures (ga) of hagfish. From Zintzen et al (2011). C, Tentacles and mouth of hagfish. From Zintzen et al (2011). D, Side view of hagfish head, showing pale eyespot. Copyright Australian Museum.
Figure 7. New Zealand Hagfish (Eptatretus cirrhatus) A, Living hagfish. From Image Quest Marine. B, Slime pores (sp) and gill apertures (ga) of hagfish. From Zintzen et al (2011). C, Tentacles and mouth of hagfish. From Zintzen et al (2011). D, Side view of hagfish head, showing pale eyespot. Copyright Australian Museum.
Hagfish are able to secrete a potent slime from a series of lateral pores (Figure 7B), and this slime functions as a highly-effective defense mechanism. The spectacular movies (Supplementary Video S1, Supplementary Video S2) obtained by Zintzen et al (53) show the rapid release of slime into the mouth of a predator at the moment that it bites a hagfish, accompanied by an almost instantaneous gagging reaction and retreat by the predator. Of 14 attacks that were filmed, none was successful, and in each case the hagfish continued swimming as if nothing had happened. The evolution of such a successful defense mechanism may have enabled hagfish to have survived in their niche virtually unaltered for hundreds of millions of years.
Hagfish eyes. Locket & Jorgensen (56) provided a comprehensive review of hagfish eyes, which had first been studied in the late nineteenth century, and then using electron microscopy in the late twentieth century. The following description for Eptatretus species is based largely on the reports of Holmberg (57, 58), Fernholm & Holmberg (59) and Locket & Jorgensen (56).
General features of the hagfish eye. At the location on the head where one would expect to find an eye, the hagfish simply has a patch of translucent (almost transparent) skin (Figure 7D). Beneath this translucent patch of skin is what in the hagfish passes for an eye (Figure 8A, Figure 8B). This organ has no extraocular muscles, no lens, and no iris, and is embedded in fat, through which a very slender optic nerve passes. The size of the eye varies considerably between individuals, but is typically around 1 – 1.5 mm in diameter, though rarely spherical in shape. The sclera/cornea is not divided into separate opaque and transparent regions, and is instead fairly uniformly translucent. No pigmentation is found in the eye, either in the sclera, choroid, or retinal epithelium, even though the skin of the animal may be darkly pigmented. Lining the sclera is a tenuous layer of capillaries, presumably comparable to the choroidal vasculature of jawed vertebrates. In the absence of a lens, much of the chamber is filled with the vitreous body. The optic nerve is thin, containing only a couple of thousand unmyelinated axons. Neither hagfish nor lampreys exhibit myelin (60, 61), and these axons project predominantly to the hypothalamus (62, 63), just as their likely homologs, the melanopsin-expressing ipRGCs, do in mammals (64).
The hagfish retina is roughly cup-shaped and lines half to two-thirds of the globe, though the choroid fissure often remains open (65). As for jawed vertebrates, the retina comprises two apposed layers, the neural retina and the retinal epithelium, though in hagfish the epithelial layer is unpigmented. Several authors have remarked that the neural retina and retinal epithelium often seem to be separated by a gap, but it is possible that this is a fixation artifact (perhaps arising from dilution of the high tonicity extracellular medium, which resembles sea-water). At the peripheral margin of the retina, the inner layer reduces to a single layer of cells and is continuous with the outer layer, though there is no extension to a ciliary body or iris.
Neural retina. The neural retina (Figure 8B) of hagfish is simpler than that of lampreys or jawed vertebrates, with only two layers of somata, comprising photoreceptor cells and projection neurons. No author has reported identifiable horizontal cells or bipolar cells, though Locket & Jorgensen (56) reported some instances of a ciliated structure resembling a Landolt club. The neural arrangement of the hagfish retina is strongly reminiscent of the pineal organ in non-mammalian vertebrates (see Section 3) and, interestingly, the hagfish lacks a pineal. It is presumed that hagfish photoreceptors make direct synaptic contact onto the projection neurons (ganglion cells), though as yet the identity of the cells that are post-synaptic at the photoreceptor synapse has not been determined. There are distinct outer and inner limiting membranes bounding the retina.
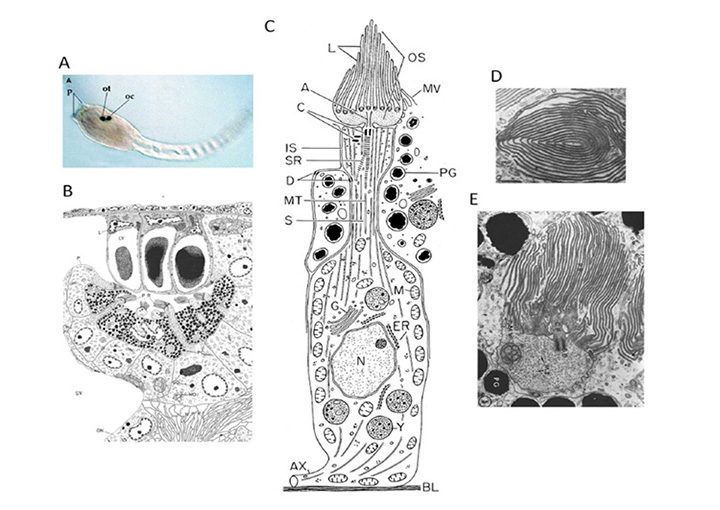
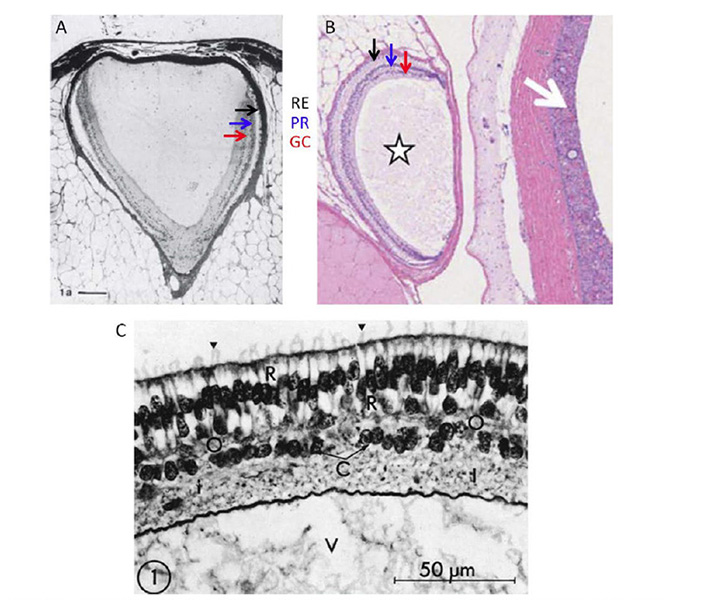
Photoreceptor morphology. The morphology of photoreceptors from the hagfish Eptatretus stoutii is illustrated in Figure 9 (57). The schematic in Figure 9A shows the general arrangement, of roughly cylindrical receptor cells (R) surrounded by glial cells (G). The region corresponding to the inner segment lies vitreal to the outer limiting membrane, in contrast to the situation in jawed vertebrates and lampreys. It contains a region corresponding to the ellipsoid, packed with mitochondria (Figure 9C), from which the cilium arises, but there is no sign of a paraboloid or myoid. The outer segment protrudes through the outer limiting membrane into the ventricular/extracellular space (ES), where it comes into contact with a non-pigmented epithelial cell (E) and the fine processes that descend from that cell. Compared with other vertebrate retinas, the outer segments are packed very sparsely in the ventricular space (Figure 8B and Figure 9A).
The electron micrographs in Figure 9B and Figure 9C show the lamellar arrangement of the outer segment membranes. According to Holmberg (57) and Locket & Jorgensen (56) the lamellae are enclosed by the plasma membrane, though this is not clear-cut from the micrographs. Some authors report the lamellae to be quite regular (Figure 9B (57)), while others do not; e.g. “These discs in Eptatretus, however, are not stacked closely, but in a loose and often disordered way” (56). The cilium has the classical 9+0 double filament structure, but unusually is located centrally, on the axis of the inner and outer segments, so that the outer segment lamellae extend roughly symmetrically on either side of the cilium (Figure 9C); this contrasts with the situation in vertebrate cones and rods where the cilium is located at the edge of the outer segment.
At its base, the receptor cell is invaginated by a synaptic contact (Figure 9A), and the synaptic zone (Figure 9D) is characterized by synaptic vesicles surrounding a ‘synaptic body’ (SB), rather than a conventional synaptic ribbon. The contact is of the dyad type, rather than the triad found in the photoreceptors of lampreys and most jawed vertebrates, though (as mentioned above) the identity of the post-synaptic elements has not been reported. Nevertheless, it is presumed that there must be direct synaptic contact from photoreceptors onto projection neurons, as no other cell types have been reported.
Electrophysiology. Single cell recordings have not been made from hagfish photoreceptors, though ERG recordings were reported by Kobayashi (55), for a species named as Myxine garmani but subsequently reported by Fernholm & Holmberg (59) to have been Eptatretus burgeri. The excised eye was used, and recordings were made between a wick electrode on the surface of the eye and a moistened cloth on which the eye sat. Dim flashes elicited a slow response of characteristic positive-then-negative shape, and the amplitude of this complex response saturated at a relatively low intensity of ~10 lux. For bright flashes a small slow negative-going wave preceded this complex response. The spectral sensitivity of the response was maximal at around 500 nm, suggestive of rhodopsin.
Although Kobayashi interpreted the complex positive-then-negative wave as analogous to the b-wave of the ERG from the vertebrate eye, there were some remarkable properties that suggest a different interpretation. First, this response exhibited a relatively long latency of ~350 ms prior to a fairly rapid climb to its positive peak in a further ~200 ms. Secondly, the form of this complex response was completely unchanged, either when the flash intensity was further increased or when the flash duration was varied from 3 ms up to 1 s. Thirdly, the response exhibited a lengthy refractory period, so that an interval of ~4 s was required after a flash of 10 lux, before any response could be elicited from a second identical flash. Fourthly, dim adapting light could completely eliminate this response, and then during subsequent dark adaptation the response reappeared fairly abruptly.
From the combination of these features I suggest the alternative interpretation that the positive-then-negative response actually reflected the synchronous firing of very slow regenerative potentials (‘action potentials’) in the projection neurons (ganglion cells). For the future, it should be possible to test this assertion by making more comprehensive electrophysiological recordings from hagfish retinal cells, including intracellular recordings from ganglion cells, suction pipette recordings from photoreceptors, and further ERG recordings.
Hagfish photoreceptors exhibit some rod-like properties. In summary, the photoreceptors of Eptatretus species exhibit a number of rod-like properties: e.g. the outer segment lamellae are reported to be disc-like, the inner segment lacks a paraboloid or myoid, and the electrical response shows peak sensitivity at around 500 nm. However, there is still no evidence as to whether these cells can respond reliably to individual photons.
Lamprey pineal (Petromyzon marinus ammocoete)
Like the retina of the lateral eyes, the pineal is an evagination of the diencephalon, though it emerges upwards on the dorsal midline (see Section 13). In non-mammalian vertebrates, the pineal contains light-sensitive ciliary photoreceptors, and all ultrastructural work has shown the existence of only three main cell types: photoreceptors, projection neurons (ganglion cells), and glial cells. The photoreceptors make ribbon synapses onto ganglion cells, which send axons to the hypothalamus.
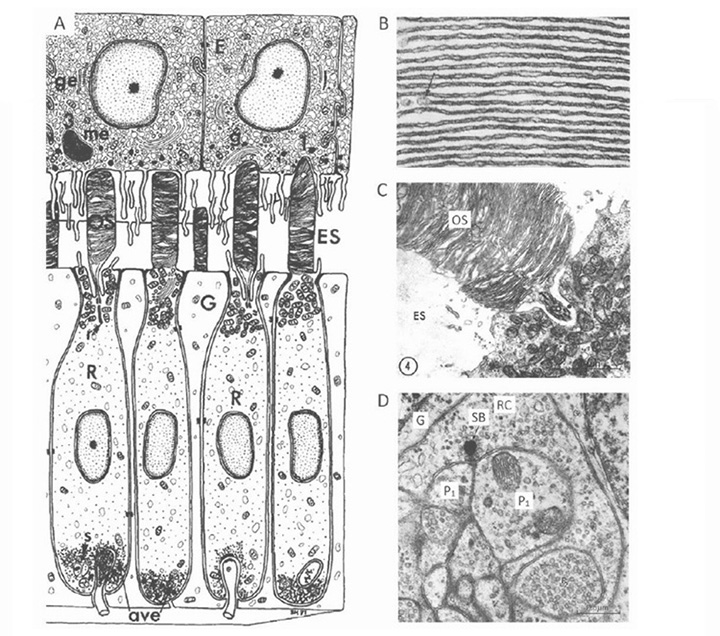
The photoreceptors in the pineal of the larval sea lamprey, Petromyzon marinus, have been investigated by Pu & Dowling (66), using light and electron microscopy as well as intracellular electrophysiological recording, and the following is based on their report. Figure 10 illustrates pineal photoreceptors and their organization within the pineal. The diagram in Figure 10A sketches the overall arrangement of the organ, while the schematic in Figure 10B illustrates the detailed features of the photoreceptor cell and its synaptic contacts.
Photoreceptor morphology. As sketched in Figure 10B (67), the photoreceptor cell is approximately cylindrical for most of its length, with a diameter approaching 10 µm, but the outer segments are often broader, with widths of 10 – 25 µm. The outer segment contains numerous lamellae, that can number more than 100 (Figure10C), which contrasts with the view of Nilsson (13) that there is “very limited membrane stacking” of pineal membranes. The lamellae are rarely flat, but usually somewhat curved, and they are reported to be somewhat less regularly stacked than for cone and rod outer segments in gnathostome retinas. The outer segment membrane is like that of cones, in being continuous with the plasma membrane (Figure 10C).
As well as the outer segment, a prominent inner segment protrudes into the lumen. At the base of the cell, synaptic contact is made with ganglion cells, predominantly at flat ribbon synapses (Figure 10B, Figure 10D), arranged either as dyads (Figure 10D) or ‘monads’; triads were never seen, and nor were feedback synapses (66).
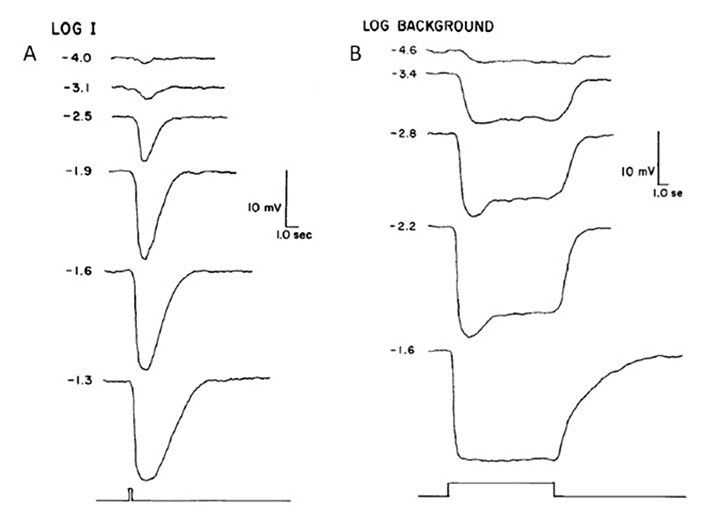
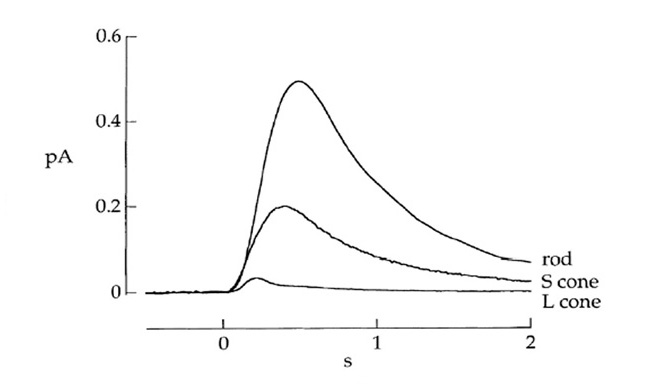
Electrical response to light. Pu & Dowling (66) made intracellular voltage recordings from these pineal photoreceptors, and response to flashes and steps of light are shown in Figure 11. The response was always a slow graded hyperpolarization, broadly similar to that recorded from vertebrate cones or rods, though without the characteristic rapid relaxation from an initial peak back to a plateau for bright stimuli. The flash responses were more than a log unit less sensitive than for cones (of the mudpuppy) and the spectral sensitivity peaked at around 545 nm. The time-to-peak for dim flashes was around 1 s (Figure 11A), much slower than for cones at room temperature, though similar to rods. The response-versus-intensity relation for flashes followed a hyperbolic saturation, I / (I+σ). In response to prolonged illumination, the response slowly sagged, except at the highest intensities, for which it remained saturated (Figure 11B). Responses to incremental flashes were desensitized, roughly according to Weber’s law, though much of the desensitization was the result of response compression, and only about 1.5 log units was due to a scaling of σ. At the cessation of steady light, the response began recovering immediately, as occurs in cones. Following intense ‘bleaching’ exposures, the sensitivity was fully recovered within 4 min, again similar to cones rather than rods.
Kusmic et al (68) obtained broadly comparable results in trout pineal photoreceptors, and additionally showed that the molecular mechanism appeared similar to that in retinal photoreceptors. Voltage clamp experiments showed that the light response was accompanied by a reduction in membrane conductance (as in retinal photoreceptors); however, bright flashes reduced the total conductance only by ~10%, which may explain a contrary report of a light-induced conductance increase by Morita et al (69). In addition, application of the phosphodiesterase inhibitor IBMX led to an increase in the size of the light responses, as in retinal photoreceptors, and consistent with a light-induced decrease in cGMP concentration. Furthermore, the response-versus-intensity relation measured at a fixed time (prior to the peak) exhibited an exponential saturation, as expected for the classic vertebrate phototransduction cascade (70).
The light adaptation behavior reported by Kusmic et al (68) differed somewhat from that reported by Pu & Dowling (66), in that the responses to steady illumination did not sag at all. Further, for superimposed test flashes the time-to-peak did not shorten in the presence of background illumination. For dim test flashes, the time-to-peak was around 1.5 s in dark-adapted conditions and it remained the same in the presence of backgrounds; likewise, responses to brighter test flashes (which had shorter times-to-peak in darkness) did not accelerate.
From the spectral sensitivity reported by Pu & Dowling (66), and from the in situ hybridization results of Koyanagi et al (71), it seems likely that the opsin in the pineal photoreceptors recorded above was rhodopsin (though with a vitamin A2-based chromophore in the larval lamprey), and the cells were probably from the ventral region of the organ. Photoreceptors in the dorsal region express parapinopsin (71) and exhibit UV-sensitive hyperpolarizations (71, 72).
Despite the fact that the pineal photoreceptors described above probably use rhodopsin as the visual pigment, their electrical responses can generally be described as resembling ‘slow and insensitive cone-like responses’, except for two properties. Firstly, light adaptation occurs without response acceleration. Secondly (and probably related), the response saturates in bright steady lights. In contrast, no matter how bright the steady light, the cones of jawed vertebrates always manage to return their circulating current and intracellular voltage to an operating point that permits them to continue responding to incremental stimuli (73).
Pineal ganglion cell responses. A typical response for a standard ‘luminosity OFF’ ganglion cell to moderately bright illumination is shown in Figure 12 (69), and compared schematically with a photoreceptor response. For sub-saturating intensities the form of the graded response in the ganglion cell closely resembles that in the photoreceptor. In other experiments it has been shown that maintained exposures reduce the firing rate in proportion to the logarithm of the intensity, over a range as great as 8 log units (74).
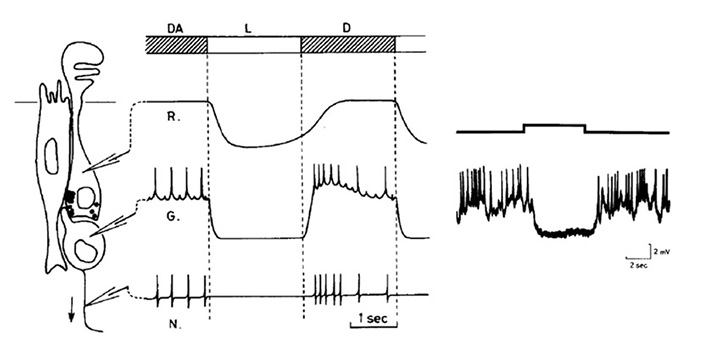 Figure 12. Light response of a pineal luminosity ganglion cell. Left, Schematic of pineal cells and recording electrodes. Middle, schematic of light responses in: R, photoreceptor (intracellular); G, ganglion cell (intracellular); N, nerve fiber (extracellular). Light monitor indicates: DA, dark-adapted; L, light; D, dark. Right, Intracellular recordings from a luminosity ganglion cell. Typical intracellular responses from photoreceptors were shown in Fig. 11. From Morita et al (1985).
Figure 12. Light response of a pineal luminosity ganglion cell. Left, Schematic of pineal cells and recording electrodes. Middle, schematic of light responses in: R, photoreceptor (intracellular); G, ganglion cell (intracellular); N, nerve fiber (extracellular). Light monitor indicates: DA, dark-adapted; L, light; D, dark. Right, Intracellular recordings from a luminosity ganglion cell. Typical intracellular responses from photoreceptors were shown in Fig. 11. From Morita et al (1985).
Lamprey lateral eye
Eye. The lateral eye of adult lampreys (Figure 13B, Figure 13C) bears a striking similarity to that of jawed fish. It is a camera-style eye, with a lens, an iris, and a set of six extraocular muscles. These extraocular muscles are in part homologous to those of jawed vertebrates (75) and interestingly an intermediate arrangement of muscles has been documented (76, 77) in a fossil placoderm, an agnathan armored fish that diverged from our lineage after the ancestors of lampreys had diverged.
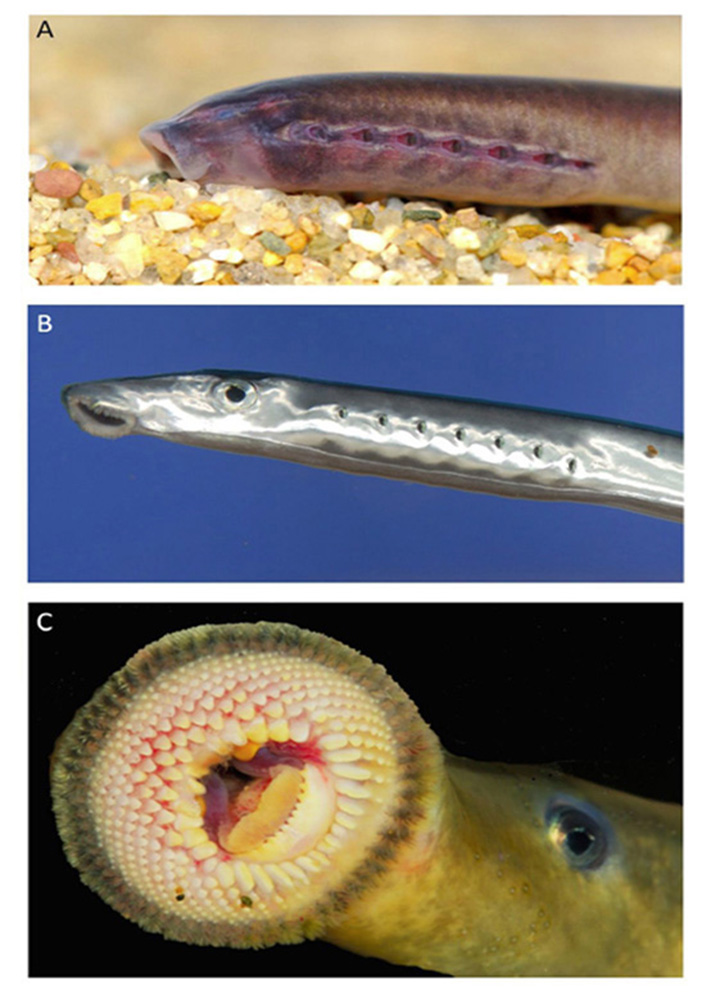 Figure 13. Lamprey (Geotria australis) and its lateral eyes. A, Ammocoete. The ammocoete’s rudimentary ‘eyes’ cannot be seen as they are embedded beneath the skin. B, Downstream migrant. C, Upstream migrant. All images courtesy of Shaun P Collin.
Figure 13. Lamprey (Geotria australis) and its lateral eyes. A, Ammocoete. The ammocoete’s rudimentary ‘eyes’ cannot be seen as they are embedded beneath the skin. B, Downstream migrant. C, Upstream migrant. All images courtesy of Shaun P Collin.
Retina. As shown schematically in Figure 14, the retina of the silver lamprey (a northern hemisphere species) appears very similar to that of gnathostomes, and contains the conventional five classes of neuron (photoreceptors, horizontal, bipolar, amacrine and ganglion cells) as well as Müller glial cells. The nuclei are distributed into three main nuclear layers, and there are two plexiform layers, though one difference between lamprey and gnathostome retinas is an apparent ‘flipping’ of the ganglion cell layer and inner plexiform layer – thus, the bulk of the retinal ganglion cells and their fibers are positioned scleral to the inner plexiform layer in the lamprey (compare Figure 14A and B) (75). Fritzsch has proposed that this arrangement in the lamprey retina (and likewise in some brain areas) is basal, and that the flipping of retinal layers in gnathostomes is derived (78, 79).
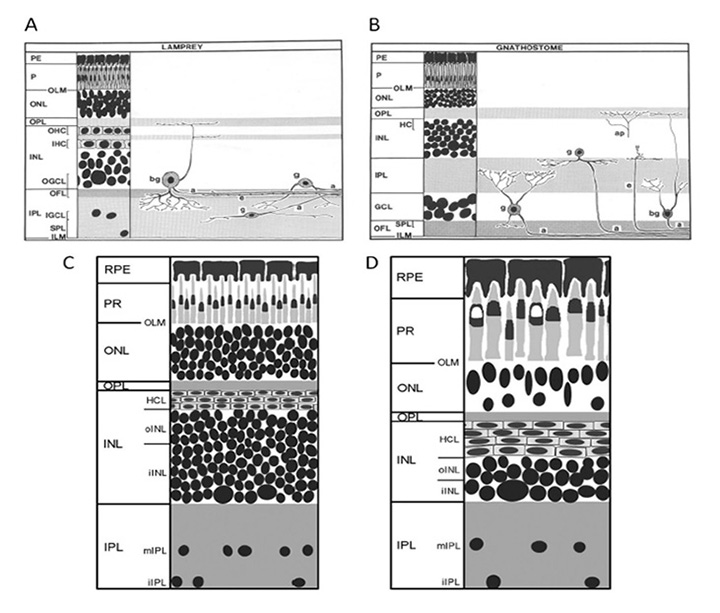 Figure 14. Comparison of lamprey and gnathostome retinas. Schematics of the organization of the retina of the lamprey lateral eye. A, Silver lamprey (Ichthyomyzon unicuspis, post-metamorphic juveniles). B, Gnathostome. The main difference is that in the lamprey the inner plexiform layer is vitreal to the majority of ganglion cells, so that most of the ganglion cells are located in the inner nuclear layer. C, D, Southern hemisphere lamprey, Geotria australis: in two of the animal’s adult forms: (C) downstream migrant phase (post-metamorphic juvenile), and (D) upstream migrant phase (ready to spawn). The retina of the downstream migrant is densely packed with cells, lying ~10 layers deep in the INL (including at least two layers of horizontal cells); the retina of the upstream migrant is much larger, and is dominated by large photoreceptors and reduced layering of cells in the INL. Abbreviations in upper case are standard, with the addition of: IGCL, OGCL, inner and outer ganglion cell layers; IHC, OHC, inner and outer horizontal cells; OFL, optic fiber layer; SPL, superficial plexiform layer. a, axon; ap, ascending process of unknown origin; bg, biplexiform ganglion cell; e, efferent fiber; g, ganglion cell. A, B, from Fritzsch & Collin (1990). C, D from Nivison-Smith et al (2013).
Figure 14. Comparison of lamprey and gnathostome retinas. Schematics of the organization of the retina of the lamprey lateral eye. A, Silver lamprey (Ichthyomyzon unicuspis, post-metamorphic juveniles). B, Gnathostome. The main difference is that in the lamprey the inner plexiform layer is vitreal to the majority of ganglion cells, so that most of the ganglion cells are located in the inner nuclear layer. C, D, Southern hemisphere lamprey, Geotria australis: in two of the animal’s adult forms: (C) downstream migrant phase (post-metamorphic juvenile), and (D) upstream migrant phase (ready to spawn). The retina of the downstream migrant is densely packed with cells, lying ~10 layers deep in the INL (including at least two layers of horizontal cells); the retina of the upstream migrant is much larger, and is dominated by large photoreceptors and reduced layering of cells in the INL. Abbreviations in upper case are standard, with the addition of: IGCL, OGCL, inner and outer ganglion cell layers; IHC, OHC, inner and outer horizontal cells; OFL, optic fiber layer; SPL, superficial plexiform layer. a, axon; ap, ascending process of unknown origin; bg, biplexiform ganglion cell; e, efferent fiber; g, ganglion cell. A, B, from Fritzsch & Collin (1990). C, D from Nivison-Smith et al (2013).
Also shown for comparison in Figures 14C, D is a schematic of the retina of the southern hemisphere lamprey, Geotria australis, in its ‘downstream’ and ‘upstream’ migratory phases; the downstream phase is just post-metamorphic, when the juveniles migrate down to the sea, while in the upstream phase the fully-grown adults migrate back upstream to spawn near where they hatched. The retina of G. australis is broadly similar to those of other lampreys. Figures 14C, D indicates the substantial increase in the size of the photoreceptors that occurs during the intervening marine phase.
The distribution of immunoreactivity for amino acid neurotransmitters and calcium-binding proteins in the retina of G. australis has recently been examined by Nivison-Smith et al (80), and shown to be generally similar to that found in jawed vertebrates. Experiments with the small organic cation agmatine were consistent with cation entry into photoreceptors and horizontal cells, again broadly similar to that seen in the jawed vertebrate retina.
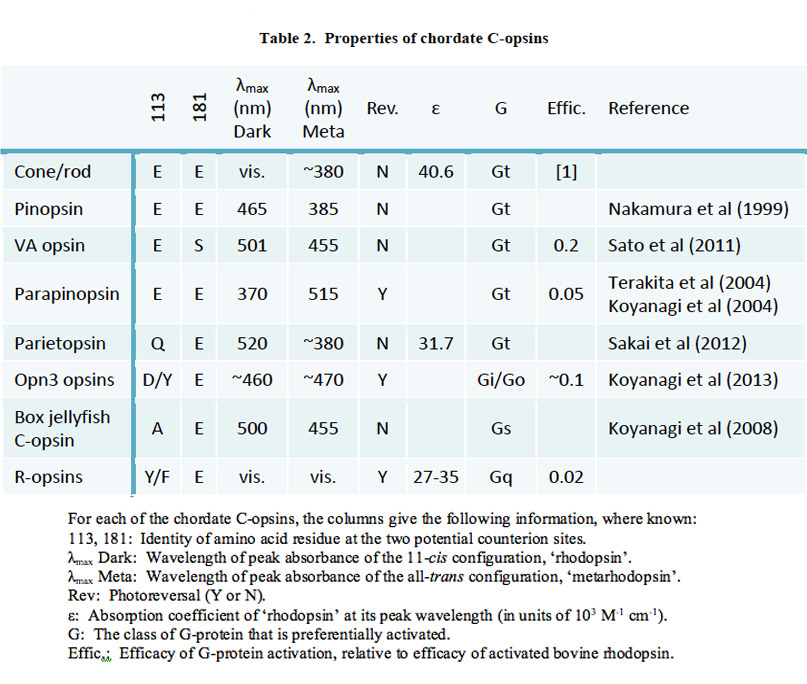
Classes of opsin and photoreceptor. Lamprey opsins fall into five classes, that appear to be homologous (or nearly so) to those of jawed vertebrates; thus, the southern hemisphere lamprey Geotria australis clearly possesses LWS, SWS1 and SWS2 opsins, and its remaining two opsins, RhA and RhB, may well be members of the Rh1 and Rh2 families, respectively (81, 82). Furthermore, this species possesses five distinct classes of photoreceptor (83). Although the distribution of expression of opsin classes amongst photoreceptor classes has not yet been determined definitively, circumstantial evidence suggests the distribution indicated in Figure 15B (Shaun P Collin, personal communication). In contrast to the case in G. australis, other species of lamprey have lost varying numbers of classes of opsin and photoreceptor; thus, another southern hemisphere species Mordacia mordax has only a single class of opsin and a single class of photoreceptor, while northern hemisphere species generally have two classes of opsin (Rh1 and LWS) and two classes of photoreceptor.
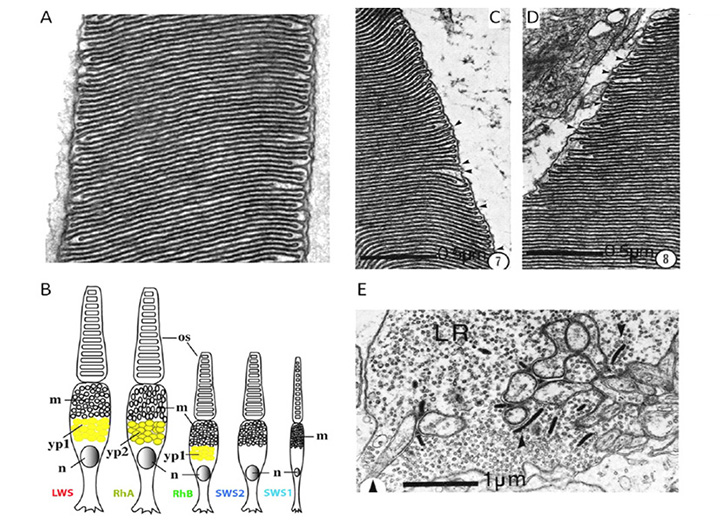 Figure 15. Lamprey retinal photoreceptors. Photoreceptors of Geotria australis, a southern hemisphere species (A, B), and of Petromyzon marinus, a northern hemisphere species (C, D, E). A, Outer segment, showing ordered stacking of sac-like membranes. From Collin & Trezise (2006). B, Schematic of G. australis photoreceptors, showing the five distinct morphologies, and making a tentative assignment of the five classes of opsins (based on personal communication from Shaun P Collin). Abbreviations: m, mitochondria; n, nucleus; os, outer segment; yp1, yp2, yellow pigments 1 and 2. Modified from Collin (2009). C, D: Outer segment ultrastructure, for long (C) and short (D) photoreceptors of P. marinus. E, Synaptic ribbons in the synaptic terminal of long photoreceptor (LR). C, D and E from Dickson & Collard (1979).
Figure 15. Lamprey retinal photoreceptors. Photoreceptors of Geotria australis, a southern hemisphere species (A, B), and of Petromyzon marinus, a northern hemisphere species (C, D, E). A, Outer segment, showing ordered stacking of sac-like membranes. From Collin & Trezise (2006). B, Schematic of G. australis photoreceptors, showing the five distinct morphologies, and making a tentative assignment of the five classes of opsins (based on personal communication from Shaun P Collin). Abbreviations: m, mitochondria; n, nucleus; os, outer segment; yp1, yp2, yellow pigments 1 and 2. Modified from Collin (2009). C, D: Outer segment ultrastructure, for long (C) and short (D) photoreceptors of P. marinus. E, Synaptic ribbons in the synaptic terminal of long photoreceptor (LR). C, D and E from Dickson & Collard (1979).
Photoreceptor morphology. In northern hemisphere lampreys, these two types of photoreceptor have been termed ‘long’ and ‘short’, based on the length of their inner segments; somewhat confusingly, their outer segments are the reverse of this. Thus the ‘long’ cells have short (~7 µm long) conical outer segments arranged in a distal layer in close contact with the retinal pigment epithelium, whereas the ‘short’ cells (which outnumber the long cells 3:1) have longer (~25 µm) cylindrical outer segments arranged in a proximal layer, and only their tips reach the RPE.
The ultrastructure of retinal photoreceptors in the lamprey Petromyzon marinus was examined by Dickson & Graves (84), who reported that both classes of cell (short and long) exhibited cone-like rather than rod-like morphology. Thus, in both cell types the outer segment membrane appeared to be continuous with the plasma membrane. Although small groups of sacs/discs were found to be surrounded by plasma membrane, there were frequent openings to the exterior, as illustrated in Figures 15C, D. Autoradiography with labeled amino acids showed that newly-synthesized protein was distributed uniformly throughout the outer segment, as found in cones. In addition, the outer segments never exhibited incisures (the deep longitudinal infoldings of the surface membrane that divide the discs of the rods of jawed vertebrates into lobules). Finally, the synaptic terminals (Figure 15E) were reported to resemble cone pedicles rather than rod spherules. Thus, on all the conventional criteria that are used to distinguish cones from rods in jawed vertebrates, the short and long receptors of P. marinus would both be classified as cones.
Broadly comparable results were obtained in the southern hemisphere lamprey, G. australis, where ultrastructural examination led to the proposal that all five classes of photoreceptor are cone-like (83, 85). In each of the five classes of photoreceptor, the outer segment membrane is continuous with the extracellular matrix (Figure 15A), and the synaptic terminals contain between one and five synaptic ribbons. Furthermore, at least three of the cell classes contain a filtering pigment in the inner segment (Figure 15B).
Proteins of phototransduction. As will be described in Section 8, the distribution of isoforms of opsin, of transducin alpha, and of PDE catalytic and regulatory subunits, has been determined for P. marinus by Muradov et al (86, 87). The long receptors express an LWS opsin, a transducin alpha subunit GαL that may be ancestral, a common PDE6 that appears ancestral, and a PDE gamma subunit that clades with the gnathostome cone isoform. The short receptors express an Rh1 rhodopsin, a rod-like transducin alpha subunit GαS that nevertheless retains cone-like features, the common PDE6 that appears ancestral, and a PDE gamma subunit that clades with the gnathostome rod isoform.
Lamprey genome. The first whole genome sequence and assembly for a lamprey has very recently been reported by Smith et al (88), for P. marinus. Analysis indicates that the ‘2R’ two rounds of whole genome duplication that occurred near the base of the vertebrate lineage had already taken place prior to the divergence of the ancestral lamprey and gnathostome lineages (i.e. prior to 5 in Figure 1).
Electrophysiology. Govardovskii & Lychakov (89) examined the ERG response properties of the long and short photoreceptors of Lampetra fluviatilis. At scotopic intensities, they observed a b-wave driven by the 517 nm pigment (known to be present in the short cells), though this ERG response was not as sensitive as seen in the frog retina. At photopic intensities the a-wave was a combination of signals from the two spectral classes, and both sets of spectral response exhibited light-adaptation and could not be saturated. Thus, while both long and short cells had cone-like morphology, the short cells behaved electrophysiologically somewhat like rods, but both classes exhibited cone-like adaptation.
Did ‘rods’ exist in the ancestral vertebrate? As discussed above, lamprey ‘rhodopsin’ RhA/Rh1 is closely homologous to jawed vertebrate rhodopsin Rh1 (82, 83, 90). Pisani et al (82) have interpreted the sequence analyses to indicate that the common ancestor of lampreys and jawed vertebrates possessed an Rh1 gene. They went on to say: “The function of Rh1 in agnathans is not yet known, but assuming its function in the vertebrate cenancestor was not dramatically different from its scotopic function in most vertebrates, this implies that both photopic and scotopic vision evolved in the stem vertebrate lineage and must have been in place in the Cambrian by about 522-518 Ma”. However, there is not yet sufficient evidence to go this far, because it is by no means certain that this Rh1 opsin was expressed in a ‘true rod’ or that the Rh1 actually mediated ‘scotopic vision’. On current evidence, it is entirely possible that the ancestral Rh1-containing photoreceptor functioned simply as a ‘slow sensitive cone’, rather than as a true rod capable of reliably detecting individual photons, as is required in order for the visual system to attain the ultimate in scotopic performance, of the kind that is attained in jawed vertebrates. All that can be said for certain from this evidence is that the common ancestor of jawed and jawless vertebrates is highly likely to have had a rod-like opsin; but this is not at all sufficient to deduce that it had scotopic vision.
Summary of lamprey photoreceptor features. In northern hemisphere lamprey species that have long and short photoreceptors, the long cells have all the features of cones in jawed vertebrates. The short cells have most of the features of cones, though they express rhodopsin as well as a somewhat rod-like transducin alpha subunit and a rod-like PDE regulatory subunit; other components of phototransduction are yet to be classified. The short cells have high sensitivity, though as yet there is no evidence that they can reliably signal individual photon hits. In southern hemisphere lampreys, there are five classes of photoreceptor that all appear to have most of the features of cones. One of these expresses rhodopsin, but as yet there is insufficient evidence to say whether it has rod-like functional properties.
Jawed vertebrate lateral eye
The eyes of all jawed vertebrates are remarkably similar, in terms of overall layout and features. The retina has the same set of cell classes, and is organized in fundamentally the same way. Furthermore, the cone photoreceptors of all gnathostomes (and likewise the rods) are closely similar in structure, apart from size differences, and their electrical responses to light are also closely comparable. In view of the period of over 400 million years that has elapsed since the emergence of the first jawed vertebrates, it is impressive how little change has occurred in the basic plan of the eye and in the structure and response properties of the photoreceptors.
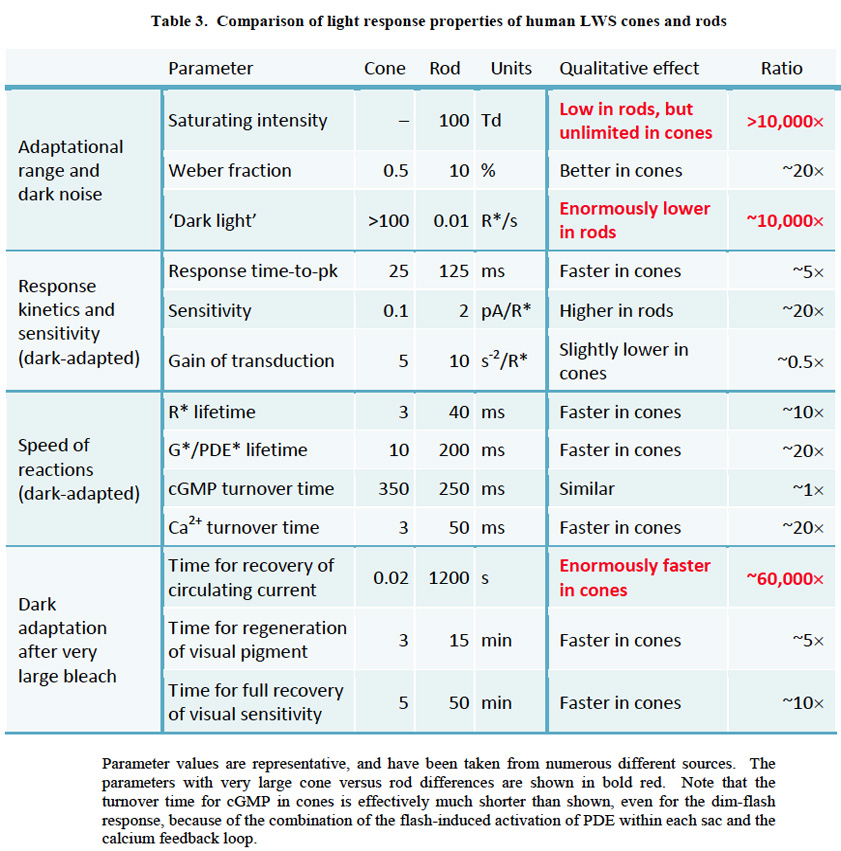
The structure and responses of cone and rod photoreceptors, as well as the process of phototransduction, in jawed vertebrates are dealt with comprehensively elsewhere (see other sections of Webvision; Ebrey and Koutalos (91); etc.), and will not be repeated here. For completeness, though, Figure 16 illustrates the main ultrastructural features of mammalian photoreceptors, for comparison with those of other chordates. The functional properties of cones and rods will be contrasted in Section 7 and the molecular components of the transduction cascade will be reviewed in Section 8.
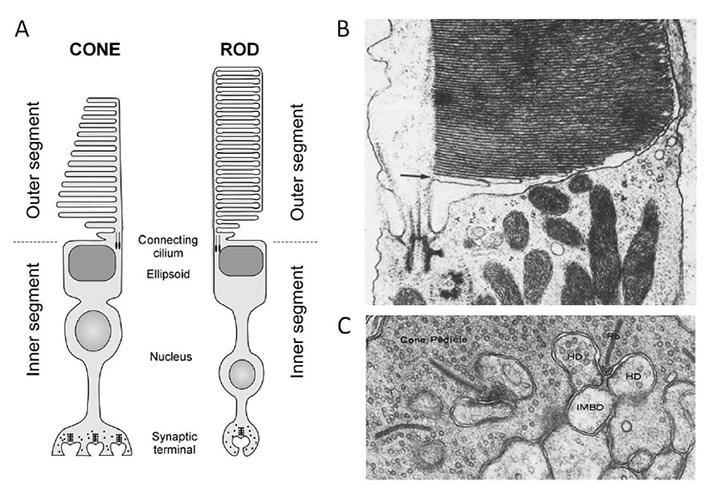 Figure 16. Jawed vertebrate retinal photoreceptors. A, Schematic of jawed vertebrate cone and rod photoreceptors. From Burns & Lamb (2003). B, Outer segment, connecting cilium, and distal inner segment of a rhesus monkey cone. From Steinberg et al (1981). C, Synaptic ribbons in the synaptic pedicle of a macaque cone. From Raviola & Gilula (1975).
Figure 16. Jawed vertebrate retinal photoreceptors. A, Schematic of jawed vertebrate cone and rod photoreceptors. From Burns & Lamb (2003). B, Outer segment, connecting cilium, and distal inner segment of a rhesus monkey cone. From Steinberg et al (1981). C, Synaptic ribbons in the synaptic pedicle of a macaque cone. From Raviola & Gilula (1975).
4 Gradations in chordate eyes: retina and photoreceptors
From the descriptions in Section 3, one can discern gradations in types of retina and types of ciliary photoreceptor, that will now be described. Likewise, one finds gradations in the properties of the opsins (C-opsins), and these will be covered in Section 6.
Categories of chordate retina
The light-sensitive neural tissue in extant chordates can be classified into three groups:
a) No ‘retina’. The ‘primitive’ chordates (cephalochordates and tunicates) do not have a retina, in the sense that the term is generally used, but rather a handful of ciliary photoreceptors associated with a pigment cell, in the frontal eye and in the ocellus, respectively. In the tunicate ocellus, the ciliary photoreceptors each give rise to an axon, but its synaptic contacts have not been determined. In the cephalochordate frontal eye, it appears that the output axons arise from a second row of cells rather than from the ciliary photoreceptors themselves.
b) Two-layered retina. The hagfish lateral ‘eye’ and the dorsal light-sensitive organs of non-mammalian jawed vertebrates (pineal / parapineal / parietal) exhibit a two-layered retina, with ciliary photoreceptors making ribbon synapse contacts onto projection neurons (ganglion cells). The photoreceptors and projection neurons are embedded amongst glial cells, but no other classes of neuron have been identified (though their existence cannot be ruled out).
c) Three-layered retina. The lateral eyes of lampreys and jawed vertebrates exhibit a three-layered retina, with bipolar cells interposed between ciliary photoreceptors and retinal ganglion cells, and with two additional classes of neuron in the form of horizontal cells and amacrine cells.
Gradations in morphological types of chordate ciliary photoreceptor
On morphological grounds, the ciliary photoreceptor cells of the chordate retinas described in Section 3 can be described as indicated schematically in Figure 17. In all cases, the light-sensitive outer segment is formed by a massive expansion of membrane surface, in the form of lamellae arising from a cilium that is characterized by 9+0 double filaments. This outer segment membrane is packed with a high concentration of visual pigment, together with lower levels of other proteins of phototransduction. The cell body is in most cases roughly cylindrical in shape, both above and below the nucleus. Synaptic output occurs at the end of the cell opposite the outer segment (i.e. at the ‘base’), which is sometimes separated from the soma by an intervening ‘axonal’ segment. These features permit classification into at least five groups, as follows:
(i) Ascidian-style. The outer segment lamellae are arranged rather like petals, lying roughly longitudinally around the centrally positioned connecting cilium. An axon leaves the base of the cell, but its synaptic contacts are not known.
(ii) Hagfish-style. The outer segment lamellae are splayed out to either side of the connecting cilium, which lies centrally. Synaptic output occurs at a basal invagination, where synaptic vesicles are arranged around a ‘spherical body’ rather than a ribbon.
(iii) Pineal-style. The outer segment lamellae are very broad, and often lie in a curve. It is generally reported that the lamellae constitute cone-like sacs rather than rod-like discs. The basal invaginating synapse exhibits synaptic ribbons, and makes a dyad (or sometimes monad) contact with ganglion cells.
(iv) Cones. The outer segment lamellae (sacs) are arranged highly regularly, and their membranes form a continuum with the plasma membrane of the inner segment. In mammals the outer segment is roughly cylindrical in shape, though in non-mammalian vertebrates it tends to be conical. The inner segment can contain a variety of specializations, including the ellipsoid (with mitochondria, and sometimes spectrally-filtering oil droplet or vesicles), the contractile myoid, and the paraboloid.
(v) Rods. Morphologically, rods are very similar to cones, though a significant exception relates to the topology of the outer segment membrane. During outer segment synthesis, cone-like sacs are generated, but these proceed to form a seal around the rim with neighboring sacs, to form discs separated from the plasma membrane, with the result that the narrow intradiscal space is isolated from the extracellular space.
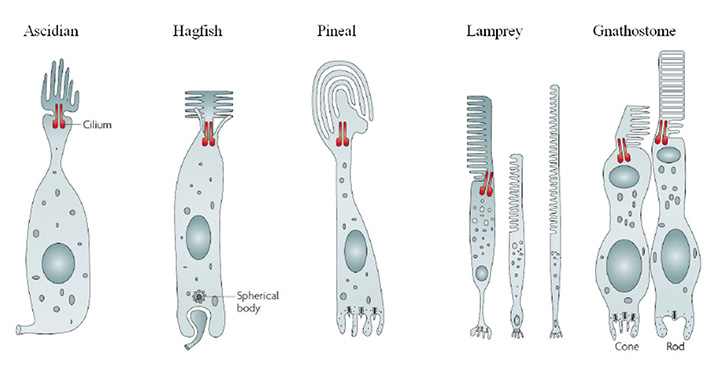 Figure 17. Schematic of transitions in ciliary photoreceptor morphology across chordates Schematic morphology of the ciliary photoreceptors of extant chordate species provides suggestive evidence of the remnants of a gradual transition in the morphology of ciliary photoreceptors during chordate evolution. Note the transitions, from left to right, towards (1) a highly organized laminar structure in the outer segment, and (2) the appearance of ribbons in the synaptic terminal. From Lamb et al (2007).
Figure 17. Schematic of transitions in ciliary photoreceptor morphology across chordates Schematic morphology of the ciliary photoreceptors of extant chordate species provides suggestive evidence of the remnants of a gradual transition in the morphology of ciliary photoreceptors during chordate evolution. Note the transitions, from left to right, towards (1) a highly organized laminar structure in the outer segment, and (2) the appearance of ribbons in the synaptic terminal. From Lamb et al (2007).
Gradations provide evidence for gradual transitions during eye evolution. The gradations in type of retina and type of photoreceptor listed above provide powerful evidence that the vertebrate eye did not ‘suddenly appear’, and instead support the view that the evolution of our eye proceeded via myriad tiny changes. This conclusion, based (up until here) primarily on the morphology of ciliary photoreceptors, is greatly strengthened by evidence from analysis of gradations in opsins (Section 5 and Section 6), from analysis of the molecular genetics of the transduction cascade (Section 8), and from studies of embryonic eye development (Section 13 and Section 14).
5 Pre-vertebrate chordate C-opsins
As a result of recent work, it has become possible to track the changes that enabled an ancestral chordate C-opsin (that exhibited many properties in common with R-opsins) to evolve into the immediate pre-cursor of modern cone and rod opsins.
Thermal stability and photoreversal
Two ways in which cone and rod C-opsins differ substantially from their R-opsin cousins (including melanopsin) relate to the thermal stability of the photo-activated all-trans ‘metarhodopsin’ state and its ability to undergo photoreversal (Figure 18).
In R-opsins, the photo-activated metarhodopsin is thermally stable (Fig. 18 right), with a half-life usually of hours or even days. This active form is rapidly inactivated by the binding of an arrestin molecule, but it remains stably in its all-trans configuration. In most cases this metarhodopsin has its peak absorption in the visible region of the spectrum, indicating that, in its enzymatically active configuration, the Schiff base bond of the all-trans retinaldehyde remains protonated. Furthermore, upon absorption of a further photon, this stable all-trans metarhodopsin (even when arrestin-bound) can undergo photoreversal back to its 11-cis rhodopsin form. Indeed, for most practical purposes this photoreversal is the only short-term mechanism available for the regeneration of visual pigment in many microvillar (rhabdomeric) photoreceptors.
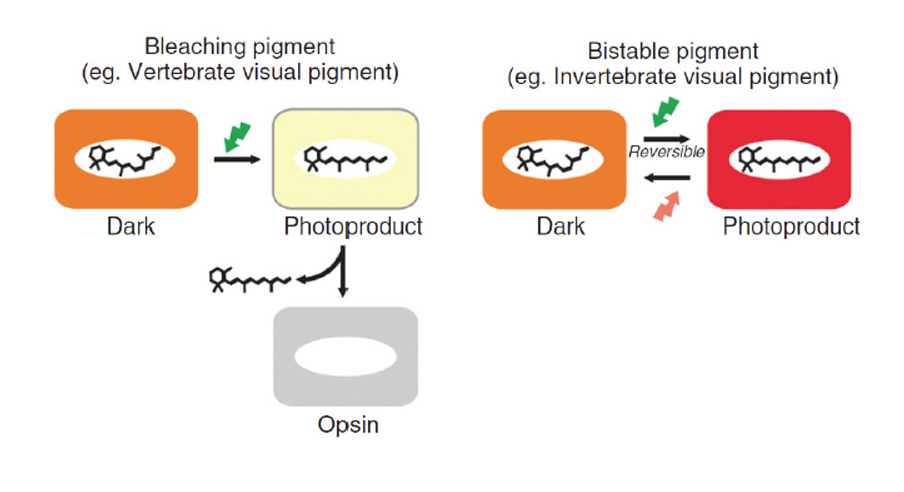 Figure 18. Thermally unstable pigment contrasted with bistable/photoreversible pigment Left: Cone pigments and rhodopsin are thermally unstable upon activation. The metarhodopsin photoproduct absorbs in the UV, so that it is colorless to human vision and hence the pigment is said to ‘bleach’ in the light. This metarhodopsin decays fairly rapidly, releasing all-trans retinal; cone metarhodopsin II decays in a matter of seconds, and rod metarhodopsin decays in minutes. Right: R-opsins and many C-opsins (other than vertebrate visual opsins) are bistable. The activated all-trans metarhodopsin is photoreversible; it absorbs in the visible part of the spectrum, and upon absorption of a photon is isomerized back to the 11-cis isomer. From Terakita et al (2012).
Figure 18. Thermally unstable pigment contrasted with bistable/photoreversible pigment Left: Cone pigments and rhodopsin are thermally unstable upon activation. The metarhodopsin photoproduct absorbs in the UV, so that it is colorless to human vision and hence the pigment is said to ‘bleach’ in the light. This metarhodopsin decays fairly rapidly, releasing all-trans retinal; cone metarhodopsin II decays in a matter of seconds, and rod metarhodopsin decays in minutes. Right: R-opsins and many C-opsins (other than vertebrate visual opsins) are bistable. The activated all-trans metarhodopsin is photoreversible; it absorbs in the visible part of the spectrum, and upon absorption of a photon is isomerized back to the 11-cis isomer. From Terakita et al (2012).
In contrast, the photo-activated metarhodopsin II state of cone and rod opsins is thermally unstable (Fig. 18, left), decaying with a half-life that is short (seconds) in cone opsins and somewhat longer (minutes) in rhodopsin (for values, see Table 2 of Imai et al (92)). Fast inactivation occurs as a result of arrestin binding, enabled by rapid phosphorylation. The active meta II absorbs in the UV (~380 nm), because the Schiff base is now un-protonated. Although the protonated meta I intermediate can undergo photoreversal to the 11-cis configuration, the active meta II state is incapable of undergoing such photoreversal, even if it absorbs a blue/UV photon (see Fig. 7 of Ritter et al (93)), and this inability is apparently a consequence of an internal molecular rearrangement that accompanies activation.
Phylogeny, and gradation in properties, of chordate C-opsins
The C-opsins comprise a large family, with representatives in cnidaria and protostomes, as well as in deuterostomes (including chordates and vertebrates). Apart from the well-known retinal cone and rod opsins, vertebrates also express several additional categories of C-opsin, including: Opn3 (encephalopsin / TMT opsin), parietopsin, parapinopsin, VA (vertebrate ancient), and pinopsin. From their molecular phylogeny, as well as from the gradation (now to be described) in functional properties of the five groups that have been investigated, these C-opsins reflect an evolutionary sequence preceding the advent of the cone and rod opsins.
 Figure 19. Abbreviated phylogeny of opsins, together with residues of interest Left: Phylogeny of a subset of opsins, comprising vertebrate C-opsins together with a selection of other opsins of interest. Note that the lengths of the lines do not indicate evolutionary distance, and so the horizontal positions of the branch points do not represent the timings of divergences. The C-opsin from Ciona intestinalis (Ci-Opsin1) is not included in this diagram, but its position in the tree is close to that of VA/VAL. Right: Residues at several locations that are known to be important for a variety of functions. Here and throughout the article, residue numbering is in accordance with bovine rhodopsin. From Davies et al (2010).
Figure 19. Abbreviated phylogeny of opsins, together with residues of interest Left: Phylogeny of a subset of opsins, comprising vertebrate C-opsins together with a selection of other opsins of interest. Note that the lengths of the lines do not indicate evolutionary distance, and so the horizontal positions of the branch points do not represent the timings of divergences. The C-opsin from Ciona intestinalis (Ci-Opsin1) is not included in this diagram, but its position in the tree is close to that of VA/VAL. Right: Residues at several locations that are known to be important for a variety of functions. Here and throughout the article, residue numbering is in accordance with bovine rhodopsin. From Davies et al (2010).
Figure 19 presents a shortened molecular phylogeny of opsins, together with a tabulation of the amino acid residues at a number of important locations (94). In the cladogram on the left, note that the lengths of the lines do not indicate evolutionary distance, and so the horizontal positions of the branch points do not represent timings of divergences. And in the tabulation on the right (as well as throughout this article), note that residues are numbered in accordance with the bovine rhodopsin sequence.
As indicated by the phylogeny in Figure 19, vertebrate C-opsins fall into the sequence (from most ancient to most recent) of: Opn3 (encephalopsin), parietopsin, parapinopsin, VA opsin, pinopsin, cone opsins, rhodopsin. For each of these C-opsins, the functional molecular properties are briefly described below and summarized in Table 2; much of this information has come from recent studies of recombinant proteins.
In evaluating the relative properties of these C-opsins, comparison will be made with a ‘typical’ R-opsin, exhibiting photoreversible transitions between two stable states. As indicated above, vertebrate cone and rod opsins do not exhibit photoreversal, and instead release their all-trans retinoid. On the other hand they exhibit stronger activation of the G-protein. We will now see that the other vertebrate C-opsins exhibit properties intermediate between these.
Opn3. The Opn3 group of opsins includes mammalian Opn3 (formerly called encephalopsin or panopsin), TMT (teleost multiple tissue) opsin, insect pteropsin, and annelid C-opsin. This group clades near the base of C-opsins, and the sequences quite closely resemble those of R-opsins. For most members of the group, the two potential sites (113 and 181) for the counterion to the protonated Schiff base have the R-opsin form, Y113 and E181, indicating that E181 serves as the counterion; however, in mammals Opn3 has D113. Mammalian Opn3 is expressed in scattered cells in deep brain areas.
The molecular properties of Opn3 opsins have only very recently been studied (95), by expression in cultured cells. Pufferfish TMT opsin was shown to bind 11-cis retinal, to form a visual pigment absorbing in the blue (~460 nm); this pigment was bistable, being isomerized to a slightly red-shifted all-trans form by short-wavelength light, and isomerized back to the 11-cis form by long-wavelength light. The light- activated form could activate Gi and Go, though with only ~10% of the efficacy of activated rhodopsin, but it did not activate Gq, Gs or Gt (transducin). Similar results were found for a mosquito Opn3 homolog, though its activity was higher. Although mammalian Opn3 itself has not yet been successfully expressed in cultured cells, Koyanagi et al (95) suggest that it is likely to exhibit similar properties.
Parietopsin. Parietopsin was first identified in photoreceptors in the parietal eye of the lizard. Its functional properties have recently been explored by Sakai et al (96), who showed that the Schiff base counterion is located in the ‘invertebrate’ position of 181, consistent with the residues Q113 and E181. The expressed pigment had its absorption peak at 520 nm, and its photosensitivity and molar extinction coefficients were marginally lower than those of cone and rod opsins, as is often characteristic of invertebrate visual opsins. On the other hand, its photochemical properties resembled those of cone and rod pigments, with the formation of decaying metarhodopsin intermediates (I, II and III), rather than the typical invertebrate photoreversible metarhodopsin.
Parapinopsin. Parapinopsin was first identified in the catfish parapineal organ. It was subsequently identified in the lamprey pineal, where it was shown to be a UV-sensitive bistable opsin (97). Koyanagi et al (71) found parapinopsin to be expressed in the ciliary photoreceptors only on the distal side of the lamprey pineal and parapineal organs, and they examined its functional properties. They discovered that illumination of parapinopsin caused the conventional 11-cis to all-trans isomerization of the chromophore, to yield a product with a spectral peak at 515 nm. However, in contrast to the case for UV-sensitive (SWS1) cone opsins, this metarhodopsin was found to be stable, and to exhibit photoreversal upon absorption of a subsequent photon; indeed it was possible to repeatedly interconvert between parapinopsin and its meta state by illumination successively with UV and then orange light. Intracellular electrical recordings from pineal photoreceptors expressing parapinopsin showed that flashes of UV light elicited slow hyperpolarizing responses, consistent with a phototransduction cascade of the vertebrate style. The efficacy of activation of G-protein by activated parapinopsin was shown to be around 20-fold lower than that by activated rhodopsin (97).
VA/VAL opsin. VA opsin was first identified in salmon retina, and then soon after in other teleost retinas, where a longer splice variant (VAL) was also found, and then in other vertebrates. The functional properties of VAL opsin have recently been investigated by Sato et al (98). Like vertebrate visual opsins, VA/VAL has E113 at the usual site for the counterion to the resting state but, uniquely, the site of the ancestral counterion location is S181. Sato et al (98) found that light activation caused cis to trans isomerization, but that the activated intermediate absorbed in the visible region, at 455 nm, indicating that the Schiff base remained protonated. Although this intermediate absorbed visible light, it did not exhibit photoreversal (as for vertebrate visual opsins). This intermediate could activate the Gi G-protein, though with an efficacy around 5-fold lower than that of activated rhodopsin. Hence, they showed that several of the properties of VA/VAL opsin are intermediate between those of parapinopsin and pinopsin (see below).
Pinopsin. Pinopsin was first cloned from the chicken pineal, and was found to clade with the LWS cone opsin near the base of the phylogenetic tree of vertebrate visual opsins (99). However, there is no clear evidence as to exactly where or how it diverged from visual opsins, and there is a distinct possibility that it could be ancestral. Its functional properties were examined by Nakamura et al (100), and found to be closely similar to those of vertebrate visual opsins. The resting dark form absorbed at 465 nm, in the blue region of the spectrum, similar to the absorption of the SWS cone opsins (e.g. SWS2, Rh2). Light absorption triggered its progression through a sequence of photoproducts (batho, lumi, meta I, meta II, meta III) analogous to those triggered by activation of rhodopsin. The lifetimes of these intermediates were comparable to those of cone opsins, except for the meta II state, which exhibited a longer lifetime more typical of rhodopsin.
Improvements in the performance of C-opsins during chordate evolution
By comparing the sequences, structures, and functional properties of chordate C-opsins, Akihisa Terakita and Yoshinori Shichida and their colleagues have elucidated a set of transitions that occurred during evolution, that endowed chordate C-opsins with improved performance (reviewed by Tsutsui & Shichida (101); Tsukamoto & Terakita (102); Terakita et al (103)). These changes included the translocation of the counterion site (for the protonated Schiff base) from residue 181 to residue 113, as well as molecular rearrangements of the protein that provided enhanced tilt of helix 6 during activation, and hence led to much stronger activation of the G- protein.
Counterion relocation. To provide stability for the positive charge on the protonated Schiff base in the resting opsin, there is a requirement for a nearby negatively charged counterion. In most opsins (including R- opsins, Go-opsins, RGR-opsins, and photoisomerases) this counterion is located at position 181 (in bovine rhodopsin numbering) which is occupied by glutamate, E181, whereas in cone and rod opsins the counterion is located at position 113, occupied by glutamate E113. Terakita et al (97) found clear evidence that the E181 location represented the ancestral position, and showed that during the evolution of chordate C-opsins the counterion site had migrated to position 113 (Figure 20).
Counterion functions. In addition to its role in stabilizing the protonated Schiff base in the resting state, the E113 counterion in cone and rod opsins has a number of other important functions (reviewed by Tsutsui & Shichida, (101)). Perhaps most importantly, it acts as an acceptor for the proton from the Schiff base during activation (see description of molecular mechanism below). Very recently it has been shown that it has two additional crucial functions, in enabling the rapid hydrolysis of the Schiff base bond after light absorption (which is important in cones) and likewise in enabling the rapid binding of 11-cis retinal (104)); these roles will be described below, and a comparison between cone and rod opsins will be made in Section 6. The counterion also exerts a powerful effect on the spectral tuning of the resting opsin state. Finally, it is also important in reducing the constitutive activity of the resting opsin; mutants that lack the E113 counterion typically exhibit substantial rates of G-protein activation in darkness. Thus, the acquisition of this alternative location for the counterion has proved enormously important in the evolution of vertebrate retinal opsins.
Increased efficacy of G-protein activation. It has been found (Table 2) that the light-activated forms of cone and rod opsins exhibit much greater efficacy of activation of the G-protein than do the active forms of R-opsins, and furthermore that there is a gradation in this property amongst chordate C-opsins. Tsukamoto et al (105) used site-directed fluorescence labeling to examine the changes in the protein that occurred during light activation, and they found a strong correlation between the degree of movement of helix 6 relative to helix 5 during activation and the strength of the opsin’s ability to activate the G-protein.
Pre-vertebrate duplication of the ancestral cone opsin. The ‘improved’ C-opsin that had emerged during chordate evolution, with a high efficacy of G-protein activation and with rapid release of all-trans retinal, became the ancestral cone opsin. Furthermore, there is strong evidence that, prior to the ‘2R’ whole genome duplications at the base of the vertebrate lineage, this ancestral cone opsin had already duplicated into the ancestral pair of SWS and LWS cone opsins. However, it is more convenient to consider that evidence later, in Section 6 on vertebrate visual opsins.
Molecular mechanism of activation in vertebrate rhodopsin
The mechanism underlying activation of the metarhodopsin state in bovine rhodopsin is now understood at a molecular level, based to a large extent on examination of the crystal structure of the activated form (106, 107). It seems very likely that fundamentally the same mechanism applies for cone opsins, and therefore that it had arisen prior to the ‘2R’ whole genome duplications. Figure 21 provides a schematic illustration of the events involved in activation (108). Within a few microseconds of photon absorption, a state (meta I) is reached, in which the excitation remains in the twist of the retinoid, and where there has been little movement in the protein. Within a few milliseconds, though, meta I relaxes thermally to the form known as meta II, which actually comprises an equilibrium between three different (though spectroscopically equivalent) forms. In the first step, a proton translocation occurs, deprotonating the Schiff base of meta I and protonating the E113 residue (109), creating meta IIa. In the second step, meta IIa undergoes an outward tilt of helix 6, opening up a crevice for interaction with the G-protein, creating meta IIb. In the third step, a proton is taken up onto E134 of the ‘ERY’ motif, which locks helix 6 in relation to helix 3. This form has been termed ‘meta IIb H+’ and is the enzymatically active form R* that activates the G- protein very efficiently. However, it is unable to undergo photoreversal back to the resting 11-cis configuration.
A schematic model for the configuration changes in the opsin/rhodopsin molecule during activation has very recently been proposed by Piechnick et al (110); see their Figure 4. On their model the protein can exist on the one hand in a ‘compact inactive’ configuration, either when it is ligand-free (as free opsin) or after binding 11cis retinal (as rhodopsin), or alternatively it can exist in an ‘opened-up active’ configuration, either upon isomerization of rhodopsin to meta II (as R*) or when ligand-free (as Opsin*). Exit or entry of alltrans or 11cis retinal is possible only from/to the opened-up active configuration, and such access appears to occur via a retinoid channel that opens between the retinoid binding pocket and the lipid membrane. A separate hydrophilic channel, that permits entry/exit of water during Schiff base hydrolysis/formation, is also proposed to open and close at the same times. This model appears capable of explaining many of the known features of retinoid entry/exit, Schiff base formation/hydrolysis, and switching of the protein’s ability to activate the G-protein. In Section 6 it will be suggested that one difference between rhodopsin and the cone opsins is that access by water is greater, and that it remains possible in the cone pigment’s ‘compact inactive’ configuration.
Rapid hydrolysis of the Schiff base bond after light activation. Recently, Chen et al (104) have shown that the presence of the relocated counterion (the equivalent of E113 in bovine rhodopsin) is crucial in enabling rapid hydrolysis of the Schiff base bond in the light-activated metarhodopsin II state (which occurs ~200× more rapidly for the cone form than for rod metarhodopsin II). As described above, in meta II the Schiff base bond is deprotonated while the counterion site is protonated. However, hydrolysis of the Schiff base bond requires its transient protonation, and Chen et al (104) showed that the proton donor is the protonated counterion residue; water is then able to attack the protonated Schiff base, breaking the covalent bond and thereby leaving all-trans retinal non-covalently attached in the retinoid-binding pocket. Hence, we can conclude that the relocation of the counterion site was instrumental in generating a visual pigment that could rapidly release its all-trans retinal, thereby contributing to the rapid shut-off of activation needed for a rapid response in cones, and also rapidly readying the opsin for binding 11-cis retinal. As will be discussed in Section 6, at a subsequent stage in evolution the Rh1 opsin (rhodopsin) found a way to prevent this rapid decay of meta II, thereby enabling activated rhodopsin to integrate the photon-triggered signal for longer times than could be achieved with the rapidly decaying meta II.
Scenario for the pre-vertebrate evolution of C-opsins
In view of the information above, the following scenario can be hypothesized for the developments that occurred during the evolution of C-opsins in the chordate lineage (modified slightly from the interpretations of Terakita, Shichida, and colleagues):
C-1) An ancestral chordate possessed a C-opsin photopigment that exhibited close homology to extant Opn3 (encephalopsin and TMT-opsin), as well as to cnidarian and protostome C-opsins and even to R-opsins. E181 served as the Schiff base counterion, and site 113 was not negatively charged, and was instead probably either Y or F. This photopigment was bistable and could undergo photoreversal from its active state. It activated a Gi protein, though at a much lower rate than modern cone or rod opsins activate Gt.
C-2) This C-opsin gained a glutamate at position 113. Subsequently this E113 adopted the role of counterion to the Schiff base in the dark resting state, while the ancient E181 may have played a role in stabilizing the protonated Schiff base in the first metarhodopsin intermediate, metarhodopsin I.
C-3) Further mutations occurred, that permitted a larger tilt of helix 6 in the active state thereby improving interaction with the G-protein. This new configuration was stabilized by protonation of the ERY motif, creating the highly-active form metarhodopsin II that is unique to vertebrate ‘visual’ opsins. This metarhodopsin state exhibits very efficient activation of the G-protein in comparison with its predecessors, but is no longer capable of undergoing photoreversal upon absorption of another quantum (and it also has its absorption maximum in the UV).
C-4) Relocation of the counterion site also expedited hydrolysis of the Schiff-base bond in the activated state, providing a short lifetime of meta II and hence a more rapid photoresponse in those early cones.
C-5) The vertebrate C-opsins Opn3, parietopsin, parapinopsin, VA opsin, and probably pinopsin, represent modern versions of intermediate forms that occurred in this evolutionary progression, and show various degrees of modification of their functional properties from those of the ancestral chordate C-opsin, that remain useful for applications in different types of light-sensitive cell.
C-6) Hence, the ancestral opsin of the first cone photoreceptor in the lateral light-sensitive organs of the proto-vertebrate exhibited highly-efficient activation of the G-protein, along with rapid shut-off; it is also likely that by this stage the G-protein of the cone had evolved to become Gt (transducin).
C-7) As will be discussed in the next Section, this first cone opsin duplicated, giving rise to what became the SWS and LWS (short- and long-wave sensitive) divisions of cone opsins.
C-8) These events all occurred prior to the ‘2R’ whole-genome duplications that occurred around the base of the vertebrate radiation.
6 Vertebrate ‘visual’ opsins
A characteristic feature of the retina of jawed vertebrates is its use of cone opsins and a cone-based pathway for intensities from twilight upwards (photopic vision) but rhodopsin and a rod-based pathway for the very lowest intensities (scotopic vision), in what has historically been termed a ‘duplex’ organization of the retina. It is of considerable interest to understand how, when, and why this division arose. Along with many of the other advents that occurred in the stem vertebrate lineage, it seems that a major factor contributing to the duality of cones and rods was the occurrence of two rounds of whole genome duplication (‘2R’), as proposed originally by Ohno (111) and subsequently confirmed in numerous studies.
By way of background, we will begin by reviewing the phylogeny of cone and rod opsins. Recently, substantial progress has been made in understanding the contribution of the ‘2R’ duplications through studies of gene arrangements on chromosomes, and here we will examine results relevant to opsins. Then we will examine the differences in functional properties between cone opsins and rhodopsin.
In addition to the opsins expressed in cones and rods, the vertebrate retina also expresses a number of other opsins, including melanopsin and VA opsin. Furthermore, it has been shown that the intrinsically-photosensitive retinal ganglion cells (ipRGCs) that express melanopsin comprise a number of subtypes with diverse functions (112, 113). Different subtypes receive synaptic input from different sub-laminae of the inner plexiform layer, and project to different brain areas. Some subtypes are able to contribute to form vision in mouse. However, the following discussion of vertebrate ‘visual’ opsins will be restricted to the opsins of cones and rods.
Phylogeny of vertebrate ‘visual’ opsins
Phylogeny. The phylogeny of opsins, including vertebrate ‘visual’ opsins, has been reviewed in a number of publications, including Terakita (25), Bowmaker (114), Shichida & Matsuyama (8), Davies et al (94), and Porter et al (12), amongst others.
Cone opsins are ancestral to rhodopsin. One of the most important discoveries about the origin of vertebrate retinal opsins was made by Okano et al (115), who showed conclusively that the rod photopigment rhodopsin had evolved from one of the pre-existing cone photopigments. Furthermore, they showed that the first split in the ancestral vertebrate visual opsins had given rise to the SWS and LWS distinction; i.e. to the long-wave-sensitive (LWS) cone opsin on the one hand, and on the other hand to all the other cone opsins plus rhodopsin (the ‘SWS’ division, that now comprises SWS1, SWS2, Rh2 and Rh1). These fundamental results, which are illustrated by the more recent phylogeny in Fig. 22, have been confirmed by all subsequent studies.
Timing of the SWS/LWS split. The evidence to be presented in the next Section (on paralogon arrangement of chromosomes) indicates that ancestral SWS and LWS opsins already existed prior to the ‘2R’ whole genome duplications that occurred at the base of the vertebrate lineage. These two cone opsins already exhibited highly effective activation of transducin, as a result of the molecular rearrangements that had followed relocation of the site for the Schiff base counterion from position 181 to 113. The advent of LWS red-shifted-sensitivity appears to have occurred as follows.
LWS opsins: Red-shift through acquisition of a chloride binding site. As residue E181 was no longer required as the counterion to the resting state, mutations were not as constrained as previously. A mutation of E181 to H181 created a chloride ion binding site that permitted a substantial red-shift in the opsin’s peak absorption, giving rise to the ancestral LWS cone opsin. (Note that this numbering is relative to bovine rhodopsin; in the frame of human OPN1LW the residues are H197/K200.) A history of investigations into the nature of this chloride-binding site is given in the recent study of Yamashita et al (116). Wang et al (117) showed that the two residues H181 and K184 contributed to the effect, with H181 being the primary residue contributing the red-shift; the K184 may contribute to enhancing the stabilization of chloride binding. Davies et al (118)and Yamashita et al (116) have recently shown that two other residues, 289 and 292, are also involved in the chloride effect.
As an aside, it is interesting that a handful of mammalian LWS cone opsins have subsequently reverted to a blue-shifted ‘MWS’ sensitivity through loss of the chloride binding site. One example is mouse ‘MWS’ opsin, which has the 181 position mutated to tyrosine (Y181); it shows no chloride effect because positions 289 and 292 have both also mutated to serine (Yamashita et al, 2013). On the other hand, guinea-pig ‘MWS’ opsin (also with Y181) retains a partial chloride effect, as positions 289 and 292 both remain occupied by alanine.
SWS opsin duplications. The ancestral SWS pigment duplicated twice, to produce four opsins with peak sensitivities in the blue/green region of the spectrum; the next Section will present evidence suggesting that this quadruplication occurred as part of the vertebrate ‘2R’ whole-genome duplications.
Vertebrate cone opsins. As a result of these duplications, the first vertebrates (jawless at that stage) possessed a complement of five distinct cone opsins, that are conventionally classified using the rather confusing terminology of SWS1, SWS2, Rh2, Rh1, and LWS (Fig. 22). Those five classes have been inherited by all the descendants of the ancestral vertebrate, though some classes of opsin have been lost in different lineages. For example, monotremes lost the SWS1 opsin (119), while placental mammals lost the SWS2 and Rh2 opsins; for more complete information, see Davies et al (120).
Where they are retained in extant vertebrate species, the four opsin classes SWS1, SWS2, Rh2, and LWS, continue to be expressed in cones. At some subsequent stage (Section 11), the cone photoreceptor that expressed the Rh1 opsin evolved to become a rod. A second type of rod exists in amphibia, utilizing the SWS2 opsin, but the timing of its origin is unclear.
Paralogon arrangement of opsin genes
In order to help determine the role of whole-genome and other large-scale duplications in the evolution of the genes encoding the opsins and associated proteins of phototransduction, Larhammar and colleagues have studied the paralogon arrangement of the genes (7, 121). The term ‘paralogon’ applies to the (usually) quartet arrangement of related chromosome regions within the genome, that still persist (though often greatly modified) some 500 million years after the two rounds of genome duplication.
Chromosomal arrangement of opsins. The results of Nordström et al (121) and Larhammar et al (7) for the paralogon arrangement of the human opsin genes (as well as the genes for transducin alpha subunits) are shown schematically in Figure 23. The genes for rhodopsin (=RHO), SWS1 (=OPN1SW), and LWS (=OPN1LW) lie in corresponding positions on chromosomes 3, 7, and X. In view of the fact that phylogenetic trees for vertebrate visual opsins always have the SWS and LWS opsins as the most basal divergence (Section 6), they proposed that the locations of the ancestral genes (prior to quadruplication) had been as indicated in the top row. Following the two genome duplications, the ancestral SWS was proposed to have evolved into the SWS1, SWS2, Rh2, and Rh1 opsins of basal vertebrates, of which Rh2 and SWS2 have been lost in mammals (crosses in first column). In addition, it appeared that the LWS opsin had been preserved in only one member of the quartet (now on the X chromosome in humans).
The chromosomal arrangement of the genes for all the other proteins involved in the phototransduction cascade will be deferred to Section 8.
Discrepancy between observed phylogenetic tree and that predicted by ‘2R’ duplications. It is important to recognize a discrepancy between the observed phylogenetic tree for vertebrate cone/rod opsins and the tree expected for whole-genome duplications. From first principles, one would expect that the pair of ‘2R’ whole-genome duplications would have produced a ‘forked’ phylogenetic tree with a double bifurcation, of the form ((SWS1,SWS2), (Rh2,Rh1)), in contrast to the ‘nested’ tree (SWS1,(SWS2,(Rh2,Rh1))) that is always observed. In fact, a doubly bifurcating tree might be expected for all gene families that survived the quadruplication event, and the general absence of such forms was originally taken by some as evidence against the occurrence of ‘2R’ duplications.
However, if (as is generally thought) the two duplication events occurred close together in time, relative to the time that has elapsed since, then a variety of influences could alter the form of the phylogenetic tree that is obtained. In general, any tendency towards different rates of incorporation of substitutions in the different branches could lead to a distortion of the extracted tree topology. For the visual opsins, which have adopted different spectral positions and different kinetic properties, it is plausible that there could have been competition between these (or other) properties, in the different branches, that in practice led to different rates of substitutions at residues in different branches.
As one example, consider what might have happened if the ancestral SWS opsin exhibited its peak absorption close to the UV, and underwent two successive duplications. After the first duplication, it seems likely that selective pressures would have caused a separation of peak wavelengths of the two opsins. However, this pressure is likely to have been asymmetric, because a shift of peak sensitivity further into the UV is unlikely to have been advantageous to the organism, whereas a shift of one of the opsins to longer wavelengths (to occupy the gap between SWS and LWS) is likely to have been advantageous. Comparable arguments would apply after a second duplication. Hence it is possible to envisage that selective pressures would initially have favored a general shifting apart of the spectral absorption peaks of the different opsins, though with a ‘barrier’ at the UV end. On this basis, whichever opsin was nearest to the UV end of the spectrum would have been under selective pressure not to alter its absorption peak, as a shift in either direction would have proved disadvantageous. As a result, it might be expected that the opsin that we now call SWS1 will have experienced a lower rate of substitutions at residues that affect its spectral tuning than have the other opsins. This could have the effect of distorting the extracted phylogenetic tree in a manner that would tend to make the SWS1 opsin appear more ancient than it really is.
It therefore seems that the observed nested phylogeny that is recovered for the visual opsins cannot necessarily be taken as a basis for rejection of the hypothesis that the four SWS opsins (SWS1, SWS2, Rh2, and Rh1) arose from the ‘2R’ pair of whole genome duplications that occurred near the base of the vertebrate lineage. But on the other hand, neither has it been proven that ‘2R’ does indeed account for the existence of the five classes of vertebrate cone and rod opsin.
Differences in functional properties between cone and rod opsins
Differences in functional properties between cone opsins and rhodopsin, and the dependence of these differences on amino acid sequence, have been reviewed by Imai et al (92). Three substantial differences involve: (i) the short lifetime of cone metarhodopsins, (ii) the rapid regeneration of cone pigments, and (iii) the high susceptibility of cone opsins to hydroxylamine attack.
Short lifetime of cone meta II. Many studies have shown that the lifetime of the meta II state is orders of magnitude shorter in cone opsins than in rhodopsin. Shichida’s group (reviewed in Imai et al (92)) examined the properties of purified extracted opsins from native membranes or from cultured opsins with point mutations, using spectrophotometry, at a range of temperatures and other conditions. For WT chicken opsins, the meta II decay times were short for cone opsins (~2, ~1, 7 and 16 s for SWS1, SWS2, Rh2 and LWS) but far longer for rhodopsin (210 s), in each case at room temperature and with extraction in CHAPS. More recently, Chen et al (104) reported a similar difference in Xenopus, where the meta II for blue/violet-sensitive (SWS1) cone opsin decayed with a time constant of ~3 s compared with ~800 s for bovine rhodopsin (Rh1), both at 20 °C and pH 6 with extraction in 0.1% dodecyl maltoside. Their method involved fluorescence microscopy measurements to monitor the release of all-trans retinal, presumed to reflect the decay of meta II.
The large observed difference in speed of meta II decay (or retinal release) is not due to extraction/purification, because Golobokova & Govardovskii (122) found a similar effect in intact individual cones and rods of the goldfish using a fast-scanning dichroic microspectrophotometer. For both the red-sensitive (LWS) and green-sensitive (Rh2) cone opsins they found a meta II lifetime of ~5 s, compared with ~330 s for rhodopsin (Rh1). Hence each of these approaches shows that the decay of meta II occurs 100× (or more) faster for cone opsins than for rhodopsin.
Role of sites 122 and 189. Yoshinori Shichida’s group has employed site-directed mutagenesis to determine the importance of the residues at sites 122 and 189 (in bovine rhodopsin numbering) for these differences in meta II lifetime, as well as for differences in pigment regeneration time (reviewed in Imai et al (92)). Residue 122 is conserved as E122 in all rhodopsins apart from those of deep-sea fish (see below), but in cone opsins it may be I, L, M or Q. Residue 189 is conserved as P in all cone opsins, but it is I in most rhodopsins. Mutations in these two residues act synergistically in determining both the lifetime of meta II and the rate of pigment regeneration. Thus, the presence of the rod forms (E122 and I189) both contributed to slow decay of meta II and to slow regeneration of visual pigment, whereas the presence of the cone forms (I/L/M/Q122 and P189) both contributed to faster kinetics. Together, the effects of these two residues accounted for all of the observed cone/rod difference in meta II kinetics. Although synergistic, the effects at the two locations were not equally strong, with the residue at position 122 being more effective in rods and the residue at position 189 being more effective in cones.
Role of the counterion site 113. The recent study of Chen et al (104) investigated the role of the counterion site in the speed of meta II decay. As mentioned in Section 5, they showed that all-trans retinal was released around 200× faster from the SWS1 cone opsin than from rhodopsin. In the Xenopus SWS1 opsin the counterion residue is aspartate (D108), and its removal (by mutation to alanine) led to extremely slow release of all-trans retinal; note that this visual pigment had an unprotonated Schiff base and hence was UV-sensitive so that it needed to be activated by UV rather than blue light. The re-introduction of aspartate at an alternative location (residue 85), also close to the Schiff base, restored absorption in the violet and in addition restored release of all-trans retinal. They also compared release of retinal from cone opsin and rhodopsin, when the counterion was removed from each (in bovine rhodopsin by mutation to glutamine); the measurements required higher temperature and longer monitoring times, but remarkably the release rate was equally slow in the two mutant opsins.
From these and further experiments Chen et al (104) concluded that the rate of hydrolysis of the Schiff base bond was similar in cone opsins and rhodopsin, and that what differed was the rate of release of retinal from the opsin after the covalent bond had been broken. This could result from either a difference in the chromophore’s interaction with the retinal binding pocket or a difference in its ability to traverse the opsin protein (i.e. its accessibility). As mentioned in Section 5, they proposed a molecular model for hydrolysis of the Schiff base, in which the protonated residue at the counterion site acted as a proton donor to transiently protonate the bond and thereby render it susceptible to attack by water.
Susceptibility to hydroxylamine attack. Cone opsins are subject to degradation by hydroxylamine, whereas most rhodopsins are not. (This applies to rhodopsins of placental mammals, though some non-mammalian and monotreme rhodopsins have been shown to be slowly degraded; (123, 124).) Hydroxylamine attacks the Schiff base bond, and is able to do so in the resting cone opsin as well as in the metarhodopsin state of activated rhodopsin. Hydroxylamine is thought to attack the Schiff base in much the same way as occurs in the normal decay of meta II, by transient protonation of the bond, so that the cone/rod opsin difference in susceptibility to attack by hydroxylamine simply reflects the accessibility of the binding site to hydroxylamine.
Molecular mechanism limiting the speed of metarhodopsin II decay and formation of rhodopsin. Decay from the metarhodopsin II state involves hydrolysis of the Schiff base bond followed by release of alltrans retinal from the chromophore pocket, and there has been controversy as to which of these represents the rate-limiting step. By combining the recent work of Chen et al (104) and Piechnick et al (110), the following is proposed. The meta II state has to be transiently protonated, and the proton donor for this is the protonated counterion residue. Water access is required, and is relatively unhindered in cone opsins, but in rhodopsin can only occur via the hydrophilic channel that opens up in the meta II state. Likewise, when 11cis retinal binds to form rhodopsin, a water molecule must be able to exit the binding site via this same route. In the molecular model of Piechnick et al (110), the hydrophilic channel in rhodopsin permits slow access/removal of water in the ‘opened-up active’ configuration, but very effectively protects the binding site in the ‘compact inactive’ state. For cone opsins it is simply necessary to postulate that access for water is much less restricted in both the active and inactive configurations.
Rhodopsin inaccessibility. Hence, the three major differences between cone opsins and rhodopsin (meta II lifetime, regeneration time, and hydroxylamine susceptibility) may all simply be manifestations of the fact that the rhodopsin molecule is much more effective at ‘shielding’ the retinal covalent binding site from small molecules, including water and hydroxylamine. Furthermore, it appears that this major cone/rod difference is contributed by the residues at sites 122 and 189. In other words, the acquisition of these residues may have permitted the rhodopsin molecule to adopt the more compact ‘shielded’ configuration that excludes water access in the resting state while permitting slow access in the meta II state (see Piechnick et al (110)). Thus, it seems entirely possible that essentially all of the important molecular differences between cone and rod opsins stem from this difference in accessibility, determined mainly by the residues at these two sites.
Evolution of this difference between cone opsins and rhodopsin. In terms of evolution, we can rationalize the above findings with the view (as explained in Section 5) that the ancestral C-opsin of cones had already evolved the relocation of its counterion site, which enabled both the increase in efficacy of G-protein activation and the release of all-trans retinal, with that latter contributing in part to a fast response. But subsequently (after the ‘2R’ duplication) the pressure for increased sensitivity provided an advantage in attaining a longer lifetime of activated meta II for one of the opsins, and this was accomplished in the Rh1 opsin when site 122 mutated to glutamate and site 189 mutated to isoleucine, their present forms in rhodopsin.
The slow decay of metarhodopsin II may additionally have provided an advantage in reducing the toxicity that could have been elicited by a massive rapid release of all-trans retinaldehyde in intense light (e.g. sunlight); if so, this would have provided pressure for the retention of E122 and I189 in rhodopsins.
Residue 122 in deep-sea fish. Interestingly, in the rhodopsins of deep-sea fish, residue 122 is often occupied by glutamine (Q122), and this provides a significant blue-shift in the absorption spectrum (125). It is conceivable that the extremely low light intensities that are experienced at great depths lead to so little retinal being released that there is never a toxicity problem with E122Q in these species, whereas there is an advantage in the shift to a shorter wavelength of peak absorption.
Similarity. One interesting similarity, rather than difference, between cone and rod photopigments is that they are all remarkably stable against spontaneous thermal isomerization to the active state. The comparative details of this stability will be treated in Section 10.
Scenario for the evolution of vertebrate ‘visual’ opsins
From the information presented up to here, the following scenario is proposed for the sequence of evolutionary events that led to the emergence of four cone opsins plus rhodopsin in the vertebrate retina:
D-1) Prior to the large-scale duplication events that occurred at the base of the vertebrate lineage, the rostral region of the central nervous system (that would expand as the diencephalon) contained neurons (some of them ciliary photoreceptors) that expressed a range of C-opsins, including members of the classes parietopsin, parapinopsin, VA opsin, pinopsin, and an ancestral cone opsin.
D-2) In the ancestral cone opsin (and some of the earlier C-opsins), the counterion site had migrated to position 113. Subsequently, changes occurred that permitted greater movement of helix 6. The new meta II form exhibited a high efficacy of activation of the G-protein, and it decayed rapidly to release all-trans retinal.
D-3) This ancestral cone opsin duplicated to form two divisions, SWS and LWS opsins, that were expressed in two separate classes of cone photoreceptor in the part of the diencephalon that was expanding laterally.
D-4) In the LWS opsin, the ancestral counterion site E181 (in bovine rhodopsin numbering) had undergone mutation to H181, to create a chloride ion binding site, thereby giving a substantial red-shift in the pigment’s peak absorption.
D-5) The genes for the SWS and LWS opsins were adjacent on a chromosome. Not far downstream were the genes for the alpha subunits of Gt (transducin) and Gi.
D-6) Two rounds of whole-genome duplication gave rise to four copies of the SWS gene, which underwent mutations to become the founding SWS1, SWS2, Rh2 and Rh1 opsins. When bound to 11-cis retinal, these visual pigments had peak spectral sensitivities ranging from the UV to the green region of the spectrum. Of the quartet of LWS genes that resulted from the duplications, only one copy survived, corresponding to the LWS (also sometimes called MWS) gene of all extant vertebrates.
D-7) Upon activation by a photon, all of these cone opsins exhibited a high efficacy of activation of the G-protein transducin; none exhibited photoreversal once they relaxed from the meta I state to meta II; and all underwent relatively rapid thermal decay from the active meta II state, releasing all-trans retinal.
D-8) In the Rh1 opsin, two mutations occurred (at residues 122 and 189) that led to slower decay of the meta II intermediate. This enabled the photoreceptor expressing the Rh1 opsin to integrate the photon signal for longer, thereby endowing it with higher sensitivity; but a trade-off was the slower re-binding of 11-cis retinal and hence slower regeneration of visual pigment. There was little else that distinguished this Rh1 ‘rhodopsin’ from the other ‘cone opsins’.
D-9) The thermal stability of these opsins against isomerization (i.e. their ability to avoid spontaneously entering the active all-trans state) was a function of the wavelength of absorption to which they were tuned (see subsequent Section 10), but all of these opsins were highly stable.
7 Vertebrate retinal cones and rods
The triumph of ciliary cells as the primary photoreceptors in vertebrates
In view of the fact that the image-forming eyes of most protostomes employ microvillar photoreceptors, it is natural to ask why vertebrate eyes instead opted for ciliary photoreceptors. Furthermore, given that a single type of fly rhabdomeric photoreceptor can reliably detect individual photons, and also respond very rapidly over an enormous range of intensities (reviewed in Yau & Hardie (126)), it is also appropriate to ask why vertebrates instead use a duplex retina with separate cone and rod divisions.
In considering the relative merits of ciliary and microvillar photoreceptors, and the likely reasons why one or other of these classes triumphed in different organisms, it is important to avoid the error of simply comparing properties between the photoreceptors of living organisms. For example, it is not appropriate to argue on the basis of a comparison between the properties of modern rhabdomeric photoreceptors in flies and modern cone and rod photoreceptors. Instead, one needs to take into consideration the properties of the photoreceptors, and the circumstances of the organisms, at the relevant stage in evolution when the cell type gained its dominance; i.e. at the time when the ‘choice’ of photoreceptor class was made. At the stage when ciliary photoreceptors first became dominant in the retinas of chordates, more than 500 Mya, the evidence suggests that rods had not yet evolved. Likewise, at the stage when microvillar photoreceptors gained their dominance in protostome compound eyes, possibly more than 550 Mya, it is unlikely that they had yet become true rhabdomeric photoreceptors or that they had yet evolved the highly specialized (and possibly unique) kind of phototransduction cascade of Drosophila eyes (127). Accordingly, one needs to investigate the relative merits of the rather simpler ciliary and microvillar photoreceptors that were likely to have existed in those ancient times.
With those points in mind, we can list a number of advantages that ciliary photoreceptors might have had over microvillar ones, at the time that the ‘choice’ between them was made:
E-1) Firstly, the polarity of response (hyperpolarizing to light and depolarizing to darkness) meant that ciliary photoreceptors would have been ideally suited for shadow detection in ancient organisms. The depolarization induced by a shadow could have triggered either an action potential or an increase in release of synaptic transmitter without additional logic, and this might have been advantageous for an animal with a simple nervous system.
E-2) Secondly, there may have been little difference in gain between the two classes of photoreceptor. Very recently, Ferrer et al (46) have shown that the gain of transduction in amphioxus microvillar photoreceptors is much lower than the gain in modern rhabdomeric photoreceptors. However, the extremely short duration of the quantal response in chordate microvillar photoreceptors (a few ms; (43)) may have been disadvantageous in comparison with the slower responses of ciliary photoreceptors.
E-3) Thirdly, it has been calculated that in a bright environment the energy consumption is lower for a hyperpolarizing ciliary photoreceptor, in which the ionic currents decrease in the light, than for a depolarizing microvillar photoreceptor, in which the ionic currents increase in the light; on the other hand the energy consumption of the two classes of photoreceptor is comparable in darkness (128). A duplex retina has a further advantage in a bright environment because the rods saturate, thereby reducing their energy consumption to a low level, and it is only the cones that continue to generate a circulating current with its consequent energy demand (11).
E-4) Fourthly, chordate ciliary opsins (but not other ciliary opsins) underwent intra-molecular changes that substantially increased the efficacy of their activation of the G-protein. As a consequence, though, they lost the ability to undergo photoreversal.
E-5) Fifthly, these new ciliary opsins exhibited quite rapid decay of the active metarhodopsin II state, releasing bound all-trans retinal, and thereby enabling the binding of 11-cis retinal. Such release would have been made essential by the inability of the pigment to undergo photoreversal; however it is also possible that this change preceded the increase in activation efficacy. In the case of bistable opsins (R-opsins and the early C-opsins), the activated pigment remains in the metarhodopsin state for an extended period after exposure to light, so that the pool of rhodopsin available to detect incident photons is depleted. In this respect, a bistable photopigment may have been severely disadvantageous when an organism moved from a bright environment to a dim environment and needed to dark-adapt rapidly. Chordate ciliary photoreceptors may thus have provided a distinct advantage under such conditions.
E-6) Finally, an additional benefit to the organism may have arisen not directly from the properties of the phototransduction process, but from the fact that the ciliary cells evolved synaptic transmission onto their microvillar counterparts (see Section 15), thereby constituting a dual photoreceptive system via a single afferent pathway.
In any case, as the discussion above shows, it is possible to point to several important ways in which ciliary photoreceptors may have proved superior to microvillar photoreceptors in ancient chordates. For one reason or another, ciliary photoreceptors did indeed triumph in the light-sensitive organs of proto-vertebrates.
Multiple classes of vertebrate retinal photoreceptor
For a review of the distribution of opsins and the variations in morphology and in transduction pathways across classes of vertebrate photoreceptors, see Ebrey & Koutalos (91).
As described in Section 6, the ancestral vertebrate possessed five classes of cone/rod opsin photopigment. In addition it possessed five morphological classes of cone/rod photoreceptor. On the basis of the norm in photoreceptors of jawed vertebrates, as well as circumstantial evidence from extant lampreys (Figure 15), it seems likely that each class of photoreceptor expressed a single class of opsin.
In jawed vertebrates, these photoreceptors comprise four classes of cone, expressing their individual cone opsins (SWS1, SWS2, Rh2, LWS), plus a single class of rod photoreceptor expressing the rod photopigment, rhodopsin (Rh1). However, at least in amphibia, the SWS2 ‘cone’ opsin can additionally be expressed in another class of rods (called ‘green rods’ because of their greenish tint). In living lampreys, all five classes of photoreceptor appear cone-like, though the class that expresses ‘rhodopsin’ has some rod-like properties (Section 3). As a result, it is plausible that ‘true’ rods had not evolved prior to point #5 in Figure 1. The timing of the emergence of rods will be discussed in Section 11.
Currently it is not clear whether the initial driving force for the multiplicity of spectral classes of cone photoreceptor was simply in order to cover more of the ‘visible’ spectrum, given that each opsin absorbs over a relatively narrow region of the spectrum, or whether it was to provide ‘color vision’. But it seems reasonable to think that, almost as soon new spectral information became available to an organism, it would have been utilized by the nervous system to provide color information.
Morphological differences between cones and rods
Details of the morphology of cone and rod photoreceptors are given in Webvision Part I, Chapter 2 ‘Photoreceptors’; here we will simply concentrate on differences between the two classes of cell.
Sac versus disc structure. The most marked morphological difference between mammalian cones and rods relates to the topology of the outer segment membrane. In cones, the entire membrane is continuous with the plasma membrane of the inner segment, whereas in rods the bulk of the outer segment membrane is sealed off from the plasma membrane in the form of discs that resemble deflated balloons. These discs are not actually ‘free floating’, but appear to be tethered to the plasma membrane by proteins including peripherin/RDS, Rom1, and the GARP domain of the cyclic nucleotide-gated channel.
In both cell types, new outer segment membrane is continually being formed in the vicinity of the ciliary neck, and bulges outwards, so that new sacs are repeatedly formed beneath recently formed ones, in a mechanism that was documented experimentally by Steinberg, Fisher & Anderson (129); see Figure 16B above. They showed that a process of rim formation occurs between the membranes of adjacent sacs, though in cones it proceeds only part way around the circumference, so that the sacs are always open to the exterior (patent). In rods, however, this process of rim formation proceeds all the way around the circumference (bi-directionally), zippering together the apposed surfaces, and thereby forming a disc pinched-off from the plasma membrane.
As a result of this ‘sealing over’ of discs in rods, newly synthesized protein is trapped in the newly formed discs. As additional discs are created, those formed earlier slowly migrate outward along the length of the outer segment, over a period of weeks, before being phagocytosed by the RPE. This localization and migration of proteins can be demonstrated experimentally by autoradiographic examination at successive times after application of labeled amino acids (130). In contrast, what is seen in cones is a uniform distribution of label over the entire outer segment, as expected if protein is able to diffuse (even very slowly) throughout the continuous plasma membrane of the outer segment.
Other morphological differences. A further morphological difference between their outer segments is the existence of one or more incisure(s) in rods; these deep longitudinal indentations into the outer segments provide an increased cross-section of cytoplasmic space that increases the effective longitudinal diffusion coefficient for intracellular messengers (see also Section 11). At the level of the inner segment, cones exhibit specializations that do not occur in rods, including the paraboloid and sometimes spectral filters (an oil droplet or vesicles, in the ellipsoid). Probably because of its high refractive index, the inner segment of cones is very effective in funneling incident light into the outer segment, and this gives rise to a marked Stiles-Crawford directional effect in cones, and a weak one in rods. At their synaptic terminals, cones and rods differ substantially, with the cones exhibiting large pedicles and the rods exhibiting smaller spherules. In addition, there are other subtle morphological differences between cones and rods.
Overview of functional differences between cones and rods
The light response properties of cone and rod photoreceptors are remarkably similar to each other (see below), and the cells exhibit just a few major differences:
(a) Rods. The defining feature in the response of the rod photoreceptor is its ability, under dark-adapted conditions, to respond reliably (i.e. with good signal-to-noise ratio) to the absorption of individual photons of light (131, 132). This single-photon detection performance is possible because the noise, expressed as ‘dark light’, is very low in rods; this dark light is many orders of magnitude lower in rods than in LWS cones, though the difference may be smaller in the case of SWS cones.
(b) Cones. The defining features in the responses of the cone photoreceptor are its ability: (i) to respond rapidly, (ii) to function over an enormous range of intensities, so that (iii) it never saturates in steady light, no matter how bright the intensity, and (iv) its ability to recover much of its responsiveness almost instantaneously when an intense light is extinguished (reviewed in Lamb (133)).
Cone/rod similarities. In most other respects, the responses of cone and rod photoreceptors are remarkably similar to each other, as shown in Table 3 for human LWS cones compared with rods. For example, the amplification of the phototransduction cascade appears to be reasonably similar in mammalian cones and rods. The observed difference in sensitivity between them (of around 20-fold) results in large part from the more rapid shut-off of the cone response, which is typically around 5-times faster than in rods under dark-adapted conditions. As illustrated in Figure 24, cone and rod responses begin rising with broadly similar gain, but the cone responses recover much sooner. It has also been shown that the shapes of the light responses (i.e. their kinetics) are closely similar, apart from an overall scaling of the time axis.
However, the gain of transduction and the rate of activation of G-protein by activated opsin are probably not exactly equal in cones and rods, and indeed there are several reports of a lower gain in cones. Kawamura & Tachibanaki (134) reviewed measurements from isolated cones and rods of fish and salamander, and concluded that the gain in the cones was considerably lower. More recently, Tachibanaki et al (135) conducted careful experiments on the rate of G-protein activation by light-activated visual pigment, and found this rate to be ~5× lower in cones than in rods. Nevertheless, a recurring difficulty in measurements of cone biochemistry is that the shut-off reactions are so fast that this creates problems in determining activation rates. Overall, it seems likely that the gain of transduction in cones lies somewhere in the range 0.2-1× that in rods.
The responses in Figure 24 also illustrate the general observation that cone photoreceptors expressing blue/green-sensitive opsins (i.e. those with the SWS1, SWS2, or Rh2 opsin) typically tend to display response properties intermediate between those of LWS cones and rods; thus, blue/green-sensitive cones are slower and more sensitive than red-sensitive cones.
Major cone/rod differences in performance. In Table 3 the parameters exhibiting major differences between cones and rods are indicated in red. Firstly, although cones and rods both exhibit classical Weber-law light adaptation, for rods this occurs only over a restricted range of intensities before they saturate, whereas for cones the adaptation continues up to indefinitely high intensities so that they never saturate in steady light. Secondly, rods typically display a very low rate of photon-like events in darkness, of the order of one event per tens or hundreds of seconds, whereas in LWS cones the ‘dark light’ may typically be of the order of hundreds of photon events per second. Although much of this difference in dark light stems from the difference in wavelength of peak absorbance (Section 10), a small part appears to be due to intrinsic differences between rhodopsin and cone opsins. Finally, following extinction of a steady light that bleaches more than 90% of the photopigment, human cones recover their circulating current within ~20 ms (136), whereas for human rods comparable recovery of circulating current may take 20 mins, which is slower by a factor of ~60,000×.
These differences will be treated according to the photoreceptor class that exhibits the superior performance. Thus, the avoidance of saturation and the speed of response will be dealt with in Section 9 on cone photoreceptors, while transduction noise and the ability to resolve individual photons will be treated in Section 10 and Section 11.
8 Evolution of the vertebrate retinal phototransduction cascade
In this Section we will investigate the evolution of the ‘vertebrate-style’ phototransduction cascade that is employed in chordate ciliary photoreceptors, with emphasis on those of the vertebrate retina. The similar cascades utilized in protostome and cnidarian ciliary photoreceptors will be mentioned only in passing. The aims will be (1) to determine the nature of the phototransduction cascade that existed in a chordate ancestor of ours, just prior to the ‘2R’ rounds of whole-genome duplication, and (2) to determine the manner in which that cascade became specialized in cones and rods. Thus, we will be examining the co-evolution of components of the cascade, initially in pre-vertebrate chordates, and thereafter in the earliest vertebrates.
Evolution of bilaterian phototransduction cascades
In Figure 25A, the major components of several phototransduction cascades are contrasted, for vertebrate photoreceptors (top), for other ciliary photoreceptors, and for microvillar photoreceptors (103). Each of these cascades appears to have evolved from a common ancestral form.
Co-evolution of cascade components. The co-evolution of molecular components across phototransduction cascades was investigated by Arendt & Wittbrodt (137), who compared the phylogenies of the genes for opsin, plus its G-protein, kinase, and arrestin, across protostomes and deuterostomes. They found clear evidence that all four of these components must have been present in the common bilaterian ancestor.
Coupling to G-proteins. The coupling of opsin type with G-protein type was examined by Koyanagi et al (138) and has recently been reviewed by Terakita et al (103). They categorized opsins into four groups according to the G-proteins to which they coupled. Chordate C-opsins couple to Gi/Gt; at least one cnidarian C-opsin couples to Gs; invertebrate ‘Go-opsins’ couple to Go; while all R-opsins couple to Gq.
Origin of cascades using CNGCs. The evolution of the coupling of opsin via its G-protein to an ion channel was investigated by Plachetzki et al (26, 27). For the downstream effector mechanism, at the level of the cell’s electrical response, Plachetzki et al (27) provided evidence that the ancestral cascade had employed CNGCs (cyclic nucleotide-gated channels), as illustrated in Figure 25B. Furthermore, the alternative TRP/TRPL (transient receptor potential-like) channel mechanism appeared to have arisen at a later stage, probably in bilaterians, and was employed in microvillar photoreceptors, where the R-opsin couples via Gq.
Molecular components of the vertebrate phototransduction cascade
The molecular and cellular mechanisms underlying activation and recovery of the (modern) vertebrate phototransduction cascade are dealt with in detail in Webvision ‘Phototransduction in Rods and Cones’, as well as in numerous reviews, including Lamb & Pugh (139), Wensel (140), Yau & Hardie (126), etc. The principal proteins involved, both in cones and rods, are shown schematically in Figure 26. For an overview of these proteins and their functions, see Wensel (140); for a review of the proteins mediating shut-off of R*, see Gurevich et al (141).
Summary of molecular mechanisms. The molecular mechanisms of phototransduction are almost identical in cones and rods. Photoisomerization of a molecule of visual pigment to its active form R* triggers the catalytic activation of the G-protein (Gt, transducin) to G*, which in turn activates the cGMP phosphodiesterase (PDE6) to E*. The increased hydrolysis of cGMP lowers its cytoplasmic concentration, causing closure of cyclic nucleotide-gated channels (CNGCs). This suppresses the circulating electrical current that had been flowing in darkness, hyperpolarizing the cell; in addition, it leads to lowered cytoplasmic Ca2+ concentration through the continued activity of a Na+/Ca2+,K+ exchanger, and this drop in Ca2+ level is important for response recovery. Response shut-off requires inactivation of each of the activated forms, as well as restoration of cGMP levels. R* is rapidly inactivated by the binding of arrestin, but this step first requires the R* to have been phosphorylated by a G-protein receptor kinase (GRK). The G*/E* complex is rapidly inactivated by hydrolysis of the terminal phosphate of the GTP bound to G*, through the action of the GTPase accelerating (GAP) activity of the RGS9-Gβ5-R9AP complex. The drop in Ca2+ concentration permits Mg2+ to bind to guanylyl cyclase activating proteins (GCAPs), thereby activating guanylyl cyclase (GC), restoring cGMP levels, and hence causing the re-opening of ion channels. This action of Ca2+ endows the photoreceptor with a powerful negative feedback loop that helps stabilize the electrical current.
Differences in isoforms and activities between cone and rod proteins
The proteins mediating the light response in cones and rods are closely similar; indeed a few of the proteins are identical in the two classes of photoreceptor, though in most cases distinct but closely related isoforms are expressed, as indicated by the gene names shown in Figure 26 and listed in Table 4. A number of online resources exist for examining the genes involved in the eye, and two useful resources are RetinaCentral.org (with the ‘retinome’, or transcriptome of the retina/RPE; (142)) and RetNet, sph.uth.edu/retnet (with retinal disease genes).
 Table 4. Genes for proteins with known function in phototransduction or retinoid cycling. List of proteins with known functions in activation and recovery of the phototransduction cascade, and in the RPE retinoid cycle. Names of the human genes are given in column 2. Where there is a clear distinction between expression in cones and rods, the genes are colored red for cones and blue for rods, while black (and ‘Both’) indicates expression in both cones and rods, as in Figs. 26 and 27. Not enough is known about the intra-retina retinoid cycle in order to list its components. Many additional proteins have important functions in cones, rods, and RPE cells, apart from involvement in phototransduction and retinoid cycling, but are not listed here. Likewise, numerous other proteins are involved in signal transmission and neural processing within the retina.
Table 4. Genes for proteins with known function in phototransduction or retinoid cycling. List of proteins with known functions in activation and recovery of the phototransduction cascade, and in the RPE retinoid cycle. Names of the human genes are given in column 2. Where there is a clear distinction between expression in cones and rods, the genes are colored red for cones and blue for rods, while black (and ‘Both’) indicates expression in both cones and rods, as in Figs. 26 and 27. Not enough is known about the intra-retina retinoid cycle in order to list its components. Many additional proteins have important functions in cones, rods, and RPE cells, apart from involvement in phototransduction and retinoid cycling, but are not listed here. Likewise, numerous other proteins are involved in signal transmission and neural processing within the retina.
When we come to compare the functional properties of cones and rods in Sections 9 to 11, we will see that it is possible to view the rod transduction cascade as a variant of the cone transduction cascade, in which the main difference is that each of the shut-off steps in the dim flash response has been slowed down. For several of the phototransduction proteins, the expression levels in cones are much higher than in rods, and this probably accounts for several instances of more rapid shut-off of the light-activated molecules. A notable example is the 10-fold higher expression level in cones of the molecular complex RGS9/Gβ5/R9AP that shuts off the activated G-protein, transducin, when it is bound to the PDE. For shutting off activated rhodopsin, cones and rods in many species employ different isoforms of the GRK (G-protein receptor kinase): in cones it is typically GRK7 whereas in rods it is GRK1; in this case, the difference in isoform probably contributes substantially to slowing the shut-off of activated rhodopsin and therefore in increasing the sensitivity of the response (134, 143, 144). For dim flashes the recovery kinetics are also determined by the turnover time in darkness for cGMP, and in rods this turnover time is slowed as a result of a lower basal activity of the rod PDE6 (145).
The overall effects of the differences in activities and expression levels of the different isoforms of phototransduction proteins have been analyzed and modeled by Kawamura & Tachibanaki (134) and Korenbrot (144), who have been able to account well for the observed differences in kinetics and sensitivity between cones and rods.
Evolution of components of the vertebrate phototransduction cascade
For vertebrate phototransduction, Hisatomi & Tokunaga (146) compared the phylogenies of the genes for eight families of the proteins involved (transducin, PDE, CNGC, GRK, arrestin, recoverin, GC, GCAP). They noted close similarity in the branching patterns of the gene dendrograms, and concluded that each of these families appeared to have evolved ‘cone’ and ‘rod’ branches, though in two cases (PDE and GCAP) there had been a further duplication in the rod branch. More recent results, examining the evolution of individual components of the phototransduction cascade, will now be presented.
Transducin. The origin of transducin alpha subunits was investigated by Muradov et al (87), who identified two isoforms in lamprey, that they named GαL and GαS, for their expression in the long (cone-like) and short (rod-like) photoreceptors, respectively. GαL is roughly equally distantly related to cone and rod transducin alpha subunits of jawed vertebrates, and might represent the ancestral form, whereas GαS clades with the rod version, though it retains certain cone-like characteristics such as the presence of the ‘hallmark’ four-residue sequence near the N-terminus. In order to determine the timing of the duplication that gave rise to these two transducin alpha subunits, there is additional information that can be obtained from analysis of the location of genes on chromosomes (see Section 8 below).
Phosphodiesterase. The phosphodiesterase (PDE6) used in vertebrate phototransduction is unique in its ability to be regulated by the gamma subunit (Pγ), and the co-evolution of these two components has been investigated by Muradov et al (86) and Zhang & Artemyev (147). Muradov et al (86) cloned both components from lamprey. For the PDE6 catalytic unit, they found that lamprey has a single isoform (as in cones), with high homology to jawed vertebrate PDE6 catalytic units, though equally distantly related to those of cones and rods, and their results were consistent with the notion that PDE6 arose from a common PDE5/6/11 ancestor in the chordate lineage. They identified a tunicate PDE that grouped with vertebrate PDE6, but they could find no other non-vertebrate sequences grouping with PDE6.
For the regulatory Pγ subunits, they found two isoforms, one cone-like and the other intermediate between cone and rod sequences; no sign of similar sequences was found in tunicate databases. Their evidence suggested that these regulatory subunits arose in the stem vertebrate lineage, and that the common ancestor of lampreys and jawed vertebrates was likely to have already possessed two isoforms. This analysis was extended by Zhang & Artemyev (147) who provided evidence that, although Pγ is a strictly vertebrate invention, the capacity of the PDE catalytic units to bind Pγ predated the emergence of the inhibitory subunit; indeed their analysis predicted that the PDE5/6-like enzymes of cnidaria should interact with vertebrate Pγ.
CNGCs. In cones the cyclic nucleotide-gated channel comprises two α subunits (CNGA3) and two β subunits (CNGB3), configured as A3-A3-B3-B3 (148), whereas in rods the channel comprises three α subunits (CNGA1) and a single β subunit (CNGB1). Nordström et al (121) found strong evidence that the duplication that gave rise to the α and β subunits of the cyclic nucleotide-gated channel took place prior to the divergence of protostomes and deuterostomes, and they also found suggestive evidence that the multiple versions of α and β subunits may have arisen in the ‘2R’ duplications.
GRKs. Mushegian et al (149) have recently investigated the origin and evolution of G-protein receptor kinases (GRKs). They found evidence that GRKs originated prior to metazoa, through the insertion of a kinase (similar to a ribosomal protein S6 kinase) into a loop in a domain with homology to RGS (regulator of G-protein signaling). During chordate evolution an ancestral GRKa split into the GRK1/7 and GRK4/5/6 lineages. Mushegian et al (149) suggest that this coincided with the first round of ‘2R’ whole-genome duplication, though it is possible that the split may have occurred earlier, as an apparently ancestral GRK1/7 is present in the tunicate Ciona intestinalis. The results of Larhammar et al (7) (see next Section) suggest that the distinction between GRK1 and GRK7 arose during the ‘2R’ duplications. However, further work is needed to resolve the origin of these isoforms.
Arrestin. The phylogeny of arrestins (including ‘visual’ arrestin) has been studied by Gurevich & Gurevich (150) and Alvarez (151), and the function of these proteins has been comprehensively reviewed by Gurevich et al (141). Arrestins arose very early in metazoan evolution, with the family comprising both the β-arrestins (named after their interaction with the β-adrenergic receptor) and the ‘visual’ arrestins, having diverged from a much larger family in pre-bilaterian times (151). A clue to the nature of the much more recent split between vertebrate visual arrestins and β-arrestins has been provided by Kawano-Yamashita et al (152) who reported that the pineal non-bleaching opsin, parapinopsin, appeared to be inactivated by a β-arrestin through a process of internalization (as in other β-arrestins). They noted that Ciona intestinalis photoreceptors apparently use a β-arrestin, and they proposed that the vertebrate-style visual arrestin had evolved specifically for use in shutting-off opsins that release their retinoid; i.e. specifically in the case of vertebrate visual opsins.
Chromosomal arrangement of phototransduction genes
The paralogon arrangement of the opsin genes was presented in Section 6. Here we consider the results of Larhammar et al (7) relating to the paralogon arrangement of other components of phototransduction.
Transducin alpha subunits. Interestingly, Larhammar et al (7) showed that the gene for the transducin alpha subunits was in the same paralogon as the opsins, and close by (see Figure 23). Recently, Lagman et al (153) have combined an analysis of the gene arrangements for each of the three subunits of the trimeric transducins with sequence-based analyses, and they have provided evidence that all three families of transducin subunit expanded during the early vertebrate tetraploidizations. They concluded that the early vertebrate tetraploidizations provided the basis for the subsequent specialization of transducin subunits leading to differential expression between cones and rods.
The original GNAT gene may have arisen by duplication within the deuterostome lineage of the ancestral GNAI/GNAT gene present in bilaterians, as some protostomes have homologs of GNAI, but none have homologs of GNAT; alternatively, the duplication could have occurred earlier, but with GNAT being lost in protostomes.
Other components of the phototransduction cascade. Larhammar et al (7) also found apparent remnants of a paralogon arrangement in a local grouping of the genes for the transducin beta subunits, for the GRKs, and for the arrestins (their Fig. 5). In addition they found evidence for ‘2R’ expansion of the genes for PDE6, and for both the alpha and beta subunits of the CNGC channel, as well as for arrestin and the GRKs.
Interpretation. For many of the proteins involved in the phototransduction cascade, there is a difference (either partial or complete) in the distribution of isoforms between cones and rods, and for at least 10 of the 13 protein components studied by Larhammar et al (7) the differentially-distributed isoforms arose as a result of the ‘2R’ duplications at the base of the vertebrate lineage. Accordingly, there seems little doubt that much of the distinction between the cone and rod transduction cascades can be traced to the reduction in constraints on gene mutation that resulted from the two whole-genome duplications.
Hypothesized nature of pre-vertebrate ciliary photoreceptor and transduction cascade
From the available evidence, it does not yet seem feasible to paint a scenario for the detailed sequence of events that transformed an ancestral bilaterian photoreceptor into a vertebrate photoreceptor. However, it is possible to propose the following for the state that had been reached, in the evolution of the ‘proto-cone’ photoreceptor and its phototransduction cascade, just prior to the ‘2R’ whole-genome duplications that occurred near the base of the vertebrate lineage:
F-1) The ancestral cone opsin of the ‘proto-cone’ photoreceptor was broadly similar to modern pinopsin and SWS and LWS cone opsins, and in particular it had evolved a high efficacy of activation of the G-protein.
F-2) Duplication of that ancestral cone opsin created a pair of retinal cone opsins: an SWS opsin with the standard E181 residue, and an LWS opsin with the H181/K184 combination that provided a chloride-binding site giving a substantially red-shifted absorption. The SWS and LWS opsins were expressed in separate, but closely similar, cones. The output signals from those cones could potentially have been utilized to provide dichromatic color vision.
F-3) The primary additional proteins involved in activation of the cascade had evolved to become: (i) the ancestral transducin Gt, with unique alpha, beta and gamma subunits; (ii) the ancestral PDE6 cyclic GMP phosphodiesterase, comprising a pair of identical catalytic subunits and a pair of identical regulatory (gamma) subunits; and (iii) a tetrameric cyclic nucleotide-gated channel composed of two classes of subunit, alpha and beta.
F-4) The Gαt had probably arisen from a common ancestral Gαi / Gαt during chordate evolution. The PDE6 had arisen from a common ancestral PDE5/6/11 during chordate evolution. The PDE regulatory subunit had arisen late in chordate evolution, after the divergence of tunicates (i.e. after #4 in Figure 1).
F-5) The primary proteins involved in response recovery and regulation had evolved to become the ancestral ‘cone’ components, specified by the genes GRK7, ARR3, RGS9, GUCY2D/F, GUCA1C, SLC24A1 and RCV1, in addition to the cone opsins themselves.
F-6) The presence of all these components suggests that response shut-off occurred in a manner fundamentally similar to that in modern vertebrate photoreceptors.
F-7) In particular, the co-existence of the components required for the Ca2+ feedback loop (GUCA1C, GUCY2D/F, CNGA3/B3) provides strong circumstantial evidence that the negative-feedback loop that stabilizes the circulating current, and assists in light adaptation, was already present.
F-8) All the components of the transduction cascade were expressed in the membranes of the lamellar sacs that radiated laterally from the cilium of the proto-cone.
F-9) Because of the release of all-trans retinoid from activated opsin, the photoreceptor’s outer segment required a source of 11-cis retinoid, and this may have been provided by the glial (Müller) cells.
F-10) The cell had evolved a simple glutamatergic synapse, permitting synaptic transmission.
9 Cones: Photoreceptors with exceptional performance
Cones exhibit high sensitivity, high speed, and high contrast sensitivity
High sensitivity. Despite frequent claims to the contrary, cone photoreceptors are highly sensitive, and the amplification of transduction appears to be quite similar to that of rods. Indeed, when one views a dim star at night, it is the cones (and the photopic system) that detect the star. For example, in viewing the Pleiades (Seven Sisters), although one will be aware of the cluster as a blur in the parafoveal field by means of scotopic vision, when one fixates the individual stars then it is the cones that are detecting them. The rod system is much better for the diffuse cluster, because of its wide spatial summation, but the cone system is better at detecting individual stars, because of its higher spatial resolution. The sensitivity of individual cones is sufficient to detect quite dim stars, though for very dim stars one may be better off using parafoveal rod vision.
High-speed response. The speed of response in cone photoreceptors is far higher than in rods, and this speed increases with increasing background intensity. In bright illumination, the human photopic visual system is able to resolve sinusoidal or square-wave flicker at frequencies of around 100 Hz (154), for which the brighter/dimmer periods are ~5 ms each. Thus, it seems that light-adapted cones are able to generate a resolvable signal when the illumination is extinguished for as short a duration as about 5 ms.
High contrast sensitivity. The human photopic (cone) system is able to detect very small fractional changes in intensity. Thus, under light-adapted conditions, an observer can detect a contrast of 0.5%; for comparison the contrast sensitivity of the scotopic (rod) system is at least an order of magnitude poorer, at >5%.
Hence, the cone system is extremely sensitive, extremely fast, and it has excellent contrast sensitivity.
Avoidance of saturation by cones: Unlimited upper operating intensity
In contrast to rod photoreceptors, which saturate at quite low intensities, cone photoreceptors are remarkable for their ability to function well during steady illumination – no matter how intense that steady background is made (155, 156). Although cones may saturate transiently at the onset of intense illumination, they rapidly recover and are able to signal increments and decrements, even when the illumination is so bright that it bleaches a large proportion of the photopigment. To account for this, Lamb & Pugh (139) compared the shut-off reactions in human cones and rods; they showed that the ability of the cones to avoid saturation can be explained in terms of the combination of the ~20-fold faster shut-off of the activated cone photopigment, and the ~20-fold faster shut-off of the activated cone Gt/PDE complex.
In human rod photoreceptors in vivo, the circulating current is halved at a steady intensity of ~70 scotopic trolands (600 R* s-1), with complete saturation occurring at ~1000 scotopic trolands (~104 R* s-1). If the activation gain of transduction is comparable in human cones and rods, then the two very short cone time constants would elevate the intensities required for half and full saturation by some ~400×, to levels of ~240,000 and ~4 × 106 R* s-1 in cones. Two additional factors are that the gain of transduction may be slightly lower in cones, and that the cGMP-gated channels of mammalian cones (in contrast to those of rods) show increased affinity for cGMP when Ca2+ falls (157); these factors would further increase the R* rate required to saturate the cone. Now, the highest steady rate of photoisomerization that can ever be reached in a human cone, by exposure to steady light, corresponds to the maximal rate of pigment regeneration, which has been shown by Mahroo & Lamb (158) to be 0.75%/s, which in a cone containing 40 million molecules of cone opsin corresponding to ~300,000 R* s-1. Hence, this maximum possible rate of steady photoisomerizations is lower than the isomerization rate calculated above to be needed to saturate the cone, meaning that the cone can never be saturated by steady light.
A more intuitive way of understanding this is to say that very high steady intensities cause the cone opsin to bleach to a level low enough to prevent saturation of the response by the steady light. As a result, the photopic visual system is able to function over (i.e. to adapt to) an enormous range of intensities, from moon-lit conditions to the brightest sun-lit scenes.
Extremely rapid recovery of cone circulating current
When the human eye is exposed to steady light sufficiently intense to bleach 90% of the photopigment in the rods, full recovery of the rod circulating current takes around 20 mins after extinction of the light (159). In contrast, a steady light that bleaches 90% of the LWS/MWS cone photopigment does not saturate the cone response (see above), and around half the circulating current remains. Upon extinction of that light, the cone circulating current recovers fully within ~20 ms, around 60,000-fold faster than for the rod (136).
As in the case of the cone’s avoidance of saturation, the molecular mechanisms that enable this extremely rapid recovery of circulating current lie in the rapidity of the inactivation steps. Kenkre et al (136) estimated that in the presence of bright light the time constants for the shut-off reactions were as follows: for R*, ~5 ms; for G*/E*, ~13 ms; and for cGMP turnover, ~4 ms. Using these parameters in a simple model of cGMP kinetics and channel activation, they were able to accurately fit their experimental measurements of the time-course of recovery of cone current. In rods, the extremely slow recovery of current is caused not by the slowness of inactivation/shut-off steps in the G-protein cascade, but instead by the slowness of the regeneration of visual pigment, combined with the fact that unregenerated opsin activates the cascade; for a full account of this slow recovery, see Lamb & Pugh (160).
Morphological features required by a cone
The operational requirements of cone photoreceptors place certain constraints on the morphological features of the cells.
High surface to volume ratio of outer segment. In order to achieve rapid response kinetics, all the recovery reactions must be fast, including the Ca2+-mediated acceleration of guanylyl cyclase activity that permits rapid re-opening of the cyclic nucleotide-gated ion channels. This means that the time constant for equilibration of cytoplasmic Ca2+ concentration (τCa) must be short. In mammalian (including human) cones, τCa has been estimated as ~3 ms (161, 162), in contrast to its value in amphibian rods of the order of 400 ms or more. Simulations showed that if τCa was not kept as short as the longest of the other shut-off time constants, then the responses became strongly biphasic or even oscillatory.
Straightforward analysis shows that τCa should be inversely proportional to the activity of the Na+/Ca2+,K+ exchanger, but directly proportional to cytoplasmic volume (see eqn (15) of Lagnado et al (163)). Accordingly, a high surface-to-volume ratio for the outer segment, that allows a large number of molecules of exchanger per unit volume of cytoplasm, will provide a short time constant τCa for Ca2+ and will therefore be advantageous for cones. In addition, the calcium buffering power of the outer segment will need to be kept low for cones.
Function of open sacs. The above requirement for a high surface-to-volume ratio no doubt provided a powerful driving force for the opsin-containing membrane of cones to remain in contact with the extracellular medium, and hence for the outer segment membrane not to seal over in the way it does in rods. In LWS cones, an additional driving force for the retention of open sacs may relate to the chloride-binding site, H197 (=H181 in the bovine rhodopsin frame), that confers a substantial red-shift in spectral sensitivity. This site is in the second extracellular loop, and in order to provide a high (and stable) chloride ion concentration on this side of the membrane, it is probably necessary for this region to remain extracellular, instead of transforming to become intradiscal as occurs in the discs of rods.
The existence of the sac structure in cone photoreceptors would appear likely to restrict both the membrane-based reactions and the cytoplasmic reactions resulting from a single photoisomerization to the domain of just a single sac; this could conceivably be either advantageous or disadvantageous, depending on the response property under consideration.
Synaptic release constraints. In order for cone photoreceptors to be able to transmit their graded (i.e. analog) signals rapidly, it is essential that the rate of release of synaptic vesicles be extremely high. This is necessary because the post-synaptic signal needs to be detected above the noise that is intrinsic to the quantal release of neurotransmitter vesicles. In order to be able to detect a change of (say) 1% in the release of vesicles, it is necessary that the mean number of vesicles released should be of the order of 1/(0.01)2, or 10,000. And in order for this to be achievable in (say) 100 ms, the rate of vesicle release would need to be around 100,000 vesicle s-1 which is enormously high. Accordingly, the cone’s synaptic terminal requires a very large area for the active release zone, in order to achieve this kind of rate of vesicle release. For comparison, in a rod the response to a single photoisomerization is considerably larger than the cell’s baseline noise, so that it may be adequate to be able to detect a change of the order of (say) 10%; in addition the rod’s response is a much slower than the cone’s. Hence, a far lower rate of vesicle release (and hence a smaller synapse) would suffice to provide a sufficiently reliable post-synaptic signal from a rod.
Scenario for the refinement of vertebrate phototransduction in cones
The following scenario is proposed for the refinement of the phototransduction cascade in chordate ciliary photoreceptors, that occurred during the early evolution of the vertebrate retina:
G-1) By the time that the chordate lateral retinas began signaling spatial (i.e. visual) information (see Section 17) there was great evolutionary pressure for rapid responses, for high sensitivity, for the ability to adapt to different intensities, and for high contrast sensitivity.
G-2) Those pressures led to acceleration of each of the recovery steps in the cascade; i.e.: shut-off of activated opsin, shut-off of G-protein/PDE, turnover time for Ca2+ concentration, and Ca2+ feedback to the re-opening of ion channels.
G-3) To compensate for the reduced sensitivity that would inevitably have accompanied the faster responses, there would have been a continuation of the pressure for increased efficacy of activation of the G-protein by the activated opsin.
G-4) Given the existence of multiple classes of cone, not all of these needed to be equally fast or equally sensitive. The systems with shorter wavelength (and hence more thermally stable) opsins could afford to become slower, in order to achieve higher sensitivity. The LWS system, with its high intrinsic noise, was better suited to achieving the fastest performance.
G-5) In response to the intense pressure to be able to transmit responses rapidly, and also to be able to signal small changes in light level (low contrasts), changes took place in the synaptic terminal that led to very high rates of release of synaptic transmitter. In this way, even brief and small changes in cone intracellular voltage led to changes in post-synaptic transmitter concentration that could be detected above the noise inherent in the vesicular nature of release.
G-6) By the time that the first (jawless) vertebrates evolved, their lateral retinas possessed five spectral classes of cone photoreceptor that exhibited exceptional responses properties, broadly similar to those of modern vertebrate cones.
10 Noise: Thermal isomerization and other sources of noise
This Section examines sources of noise (fluctuations) in the responses of photoreceptors, beginning with intrinsic properties of the opsins and then within the transduction cascade.
Thermal isomerization of cone opsins and rhodopsin
In considering the differences between cones and rods, one major disparity relates to the level of noise: LWS cones are many orders of magnitude noisier than rods (Table 3). Here we will examine the extent to which this difference in noise is a function of the stability of opsin molecules with different peak wavelengths.
More than 60 years ago, Stiles (164) developed a model describing the long-wavelength decline in spectral sensitivity of visual pigments, later expanded by Lewis (165), invoking the concept that the thermal energy of vibration in the molecule could add to the energy of a photon in order to trigger activation. Barlow (166) extended this concept, with the proposal that spontaneous thermal activation could occur, at a very low rate, even in the absence of light, and he predicted reduced stability (greater noise) for pigments of longer wavelength. Barlow’s prediction has been borne out in numerous experimental studies (167, 168), and theoretical models of varying complexity have been proposed to account for the exact relationship between peak wavelength and rate of thermal activation (165, 168-170).
One important observation is that ‘cone’ opsins can be incredibly stable, and this is most obvious when that particular opsin is expressed in a rod. In amphibia, the so-called ‘green rods’ (that absorb in the blue, and hence appear green) express a blue-sensitive pigment, which has been shown to be the SWS2 opsin that is also expressed in blue-sensitive cones in the same retina (171). Early electrophysiological experiments showed that green rods of the toad exhibit a very low rate of spontaneous thermal isomerizations (172), and more recent experiments have shown this rate to be amazingly low, at <10-14 s-1 at 23 °C (168). This means that the SWS2 (cone) pigment, when expressed naturally in a toad rod, only activates spontaneously on average once every ~1014 s, or ~4000 years. Thus, this ‘cone’ pigment is the most stable of all known opsins, including conventional rhodopsins!
Cones: Dark noise
Intracellular voltage recordings from LWS cones in turtle have shown very high levels of noise (173). Usually, this noise is reduced in amplitude by the ‘averaging out’ that results from the extensive lateral spread of signals across the electrically coupled network that is created by the gap junctions that link neighboring cones (174). But in a cone that is not coupled to its neighbors (an ‘electrically isolated’ cone), the observed amplitude of dark noise, of around 3000 µV peak-to-peak, is truly enormous when compared with the mean single-photon response amplitude of only 25 µV, and utterly precludes the resolution of individual photon events. Calculations indicate that this dark noise is equivalent to the quantal fluctuations that would be produced by a real light of a little over 2000 photoisomerizations s-1; this parameter is termed the ‘dark light’ experienced by the cone. Experiments with backgrounds showed that application of a real light of this intensity roughly halved the flash sensitivity of the cone. Subsequently, in intracellular measurements from macaque LWS/MWS cones, Schneeweis & Schnapf (175) found dark noise that they calculated to be equivalent to >3000 R* s-1per cone.
The source of these dark fluctuations in the intracellular voltage of LWS cones has not been established. In turtle cones, a significant contribution would be expected to come from thermal activation of the opsin (see above), because the wavelength of peak absorption is exceptionally long, at around 620 nm; indeed this appears to be the longest known wavelength of peak sensitivity of any opsin. If all the dark noise originated from thermal isomerizations, then the cone’s opsin content of ~108 molecules divided by the event rate of 2000 s-1 would yield a mean thermal lifetime of 0.5 × 105 s, which is not far from the prediction of Luo et al (168); their Fig. 4C) for a cone opsin of this wavelength. In addition to photopigment noise, the transduction process is also expected to contribute noise (see below). And finally, the chattering activity of ion channels in the synaptic terminal should contribute additional noise. To date, the relative contribution of these potential sources has not been evaluated. One way to investigate the contributions would be to use suction pipette experiments, though a potential drawback is that separation of the cone outer segment from its normal enmeshment amongst the processes of the RPE cells might conceivably alter the cone’s performance compared with that in the intact retina.
Rods: Transduction noise and variability of the single-photon response
Transduction noise in rods. In rods, it is possible to separate noise in the transduction process (and subsequently) from the noise caused by thermal isomerization of rhodopsin, because of the fact that the spontaneous photon-like events are individually resolvable (at least, under dark-adapted conditions). In dark-adapted toad rods, Baylor et al (176) reported that the residual ‘continuous’ noise corresponded to shot events with an amplitude around 1/400 of the single-photon event and occurring randomly at a mean rate of around 6000 events s-1. In functional terms, this source of noise can be considered low, simply on the basis that the great majority of single-photon responses are resolvable despite its presence. It seems likely that the proteins of the rod transduction cascade have evolved greater stability against spontaneous activation than have those of the cone cascade, as exemplified by the results of Muradov et al (145) for PDE6, which showed that the rod form has a lower basal hydrolytic activity in conjunction with a weaker affinity for transducin (see also Section 8).
Variability of the rod single-photon response. Despite the contrary view that is painted in most publications on the subject, the single-photon response of rods is quite variable, especially in terms of its time-course, yet most authors describe the kinetics as ‘reproducible’. For toad rods, Whitlock & Lamb (177) illustrated the wide variability in time-course of the single-photon response visually, by matching individual responses that began rising with a similar slope, and showing how different the peaks and falling phases could be. When they estimated the variation in a single shut-off step that was needed to account for the experimental measurements, they found a coefficient of variation (standard deviation divided by mean) of ~0.4. This is sufficiently high that it is not reasonable to describe the quantal responses as exhibiting ‘reproducible’ kinetics. The substantial variability in time-course, and also in amplitude, is a perfectly normal (and expected) feature of the known shut-off steps in the rod photoresponse, and has been accurately modeled by Hamer et al (178, 179) and Gross et al (180).
Magnitude of the rod single-photon response. It has recently been shown that mouse rods in the retinal slice preparation exhibit considerably larger single-photon responses than reported previously using the suction pipette approach, with mean amplitudes of 2-3 mV in cells with ~20 mV maximal responses (181). With a single-photon response as large as this, reliable synaptic transmission of that response to rod bipolar cells is far less problematic than has frequently been suggested in the literature.
11 Rod specializations and the origin of rods
As discussed in Section 7, the defining feature that distinguishes a rod photoreceptor from a cone photoreceptors is its ability, under dark-adapted conditions, to respond reliably (i.e. with good signal-to-noise ratio) to the absorption of individual photons of light (131, 132). Although numerous differences between cones and rods have been catalogued, in terms of morphology, molecular components, and response properties, there has never been a compelling account of what it is that enables vertebrate rods to achieve their photon-resolving performance.
This Section first analyzes structural features that contribute to the ability of rods to reliably signal individual photoisomerizations. It then examines two unusual types of ‘rod’: those of the nocturnal gecko, where the photoreceptors exhibit rod-like properties, yet are relatively recently descended from cones; and those in the all-rod retina of the skate, where the rods exhibit certain cone-like properties. Thereafter the emergence of rods in early vertebrates is considered, and finally a scenario is painted for the evolution of rod photoreceptors.
Structure as a factor in the rod’s ability to signal individual photoisomerizations
In order to achieve a single-photon response amplitude of reasonable amplitude (say 5% of the circulating dark current), ion channels must be affected over a moderate length of the outer segment, and this necessitates longitudinal spread of the cytoplasmic messenger cGMP along the outer segment. The situation in a typical cone, where the cytoplasmic message is restricted primarily to the interior of a single sac during the short duration of the cone response, cannot provide a single-photon signal of more than 1/N of the circulating current, where N is the number of sacs in the outer segment (typically >500).
More effective longitudinal spread (a higher longitudinal diffusion coefficient) can be achieved by increasing the cross-sectional area available for longitudinal diffusion, as was originally modeled by Lamb et al (182). In a cone outer segment, only a tiny proportion of the circumference of the sac is available as an intracellular route for longitudinal communication, and hence the effective longitudinal diffusion coefficient is low. But, by enclosing the sac membranes within the plasma membrane, through the formation of pinched-off discs, then essentially the entire circumference is available as a longitudinal conduit. The longitudinal diffusion coefficient can be further increased by the use of one or more incisures (longitudinal infoldings of the outer segment membrane), as this will increase the total length of the gap that encircles the discs, and hence will increase the cross-sectional area of the cytoplasmic path along the outer segment.
In the large rods of toads, Lamb et al (182) measured the longitudinal spread of activation to have a length constant of the order of 3 µm at the time of the peak of the single-photon response, and they measured the steady-state spread of desensitization during light adaptation to have a length constant of around 6 µm. Subsequent studies have confirmed and extended these observations to mammalian rods.
With this extent of longitudinal spread of cytoplasmic messenger in the outer segment, the rod is readily able to achieve an amplitude for the single-photon response of 5% of the circulating dark current (provided that its shut-off reactions have been made sufficiently slow). The effect of such longitudinal diffusion on the time-course of the single-photon response was modeled by Lamb & Pugh (70); their Appendix B).
Nocturnal gecko: Cones exhibiting rod-like properties
The photoreceptors of nocturnal species of gecko provide an important test of what is needed to make a ‘rod’, because in essence they are cones with a few rod-like features, yet they exhibit rod-like response properties. Walls (183, 184) proposed that the photoreceptors of nocturnal geckos had been ‘transmuted’ from cones into rods. He suggested that extant geckos were derived from a diurnal ancestor that had possessed an all-cone retina (i.e. one from which the rods had been completely lost). He suggested that nocturnal species of gecko had adapted by evolving rod-like outer segments, while retaining other morphological features of cones together with the organization of an all-cone retina. In more recent literature, most authors have referred to the retina of the nocturnal gecko (e.g. Gekko gekko) as being ‘all-rod’, and the photoreceptors as being ‘rods’, but this is not the case (see below).
Light responses. Intracellular recordings of light responses were made from photoreceptors of Gekko gekko by Kleinschmidt & Dowling (185), who reported response properties broadly similar to those found in the rods of other species. The responses to dim flashes had a slow time-to-peak, of around 700 ms at 25 °C. Although those responses appeared very sensitive (and hence rod-like), I have not been able to extract a quantitative value for their sensitivity. The family of responses to flashes of increasing intensity is reminiscent of rod families. The response to a bright flash had an amplitude of ~20 mV, and exhibited a rapid sag from a peak to a plateau; this sag was eliminated by treatment with 100 mM aspartate, suggesting that it arose through feedback from horizontal cells; if so this aspect would be cone-like rather than rod-like, because rods typically display a rapid sag originating in the inner segment. In the presence of steady backgrounds, the photoreceptor’s response light-adapted, over a range of ~4 log10 units, before saturating; such saturation is rod-like.
Rispoli et al (186) reported voltage-clamp electrical recordings from isolated outer segments of Gekko gekko photoreceptors, that had been detached at the connecting cilium and then dialyzed with an energy-rich intracellular solution. The mean dark current was ~67 pA, and the family of responses to flashes of increasing intensity was remarkably similar to that seen in rods of other species. Responses to dim flashes had a time-to-peak of ~1.1 s at 17 °C, with kinetics of the form reported for rods. The dark-adapted flash sensitivity was calculated (from intensity measurements) to correspond to a single-photon response amplitude of ~0.8 pA, while fluctuation analysis (ensemble variance divided by mean) gave a marginally smaller value of ~0.6 pA. This indicates that the single-photon response corresponded to ~1% of the dark current, far larger than in conventional cones though somewhat smaller than in most rods.
Taken together, the results of these two studies indicate that the electrical responses of Gekko gekko photoreceptors to illumination exhibit predominantly rod-like properties, though the amplitude of the single-photon response may not be as large as in true rods of other species.
Photoreceptor morphology. In an ultrastructural study, Röll (187) showed conclusively that almost every morphological feature of the photoreceptors of nocturnal geckos is actually cone-like, confirming the original view of Walls (183, 184), and contradicting more recent assertions that they are rods. The only features that are rod-like relate to the outer segment, and are: (1) the large size of the outer segment, (2) the existence of outer segment ‘discs’ that for the most part are enclosed by the plasma membrane, and (3) the existence of an incisure. The cone-like features that she demonstrated in the photoreceptors from all species of gecko studied (both nocturnal and diurnal) were as follows: the connecting cilium was short; the inner segment contained a glycogen-rich paraboloid, and an ellipsoid (with oil droplets in diurnal species); the nuclei contained dispersed chromatin; and the synaptic terminals closely resembled cone pedicles. She concluded that “the retinae of nocturnal geckos have definitely to be classified as cone retinae”.
The disc-like structure of the photoreceptor outer segment in the nocturnal gecko had previously been reported by Yoshida (188), and the study of Röll (187) was in close agreement. Yoshida (188) stated that “as in other vertebrate rods, the stack of double membrane discs of the outer segment of the photoreceptor cells in the retina of adult [nocturnal] geckos is enclosed by the plasma membrane, except for the proximal zone of the outer segment”. Both studies found the great majority of discs to be enclosed by plasma membrane, but both also reported frequent cone-like openings to the exterior, scattered along the length of the outer segment. Both also reported the existence of one (or rarely two) rod-like incisures.
Gekko opsins. The differences between cone and rod opsins were presented in Section 6. The two primary opsins of Gekko gekko have been shown to clade with the LWS and Rh2 cone opsins of other vertebrates, and to exhibit cone-like biochemical properties (189). In particular, the gecko Rh2 opsin has the Q122 and P189 that have been reported to underlie the cone visual pigment properties of fast meta II decay and fast pigment regeneration (92), Section 6).
Gecko phototransduction cascade. The proteins of the transduction cascade in Gekko gekko were investigated by Zhang et al (190) and, in all the proteins that they were able to identify, the sequences were cone-like rather than rod-like; those identified proteins comprised the α subunit of Gt (GNAT2), the catalytic and inhibitory subunits of the PDE6 (PDE6C and PDE6H), the α subunit of the cyclic nucleotide-gated channel (CNGA3), and arrestin (ARR3). Although these proteins were predominantly cone-like, a few residues were identified as being rod-like. On the other hand, in measuring expression levels and activities, they found that RGS9 was expressed at the low level expected for rods rather than the high level characteristic of cones, and the resulting GAP activity was correspondingly low; likewise, the low dark basal activity of the PDE was typical of rods rather than of cones.
Taken together, these results on the opsin and the cascade appear to indicate that the proteins of Gekko gekko photoreceptors are overwhelmingly cone-like, but that the expression levels and/or activities of at least two of the proteins that are important in generating slow responses (GAP activity and basal PDE activity) are instead rod-like.
Skate: Rods that can exhibit cone-like properties
Some deep-water species of skate have been shown to possess pure rod retinas, but their rods are able to function in a cone-like manner at intensities that would normally saturate rods (191). The outer segments of the rods in these species have all the morphological and other features of classical rods, in terms of large cylindrical outer segment, sealed-off discs, microspectrophotometric peak at 500 nm, and band-like incorporation of labeled amino acid. On the other hand, the ultrastructure of the synaptic terminal is intermediate between that of conventional rods and cones, with multiple synaptic ribbons and contacts at both invaginating and flat contacts (192).
The electrical responses of these cells were recorded by Dowling & Ripps (193) using the aspartate-isolated trans-retinal potential from the isolated retina, which reflects photoreceptor activity. Under dark-adapted conditions the electrical responses closely resembled those of conventional rods. Furthermore, application of steady background illumination led to saturation at relatively low intensities, as in other rods. However, when these backgrounds were left on for extended periods the rods slowly recovered and were able to respond to incremental stimuli. For backgrounds of moderate intensity, a steady state of adaptation was reached within 5-10 min, but for more intense backgrounds the rods took 20 min or more to recover from saturation and to reach a steady adaptational state. Those findings from trans-retinal recordings were subsequently confirmed by suction pipette recordings from skate rods, under dark-adapted conditions and at a single moderately bright background intensity (194). The suction pipette measurements showed that the acceleration of the flash response that occurs when the cell recovers from the initial saturation involves speeding-up of each of the shut-off reactions, in much the same way as occurs in a conventional rod exposed to steady backgrounds or to temperature changes.
These results are consistent with the notion that rods in the skate all-rod retina function as conventional rods under fully dark-adapted conditions and in dim illumination. However, upon exposure to brighter steady backgrounds, the skate rods initially saturate but then recover after tens of minutes and display adaptational properties that are reminiscent of those of cones. Hence it would appear that, in this retina that lacks cones, the rods have become modified in a way that permits them to slowly change their response properties from rod-like to somewhat more cone-like. At present, the cellular and molecular mechanisms that enable this transition are not known.
In a conventional ‘duplex’ retina, containing both rods and cones, there is no need for the rods to function at moderate to high background intensities. Indeed, there is a distinct advantage in the rods becoming saturated at the intensities at which cones operate, because this reduces the metabolic load on the outer retina and permits the oxygen tension to be maintained at a reasonable level for the cones. But in the all-rod skate retina, it seems that the rods have had to forgo this energy-saving trick in order to enable the animal to continue to see at intensities that would otherwise be blinding.
What is needed to make a ‘true rod’?
In light of the results presented in Section 10 and up to here in Section 11, the following interpretations are drawn regarding the crucial features that are needed to make a ‘true rod’, defined as one that reliably signals the occurrence of individual photoisomerizations, as distinct from a photoreceptor with cone-like properties:
H-1) First, the photoreceptor needs to express an opsin with a peak spectral sensitivity shorter than about 520 nm, in order to achieve a sufficiently low rate of thermal isomerizations; hence, LWS opsins are effectively ruled out.
H-2) The amplification of the phototransduction cascade does not need to differ significantly from that in a conventional cone.
H-3) One essential change is that all of the shut-off reactions underlying recovery of the dim-flash response need to be slow enough that the response to a single photoisomerization can build up sufficiently to suppress at least 1% (and preferably more) of the circulating dark current.
H-4) In comparison with typical cone shut-off, the required slowing necessitates concerted changes that: (1) slow the shut-off of R*; (2) slow the shut-off of G*/E*; (3) slow the cGMP turnover time in darkness; and (4) slow the Ca2+-mediated negative feedback loop so that the recovery of cGMP levels and the re-opening of ion channels are slowed. It is not crucial whether these changes are achieved by alteration in the activities of the proteins, or by altered expression levels.
H-5) The way that this slowing was actually achieved appears to have included: slowing the rate at which the GRK and arrestin could phosphorylate and bind to R*; slowing the GAP activity by lowering the concentration of the RGS9 complex; and slowing the cGMP turnover time in darkness by lowering the basal activity of the PDE6.
H-6) Secondly, the cytoplasmic messengers (cGMP and Ca2+) need to be able to diffuse substantial distances axially along the outer segment, and this requires a significant change in outer segment morphology: the sac membranes need to be enclosed by the plasma membrane (in effect forming discs) in a manner that provides an enlarged conduit for axial movement. In addition, one or more longitudinal incisures in the outer segment can further assist in axial diffusion.
H-7) An additional change that will assist in rendering the (now) large single-photon response detectable above the background noise is a lowering of the intrinsic fluctuations in the phototransduction process through increased stability of the proteins of the cascade.
H-8) Through the combination of all the above changes, it should be possible to convert a cone photoreceptor that is specialized for high-performance rapid photopic vision into a rod photoreceptor that can reliably signal individual photoisomerizations under dark-adapted conditions. An inevitable consequence of the slowing of shut-off reactions, though, will be that this cell saturates at low to moderate background intensities.
Timing of the emergence of rods
In Section 10 it was shown that modern SWS2 ‘cone’ opsins have an exceedingly low rate of thermal isomerization, making them exceptionally well adapted to act as the photopigment in the green rods of amphibia. We cannot be certain that the ancestral SWS2 opsin was equally as stable as this. But, even if its thermal stability had been several orders of magnitude poorer, then that would have been entirely adequate to support reliable single-photon detection if that opsin had been expressed in a ‘rod’. We can conclude that cone opsins (at least of the SWS2 variety) already exhibited thermal stability sufficient to enable single-photon detection before rhodopsin evolved, and therefore presumably before rods evolved. Hence, it would appear that thermal stability of the opsin molecule was not the limiting factor in the evolution of rods (at least, when ‘rods’ are characterized by their ability to detect single photons).
It seems clear that photoreceptors with certain rod-like features had evolved prior to the divergence of cyclostomes from the lineage leading to jawed vertebrates. Thus, hagfish photoreceptors have been reported to have discs enclosed by plasma membrane, and their peak spectral sensitivity is at 500 nm (Section 3). Likewise, one class of lamprey photoreceptor has a number of rod-like features, including the expression of an opsin that appears homologous with Rh1, though the cell also retains other cone-like features, both anatomically and electrophysiologically (Section 3). Unfortunately, though, it has not yet been discovered whether these hagfish and lamprey photoreceptors are able to respond reliably to the absorption of individual photons of light ‐ in other words whether they achieve the ultimate sensitivity that is the defining feature of the ‘true rods’ of jawed vertebrates. If future experiments were to show that both hagfish and lamprey photoreceptors indeed meet this criterion, then this would be evidence in support of the notion that their common ancestor likewise possessed true rods.
Until this issue can be resolved, it is not possible to confidently go further than to say, firstly, that it seems highly probable that the last common ancestor of hagfish and lampreys possessed a class of photoreceptor with a number of rod-like features and, secondly, that ‘true rods’ had definitely evolved by the time that the first jawed vertebrates appeared.
Scenario for the emergence of ‘true rod photoreceptors’
The following steps are proposed as the likely means by which rod photoreceptors emerged from their cone photoreceptor forerunners:
I-1) Following the ‘2R’ rounds of whole-genome duplication, the lateral retina of an early vertebrate possessed five classes of cone-like photoreceptor cell, each expressing one of the five opsin classes (LWS, SWS1, SWS2, Rh2, Rh1).
I-2) In the photoreceptor expressing the Rh1 opsin, a number of changes gradually occurred, that led to a slowing of the response and concomitantly to an increase in its sensitivity.
I-3) These changes in the photoreceptor expressing Rh1 included slowing of the three shut-off reactions and slowing of the negative feedback loop. Some of these changes involved specialization of the shut-off proteins expressed in that cell, some involved altered expression levels, and some involved changes in morphology.
I-4) R* shut-off became slower, in part because of the slower decay of metarhodopsin II due to the mutations at residues 122 and 189, and in part through changes in the kinase and arrestin (GRK1 and SAG).
I-5) G*/E* shut-off became slower primarily as a result of lowered levels of the RGS complex (RGS9-Gβ5-R9AP).
I-6) The turnover time for cGMP in darkness lengthened as a result of lowered resting phosphodiesterase activity (PDE6A, B).
I-7) The time constant for equilibration of cytoplasmic Ca2+ concentration increased in parallel, through a reduction in the number of Na+/Ca2+,K+ exchangers per unit volume of outer segment, in part as a result of lowered surface-to-volume ratio, and in part through an increase in cytoplasmic Ca2+-buffering power.
I-8) Changes at the rim of the outer segment lamellae led to the plasma membrane enclosing groups of lamellae (which thereby became groups of discs), providing an enhanced cross-sectional area for axial diffusion in the cytoplasm.
I-9) With these changes occurring gradually, but in concert, the photoreceptor expressing the Rh1 opsin would, over time, have become steadily slower and more sensitive.
I-10) Eventually almost the entire outer segment became sealed over, providing extensive longitudinal spread, and the shut-off reactions became sufficiently slow that the response to a single photoisomerization became large enough to exceed the level of noise in the cell.
I-11) Any further slowing of the shut-off reactions would then have been disadvantageous.
I-12) The synapse of this cell had a less demanding role, because the signal-to-noise ratio was higher and the kinetics were slower, and hence the synapse was able to function satisfactorily with less expenditure on synaptic machinery (i.e. with less extensive ribbon and fewer vesicles).
I-13) The eventual outcome of these combined gradual changes was the emergence of a ‘true rod’ photoreceptor, that reliably signaled individual photoisomerizations.
12 Retinoid re-isomerization in darkness
As discussed in Section 5, a major difference between vertebrate retinal photopigments and those of invertebrates is that the vertebrate visual opsins release their all-trans retinoid following light activation. The vertebrate retina therefore requires a continual supply of 11-cis retinal, in order to silence the residual activity of the free opsin and at the same time regenerate native rhodopsin for further signaling by light. This is accomplished by two biochemical pathways that operate in darkness (Figure 27; reviewed in Lamb & Pugh, (160); Wang & Kefalov, (195); Saari, (196)). One is the classical ‘retinoid cycle of vision’ (Figure 27B), in which all-trans retinoid is transported to the RPE, isomerized to the 11-cis isomer, and then transported back to the retina. The second is a less well understood pathway, internal to the retina, involving Müller cells and cone inner segments, that is thought to contribute to the regeneration of cone visual pigment.
Intra-retina retinoid cycle
Although most work on retinoid recycling over the past four decades has concentrated on the RPE cycle, the fact that cone photoreceptors pre-date rod photoreceptors raises the distinct possibility that the ancestral retinoid cycle of the vertebrate eye may have been the intra-retina cycle involving the cones and Müller cells, with the RPE cycle having evolved subsequently. The intra-retina cycle appears simpler, in that it involves isomerization of all-trans retinol (vitamin A) directly to 11-cis retinol (bottom of Figure 27A), via what has been termed ‘isomerase 2’ in Müller cells, without requiring initial esterification followed by an isomerohydrolase reaction as in the RPE (see below). A very recent report has indicated that this second isomerase might be the desaturase, DEGS1 (197), but the identity of the other enzymes that contribute to this intra-retina cycle are only just beginning to emerge. Unfortunately, as this cycle has been less intensively studied, it has not yet proven possible to investigate its evolutionary roots.
RPE retinoid cycle
Two recent papers have made important advances in determining the origin of the classical RPE retinoid cycle. Albalat (198) and Poliakov et al (199) have shown that this cycle is present only in vertebrates, as cephalochordates and tunicates do not possess the required enzymes, implying that the cycle evolved during the 100 million year interval from #4 to #5 in Figure 1.
Two crucial enzymes in the retinoid cycle are LRAT (lecithin:retinol acyltransferase) and RPE65. LRAT esterifies vitamin A (all-trans retinol) to all-trans retinyl ester, which is the substrate for RPE65, which actually performs the isomerization while at the same time cleaving the ester bond, in a so-called ‘isomerohydrolase’ reaction. This step generates 11-cis retinol which is subsequently oxidized to the aldehyde, 11-cis retinal, and then transported to the retina.
Albalat (198) undertook an in silico search for the genetic machinery of retinoid processing amongst invertebrates, and he analyzed the likely function of the components that he found. He concluded that “genome surveys, phylogenetic reconstructions and structural analyses of invertebrate components similar to those of the vertebrate retinoid cycle ‐ that is, Rdh8, Rdh12, Lrat, Rpe65, Rdh5, Rlbp1, and Rbp3 ‐ did not provide any evolutionary or functional support for the existence of the genetic machinery of the retinoid cycle outside vertebrates”.
Poliakov et al (199) extended that approach, by experimentally determining the functional activity of the key enzymes LRAT and RPE65 (and similar molecules) in lamprey and in tunicate. In lamprey (Petromyzon marinus), they showed that LRAT and RPE65 are both present and functional, and further that the key sites for RPE65’s ability to act an isomerohydrolase are remarkably similar to those of jawed vertebrates. On the other hand, their phylogenetic analyses confirmed that neither RPE65 nor LRAT have orthologs in the cephalochordate (amphioxus) or the tunicate (Ciona intestinalis). They showed that an enzyme previously proposed (and named) as a Ciona ortholog of RPE65 by Takimoto et al (200) has no isomerohydrolase activity, confirming the report of Kusakabe et al (9), and they showed that it functions instead as a BCMO (β-carotene mono-oxygenase).
Taken together, these results show that both the crucial transition from a BCMO to a true RPE65 isomerohydrolase, and also the origin of LRAT, occurred only in the lineage leading to vertebrates (jawless and jawed), after the divergence of tunicates. Hence, the RPE retinoid cycle evolved only in vertebrates.
In a number of invertebrate species, it has been known for many years that rhodopsin can be regenerated in darkness (201), but there is scant evidence as to how this occurs. In Drosophila, evidence has recently been obtained for the likely existence of a retinoid cycle, though rather different from that in the RPE (202).
Scenario of the origin of retinoid recycling in the vertebrate retina/RPE
From our current knowledge of the intra-retinal and RPE retinoid cycles, it is possible to hypothesize the following scenario for the evolution of retinoid processing in the vertebrate eye:
J-1) At around the time that chordate ciliary opsins lost their bistable (photoreversible) properties and instead released their all-trans retinoid, an intra-retinal retinoid processing cycle arose, that utilized an isomerase (currently termed isomerase 2) in the Müller cells to isomerize retinol from its all-trans to its 11-cis form.
J-2) Subsequently, around the time that the RPE became specialized as a monolayer apposed to the retina, the two crucial enzymes LRAT and RPE65 evolved, and the RPE was able to adopt an additional role in retinoid re-isomerization.
13 Development of the retina at a macroscopic level
Clues to the evolution of the vertebrate eye can be obtained by studying its embryonic development, though caution is required. Nearly 200 years ago, Karl Ernst von Baer (203) observed that organisms with widely different adult forms have embryos that resemble each other closely, and he suggested that the developmental stages through which the embryo passes might to some extent reflect the evolutionary history of the organism; for a recent evaluation of von Baer’s ideas, see Brauckmann (204).
This concept was extended by Ernst Haeckel (205) to the extreme view that ‘ontogeny recapitulates phylogeny’; i.e. that each step in development reflects a corresponding step in the evolutionary sequence. The problem with this strict relationship is that it implies that evolution can work only by altering or adding to the final stage of embryogenesis, and this is clearly not correct. Nevertheless, despite the fact that all stages of development are subject to evolution, there is a marked tendency for more ancient steps to be preserved, so that the fundamental body plan that was established early in evolution appears seldom to be altered in a major way during embryogenesis, and instead there appears to be a tendency for newly-evolved features to be ‘tacked-on’ to later stages of embryogenesis.
Because of the likelihood that events that occurred in the distant past during eye evolution have left remnant traces in the process of eye development, we shall now examine several aspects of eye embryology: firstly, the overall sequence of eye development across jawed vertebrates, lampreys and hagfish; secondly, the sequential differentiation of cell classes in the vertebrate retina; and thirdly, the maturation of photoreceptors and the formation of their neural connections. Inevitably, this leads on to the nature and origin of retinal bipolar cells. Analysis of each of these topics provides important clues to the evolution of the retina.
Development of diencephalic light-sensitive organs in jawed vertebrates
During vertebrate embryonic development, a small region of the anterior neural plate (the eye fields) contains cells that will give rise to several distinct light-sensitive organs: the paired lateral eyes, the pineal organ, and the parapineal organ or the parietal eye, though not all are present in all vertebrate species (Figure 28; (206, 207)). Each of these organs arises as an evagination from the developing diencephalon; the dorsal evagination begins as a single bulge, but then becomes constricted, dividing into paired evaginations that take up rostral and caudal positions, with the caudal vesicle becoming the pineal organ and the rostral vesicle becoming either the parapineal organ or parietal eye (206). These organs share close homologies with each other, and the evolutionary significance of these parallels have been highlighted in a number of studies, including Reichenbach and Robinson (208), Vigh et al (207, 209), Ekström & Meissl (210), Klein (211), Mano & Fukada (212).
Polarity of the developing diencephalic vesicles. The developing light-sensitive outgrowths from the diencephalon (retina, pineal, parapineal, parietal) become rather flattened – compare Figure 10A for the lamprey pineal with Figure 29f for the lateral retina and RPE. One significant difference between the organs concerns the side of the vesicle that develops as the retina (or its homolog). In the pineal and parapineal organs, and in the parietal eye, it is primarily the proximal side of the vesicle (nearest the brain) that develops as a 2-layered retina, so that the photoreceptor outer segments (which project into the lumen) point out towards the external world. As the projection neurons are on the outer surface, their axons have no need to penetrate the organ, and are able to run directly to the CNS. The distal side of the organ (facing the external world) develops differently, in some cases as non-photoreceptive tissue; e.g. in the parietal eye as a so-called ‘lens’, and in the pineal as a thinner layer either containing either occasional photoreceptors or sometimes UV-sensitive photoreceptors (71). In comparison with these dorsal organs, the polarity is reversed in the lateral eyes, with the proximal side developing as a monolayer of RPE cells, and with the distal side differentiating as the multi-layered retina. It will be suggested below that the signal for this inverted polarity came from an ancestral placode in the stem vertebrate, that subsequently evolved to become the lens placode.
In the parietal eye, and also in the pineal organ of non-mammalian vertebrates, the glial cells (amongst which the photoreceptors are embedded) are pigmented; in the parietal eye, these glia contain black pigment granules (213), whereas in the pineal organ they contain reflective organelles that give a slate gray appearance.
Lateral eye development at a macroscopic level. The paired lateral evaginations of the developing diencephalon give rise to the eyes, in a sequence (Figure 29 and the animation below) that was described nicely by Walls (184) and that has been reviewed recently in Martinez-Morales & Wittbrodt (214). On each side of the rostral neural plate a depression forms, termed the optic groove (Figure. 29b). As the neural plate folds upwards and inwards (Figures 29b,c), meeting and closing over to form the neural tube, these growing regions bulge outwards (Figure 29d) and are now termed optic vesicles. However, it appears that the luminal space is minimal, so that the interior surfaces of the vesicle remain effectively in contact with each other, somewhat like a deflated balloon. Contact between the expanding optic vesicle and the surface ectoderm (Figure 29e), at a region termed the lens placode, induces changes in both, with the ectoderm thickening progressively and eventually forming the lens (Figure 29g). Although it has sometimes been suggested that invagination of the optic cup is a physical result of growth of the lens, this cannot be the case, because a normal eye-cup and retina can develop in the absence of a lens, as in human congenital primary aphakia (215). Remarkably, such development can also occur in cell culture, in the absence of ectoderm or lens tissue (216), see below).
Click below for an animation of vertebrate eyecup development:
Lamb et al (3) Supplementary Animation S1
Specification of retina and RPE. The initial polarization of the lateral eye vesicles into RPE and retina is under the control of a number of extracellular signaling molecules, including FGFs released from the surface ectoderm, but also involving Bmp, Activin and Wnt. Very recently, evidence has been found for a positive feedback circuit involving Pax6 whereby, once the initial specification of RPE and retina has begun, the signals within the retina/RPE become self-reinforcing (217). These authors propose that, when expressed in conjunction with Mitf or Tfec, Pax6 acts as a pro-RPE (i.e. anti-retina) factor, whereas in conjunction with other retinogenic genes it acts as a pro-retina factor. Thus, once there is a bias towards an initial retina/RPE polarity, this bias becomes self-reinforcing by signaling events that are internal to the developing retina/RPE.
A further exciting and intriguing recent finding is that when aggregates of mouse embryonic stem cells are grown entirely in vitro, as vesicles in 3D culture, they are capable of autonomously organizing themselves into eye-cups with appropriately oriented retina and RPE (216), as illustrated in the movie linked below. This observation is again consistent with the notion of a signaling system internal to the retina/RPE that can regulate its development in the absence of external signals.
Click below for movies of spontaneous formation of eye-cup in vitro:
Eiraku et al (2011) Supplementary information , especially Movie 2
Coordinated migration of cells. The early steps in formation of the zebrafish eye vesicle have been monitored in several studies that have used time-lapse video imaging (218-220), so that individual cells could be visualized and their migration tracked. The movies (linked below) from each of these studies provide impressive visualizations of the coordinated cell migrations that occur in zebrafish. Thus, the macroscopic development (indicated, for example, in Figure 29) can be visualized as the coordinated migration of individual cells, firstly as primarily outward migrations, and then through a combination of inward migration of those cells near the locus of contact with the placode, together with continued outward migration of new cells into the eye vesicle (see movies).
Click below for movies of cell migrations in formation of zebrafish eye vesicle and eye cup:
England et al (218) Supplementary material, especially Movie S1
Rembold et al (219) Supporting online material, especially Movie S1
Kwan et al (220) Supplementary material, especially Movie S1
Scenario for the evolution of chordate diencephalic light-sensitive structures
It seems reasonable to think that the events in vertebrate embryonic development described above may represent a shadow of the sequence of steps that occurred during chordate evolution, in the interval between the divergence of tunicates and the emergence of vertebrates (i.e. between #4 and #5 in Figure 1). Accordingly, the following steps (also explained in the schematic in Figure 30) are suggested to have occurred:
K-1) The anterior neural tube of an early chordate contained a light-sensitive region that sent axons to relevant parts of the nervous system (e.g. to the forerunner of the hypothalamus).
K-2) The inner surface (in contact with the CSF) contained ciliary photoreceptors, while microvillar photoreceptors (that later simply became projection neurons) were located on the outer side (Figure 30A). Pigment-containing cells were also present.
K-3) This light-sensitive region expanded, possibly initially simply as a means of increasing the number of photoreceptors and thereby increasing the organism’s ability to catch light. In doing so, it bulged outwards in different directions, dorsally and laterally (Figure 30B).
K-4) As these regions expanded, the somata of the ciliary photoreceptors and the microvillar photoreceptors (i.e. projection neurons) remained in close proximity to each other, while the axons of the projection neurons lengthened so that they continued to reach their targets in the CNS; thus the cell bodies remained close together but the axons of the projection neurons ‘stretched’.
K-5) The interior of each bulge (the luminal space, containing CSF) remained relatively small, so that the inner surface of the epithelium (with ciliary photoreceptors and pigment cells) became folded in upon itself, resembling a deflated balloon rather than an inflated one.
K-6) Initially, this double layer of epithelial tissue was probably spatially uniform, with ciliary photoreceptors, pigment cells and projection neurons distributed across the entire surface of the organ (not shown in Figure 30).
K-7) In due course each of the organs (dorsal and lateral) tended to develop a polarity, with one side remaining predominantly light-sensitive and with the other side adopting a different role (e.g. as a lens in the parietal eye).
K-8) In the lateral bulges, but not in the dorsal bulges, the orientation of this polarization was such that only the distal (outer) side remained light sensitive, and only the proximal side (closer to the brain) made pigment cells (see Fig. 30B). In this way, ciliary photoreceptors occupied the entire cross-sectional area struck by incident light, and the pigmented cells were behind the photoreceptors yet still in close contact with them.
K-9) In these lateral organs, the axons continued to run across the outer surface, which now comprised the vitreal surface of the retina. As they headed centrally they converged as a bundle running along a groove under the optic stalk (Figure 30C). With continued lateral and downward expansion of the retina/RPE, the narrowing gap around the optic stalk became the optic fissure. At some (possibly later) stage in evolution this fissure sealed over, so that the optic nerve penetrated the lateral eyecup.
Eye development in lampreys and hagfish
Lamprey eye development. Lampreys have a larval form (the ammocoete, Figure 13A) that develops slowly over a period of 5 years or more, before metamorphosing into the adult (see for example Dickson & Collard (84)). The ammocoete is effectively blind, and its eyes resemble those of the hagfish, in being small and buried beneath skin. The initial stages of eye development occur at an early age in the ammocoete, and appear comparable to the initial stages of eye development in jawed vertebrates. However, eye development then becomes arrested; for the remainder of the ammocoete phase (i.e. perhaps 5 years) the main change to the eye is that it (and its lens) slowly grows in size as the animal grows.
The bulk of the ammocoete retina is undifferentiated, with a thick neuroblastic layer, though a narrow central region (~50 µm wide, adjacent to the optic nerve) is differentiated (84, 221-223). Although it has been reported that all the conventional layers of the vertebrate retina are present, re-examination of those micrographs suggests that bipolar cells may not in fact be present in the ammocoete retina. A slow process of neural differentiation occurs, over a period of years, and this accelerates as metamorphosis approaches. The area of differentiated cells spreads outward, and the cells differentiate in the following sequence: ganglion cells, amacrine and horizontal cells, photoreceptors, and finally bipolar cells (221, 222); i.e. in broadly the same order as occurs much more rapidly during embryogenesis in jawed vertebrates (see Section 14).
Then, at metamorphosis, development of the lamprey’s eye resumes, apace. The eye and lens grow rapidly in size, differentiation of the retina reaches completion, the cornea splits into scleral and dermal layers (allowing the eye to move with respect to the epidermis, as in jawed fish; (224)), extraocular muscles develop, and the eye erupts at the surface, as a vertebrate-style visual organ (Figure 13B).
Hagfish eye development. The development of hagfish embryos was first studied by Price (225), and comprehensive accounts were given by Dean (226, 227) and von Kupffer (228), though there was only limited data on the eye in any of these. The embryonic development of hagfish eyes was investigated by Allen (65) and Stockard (229), with particular reference to the origin (or the absence) of a lens. The combined results of those early studies are consistent with the following description.
The developing eye is always very small in comparison with the size of the embryo. At a very early stage the optic vesicle contacts the surface ectoderm and a definite ‘lens placode’ is seen, though even at its thickest (in a 15 mm embryo) this placode is only a few cells deep (229); thereafter the lens placode thins and disappears. Allen (65) concluded by suggesting that, if the thickening of the lens placode represented a rudimentary lens, then “we should have a clear case of arrested development resulting in the continuance of an embryonic condition into adult life. This view is strongly supported by the condition of the retina with its space of separation between the inner and outer layers of the optic-cup, by the rudimentary iris, and by the often persistent choroid fissure”.
Following those studies, it did not prove possible to obtain viable fertilized hagfish eggs for about 100 years; it would be exciting to continue those earlier studies using the procedures recently developed in Shigeru Kuratani’s laboratory (230).
Implications of cyclostome eye development for vertebrate eye evolution
In view of the comparative aspects of cyclostome and jawed vertebrate eye development described above, the following implications for evolution of the vertebrate eye are hypothesized (3):
L-1) The ‘arrested development’ of the hagfish eye (65) may reflect a failure of hagfish eye development to proceed beyond a point corresponding to an earlier stage in vertebrate eye evolution. Hagfish do not undergo metamorphosis, and in the absence of the developmental changes that occur at metamorphosis, perhaps the hagfish eye has been ‘frozen’ in its earlier evolutionary state. In other words, eye development (and perhaps all development) in hagfish might correspond to a kind of neoteny.
L-2) If this is correct, then the hagfish eye and retina provide an invaluable window into an important stage of vertebrate eye evolution, with the following additional implications.
L-3) The retina of the stem vertebrate would, at some stage of evolution, have resembled that of the hagfish, with photoreceptors directly contacting projection neurons (ganglion cells). Further evidence relating to this notion will be presented in Section 14 on maturation of retinal connectivity.
L-4) The failure of the hagfish ‘lens’ placode to develop into a lens would suggest that the ancestral function of this placode may have been to influence the orientation of polarization of the retina/RPE (see above), rather than to form a lens. On this basis, the evolution of the lens would have occurred subsequent to the evolution of the paired lateral ‘eye-cups’ that comprised infolded, two-layered retinas.
L-5) Likewise, the extraocular muscles would not have evolved until after the lateral eye-cups were already present.
L-6) Taken together, these interpretations further suggest that the ancestral function of the paired lateral retinas was not for image-forming vision, because the evidence suggests that these retinas were present before the evolution of a lens, of extraocular muscles, or of anything more than the most basic computational power in the retina. Instead, these lateral counterparts of the pineal organ may have subserved a function in circadian and/or seasonal timekeeping, or perhaps in shadow detection, or even in control of axial orientation (i.e. roll).
L-7) Hence, it is suggested that at an early stage in vertebrate evolution, after the ‘2R’ genome duplications but before the divergence of cyclostomes from the jawed lineage, a pair of primitive lateral retinas with RPE had evolved in the stem vertebrate. These retinas possessed early forms of the five ‘visual’ ciliary opsins, expressed in cones and in a rod-like photoreceptor. Bipolar cells had not evolved, nor had a lens or extraocular muscles. It is suggested that the ancestral function of the ‘lens placode’ was to specify the polarity of the lateral eyecup, possibly by releasing molecules like fibroblast growth factors (FGFs).
L-8) It is suggested that a state resembling this is retained in the eyes of living hagfish, possibly as a result of neoteny. In lampreys (and in their ancestors) the subsequent stages of eye development occur (and may have occurred) slowly, predominantly during metamorphosis. But in living jawed vertebrates those subsequent stages of eye development occur rapidly, and inseparably from the earlier stages.
14 Molecular signatures and cell differentiation in the vertebrate retina
Molecular signatures of neurons in the developing vertebrate retina
In the developing nervous systems of both protostomes and deuterostomes the specification of neuronal cell fate appears to be determined by the combinatorial code of transcription factors (mainly of the bHLH and homeodomain superfamilies) that are expressed. In the differentiating cell, the particular combination of transcription factors regulates the programs controlling cellular morphology, outgrowth of axons, and the expression of effector molecules (such as members of transduction cascades, neurotransmitter-synthesizing enzymes, ion channels, etc.). For a review of the deployment of transcription factors in eye development across phyla, see Vopalensky & Kozmik (6).
A comparison of the molecular factors that underlie the differentiation of cell types has been applied to the retina, both within an organism and between organisms, by Detlev Arendt and his colleagues, in order to gain insight into the evolutionary history of the component cells (231). Arendt refers to the approach as ‘comparative molecular cell biology’.
Sister and homologous cell types. Arendt (231) defined ‘sister’ and ‘homologous’ cell types as follows. Within an organism, ‘sister cell types’ are those that have evolved from one common precursor, by diversification of cell type. And across the evolutionary tree, ‘homologous cell types’ are those that have evolved from the same type of precursor cell in the last common ancestor of the groups being compared. Using these definitions he argued that, within any vertebrate species, the horizontal cells, amacrine cells, and ganglion cells are ‘sister’ cell types, having descended from a single ancestral cell type. And across phyla, he argued that rhabdomeric photoreceptors and vertebrate retinal ganglion cells are ‘homologous’ cell types, having descended from a microvillar photoreceptor in the last common (bilaterian) ancestor.
The evidence supporting the former assertion is illustrated in Figure 31 which compares the transcription factors and effector genes expressed in the different cell types of the vertebrate retina. Cone and rod photoreceptors are clearly sister cell types, having descended from a common ancestral ciliary photoreceptor (see Section 11). Although Arendt was equivocal about the origin of retinal bipolar cells, it will be argued subsequently (Section 16) that the bipolar cells are also sister cells of ciliary photoreceptors.
The lower part of Figure 31 shows that the transcription factors and effector genes expressed in horizontal, amacrine, and ganglion cells are closely similar (for detailed comparisons, see Arendt (231)). Furthermore, most notably in the case of ganglion cells, these factors are fundamentally the same as the factors that underlie differentiation and that specify phototransduction in the rhabdomeric photoreceptors of Drosophila. By Arendt’s analysis, horizontal, amacrine, and ganglion cells appear to have descended from an ancestral microvillar photoreceptor, so not only are they ‘sister’ cell types (to each other) but they are also each ‘homologous’ cell types to protostome rhabdomeric photoreceptors.
Complications. Two observations that complicate this relatively simple account have been pointed out by Davies et al (94). Firstly, despite their postulated common origin as microvillar photoreceptors, some horizontal cells and some ganglion cells express a C-opsin (VA/VAL). Secondly, it appears that this C-opsin VA/VAL is frequently co-expressed with the R-opsin melanopsin. What might lead to expression or co-expression of a C-opsin in a cell that has descended from an ancestral microvillar photoreceptor? In addition to this complication with the opsins, it is now known that the set of transcription factors is more extensive than shown in Figure 31, and as a result the grouping of cell types is less distinct.
Solution? One possible solution to this conundrum (as well as to other issues) would be to take the view that all vertebrate retinal and RPE cells are descended from a single pre-bilaterian cell type that combined the features of a photoreceptor cell, a pigment cell, and a glial cell; i.e. from an ancestral cell of the kind envisaged in the ‘division of labor’ model proposed by Arendt et al (5). Such a view seems consistent with knowledge of cell differentiation in the vertebrate retina (see next sub-Section), where it has been shown that progenitor cells are initially unrestricted in their cell fate, and are potentially able to form any type of retinal cell. By taking this viewpoint, one would not need to apply the restriction that all vertebrate retinal/RPE cell types had proceeded through antecedents that were either strictly ‘ciliary photoreceptor type’ or strictly ‘microvillar photoreceptor type’ before reaching their current forms. Instead, some cell types (such as horizontal cells) might be descended from intermediate cell type(s) that had not committed to either a ciliary or a microvillar form of photoreceptor. If this were the case, then modern cells in the retina and RPE might share certain features with one more of the ancestral ciliary, microvillar, pigment, or glial categories, in what might resemble a mix-and-match fashion.
This concept will be followed up after a brief description of cell differentiation in the vertebrate retina.
Cell differentiation in the vertebrate retina
For reviews of cell differentiation and neuronal development in the retina, the reader is referred to Webvison “Retinal neurogenesis” as well as to the chapters in Sernagor et al (232), and to Agathocleous & Harris (233), Swaroop et al (234), Reese (235) and Chen et al (21). Here the briefest of summaries is given.
Cells in the neuroepithelium of the optic vesicle, that will generate the retina, proceed through the standard cycle of cell replication in much the same way as in other regions of the brain. The soma of a progenitor cell migrates vertically within the neuroepithelium, between the outer (ventricular) and inner (vitread) surfaces, in a characteristic pattern, and when a daughter cell exits the cell cycle its soma then migrates towards its final position within the retinal layer. Differentiation begins in the central retina and proceeds outwards to the periphery.
Fate, competence, and birth order. Initially, progenitor cells are unrestricted in fate, with the capacity to form any class of retinal cell. Nevertheless, the order in which different classes of retinal cell are ‘born’ (i.e. exit the cell cycle) is well defined within a species, as illustrated in Figure 32 and well conserved across vertebrate species. In particular, most cone photoreceptors are born before most rod photoreceptors, and most bipolar cells are born very late, though there is temporal overlap in the generation of different classes (236, 237).
A number of models have been put forward to explain the experimental results that have been amassed on the determination of retinal cell fate, and three are illustrated schematically in Figure 33. One model, termed serial competence (Figure 33A(a)), proposes that at any time a given progenitor has competence to produce cells of only one or a few types, and that the progenitors proceed through multiple states of this kind. Another model, progressive restriction (Figure 33A(b)), proposes that early progenitors have competence to produce all cell fates and that this competence is steadily lost (restricted) over time.
An explicit scheme under the latter model is shown in Figure 33B, from Reese (235). This scheme is based on the results of Wong & Rapaport (238), which suggested that restriction of competence represents a unidirectional sequence through which progenitors progress. The diagram illustrates the (unusual) case in which a single progenitor cell generates the entire set of retinal cell classes. As we shall see below, though, not every division produces one post-mitotic daughter cell plus one continuing progenitor cell; some divisions can produce a pair of post-mitotic daughter cells (e.g. a ganglion cell and a cone, or a pair of horizontal cells), while some can produce a Müller cell and a progenitor, or a pair of progenitors. Importantly, though, in the scheme of Wong & Rapaport (238), at every division the competence of the progenitor is progressively restricted, so that cells corresponding to an ‘earlier’ stage can no longer be created. In Figure 33B, the presumed influence of signaling molecules is indicated by the broader shaded arrows: diagonal colored arrows indicate signals from the different classes of newly-born cells, while vertical gray arrows indicate Notch-Delta mediated lateral inhibition.
Alternative stochastic scheme. Recently, Gomes et al (239)proposed an alternative stochastic model (Figure 33C) in which the sequential order of cell birth in the overall population results from random choices of cell fate at each cell division, and both they and He et al (240) have obtained evidence in support of this stochastic model. Intuitively, it may be difficult to envisage how it could be possible for random divisions to lead to a deterministic make-up of cell classes, but He et al (240) provide numerical simulations that support their contention.
Three modes of cell division are possible (Figure 33C). In the first mode, termed ‘PP’, the progenitor cell gives rise to a pair of progenitors, resulting in an expansion of the progenitor population. In the second mode, ‘PD’, it gives rise to one continuing progenitor and one daughter cell that exits the cell cycle. In the third mode, it gives rise to two daughter cells, ‘DD’, thereby terminating the lineage. The analysis of He et al (240) showed that as the progenitors proceeded through multiple mitoses, they exhibited a shift from the initially preferred PP mode to the PD mode and finally to the DD mode. Furthermore, the types of daughter cell appeared to be correlated with the PD and DD division modes. In particular, most RGCs arose from PD mode divisions; amacrine cells arose from either PD or DD modes; but horizontal cells, photoreceptors, and bipolar cells mostly arose from DD divisions. They also showed that Ath5 was required both for the specification of RGCs and also for the PD mode of division, explaining why RGC differentiation is always earlier than that of other cell types.
Through the combination of these (and probably additional) factors influencing the probabilities of individual stochastic divisions, the overall effect at a macroscopic level is that the cells of the retina are born (on average) in a well-defined sequence, and also that the final composition of retinal cell types is determined appropriately.
Horizontal cells. In spite of the general understanding that retinal precursors undergo mitosis at the outer (ventricular) surface, and that the daughter cells migrate vertically to their final position in the retina, an exception applies to horizontal cells (at least in the zebrafish retina). In the case of zebrafish horizontal cells, the great majority of precursor cells (90% of the final population) undergo a symmetrical ‘DD’ mode of mitosis at the laminar position of the mature horizontal cells, giving rise to a pair of horizontal cell daughters (241). This mode of division at the final laminar position may assist in the formation of the layer of horizontal cells, and in achieving lateral connectivity between these cells.
Photoreceptors. The birth and differentiation of photoreceptors is influenced by a plethora of transcription factors and other messengers, as reviewed in Swaroop et al (234). Interestingly, the default situation for a newly-born mammalian photoreceptor cell is that, in the absence of other influences, it will become an S-cone (expressing the SWS1 opsin). In the absence of exposure to the factors NRL/NR2E3 the generic photoreceptor will become a cone, and in the absence of the factor TRβ2 it will become an S-cone. Exposure of the cone to TRβ2 will cause it to express an LWS opsin, with the choice (in human) between L- and M- (via the tandem OPN1LW and OPN1MW genes) being under control of an upstream locus control region. Exposure of the generic photoreceptor to NRL/NR2E3 will commit the cell to becoming a rod.
The default condition, of producing an S-cone, makes sense if one assumes that the ancestral cone type was the SWS variety. At the stage in chordate evolution, prior to ‘2R’, when this ancestral cone and its opsin duplicated, an additional signal would have been needed to specify the alternative LWS fate. Likewise, at the later stage of vertebrate evolution when rods emerged, an appropriate signal would have been needed to specify that fate.
Hypotheses for the evolutionary origin of cells in the vertebrate retina/RPE
On the basis of the observations in the preceding sections, the following hypotheses are advanced for the evolutionary origin of cells in the vertebrate retina/RPE:
M-1) All the cell types in the vertebrate retina/RPE are descended from a single ancestral type of multifunctional cell, that existed in a pre-bilaterian organism, and that functioned as a photoreceptor and that also contained shielding pigment.
M-2) That cell expressed the ancestral opsin and activated a G-protein cascade, and it probably employed cyclic nucleotide-gated channels to generate its electrical response; it might also have had additional (e.g. motile) functions.
M-3) During evolution, subsequent duplications and division of labor gave rise to separate ciliary and microvillar photoreceptors, as well as (in some cases) to separate pigment cells and separate glial cells, in bilaterian organisms.
M-4) Since ancient times, development of the light-sensitive region has involved divisions of a multipotent progenitor cell. In early bilaterians, that progenitor might have given rise only to ciliary photoreceptors, microvillar photoreceptors, pigment cells, and glial cells.
M-5) In the vertebrate lineage, probably after the time of the ‘2R’ duplications but before the divergence of the jawed and jawless lineages, other cell types emerged, as variants of the pre-existing cells (including the microvillar and ciliary photoreceptors). In due course these new cells became horizontal cells, amacrine cells, rod photoreceptors, and bipolar cells.
M-6) In the expanding vertebrate lateral vesicles, a polarity arose, possibly triggered by a signal from the ancestral ‘lens placode’, causing the outer layer to generate only epithelial cells (RPE), and causing the inner layer to generate all the other classes of cells (the retina). In the dorsal vesicles (pineal, parapineal and parietal) that lacked the placode signal, the same polarization did not occur, leading to a somewhat different distribution of cell classes.
In considering the data on the order of cell birth in the retina, Dowling (242) has suggested that the early birth of ganglion cells, cones, and horizontal cells may indicate that these were the original classes of cells in the ancestral retina. He notes that horizontal cells share many properties with glial cells, and suggests that the original glia took on neuronal properties and thereby became horizontal cells, with a new class of glia (the Müller cells) emerging; thereafter amacrine cells, rods, and bipolar cells were added. Dowling’s scheme represents an explicit proposal for the origin of horizontal cells in step M-5) above.
Uncoupling of differentiation and lateral expansion. As a final point, it is interesting to note that the differentiation of retinal neurons is not obligatorily coupled to the lateral expansion of the eye vesicles. Thus, Manns & Fritzsch (243)showed that application of retinoic acid to Xenopus embryos could prevent the formation of eye vesicles, yet in this case an apparently normal set of retinal and RPE cells could differentiate in the dorsal region of the forebrain, with the photoreceptors protruding into the third ventricle.
15 Synaptic transmission from photoreceptors to ganglion cells
Development of photoreceptor connectivity in the mammalian retina
The process of development of photoreceptor neural connectivity is intriguing, and may represent an informative relic of retinal evolution. The process is probably common across mammalian species, though it has been studied most comprehensively (244, 245) in the ferret, which has proved convenient as the young are born at a relatively immature stage of retinal development, and eye-opening does not occur for a further 2 weeks. The process is illustrated by the micrographs and the schematized sequence in Figure 34.
Initially (during the first post-natal week in the ferret) the photoreceptors have a simple bipolar form, making it almost impossible to identify them on purely morphological grounds. But even these very young cells express markers specific for photoreceptors (e.g. recoverin, vGluT1, rhodopsin), and they do so long before they exhibit the characteristic morphology of mature photoreceptors. In the immunofluorescence image of Figure 34A, the young rods are labeled green as a result of reactivity for rhodopsin, which is found to be distributed throughout the entire cytoplasm of the rods before the outer segments have differentiated.
Transient contacts at the inner plexiform layer. At very early times each immature cone or rod photoreceptor sends a process directly to the inner plexiform layer (schematic in Figure 34C). Over the course of the first two post-natal weeks, more and more photoreceptors form, and their processes continue to penetrate the location of the nascent outer plexiform layer, even though the horizontal cells with which they will eventually connect are already in their final positions and have begun to extend their processes laterally (schematic in Figure 34D). Within the IPL, the processes of the young photoreceptors terminate in two discrete sub-layers, coincident with the stratifying processes of cholinergic amacrine cells (micrographs of Figure 34A,B and schematic in Figure 34E). The photoreceptor terminals express the synaptic proteins synaptophysin and synaptotagmin, and have the morphological appearance of functional synapses (245). However, it has not been established whether the post-synaptic processes that they contact are amacrine or ganglion cells.
Mature contacts at the outer plexiform layer. Subsequently (around post-natal day 14 in the ferret), these transient processes retract from the IPL, and the photoreceptors instead make synaptic contacts within the developing OPL (schematic in Figure 34F). This phase of retraction coincides in time with maturation of the bipolar cells and with continued maturation of horizontal cells and the OPL. At the developing OPL, each photoreceptor terminal develops a ribbon synapse which first forms a dyad contact with a pair of horizontal cell processes, and is thereafter contacted by a bipolar cell process, giving rise to the adult triad arrangement of the ON pathway. At around the same time, the distal process of each photoreceptor penetrates the outer limiting membrane, and begins to form an inner segment and an outer segment.
The above developmental sequence, whereby the route for signal transmission from the photoreceptor initially occurs via direct synaptic contact onto the output layer, but is subsequently transformed by the insertion of a bipolar cell into the afferent pathway, is consistent with the notion that a comparable transformation occurred during the evolution of the retina.
Photoreceptor connectivity in organs other than gnathostome retina
Pineal organ: Photoreceptor connectivity to ganglion cells. In non-mammalian vertebrates, pineal photoreceptor cells make direct synaptic contact via ribbon synapses onto projection neurons (‘ganglion cells’), as indicated previously in Figure 10A,D and reviewed in Dodt (246) and Ekström & Meissl (247). It is generally thought that the pineal organ contains only photoreceptors, ganglion cells, and glial cells, and that it completely lacks the other classes of neuron that characterize the lateral retinas; however, Ekström & Meissl (247) reported the occurrence of a single case of a presumed interneuron that exhibited electrical response properties closely similar to those of the photoreceptors.
Pineal ganglion cell responses are predominantly ‘luminosity OFF’ units, showing sustained firing in darkness and a graded inhibition of firing during illumination at any wavelength (69, 74, 246, 248). Typical responses for a pineal photoreceptor and a standard ‘luminosity OFF’ pineal ganglion cell to moderately bright illumination were indicated schematically in Figure 12. For sub-saturating intensities the form of the graded response in the ganglion cell closely resembles that in the photoreceptor. For maintained exposures, the firing rate is reduced in proportion to the logarithm of the intensity, over a range as great as 8 log units (74).
In addition, some luminosity cells are transient, showing little spike activity in darkness but giving a burst of firing at the cessation of a light stimulus. Occasionally, chromatic responses are seen, that involve antagonism between UV-sensitive photoreceptors on the distal side of the organ and the main rhodopsin-containing photoreceptors.
The interpretation of these results is as follows. In the light-sensitive pineal organ of non-mammalian vertebrates, the photoreceptor cells make direct synaptic contact onto ganglion cells via ribbon synapse junctions. The high rate of release of glutamate in darkness leads to a sustained depolarization and steady firing in the ganglion cells. In response to light, the photoreceptors hyperpolarize, reducing their release of glutamate, and eliciting graded responses of similar form in the ganglion cells. The resulting sign-preserving responses in the ganglion cells are accordingly of ‘OFF’ polarity; they are maintained during steady illumination and show a logarithmic relation to intensity. There is no ‘ON’ pathway, and no evidence for the involvement of cells other than the photoreceptors and ganglion cells. Given that the pineal organ may well have antedated the lateral retinas of vertebrates, these results suggest that the ancestral pathway for signal flow in the vertebrate retina could conceivably have been directly from cones to OFF retinal ganglion cells.
Hagfish ‘eye’: Photoreceptor connectivity to ganglion cells. In the hagfish eye, the only known classes of neuron are ciliary photoreceptors and projection neurons. The synaptic terminals of the photoreceptor cells have been shown to form dyad contacts with post-synaptic elements, though to date the identity of those post-synaptic processes has not been determined; nevertheless, it is presumed that they are the projection neurons. Hence the presumption is that the ciliary photoreceptors make direct synaptic contact onto the projection neurons in hagfish.
Contact between ciliary and microvillar cells in amphioxus? The kind of molecular homologies discovered by Arendt and his colleagues, between the vertebrate and Drosophila retinas, have recently been extended to the frontal eye of amphioxus. As presented in Section 3, Vopalensky et al (47) have shown a close homology between ciliary photoreceptors in Row 1 of the amphioxus frontal eye and the cone and rod photoreceptors of the vertebrate retina; in addition, it is possible that the Row 2 cells might be homologous to retinal ganglion cells. Elsewhere in the brain of amphioxus, microvillar photoreceptors (Joseph cells) are immediately adjacent to the cells of the lamellar organ, with the Joseph cells ‘capping’ the lamellate cells (37, 249, 250). Although there is no evidence that the lamellate cells are actually photoreceptors, these reports at least show that during evolution microvillar photoreceptors have come into very close apposition with lamellate ciliary cells.
Scenario for origin of synaptic transmission from photoreceptors to ganglion cells
In light of these observations, the following scenario is proposed for the origin of synaptic transmission from ciliary photoreceptors onto projection neurons in a proto-vertebrate retina (see Figure 35):
N-1) At an early stage in chordate evolution, ciliary photoreceptors and microvillar photoreceptors (that had descended from a common ancestral cell) lay adjacent to each other. They responded to light with hyperpolarizing and depolarizing responses, respectively. These photoreceptor cells were embedded amongst pigmented cells, and possibly separate glial cells, that had likewise descended from the common ancestral cell.
N-2) At this stage, the ciliary photoreceptors conveyed their output via a ‘local circuit’ or paracrine mechanism, probably releasing melatonin, and possibly also glutamate. This paracrine release may well have had a circadian or seasonal time-keeping function.
N-3) The microvillar photoreceptors signaled their output to other regions of the CNS via axon terminals that released glutamate as synaptic transmitter.
N-4) Over time, the microvillar photoreceptors additionally came to express glutamate receptors and thus were able to respond to signals from the ciliary photoreceptor, and in due course full-blown synaptic contacts evolved. At this stage, the microvillar photoreceptors acted as projection neurons and could transmit the responses of the ciliary photoreceptors rapidly, via their axons and synaptic outputs.
N-5) At some stage (probably coinciding with the improvements in C-opsin performance and the invention of retinoid re-isomerization in darkness), the signals from the ciliary photoreceptors provided virtually all the information that was needed by the organism, and the phototransduction machinery in most of the projection neurons became redundant and withered away.
N-6) The region in which these cells were located was the forerunner of the ‘eye fields’ that in the future would give rise to the several diencephalic light-sensitive organs. Its expansion occurred as described above in Section 13.
16 Retinal processing: Bipolar cells and the photopic and scotopic pathways
The nature and origin of retinal bipolar cells
Retinal bipolar cells share a large repertoire of features with cone and rod photoreceptors. Structurally, retinal bipolar cells have the same ‘bipolar’ shape as is initially exhibited by developing photoreceptors, prior to the differentiation of their inner and outer segments.
Landolt’s club. Some bipolar cells possess an appendage, the Landolt’s club (Figure 36) that bears a striking similarity to the inner segment of the photoreceptor (251-253). This organelle has microtubules organized in the 9+0 pattern characteristic of non-motile cilia, and is located at the outer limiting membrane, though it differs from the inner segment in not giving rise to an outer segment. In the chick retina, Quesada & Genis-Galvez (254) have reported that in early embryonic stages all of the bipolar cells are connected to the outer limiting membrane by a Landolt’s club, and that during development many of these processes retract.
Transduction. In terms of transduction mechanisms, the G-protein cascade of the ON bipolar cells bears a remarkable similarity to the phototransduction cascade in vertebrate photoreceptors. In addition, the output synapse of the bipolar cell is very similar to that of the photoreceptor, employing synaptic ribbon structures that are not found in any other types of retinal neuron. Finally, many proteins expressed in retinal bipolar cells are either identical to, or else represent isoforms of, proteins found in cone and rod photoreceptors; for example, recoverin, potassium channels, and the molecular machinery of ribbon synapses.
In view of these close similarities, as well as the order of differentiation of retinal cells (Figure 33) it is possible to view retinal bipolar cells as probable ‘descendants’ of retinal ciliary photoreceptors, in the sense that they appear to have evolved as a kind of variant of the established ciliary photoreceptor theme and have thereby inherited a great many of the properties of those photoreceptors. If this is indeed the case then one might anticipate that cone ON bipolar cells would have descended from cones, and that rod bipolar cells might have descended from rods rather than from cone bipolar cells.
An alternative (though perhaps less likely) possibility that might account for these observations is that photoreceptors and bipolar cells are independent descendants of the ‘proto-neuron’ that has been hypothesized to have been the most ancient type of vertebrate nerve cell (208, 209, 255). However, this hypothesis would not seem to sit well with the evidence that cone and rod photoreceptors are descended from an ancestral ciliary photoreceptor. An extant representative of the presumed ancestral proto-neuron has been suggested to be the sensory ‘cerebrospinal fluid (CSF)-contacting neuron’ present in the CNS of amphioxus, hagfish (256) and lampreys (257), as well as jawed vertebrates.
Scenario for evolution of the photopic signaling pathway via bipolar cells
From the observations in Section 14 on the development of photoreceptor contacts in the embryonic retina, and the observations above on the nature of bipolar cells, as well as the findings in Section 14 on the timing of retinal development, the following is proposed as the likely sequence of events that occurred during evolution of the afferent pathway in the vertebrate retina:
O-1) At the stage of eye evolution where the eye vesicles had bulged laterally to form the ancestral retina/RPE, the photoreceptors already made synaptic contacts directly onto ganglion cells, which projected axons to the CNS.
O-2) Horizontal cells evolved, and made synaptic contacts with photoreceptors at ribbon synapses in a dyad structure; they provided spatial integration of light signals, and fed this spatially-averaged signal back (inverted) onto the photoreceptor terminals thereby generating an element of spatial contrast.
O-3) Subsequently, a modified class of ‘pseudo photoreceptor’ cell evolved, that had lost the capability of expressing visual pigment and an outer segment, but that expressed metabotropic glutamate receptors.
O-4) During retinal development, this new class of cell, the retinal ON bipolar cell, inserted its dendritic terminals into photoreceptor dyads, to form triads, while its afferent process made the same journey to the IPL that its predecessor (the photoreceptor) had made.
O-5) A variant of this cell expressed ionotropic glutamate receptors and became the OFF bipolar cell.
O-6) Overall, a new twin-set of neural elements had been incorporated into the ancestral two-layered retina, greatly expanding the retina’s capacity to perform spatiotemporal processing computations.
Dedicated scotopic pathway for rod signals
The existence of unexpected retinal circuitry in the rod pathway of mammals was first described by Kolb & Famiglietti (258), Nelson (259) and Kolb and Nelson (260); see chapters in webvision on “Circuitry for rod signals”, “AII amacrine cells” and “Bipolar cell pathways”. The details of this circuitry were expanded upon by Strettoi et al (261) and Vaney et al (262), and shortly afterwards Strettoi et al (263) set out the concept that the mammalian scotopic (rod-driven) retinal pathway ‘piggybacks’ on a pre-existing photopic (cone-driven) pathway. Coincidentally, this was the same year that Okano et al (115) discovered that rod opsins had evolved from cone opsins.
In functional terms, the main advantage of this piggyback arrangement is that the duplex cone-plus-rod system does not require duplicate pathways for neural processing in the retina, and nor does it require a duplicate set of nerve fibers to the brain. Instead, the rod signals enter the pre-existing sophisticated cone neural processing circuitry, where they can be seamlessly integrated. For visual perception, this means that it is almost impossible for one to distinguish whether it is one’s scotopic or photopic system that is being utilized.
The manner in which the rod signals are injected into the mammalian cone system looks complicated (Figure 37), but it cleverly avoids introducing major compromises, especially by avoiding the generation of additional noise. From mesopic (twilight) light levels down to high scotopic (moonlight) levels, the rod signals pass via gap junctions onto cones (denoted ON2 and OFF2 in Figure 37) thereby employing the photopic pathway in its entirety. But at very low scotopic (starlight) levels, a separate dedicated system comes into operation, utilizing the rod bipolar cell and the AII amacrine cell.
For the ON system, illustrated on the left side of Figure 37, the photopic pathway uses a sign-inverting synapse (red arrow) from cone photoreceptor to cone ON bipolar cell (via a metabotropic glutamate receptor mechanism), followed by a sign-preserving synapse (green) from cone ON bipolar cell to ON ganglion cell. Thus, at low light levels, when the cones are contributing little in the way of signals, the ON bipolar cell is hyperpolarized and the synapse from ON bipolar cell to ganglion cell is quiescent. At low scotopic levels (indicated ON1), rod activity is signaled via a sign-inverting synapse onto the rod bipolar cell, and thence via a sign-preserving synapse to the AII amacrine cell, so that dim scotopic illumination causes depolarization of the AII amacrine cells (i.e. the same polarity as any light-stimulated activity in the cone ON bipolar cells). The scotopic signals are then coupled into the cone pathway, with no additional noise contribution, via gap junction electrical connections from the AII amacrine cells onto the cone ON bipolar cells (indicated in orange). In bright light, when the rods are saturated, and the rod bipolar cells and AII amacrine cells are strongly depolarized, it is possible that the gap junctions close, to prevent the intrusion of unwanted signals. If instead the rod bipolar cells had connected directly onto the ON ganglion cells synaptically (as for cone bipolar cells), a major disadvantage would have been the injection of large levels of synaptic noise into the photopic system when the rods were saturated.
For the OFF system, illustrated on the right of Figure 37, the photopic pathway uses a sign-preserving synapse (green arrow) from cone to OFF bipolar cell (via an ionotropic glutamate receptor mechanism), followed by a second sign-preserving synapse (green) from cone OFF bipolar cell to OFF ganglion cell. As a result, in the quiescent dark state, the cone OFF bipolar cell is depolarized and is continually releasing synaptic transmitter (glutamate) onto the OFF ganglion cell at a high rate. How, then, could activity from the scotopic system be injected into the cone OFF pathway, in the presence of this continual synaptic input? The answer that has been adopted is to employ a sign-inverting synaptic input from the AII amacrine cell onto the pre-synaptic terminal of the cone OFF bipolar cell (indicated OFF1). In this way, dim scotopic light leads to hyperpolarization of the cone OFF bipolar cell’s synaptic terminal, thereby suppressing its on-going high rate of release of synaptic transmitter, just as occurs for signals that come from the cones.
This analysis shows that, by the time that mammals appeared, evolution had found a means by which to superimpose the single-photon detection capabilities of the more recently evolved rods onto a pre-existing cone neural processing pathway, in a seamless manner, with minimal additional wiring, and in a way that introduced minimal additional noise and that minimally perturbed the sophisticated signal processing functions of the cone circuitry. However, it is not yet clear whether this was the first solution that had been adopted in the early vertebrate retina.
Thus, it will be important to discover whether similar AII amacrine cell circuitry is employed in the retinas of vertebrates other than mammals, but so far a definitive answer to this question does not appear to have been established. In non-mammalian vertebrate retinas, it is clear that scotopic signals are processed via bipolar cells that are highly sensitive and that display response properties very similar to those of mammalian rod bipolar cells; e.g. Ashmore & Falk (264) in dogfish. Likewise, ERG recordings in lamprey retina have show a sensitive b-wave signal at very low light intensities However, the circuitry by which rod and cone signals are combined is not entirely clear. One possibility is that in non-mammalian retinas rod and cone signals are merged solely at the level of the outer plexiform layer, via gap junctions between rods and cones, and possibly via synaptic input from both rods and cones onto a single class of bipolar cell. In this case the bipolar cells would be of the mixed rod-cone type, and thereafter the signals would be processed by the conventional cone pathway, comprising bipolar cell to ganglion cell. Alternatively, it is possible that circuitry analogous to that of the AII amacrine cell exists in the inner retina. Until the details of the scotopic circuitry of non-mammalian vertebrate species have been elucidated, it may not be possible to ascertain the extent to which the ancestral vertebrate retina employed the outer versus the inner plexiform layer to inject its rod-derived signals into the cone pathway. But in either case it is clear that, as a minimum, the output (ganglion cell) stage of the pre-existing cone system was utilized.
At this stage, there is insufficient information available to justify the proposal of explicit steps in the evolution of the dedicated scotopic pathway.
17 When did spatial (imaging) vision arise in the chordate lineage?
It was argued above, in Section 13, that the retina of the vertebrate lateral eyes evolved first as a non-imaging light-sensitive organ, with many similarities to the two-layered retina in the dorsal organs (pineal, parapineal, and parietal eye) of extant non-mammalian vertebrates. But when did that two-layered retina first emerge? And when, and by what means, did that pair of ‘lateral pineal-like organs’ evolve to become imaging organs for a visual system?
There is no evidence to suggest that the last common ancestor that we share with tunicates (at point #4 in Figure 1) could have possessed anything resembling a retina. On the other hand, our last common ancestor with lampreys (at point #5 in Figure 1) seems almost certain to have possessed a fully-fledged vertebrate-style eye, with three-layered retina, lens, extraocular muscles, and so on. Unfortunately, none of the numerous organisms that diverged from our own lineage (represented by the red curve in Figure 1) between those times has survived to the present day. Several extinct species that diverged during that interval are known from the fossil record. Although these fossil species have been reported to have possessed ‘eyes’, the preservation of their soft tissues is so imperfect that it is not even known whether those organs contained a lens. It seems that the fossilized lateral impressions described as ‘eyes’ tend to be black, or at least dark, which might be consistent with their having contained pigment.
Accordingly, it does not currently seem possible to be more specific than to say that the lateral two-layered retina arose some time after point #4 in Figure 1 and that the full-blown lateral eyes arose subsequently, at some time prior to point #5 in Figure 1. On the basis of the dates put forward by Erwin et al (20), these divergences occurred 600 Mya and 500 Mya, respectively. So, unfortunately, we do not appear to be in a position to tie down the relevant times of evolution of these important features any more precisely than within that interval of 100 million years.
If it were possible to date with any precision the timing of the occurrences of the ‘2R’ pair of whole genome duplications, then it might be possible to narrow down the timing of the events of interest in eye evolution. Thus, the ciliary opsin quadruplication did not appear until after ‘2R’, and hence the present five classes of ciliary opsin could not have appeared before that time, so that (for example) rods could not have appeared before that time.
A calculation has been made by Nilsson & Pelger (265) of the time required for a lens to evolve. This calculation assumed that a fully functional retina was already present, and that it was already connected appropriately to the brain, but the calculation did not take account of other changes that might be required, such as the emergence of a system of muscles to orient the eye. Hence, the time that they calculated, of approaching 1 million years, would appear to enormously underestimate the time required to transform a handful of photoreceptors into a functional eye.
Instead, we can expect that the transitions described above might have taken far longer, because each must have involved a long sequence of changes. To transform a handful of ciliary and microvillar photoreceptors into a pineal-like retina would have required numerous steps. Thereafter, to add bipolar cells, to add a lens and cornea, to add extraocular muscles, and (possibly most demandingly) to create a processing center in the brain that could deal with this spatial information, would have required enormous numbers of parallel steps. It does not seem unreasonable to contemplate that the time taken to implement the combination of these major transformations (first to non-imaging retinas, and then to imaging eyes) might have occupied a very substantial proportion of the 100 million year interval that is currently thought to have been available.
18 Conclusions: Scenario for the evolution of photoreceptors and the vertebrate retina
The following sub-sections set out a proposed scenario for the sequence of the more important of the numerous steps that were involved in the evolution of photoreceptors cells and of the vertebrate retina, using the epoch numbering scheme from Figure 1.
Early metazoans: Prior to point #1 (>700 Mya)
Sensory cell. The sensory cell from which all animal photoreceptors evolved employed a G-protein coupled receptor (GPCR), which triggered a G-protein cascade that is likely to have employed cyclic nucleotide gated channels (CNGCs).
Opsins. The original opsin arose during the relatively short interval (of ~10 million years), just prior to 700 Mya (see Figure 2), between the divergence first of placozoans and then of cnidarians from our own lineage. Prior to this, mutations in a GPCR had changed its binding specificity so that the ligand that activated it became retinaldehyde, and this modified GPCR thereby became a retinaldehyde receptor; as in other GPCRs the ligand bound non-covalently. (One of the close relatives of this GPCR appears to have given rise to the melatonin receptors of vertebrates.) After placozoans had diverged, mutation of this retinaldehyde receptor gave it a lysine residue in its seventh transmembrane helix (corresponding to K296), so that the retinaldehyde ligand could now bind covalently via a Schiff base bond. A further mutation added a negatively charged residue nearby (corresponding to E181) that acted as a counterion, enabling the Schiff base to be protonated. This molecule thus became the ancestral opsin. It would have absorbed at the short-wavelength end of the spectrum, though we cannot be sure whether its peak would have been in what we call the ‘visible’ part of the spectrum or in the UV. Subsequently, this ancestral opsin duplicated twice, prior to the divergence of cnidaria, giving rise to three major branches of opsins, namely C-, R-, and RGR/Go-opsins.
Photoreceptor cells and phototransduction cascades. Once that ancestral opsin appeared, the ancestral photoreceptor had been born. It seems likely that this original photoreceptor cell resembled the ciliary category, with the opsin expressed in the membrane of the cilium and coupling via a Gi and/or Gs cascade to modulate cyclic nucleotide levels and CNGC activity. It is possible that this ancestral photoreceptor cell possessed the components of more than one G-protein signaling cascade, but at present there is too little information available to be sure about its transduction cascade(s). In the short time before cnidaria diverged, it is possible that two classes of opsin-containing cell had come into existence, with one expressing the ancestral C-opsin in its ciliary surface membrane and the other expressing the RGR/Go-opsin in the membrane of intracellular organelles. One likely function of the ancestral RGR/Go-opsin was as a retinoid photoisomerase that used light to convert the all-trans isomer to the 11-cis isomer. While it is possible that separate microvillar photoreceptors using the R-opsin had also emerged, and that they later disappeared in cnidaria, it is suggested that the microvillar class of photoreceptor arose only in bilaterians.
Other components of the ancestral light-sensitive cells. It is likely that the ancestral light-sensitive cells in those early metazoa were multifunctional, additionally expressing pigment that could act as a light shield, and it is also possible that these cells had motile functions (79). The animals possessing these cells would have been very small and would not have had a recognizable nervous system. Their cells would have been able to signal to each other by means of diffusible chemical messengers; i.e. by paracrine release. A chemical messaging system to control the basic features of differentiation in these cells would need to have been laid down by this time. Thus, the ancestral system of transcription factors specific to light-sensitive tissue was probably already in place.
Division of labor. As proposed by Arendt et al (5), it seems likely that processes of gene duplication and evolution led to the emergence of more specialized daughter cells, that shared out between them roles that had originally been performed by a single cell. If, as is suggested above, an ancient multifunctional photoreceptor cell had expressed more than one class of opsin and transduction cascade, and also functioned as a pigment cell and had a motile role, then over time these functions may have been delegated to separate specialized cells that inherited only the components relevant to a subset of the original functions. Thus, motile cells and pigment cells became separate from those that performed phototransduction; likewise, the ‘photoisomerase’ opsins could have been relegated to separate cells (for example, to the pigment cells). Similarly, the different opsins and their transduction cascades became separated into distinct cell types.
Bilaterians, protostomes, and early deuterostomes
Early bilaterians. In the bilaterian lineage (i.e. after the divergence of cnidaria), at least two variants of photoreceptor cell were present: a ciliary photoreceptor class expressing a C-opsin, and a microvillar photoreceptor class expressing an R-opsin. It seems likely that these two classes of cell came to be utilized for different functions. One possibility is that the ciliary photoreceptors adopted a role in diurnal and seasonal time-keeping functions, whereas microvillar photoreceptors adopted a role in phototactic behavior, providing the signal for the organism to move either towards or away from light (e.g. up or down in the water column).
Protostomes. Following the split between protostomes and deuterostomes (at point #2, Figure 1), it would appear that some protostomes evolved a lens in front of a handful of microvillar photoreceptors, in what would have become an ocellus or a single ommatidium. Perhaps signals evolved that led to the duplication/multiplication of a number of such adjacent ommatidia, leading to the formation of compound eyes. At some later stage, the microvillar photoreceptors in some protostome lineages developed a highly organized sub-cellular structure (the rhabdomere) and a highly-specialized version of the transduction cascade, and gave rise to rhabdomeric photoreceptors. Until the advent of full-blown rhabdomeric photoreceptors in those particular lineages, it is entirely plausible that microvillar photoreceptors may not have been very sensitive. In the chordate lineage, for example, microvillar photoreceptors in amphioxus have much lower sensitivity than their protostome counterparts that evolved rhabdomeric structure (46).
Early deuterostomes. No such developments (in the form of either a lens or a rhabdomere structure) appear to have occurred in the deuterostome lineage. Thus, although microvillar photoreceptors persisted in deuterostomes, they remained as relatively simple cells, sparsely distributed, sometimes in association with pigmented cells. Likewise, relatively little seems to have occurred in the way of changes to the ciliary photoreceptors in the early deuterostome lineage.
Chordates – Early events: From point #3 onwards (Since ~650 Mya)
Refinement of C-opsins. The transformation of an ancestral C-opsin into vertebrate visual opsins appears to have involved a prolonged sequence. One of the most important changes was the re-location of the counterion site (from 181 to 113), as this allowed the meta II state to be modified in a way that achieved a higher rate of activation of the G-protein and it also allowed the meta II state to decay fairly rapidly, releasing all-trans retinal.
In the phylogenetic tree of C-opsins (Figure 19), there are several extant opsins that clade between the most basal C-opsin and the cone opsins, and these include Opn3, parietopsin, parapinopsin, VA/VAL-opsin, and possibly pinopsin. The functional properties of these opsins also tend to follow a sequence (Section 5), and it seems clear that the performance of C-opsins was steadily enhanced during chordate evolution, reaching a high level by the time that the first cone opsin emerged (Section 5) Thereafter this ancestral cone opsin duplicated to form both an SWS and an LWS opsin. The tunicate Ciona intestinalis, which diverged at point #4 (Figure 1), possesses a single C-opsin, Ci-Opsin1, that clades close to VA/VAL (though this is not shown explicitly in Figure 19. Accordingly it seems plausible that the refinement of chordate C-opsins that ultimately gave rise to cone opsins was only partway through by the time that tunicates diverged, and perhaps that the entire process of refinement may have stretched out over much of the 150 million year period from #3 to #5 in Figure 1.
Refinement of the phototransduction cascade. Over the course of chordate evolution, it appears that refinements also occurred in the phototransduction cascade. These changes include: the emergence of a Gt variant of the G-protein alpha subunit (i.e. transducin), apparently through duplication of a common Gαi / Gαt ancestor; the emergence of PDE6 specific for cGMP; the emergence of its regulatory PDEγ subunits; and so on. While the exact sequence of these developments remains obscure, and the chain of events was no doubt prolonged, it seems clear that, prior to the ‘2R’ whole-genome duplications, the phototransduction cascade in the ancestral cone photoreceptor had already reached a state that would have been fundamentally similar to that in modern vertebrate cones.
Synaptic contact from ciliary photoreceptors onto microvillar photoreceptors. There are few landmarks to indicate when it was that synaptic contact became established between ciliary photoreceptors and their microvillar counterparts (most of which subsequently lost their light sensitivity). Amphioxus possesses microvillar photoreceptors (Joseph cells) that are immediately adjacent to cells of the lamellar organ, with the microvillar cells ‘capping’ the lamellate cells (Section 15) although these lamellate cells have generally been presumed to be ciliary photoreceptors, this has not been confirmed through electrophysiological recording and nor has an opsin yet been identified in them. In the ‘frontal eye’ of amphioxus, it has recently been shown that ciliary photoreceptors (Row 1 cells) are positioned immediately adjacent to projection neurons (Row 2 cells) that may conceivably be homologous to retinal ganglion cells (6). Hence it is possible that the last common ancestor we share with amphioxus already had ciliary photoreceptors making synaptic contact onto microvillar photoreceptors.
Chordate to vertebrate transition: Interval #4 – #5 (~600 – 500 Mya)
In the chordate lineage, it would seem that most of the transformations that distinguish an ‘eyespot’ from an ‘eye’ are likely to have occurred during the 100 million year interval from #4 to #5 (Figure 1), around 600-500 Mya. In the light-sensitive tissue itself, these transformations included: massive enlargement of the tissue to form a retina; the invention of a means of re-isomerization of retinoid in darkness; the elaboration of retinal processing power, including the invention of bipolar cells; and the transformation of one class of cone into more rod-like cells. Outside the retina, the transformations included: the formation of accessory structures, including the lens, the cornea, the iris, and intra- and extra-ocular muscles, as well as the formation of brain regions to process the visual information and to control the motor system.
Expansion of diencephalic light-sensitive region. The region comprising the light-sensitive tissue expanded, both dorsally and laterally, at some stage during chordate evolution; most of this expansion appears to have occurred after the divergence of tunicates. The initial driving force for expansion may simply have been the need to achieve greater sensitivity, and that may have led to the deployment of a larger area for absorption of incident light. One factor favoring expansion in the lateral direction may have been the shielding of down-welling light that accompanied the expansion of the primitive brain and the formation of a cartilaginous cranium. The advantages to the organism would have been not only increased light sensitivity, but the potential for shadow detection and a crude comparison of the direction of incident illumination.
In the tissue expansion that occurred, it seems that only the microvillar photoreceptor cells extended axons to their pre-existing targets in more central brain regions, and that the ciliary photoreceptor cells continued to signal in a purely local manner. At some stage, their signaling mechanism became synaptic, rather than paracrine, though the ciliary photoreceptors have retained to this day the capacity to release melatonin.
The region that bulged appears to have had minimal luminal space, so that its apposing luminal (interior) surfaces were in contact with each other; the innermost surface of each side contained ciliary photoreceptors embedded amongst pigment cells and glial cells. This expanding vesicle developed a ‘polarity’, that rendered the two sides different, and that in due course gave rise to a complete distinction between ‘retina’ on one side and ‘RPE’ on the other; the signal for this development of polarity may have been generated by the ancestral placode that subsequently evolved into the lens placode. As a result of the polarization, pigment cells no longer formed on the side facing the external world, while neural elements formed only on that side. This afforded a major advantage to the organism, in terms of increased light sensitivity, because the entire cross-sectional area of the organ was now tiled with photoreceptors, whereas previously a substantial proportion of that area had been occupied by pigment cells; instead, the pigment cells were now effectively placed behind the photoreceptors (in terms of the incident light path) but nevertheless still in physical contact with them.
This expansion and bulging, combined with the retention of minimal luminal volume and the development of a polarity to the organ, is what gave rise to the ‘inside-out’ vertebrate retina.
At this stage of chordate evolution, it is suggested that the rudimentary ‘eye’ had not yet evolved optical components (lens, etc.), or sophisticated neural processing power (bipolar cells, etc.), or a motor system for orienting it, and that instead it probably bore a close resemblance to the ‘eye’ of extant hagfish.
Dark re-isomerization of retinoid. Concomitant with the improvement in efficacy of G-protein activation that evolved in chordate C-opsins was the loss of ability of the active metarhodopsin to undergo photoreversal to the 11-cis isomeric form (Section 5). This necessitated release of the all-trans retinoid and its re-isomerization via some other route. Potentially, that could have utilized a separate photoisomerase, along the lines of the retinochrome that is used in the squid eye – and possibly such an isomerase was at some stage used, given the presence in the RPE of RGR-opsin that is capable of such trans-to-cis photoisomerization. However, it appears that what was of greater importance in the chordate lineage was a chemical route for isomerization in darkness (Section 12). In living vertebrate species there is clear evidence for the existence of an intra-retina retinoid cycle, available to cones, though the details are obscure and the origins unknown – but it is entirely possible that this intra-retina cycle might represent the ancestral pathway for isomerization of the all-trans form to the 11-cis isomer in darkness. The better known pathway, involving retinoid transport to the RPE and back, evolved after the divergence of tunicates, judging from the absence of a true RPE65 or LRAT in Ciona.
Triumph of ciliary photoreceptors. The advent of a chemical route for re-isomerization in darkness is likely to have invested chordate ciliary photoreceptors with a major advantage over microvillar photoreceptors that utilized bistable photopigments. Whenever the animal moved from a brightly-lit environment to a dimly-lit one, the microvillar photoreceptors would have been burdened by a large complement of stable metarhodopsin, with no way to rapidly convert it back to rhodopsin; hence the content of useful rhodopsin would have remained low, and these photoreceptors would have exhibited a prolonged reduction in sensitivity. In contrast, the ciliary photoreceptors would have been able to ‘dark adapt’ through release of all-trans retinoid and re-binding of 11-cis retinal to form rhodopsin; to maintain this ability in the long-term, the released all-trans retinoid needed to be chemically isomerized back to the 11-cis form in darkness. As a result, pre-vertebrate chordates are likely to have had a distinct advantage over organisms with stable photopigments, when they entered dim environments during the daytime, as well as at the fading of sunlight. Likewise, the ciliary photoreceptors may have enjoyed a sufficiently large advantage over their microvillar companions within the same retina, in terms of higher sensitivity and the ability to dark adapt, that there was little to be gained in retaining both sets of photoreceptor. Instead, the microvillar cells for the most part lost their photoreceptive properties and instead became specialized as projection neurons, though some retained a role in signaling light for circadian entrainment and other ‘non-visual’ purposes.
Multiple classes of retinal opsin and photoreceptors. Prior to the ‘2R’ rounds of whole-genome duplication, there appear to have been just two types of cone opsin (SWS and LWS) that were expressed in two classes of cone. Then, as a result of the ‘2R’ duplications, the SWS opsin quadruplicated into SWS1, SWS2, Rh2 and Rh1 opsins, expressed in what originally would have been four classes of short-to-mid-wave-sensitive cone photoreceptors. However, the pressure for high sensitivity at low light levels would have made it advantageous for the meta II lifetime to have lengthened in (at least) one of these opsins, so that the light response could be integrated for longer. This appears to have led to mutations at sites 122 and 189 in the Rh1 opsin, that lengthened the meta II lifetime by partly shielding the retinoid binding site from access by water. The same pressure additionally led to the slowing of other recovery steps in the cells expressing the Rh1 opsin, so that modified versions of the GRK, arrestin and PDE6 arose. Although the LWS opsin is likewise assumed to have quadruplicated, only a single copy has survived.
Even by the stage at which lampreys diverged from our own lineage (i.e. #5 in Figure 1), it seems unlikely that ‘true rods’ had evolved; thus, although the short photoreceptors of northern hemisphere lampreys express an Rh1 opsin and are quite sensitive, they exhibit a number of cone-like properties and it remains to be determined whether they are capable of reliably signaling individual photon hits. True rods, of the kind we recognize in jawed vertebrates, may not have been perfected until after that divergence.
Evolution of retinal bipolar cells. In vertebrate light-sensitive regions other than the lateral retinas (i.e. in the pineal organ, the parapineal organ, and the parietal eye), and also in the retina of the hagfish eye, the ciliary photoreceptors make synaptic contact directly onto projection neurons which, as argued above, are likely to represent descendants of microvillar photoreceptors. In contrast, the cone and rod photoreceptors of the vertebrate retina communicate with their projection neurons via an intervening bipolar cell. Examination of retinal development shows that these bipolar cells differentiate later than all other retinal neurons, and furthermore that the developing bipolar cells become interposed into a circuit that initially comprises direct contact from photoreceptors onto ganglion cells.
These findings are consistent with the notion that retinal bipolar cells evolved late, after a two-layered retina had already been established (comprising ciliary photoreceptors connected to projection neurons) and that these newer cells became inserted into the pre-existing neural pathway. That new arrangement provided an enormous increase in computational power in the retina, allowing the pooling of photoreceptor signals, as well as comparisons of different signals, as in center-surround processing and spectral (color) comparisons.
Accessory structures (lens, cornea, muscle). The accessory structures that enable a retina to function as the ‘sensor’ in a camera-style eye include the lens, the cornea and sclera, the iris, and the intra- and extra-ocular muscles. In addition, and arguably most importantly, there needs to be a neural processing center in the brain to interpret the information and act on it, as well as to control the movement of the eyes. Although it is clear that these structures must have evolved in parallel with the evolution of the retina, unfortunately any account of their evolution is beyond the scope of this article.
Jawed vertebrates: After point #5 (Since 500 Mya)
After the divergence of cyclostomes, the fundamental bauplan of the eyes of jawed vertebrates did not change substantially. Nevertheless, numerous refinements occurred, many of which relate to the optics of the eye, and especially to the optics required with an air interface once tetrapods emerged. Those changes will not be discussed here.
One major change that evolved within the jawed vertebrate retina involved scotopic pathways. As far as we know, neither lampreys nor hagfish possess a ‘duplex’ retina of the kind that evolved in jawed vertebrates, with true rods capable of reliably detecting single photon hits and with a dedicated scotopic visual system. Instead, lamprey photoreceptors that express the rod-like Rh1/RhA opsin retain a variety of cone-like features. On current evidence I suggest that the ultimate scotopic sensitivity exhibited by modern vertebrates, that is made possible by the retina’s ability to ‘count’ individual photons hitting ‘true rods’, did not evolve until after our ancestors had diverged from the ancestors of lampreys and hagfish. It will be important to test this assertion through future research.
19 Summary
The following is an abbreviated summary of the main events hypothesized to have contributed to the evolution of vertebrate cone and rod photoreceptors and their transduction cascades, and to the evolution of the vertebrate retina:
1) An ancestral cyclic nucleotide version of a G-protein cascade arose very early in metazoan evolution. In that cascade a (non-light-sensitive) GPCR activated a G-protein that coupled to an effector enzyme that modulated cyclic nucleotide levels, thereby modulating the opening of CNGCs.
2) One type of GPCR evolved the ability to bind retinaldehyde non-covalently, as its ligand, and hence effectively became a retinaldehyde receptor. This occurred prior to the divergence of placozoans. Subsequently, the lysine 296 residue evolved, so that the binding of retinoid became covalent, via a Schiff base bond. A counterion site (e.g. E181) also evolved, enabling the Schiff base bond to be protonated, and generating the ancestral opsin that would have had its peak absorption near the shortwave end of the spectrum.
3) The cell that expressed this first opsin thereby became the ancestral metazoan photoreceptor cell. It employed a G-protein cascade broadly comparable to that of modern ciliary photoreceptors, and in particular it used a cyclic nucleotide as cytoplasmic messenger to modulate the opening of cyclic nucleotide-gated ion channels.
4) The organism in which this photoreceptor cell evolved was very small, and did not yet possess what could be described as a nervous system. The cell signaled its response to other cells by the local release of one of more chemical messengers, probably melatonin and glutamate.
5) This cell probably did not require a source of 11-cis retinal, because the opsin apoprotein could bind all-trans retinal, and because the resulting visual pigment could undergo photoreversal from the active metarhodopsin state to the ground state rhodopsin (as can most opsins, with the exception of vertebrate cone and rod opsins).
6) Variants of this cell arose, through a number of duplications, leading to different cell types that expressed a C-opsin, an R-opsin, or an RGR-opsin.
7) In the cell expressing the ancestral C-opsin, the opsin trafficked to the plasma membrane of the cilium. In time, mechanisms evolved for the massive expansion of this ciliary membrane. At first, these expansions were poorly organized; often they tended to align parallel to the axis of the cilium (longitudinally).
8)In the deuterostome/chordate lineage, these expansions of the ciliary membrane progressively evolved greater degrees of organization, becoming flattened (lamellar) by the time that cephalochordates diverged, and as rather bud-like lamellar petals by the time that ascidia diverged, and then subsequently being splayed-out at right angles to the ciliary axis by the time that vertebrates appeared.
9) During early chordate evolution there was probably just a single type of C-opsin, probably of the Opn3 family, that in many respects did not differ greatly from R-opsins. But during early vertebrate evolution a number of variants of this C-opsin arose, of which residual members in living vertebrates include parietopsin, parapinopsin, VA opsin, and pinopsin.
10) During this progression, the counterion for the Schiff base migrated from site 181 to site 113, and this re-location turns out to have been important in at least three ways. First, it permitted release of all-trans retinal and hence the rapid regeneration of visual pigment in darkness (from a store of 11-cis retinal); secondly, it improved the time resolution of the cone by shortening the lifetime of active meta II; and thirdly, it paved the way for the achievement of a higher efficacy of G-protein activation by enabling further intra-molecular rearrangements that led to a large tilt in helix 6 in the meta II state. That last improvement prevented photoreversal of meta II, and made the release of all-trans retinal essential.
11) Hence the ancestral cone photoreceptor utilized an opsin (the ancestral cone opsin) that was expressed in lamellar sac membranes that radiated laterally from the cilium. It required a source of 11-cis retinoid, which (by analogy with modern cones) was probably provided by the Müller cells.
12) The primary proteins involved in activation of the cascade had evolved to become: (i) the ancestral transducin, with its Gt alpha subunit duplicated and modified from an earlier Gi, and with a ubiquitous beta and unique gamma subunit; (ii) the ancestral PDE6 cyclic GMP phosphodiesterase, comprising a pair of identical catalytic subunits and a pair of regulatory (gamma) subunits; and (iii) a tetrameric cyclic nucleotide-gated channel composed of two classes of subunit, alpha and beta.
13) The primary proteins involved in response recovery and regulation had evolved to become the ancestral ‘cone’ components: GRK7, ARR3, RGS9, GUCY2D, GUCA1C, SLC24A1, RCVRN.
14) Duplication of the first cone opsin gave rise to a pair of retinal cone opsins: an SWS opsin with the standard E181 residue, and an LWS opsin with the H181/K184 combination that provided a chloride-binding site and a substantially red-shifted absorption. These two opsins were expressed in separate, but closely similar, cone photoreceptors. Their outputs provided signals that could have been utilized to provide dichromatic processing.
15) Also present nearby were microvillar photoreceptors that had descended from a common ancestral photoreceptor. The paracrine release mechanism of the ciliary photoreceptors evolved into synaptic contacts onto the neighboring microvillar photoreceptors. At some stage, the performance of the ciliary photoreceptors (as transmitted to the cells of microvillar origin) became sufficient to signal all the features of illumination that were important to the organism, and thereafter the majority of the microvillar photoreceptors dispensed with their opsin and phototransduction cascade, and specialized as projection neurons.
16) All the above events took place prior to the ‘2R’ two rounds of whole-genome duplication that occurred at the base of the vertebrate lineage.
17) As a result of the two genome duplications, the quadruplication of the SWS opsin gave rise to the vertebrate SWS1, SWS2, Rh2, and Rh1 opsin classes. Only a single copy of the LWS quadruplication survived. These five opsins were expressed in five classes of cones.
18) The genome duplications additionally gave rise to the cone versus rod differences in isoforms of many of the components of the phototransduction cascade.
19) In the cone photoreceptor that expressed the Rh1 opsin, the confluence of a number of changes led to a slowing of response shut-off, which elicited a larger response per photoisomerization. One of these changes was an increase in lifetime of meta II that occurred as a result of mutations at sites 122 and 189. This also reduced the rate at which rhodopsin could be regenerated, and probably explains the resistance of rhodopsin to hydroxylamine.
20) Ultrastructural changes in the outer segment of this cell, including the enclosure of lamellae within the plasma membrane, permitted more extensive longitudinal spread of messengers.
21) In due course, the combination of these changes gave rise to a photoreceptor that was capable of reliably signaling individual photoisomerizations, and that thereby deserved the title of ‘first true rod’. Whether this stage was reached before or after the divergence of cyclostomes is not yet certain, but it seems likely to have been after.
22) At around the time that chordate C-opsins lost their ability to undergo photoreversal, one or more mechanisms arose for the re-isomerization of retinoid in darkness. It is possible that the first of these mechanisms was the intra-retina cycle that modern cones have access to. The other cycle, involving transport of retinoid to and from the RPE, had not arisen by the time that tunicates diverged, and hence (among extant species) is a purely vertebrate phenomenon.
23) The light-sensitive region in the anterior neural tube of an ancestral chordate expanded dorsally and laterally. The ciliary photoreceptors and the projection neurons remained in close proximity to each other, and what ‘stretched’ was the axon of the projection neuron that conveyed information to other areas of the brain.
24) The lateral vesicles developed a sidedness, such that the side facing outwards made only the cells of the neural retina whereas the proximal side made only the epithelial cells that provided supportive, nutritional, and retinoid processing functions, as well as pigment to absorb unwanted light. At that stage of evolution the lateral retinas broadly resembled the retinas of modern hagfish.
25) As a consequence of the genome duplications, two new classes of retinal cells appeared. These horizontal cells and amacrine cells made synaptic connections with ciliary photoreceptors and ganglion cells, respectively, in a manner that mediated lateral interactions between cells and possibly mediated extraction of simple spatial contrast.
26) Also as a consequence of the genome duplications, another new class of cell, the retinal bipolar cell, evolved. It closely resembled the ciliary photoreceptor cells in many ways, though instead of making an outer segment it expressed glutamate receptors. This new cell became incorporated into the neural pathway from ciliary photoreceptors to ganglion cells, and thereby permitted a great increase in the computational ability of the retina.
27) Relics of the evolution of retinal cells and the evolution of retinal wiring are retained in various features of retinal cell differentiation and the early connectivity of retinal cells.
28) Spatial vision in chordates arose gradually, through parallel changes not just in the photoreceptors and the retina, but also through the formation of an imaging element (the lens) and other ancillary structures such as the cornea/sclera, the iris, and intra- and extra-ocular muscles, as well as through elaboration of brain structures that could make use of spatial information.
29) The advent of spatial vision provided immense survival value to the organism, but the process occurred slowly, over countless steps, with the transition from a simple eye spot in an early chordate to the vertebrate-style camera eye possibly taking as long as 100 million years.
I should emphasize that the brief summary painted above represents my best guess at the events underlying the evolution of vertebrate photoreceptors and retina, distilled from a large body of experimental data in the literature. The purpose of presenting an explicit proposal for events that occurred over 500 million years ago is to stimulate further experiment, in order to test and thereby refine or reject specific proposals. I look forward to updating the ideas that I have presented above, in the light of the results of future experiments.
Acknowledgements
I am extremely grateful to Helga Kolb for stimulating me to undertake this project, to Nansi Colley, Tom Cronin, John Dowling, Martin Heck, Dan Larhammar, Robert Marc, Ralph Nelson and Yoshinori Shichida for helpful comments on a draft of this article, and to Shaun Collin for the use of images. Supported by grants CE0561903 and DP110103294 from the Australian Research Council.
1. Dobzhansky, T., Nothing in biology makes sense except in light of evolution. American Biology Teacher, 1973. 35: p. 125-129. [PBS.org]
2. Darwin, C., On the origin of species by means of natural selection. 1859, London,: J. Murray. 502 p. [Google Books]
3. Lamb, T.D., S.P. Collin, and E.N. Pugh, Jr., Evolution of the vertebrate eye: opsins, photoreceptors, retina and eye cup. Nat Rev Neurosci, 2007. 8(12): p. 960-76. [PubMed]
4. Lamb, T.D., Evolution of vertebrate retinal photoreception. Philos Trans R Soc Lond B Biol Sci, 2009. 364: p. 2911-24.
5. Arendt, D., H. Hausen, and G. Purschke, The ‘division of labour’ model of eye evolution. Philos Trans R Soc Lond B Biol Sci, 2009. 364: p. 2809-17. Philosophical Transactions of the Royal Society of London B 364, 2809-2817. doi:10.1098/rstb.2009.0104
6. Vopalensky, P. and Z. Kozmik, Eye evolution: common use and independent recruitment of genetic components. Philos Trans R Soc Lond B Biol Sci, 2009. 364: p. 2819-32. [PubMed]
7. Larhammar, D., K. Nordstrom, and T.A. Larsson, Evolution of vertebrate rod and cone phototransduction genes. Philos Trans R Soc Lond B Biol Sci, 2009. 364: p. 2867-80. [PubMed]
8. Shichida, Y. and T. Matsuyama, Evolution of opsins and phototransduction. Philos Trans R Soc Lond B Biol Sci, 2009. 364: p. 2881-95. [PubMed]
9. Kusakabe, T.G., et al., Evolution and the origin of the visual retinoid cycle in vertebrates. Philos Trans R Soc Lond B Biol Sci, 2009. 364: p. 2897-910. [PubMed]
10. Collin, S.P., et al., The evolution of early vertebrate photoreceptors. Philos Trans R Soc Lond B Biol Sci, 2009. 364: p. 2925-40. [PubMed]
11. Fain, G.L., R. Hardie, and S.B. Laughlin, Phototransduction and the evolution of photoreceptors. Curr Biol, 2010. 20(3): p. 114-24. [PubMed]
12. Porter, M.L., et al., Shedding new light on opsin evolution. Proc R Soc Lond [Biol], 2012. 279: p. 3-14. [PubMed]
13. Nilsson, D.E., Eye evolution and its functional basis. Vis Neurosci, 2013. 30(1-2): p. 5-20. [PubMed]
14. Schwab, I.R., R.R. Dubielzig, and C. Schobert, Evolution’s witness : how eyes evolved. 2012, New York: Oxford University Press. ISBN 978-0-19-536974-8. 306 p.
15. Butler, A.B., Chordate evolution and the origin of craniates: an old brain in a new head. Anat Rec, 2000. 261(3): p. 111-25. [PubMed]
16. Butler, A.B. and W. Hodos, Comparative vertebrate neuroanatomy : evolution and adaptation. 2nd ed. 2005, Hoboken, N.J.: Wiley-Interscience. 715 p.
17. Salvini-Plawen, L. and E. Mayer, On the evolution of photoreceptors and eyes. Evol Biol, 1977. 10: p. 207-263.
18. Jacob, F., Evolution and tinkering. Science, 1977. 196: p. 1161-6. [Pubmed]
19. Goldsmith, T.H., Evolutionary tinkering with visual photoreception. Vis Neurosci, 2013. 30(1-2): p. 21-37. [PubMed]
20. Erwin, D.H., et al., The Cambrian conundrum: early divergence and later ecological success in the early history of animals. Science, 2011. 334: p. 1091-7. [PubMed]
21. Chen, J.-Y., Evolutionary scenario of the early history of the animal kingdom: Evidence from Precambrian (Ediacaran) Weng’an and Early Cambrian Maotianshan biotas, China., in Earth and Life, Springer, J.A. Talent, Editor. 2012, Springer. doi:10.1007/978-90-481-3428-1_10
22. Land, M.F. and D.-E. Nilsson, Animal eyes. Oxford animal biology series. 2002, New York: Oxford University Press. 221 p.
23. Paterson, J.R., et al., Acute vision in the giant Cambrian predator Anomalocaris and the origin of compound eyes. Nature, 2011. 480: p. 237-40. [PubMed]
24. Plotnick, R.E., S.Q. Dornbos, and J. Chen, Information landscapes and sensory ecology of the Cambrian Radiation. Paleobiology, 2010. 36: p. 303–317. [Paleobiology]
25. Terakita, A., The opsins. Genome Biol, 2005. 6(3): p. 213. [PubMed]
26. Plachetzki, D.C., B.M. Degnan, and T.H. Oakley, The origins of novel protein interactions during animal opsin evolution. PLoS One, 2007. 2(10): p. e1054. [PubMed]
27. Plachetzki, D.C., C.R. Fong, and T.H. Oakley, The evolution of phototransduction from an ancestral cyclic nucleotide gated pathway. Proc R Soc Lond [Biol], 2010. 277: p. 1963-9. [PubMed]
28. Kozmik, Z., et al., Assembly of the cnidarian camera-type eye from vertebrate-like components. Proc Natl Acad Sci U S A, 2008. 105(26): p. 8989-93. [PubMed]
29. Suga, H., V. Schmid, and W.J. Gehring, Evolution and functional diversity of jellyfish opsins. Curr Biol, 2008. 18(1): p. 51-5. [PubMed]
30. Feuda, R., et al., Metazoan opsin evolution reveals a simple route to animal vision. Proc Natl Acad Sci U S A, 2012. 109(46): p. 18868-72. [PubMed]
31. Mason, B., et al., Evidence for multiple phototransduction pathways in a reef-building coral. PLoS One, 2012. 7(12): p. e50371. [PubMed]
32. Krishnan, A., et al., The origin of GPCRs: identification of mammalian like Rhodopsin, Adhesion, Glutamate and Frizzled GPCRs in fungi. PLoS One, 2012. 7(1): p. e29817. [PubMed]
33. Eakin, R.M., Evolution of photoreceptors. Cold Spring Harb Symp Quant Biol, 1965. 30: p. 363-70.
34. Eakin, R.M., Morphology of invertebrate photoreceptors. Methods Enzymol, 1982. 81: p. 17-25. [PubMed]
35. Vanfleteren, J.R. and A. Coomans, Photoreceptor evolution and phylogeny. J Zool Syst Evol Res, 1976. 14: p. 157-168. [Wiley]
36. Vanfleteren, J.R., A monophyletic line of evolution? Ciliary induced photoreceptor membranes., in Visual cells in evolution, J.A. Westfall, Editor. 1982, Raven Press: New York. p. 107-136.
37. Ruiz, M.S. and R. Anadon, Some considerations on the fine structure of rhabdomeric photoreceptors in the amphioxus, Branchiostoma lanceolatum (Cephalochordata). J Hirnforsch, 1991. 32(2): p. 159-64. [PubMed]
38. Brandenburger, J.L., R.M. Woollacott, and R.M. Eakin, Fine structure of eyespots in tornarian larvae (phylum: Hemichordata). Z Zellforsch Mikrosk Anat, 1973. 142(1): p. 89-102. [PubMed]
39. Blumer, M., Alterations of the eyes during ontogenesis in Aporrhais pespelecani (Mollusca, Caenogastropoda). Zoomorphology, 1996. 116: p. 123-131. Zoomorphology 116, 123-131. doi:10.1007/bf02526944
40. Ready, D.F. and U. Tepass, Crumbs-dependent epithelial organization in retinal morphogenesis and disease, Volume 10, in Recent Advances in Human Biology D.S. Williams, Editor. 2004, World Scientific Publishing: River Edge, NJ. p. 7-27.
41. Lacalli, T.C., Frontal eye circuitry, rostral sensory pathways and brain organization in amphioxus larvae: Evidence from 3D reconstructions. Philos Trans R Soc London [Biol], 1996. 351: p. 243-263. [Royal Society]
42. Lacalli, T.C., Sensory systems in amphioxus: a window on the ancestral chordate condition. Brain Behav Evol, 2004. 64(3): p. 148-62. [PubMed]
43. Gomez, M.P., J.M. Angueyra, and E. Nasi, Light-transduction in melanopsin-expressing photoreceptors of Amphioxus. Proc Natl Acad Sci U S A, 2009. 106(22): p. 9081-6. [PubMed]
44. Koyanagi, M., et al., Cephalochordate melanopsin: evolutionary linkage between invertebrate visual cells and vertebrate photosensitive retinal ganglion cells. Curr Biol, 2005. 15(11): p. 1065-9. [PubMed]
45. Angueyra, J.M., et al., Melanopsin-expressing amphioxus photoreceptors transduce light via a phospholipase C signaling cascade. PLoS One, 2012. 7(1): p. e29813. PLoS One 7, e29813. doi:10.1371/journal.pone.0029813
46. Ferrer, C., et al., Dissecting the determinants of light sensitivity in amphioxus microvillar photoreceptors: possible evolutionary implications for melanopsin signaling. J Neurosci, 2012. 32(50): p. 17977-87. [PubMed]
47. Vopalensky, P., et al., Molecular analysis of the amphioxus frontal eye unravels the evolutionary origin of the retina and pigment cells of the vertebrate eye. Proc Natl Acad Sci U S A, 2012. 109(38): p. 15383-8. [PubMed]
48. Sorrentino, M., Evolution of cerebral vesicles and their sensory organs in an ascidian larva. Acta Zool-Stockholm, 2000. 81(3): p. 243.
49. Barnes, S.N., Fine structure of the photoreceptor and cerebral ganglion of the tadpole larva of Amaroucium constellatum (Verril) (subphylum: Urochordata; class: Ascidiacea). Z Zellforsch Mikrosk Anat, 1971. 117(1): p. 1-16. [PubMed]
50. Eakin, R.M. and A. Kuda, Ultrastructure of sensory receptors in Ascidian tadpoles. Z Zellforsch Mikrosk Anat, 1971. 112(3): p. 287-312. [PubMed]
51. Gorman, A.L., J.S. McReynolds, and S.N. Barnes, Photoreceptors in primitive chordates: fine structure, hyperpolarizing receptor potentials, and evolution. Science, 1971. 172: p. 1052-4. [PubMed]
52. Heimberg, A.M., et al., microRNAs reveal the interrelationships of hagfish, lampreys, and gnathostomes and the nature of the ancestral vertebrate. Proc Natl Acad Sci U S A, 2010. 107(45): p. 19379-83. [PubMed]
53. Zintzen, V., et al., Hagfish predatory behaviour and slime defence mechanism. Sci Rep, 2011. 1: p. 131. [PubMed]
54. Newth, D.R., On the reaction to light of Myxine glutinosa L. J Exp Biol, 1955. 32(1): p. 4.
55. Kobayashi, H., On the photo-perceptive function in the eye of the hagfish, Myxine garmani Jordan et Snyder. Journal of National Fisheries University (formely Journal of Shimonoseki Fisheries University), 1964. 13: p. 67-83. http://www.fish-u.ac.jp/kenkyu/sangakukou/kenkyuhoukoku/13/13-2-9.pdf
56. Locket, N.A. and J.M. Jorgensen, The biology of hagfishes, in The biology of hagfishes, J.M. Jørgensen, et al., Editors. 1998, Chapman & Hall: London ; New York. p. 541-546.
57. Holmberg, K., The hagfish retina: electron microscopic study comparing receptor and epithelial cells in the Pacific hagfish, Polistotrema stouti, with those in the Atlantic hagfish, Myxine glutinosa. Z Zellforsch Mikrosk Anat, 1971. 121(2): p. 249-69. [PubMed]
58. Holmberg, K., The cyclostome retina. Handbook of Sensory Physiology, 1977. 7(5): p. 47-66.
59. Fernholm, B. and K. Holmberg, The eyes in three genera of hagfish (Eptatretus, Paramyxine and Myxine)–a case of degenerative evolution. Vision Res, 1975. 15(2): p. 253-9. [PubMed]
60. Bullock, T.H., J.K. Moore, and R.D. Fields, Evolution of myelin sheaths: both lamprey and hagfish lack myelin. Neurosci Lett, 1984. 48(2): p. 145-8. [PubMed]
61. Schultz, R., E.C. Berkowitz, and D.C. Pease, The electron microscopy of the lamprey spinal cord. J Morphol, 1956. 98(2): p. 251-273.
62. Kusunoki, T. and F. Amemiya, Retinal projections in the hagfish, Eptatretus burgeri. Brain Res, 1983. 262(2): p. 295-8. [PubMed]
63. Wicht, H. and R.G. Northcutt, Retinofugal and retinopetal projections in the Pacific hagfish, Eptatretus stouti (Myxinoidea). Brain Behav Evol, 1990. 36(5): p. 315-28. [PubMed]
64. Hattar, S., et al., Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J Comp Neurol, 2006. 497(3): p. 326-49. [PubMed]
65. Allen, B.M., The eye of Bdellostoma stouti. Anat. Anz, 1905. 26: p. 208-211. In http://www.biodiversitylibrary.org/ia/anatomischeranze26anat
66. Pu, G.A. and J.E. Dowling, Anatomical and physiological characteristics of pineal photoreceptor cell in the larval lamprey, Petromyzon marinus. J Neurophysiol, 1981. 46(5): p. 1018-38. [PubMed]
67. Eakin, R.M., The third eye. 1973, Berkeley,: University of California Press. 157 p. [Google Books]
68. Kusmic, C., P. Marchiafava, and E. Strettoi, Photoresponses and light adaptation of pineal photoreceptors in the trout. Proc R Soc Lond [Biol], 1992. 248: p. 149-157. [Royal Society]
69. Morita, Y., M. Tabata, and S. Tamotsu, Intracellular response and input resistance change of pineal photoreceptors and ganglion cells. Neurosci Res Suppl, 1985. 2: p. S79-88. [PubMed]
70. Lamb, T.D. and E.N. Pugh, Jr., A quantitative account of the activation steps involved in phototransduction in amphibian photoreceptors. J Physiol, 1992. 449: p. 719-58. [PubMed]
71. Koyanagi, M., et al., Bistable UV pigment in the lamprey pineal. Proc Natl Acad Sci U S A, 2004. 101(17): p. 6687-91. [PubMed]
72. Uchida, K. and Y. Morita, Intracellular responses from UV-sensitive cells in the photosensory pineal organ. Brain Res, 1990. 534(1-2): p. 237-42. [PubMed]
73. Burkhardt, D.A., Synaptic feedback, depolarization, and color opponency in cone photoreceptors. Vis Neurosci, 1993. 10(6): p. 981-9. [PubMed]
74. Meissl, H., T. Nakamura, and G. Thiele, Neural response mechanisms in the photoreceptive pineal organ of goldfish. Comp Biochem Physiol A Comp Physiol, 1986. 84(3): p. 467-73. [PubMed]
75. Fritzsch, B. and S.P. Collin, Dendritic distribution of two populations of ganglion cells and the retinopetal fibers in the retina of the silver lamprey (Ichthyomyzon unicuspis). Vis Neurosci, 1990. 4(6): p. 533-45. [PubMed]
76. Young, G.C., Number and arrangement of extraocular muscles in primitive gnathostomes: evidence from extinct placoderm fishes. Biology Letters, 2007. 4: p. 110-114. [PubMed]
77. Young GC (2008). Early evolution of the vertebrate eye: fossil evidence. Evolution: Education and Outreach 1, 427-438. doi:10.1007/s12052-008-0087-y [Springer]
78. Fritzsch, B., Ontogenetic clues to the phylogeny of the visual system, in The changing visual system : maturation and aging in the central nervous system, P. Bagnoli and W. Hodos, Editors. 1991, Plenum Press: New York. p. 33-49. [Springer]
79. Fritzsch, B. and J.C. Glover, Evolution of the deuterostome central nervous system: an intercalation of developmental patterning processes with cellular specification processes, Volume 2, in Evolution of nervous systems : a comprehensive reference, J.H. Kaas, Editor. 2007, Elsevier Academic Press: Amsterdam ; Boston.
80. Nivison-Smith, L., et al., Retinal amino acid neurochemistry of the southern hemisphere lamprey, Geotria australis. PLoS One, 2013. 8(3): p. e58406. [PubMed]
81. Collin, S.P., et al., Ancient colour vision: multiple opsin genes in the ancestral vertebrates. Curr Biol, 2003. 13(22): p. R864-5. [PubMed]
82. Pisani, D., et al., Molecular evidence for dim-light vision in the last common ancestor of the vertebrates. Curr Biol, 2006. 16(9): p. R318-9; author reply R320. [PubMed]
83. Collin, S.P., et al., Morphology and spectral absorption characteristics of retinal photoreceptors in the southern hemisphere lamprey (Geotria australis). Vis Neurosci, 2003. 20(2): p. 119-30. [PubMed]
84. Dickson, D.H. and T.R. Collard, Retinal development in the lamprey (Petromyzon marinus L.): premetamorphic ammocoete eye. Am J Anat, 1979. 154(3): p. 321-36. [PubMed]
85. Collin, S.P. and A.E.O. Tresize, Evolution of colour discrimination in vertebrates and its implications for visual communication, in Communication in fishes, F. Ladich, Editor. 2006, Science Publishers: Enfield, NH. p. 303-335.
86. Muradov, H., et al., PDE6 in lamprey Petromyzon marinus: implications for the evolution of the visual effector in vertebrates. Biochemistry, 2007. 46(35): p. 9992-10000. [PubMed]
87. Muradov, H., et al., Unique transducins expressed in long and short photoreceptors of lamprey Petromyzon marinus. Vision Res, 2008. 48(21): p. 2302-8. [PubMed]
88. Smith, J.J., et al., Sequencing of the sea lamprey (Petromyzon marinus) genome provides insights into vertebrate evolution. Nat Genet, 2013. 45(4): p. 415-21, 421e1-2. [PubMed]
89. Govardovskii, V. and D. Lychakov, Visual cells and visual pigments of the lamprey, Lampetra fluviatilis. J Comp Physiol [A], 1984. 154(2): p. 279-286. [Springer]
90. Davies, W.L., et al., Functional characterization, tuning, and regulation of visual pigment gene expression in an anadromous lamprey. FASEB J, 2007. 21(11): p. 2713-24. [PubMed]
91. Ebrey, T. and Y. Koutalos, Vertebrate photoreceptors. Prog Retin Eye Res, 2001. 20(1): p. 49-94.
92. Imai, H., et al., Molecular properties of rod and cone visual pigments from purified chicken cone pigments to mouse rhodopsin in situ. Photochem Photobiol Sci, 2005. 4(9): p. 667-74. [PubMed]
93. Ritter, E., et al., Transition of rhodopsin into the active metarhodopsin II state opens a new light-induced pathway linked to Schiff base isomerization. J Biol Chem, 2004. 279(46): p. 48102-11. [PubMed]
94. Davies, W.L., M.W. Hankins, and R.G. Foster, Vertebrate ancient opsin and melanopsin: divergent irradiance detectors. Photochem Photobiol Sci, 2010. 9(11): p. 1444-57. [PubMed]
95. Koyanagi, M., et al., Homologs of vertebrate Opn3 potentially serve as a light sensor in nonphotoreceptive tissue. Proc Natl Acad Sci U S A, 2013. 110(13): p. 4998-5003. [PubMed]
96. Sakai, K., et al., Photochemical nature of parietopsin. Biochemistry, 2012. 51(9): p. 1933-41. [PubMed]
97. Terakita, A., et al., Counterion displacement in the molecular evolution of the rhodopsin family. Nat Struct Mol Biol, 2004. 11(3): p. 284-289. [PubMed]
98. Sato, K., et al., Vertebrate ancient-long opsin has molecular properties intermediate between those of vertebrate and invertebrate visual pigments. Biochemistry, 2011. 50(48): p. 10484-90. [PubMed]
99. Okano, T., T. Yoshizawa, and Y. Fukada, Pinopsin is a chicken pineal photoreceptive molecule. Nature, 1994. 372: p. 94-7. [PubMed]
100. Nakamura, A., et al., Chimeric nature of pinopsin between rod and cone visual pigments. Biochemistry, 1999. 38(45): p. 14738-45. [PubMed]
101. Tsutsui, K. and Y. Shichida, Multiple functions of Schiff base counterion in rhodopsins. Photochem Photobiol Sci, 2010. 9(11): p. 1426-34. [PubMed]
102. Tsukamoto, H. and A. Terakita, Diversity and functional properties of bistable pigments. Photochem Photobiol Sci, 2010. 9(11): p. 1435-43. [PubMed]
103. Terakita, A., E. Kawano‐Yamashita, and M. Koyanagi, Evolution and diversity of opsins. Wiley Interdisciplinary Reviews: Membrane Transport and Signaling, 2012. 1(1): p. 104-111.
104. Chen, M.H., et al., Rapid release of retinal from a cone visual pigment following photoactivation. Biochemistry, 2012. 51(20): p. 4117-25. [PubMed]
105. Tsukamoto, H., et al., The magnitude of the light-induced conformational change in different rhodopsins correlates with their ability to activate G proteins. J Biol Chem, 2009. 284(31): p. 20676-83. [PubMed]
106. Choe, H.W., et al., Crystal structure of metarhodopsin II. Nature, 2011. 471(7340): p. 651-5. [PubMed]
107. Scheerer, P., et al., Crystal structure of opsin in its G-protein-interacting conformation. Nature, 2008. 455: p. 497-502. [PubMed]
108. Hofmann, K.P., et al., A G protein-coupled receptor at work: the rhodopsin model. Trends Biochem Sci, 2009. 34(11): p. 540-52. [PubMed]
109. Yan, E.C., et al., Retinal counterion switch in the photoactivation of the G protein-coupled receptor rhodopsin. Proc Natl Acad Sci U S A, 2003. 100(16): p. 9262-7. [PubMed]
110. Piechnick, R., et al., Effect of channel mutations on the uptake and release of the retinal ligand in opsin. Proc Natl Acad Sci U S A, 2012. 109(14): p. 5247-52. [PubMed]
111. Ohno, S., Evolution by gene duplication. 1970, Berlin, New York,: Springer-Verlag. xv, 160 p.
112. Ecker, J.L., et al., Melanopsin-expressing retinal ganglion-cell photoreceptors: cellular diversity and role in pattern vision. Neuron, 2010. 67(1): p. 49-60. [PubMed]
113. Schmidt, T.M., S.K. Chen, and S. Hattar, Intrinsically photosensitive retinal ganglion cells: many subtypes, diverse functions. Trends Neurosci, 2011. 34(11): p. 572-80. [PubMed]
114. Bowmaker, J.K., Evolution of vertebrate visual pigments. Vision Res, 2008. 48(20): p. 2022-41. [PubMed]
115. Okano, T., et al., Primary structures of chicken cone visual pigments: vertebrate rhodopsins have evolved out of cone visual pigments. Proc Natl Acad Sci U S A, 1992. 89(13): p. 5932-6. [PubMed]
116. Yamashita, T., et al., Chloride-dependent spectral tuning mechanism of L-group cone visual pigments. Biochemistry, 2013. 52(7): p. 1192-7. [PubMed]
117. Wang, Z., A.B. Asenjo, and D.D. Oprian, Identification of the Cl(-)-binding site in the human red and green color vision pigments. Biochemistry, 1993. 32(9): p. 2125-30. [PubMed]
118. Davies, W.I., et al., Anion sensitivity and spectral tuning of middle- and long-wavelength-sensitive (MWS/LWS) visual pigments. Cell Mol Life Sci, 2012. 69(14): p. 2455-64. [PubMed]
119. Davies, W.L., et al., Visual pigments of the platypus: a novel route to mammalian colour vision. Curr Biol, 2007. 17(5): p. R161-3. [PubMed]
120. Davies, W.I., S.P. Collin, and D.M. Hunt, Molecular ecology and adaptation of visual photopigments in craniates. Mol Ecol, 2012. 21(13): p. 3121-58. [PubMed]
121. Nordstrom, K., T.A. Larsson, and D. Larhammar, Extensive duplications of phototransduction genes in early vertebrate evolution correlate with block (chromosome) duplications. Genomics, 2004. 83(5): p. 852-72. [PubMed]
122. Golobokova, E.Y. and V.I. Govardovskii, Late stages of visual pigment photolysis in situ: cones vs. rods. Vision Res, 2006. 46(14): p. 2287-97. [PubMed]
123. Kawamura, S. and S. Yokoyama, Functional characterization of visual and nonvisual pigments of American chameleon (Anolis carolinensis). Vision Res, 1998. 38(1): p. 37-44. [PubMed]
124. Bickelmann, C., et al., Functional characterization of the rod visual pigment of the echidna (Tachyglossus aculeatus), a basal mammal. Vis Neurosci, 2012. 29(4-5): p. 211-7. [PubMed]
125. Hunt, D.M., et al., The molecular basis for spectral tuning of rod visual pigments in deep-sea fish. J Exp Biol, 2001. 204(Pt 19): p. 3333-44. [PubMed]
126. Yau, K.W. and R.C. Hardie, Phototransduction motifs and variations. Cell, 2009. 139(2): p. 246-64. [PubMed]
127. Hardie, R.C. and K. Franze, Photomechanical responses in Drosophila photoreceptors. Science, 2012. 338: p. 260-3. [PubMed]
128. Okawa, H., et al., ATP consumption by mammalian rod photoreceptors in darkness and in light. Curr Biol, 2008. 18(24): p. 1917-21. [PubMed]
129. Steinberg, R.H., S.K. Fisher, and D.H. Anderson, Disc morphogenesis in vertebrate photoreceptors. J Comp Neurol, 1980. 190(3): p. 501-8. [PubMed]
130. Young, R.W., Visual cells and the concept of renewal. Invest Ophthalmol Vis Sci, 1976. 15(9): p. 700-25. [IOVS]
131. Hecht, S., S. Shlaer, and M.H. Pirenne, Energy, Quanta, and Vision. J Gen Physiol, 1942. 25(6): p. 819-40. [PubMed]
132. Baylor, D.A., T.D. Lamb, and K.W. Yau, Responses of retinal rods to single photons. J Physiol, 1979. 288: p. 613-34. [PubMed]
133. Lamb, T.D., Phototransduction: Adaptation in cones., Volume 3, in Encyclopedia of the eye, D.A. Dartt, J.C. Besharse, and R. Dana, Editors. 2010, Academic Press: Oxford. p. 354-360.
134. Kawamura, S. and S. Tachibanaki, Rod and cone photoreceptors: molecular basis of the difference in their physiology. Comp Biochem Physiol A Mol Integr Physiol, 2008. 150(4): p. 369-77. [PubMed]
135. Tachibanaki, S., et al., Low activation and fast inactivation of transducin in carp cones. J Biol Chem, 2012. 287(49): p. 41186-94. [PubMed]
136. Kenkre, J.S., et al., Extremely rapid recovery of human cone circulating current at the extinction of bleaching exposures. J Physiol, 2005. 567(Pt 1): p. 95-112. [PubMed]
137. Arendt, D. and J. Wittbrodt, Reconstructing the eyes of Urbilateria. Philos Trans R Soc London [Biol], 2001. 356: p. 1545-1563. [PubMed]
138. Koyanagi, M., et al., Jellyfish vision starts with cAMP signaling mediated by opsin-Gs cascade. Proc Natl Acad Sci U S A, 2008. 105(40): p. 15576-15580. [PubMed]
139. Lamb, T.D. and E.N. Pugh, Jr., Phototransduction, dark adaptation, and rhodopsin regeneration the proctor lecture. Invest Ophthalmol Vis Sci, 2006. 47(12): p. 5137-52. [IOVS]
140. Wensel, T.G., Signal transducing membrane complexes of photoreceptor outer segments. Vision research, 2008. 48(20): p. 2052-2061. [PubMed]
141. Gurevich, V.V., et al., The functional cycle of visual arrestins in photoreceptor cells. Prog Retin Eye Res, 2011. 30(6): p. 405-430. [PubMed]
142. Schulz, H.L., et al., The Retinome–Defining a reference transcriptome of the adult mammalian retina/retinal pigment epithelium. BMC genomics, 2004. 5(1): p. 50. [PubMed]
143. Vogalis, F., et al., Ectopic expression of cone‐specific G‐protein‐coupled receptor kinase GRK7 in zebrafish rods leads to lower photosensitivity and altered responses. J Physiol, 2011. 589(9): p. 2321-2348. [PubMed]
144. Korenbrot, J.I., Speed, sensitivity, and stability of the light response in rod and cone photoreceptors: Facts and models. Prog Retin Eye Res, 2012. 31(5): p. 442-466. [PubMed]
145. Muradov, H., K.K. Boyd, and N.O. Artemyev, Rod phosphodiesterase-6 PDE6A and PDE6B subunits are enzymatically equivalent. J Biol Chem, 2010. 285(51): p. 39828-34. [PubMed]
146. Hisatomi, O. and F. Tokunaga, Molecular evolution of proteins involved in vertebrate phototransduction. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 2002. 133(4): p. 509-522. [PubMed]
147. Zhang, Z. and N.O. Artemyev, Determinants for Phosphodiesterase 6 Inhibition by Its γ-Subunit. Biochemistry, 2010. 49(18): p. 3862-3867. [PubMed]
148. Peng, C., E.D. Rich, and M.D. Varnum, Subunit configuration of heteromeric cone cyclic nucleotide-gated channels. Neuron, 2004. 42(3): p. 401-410. [PubMed]
149. Mushegian, A., V.V. Gurevich, and E.V. Gurevich, The origin and evolution of G protein-coupled receptor kinases. PloS one, 2012. 7(3): p. e33806. [PubMed]
150. Gurevich, E.V. and V.V. Gurevich, Arrestins: ubiquitous regulators of cellular signaling pathways. Genome biology, 2006. 7(9): p. 236. [PubMed]
151. Alvarez, C.E., On the origins of arrestin and rhodopsin. BMC evolutionary biology, 2008. 8(1): p. 222. [PubMed]
152. Kawano-Yamashita, E., et al., Beta-Arrestin Functionally Regulates the Non-Bleaching Pigment Parapinopsin in Lamprey Pineal. PloS one, 2011. 6(1): p. e16402. [PubMed]
153. Lagman, D., et al., Expansion of transducin subunit gene families in early vertebrate tetraploidizations. Genomics, 2012. [PubMed]
154. Tyler, C.W. and R.D. Hamer, Analysis of visual modulation sensitivity. IV. Validity of the Ferry-Porter law. JOSA A, 1990. 7(4): p. 743-758. [PubMed]
155. Normann, R.A. and F.S. Werblin, Control of retinal sensitivity I. Light and dark adaptation of vertebrate rods and cones. J Gen Physiol, 1974. 63(1): p. 37-61. [PubMed]
156. Burkhardt, D.A., Light adaptation and photopigment bleaching in cone photoreceptors in situ in the retina of the turtle. J Neurosci, 1994. 14(3 Pt 1): p. 1091-105. [PubMed]
157. Rebrik, T.I., et al., CNG-modulin: a novel Ca-dependent modulator of ligand sensitivity in cone photoreceptor cGMP-gated ion channels. J Neurosci, 2012. 32(9): p. 3142-53. [PubMed]
158. Mahroo, O. and T. Lamb, Recovery of the human photopic electroretinogram after bleaching exposures: estimation of pigment regeneration kinetics. J Physiol, 2004. 554(2): p. 417-437. [PubMed]
159. Thomas, M. and T. Lamb, Light adaptation and dark adaptation of human rod photoreceptors measured from the a-wave of the electroretinogram. J Physiol, 1999. 518(2): p. 479-496. [PubMed]
160. Lamb, T. and E. Pugh, Dark adaptation and the retinoid cycle of vision. Prog Retin Eye Res, 2004. 23(3): p. 307-380. [PubMed]
161. van Hateren, H., A cellular and molecular model of response kinetics and adaptation in primate cones and horizontal cells. J Vis, 2005. 5(4). [PubMed]
162. Van Hateren, J. and T. Lamb, The photocurrent response of human cones is fast and monophasic. BMC neuroscience, 2006. 7(1): p. 34. [PubMed]
163. Lagnado, L., L. Cervetto, and P. McNaughton, Calcium homeostasis in the outer segments of retinal rods from the tiger salamander. J Physiol, 1992. 455(1): p. 111-142. [PubMed]
164. Stiles, W., The physical interpretation of the spectral sensitivity curve of the eye, in Transactions of the Optical Convention of the Worshipful Company of Spectacle Makers. 1948, Spectacle Makers’ Company: London. p. 97-107.
165. Lewis, P., A theoretical interpretation of spectral sensitivity curves at long wavelengths. J Physiol, 1955. 130(1): p. 45. [PubMed]
166. Barlow, H., Purkinje shift and retinal noise. Nature, 1957. 179: p. 255-256. [PubMed]
167. Ala-Laurila, P., et al., On the relation between the photoactivation energy and the absorbance spectrum of visual pigments. Vision Res, 2004. 44(18): p. 2153-2158. [PubMed]
168. Luo, D.-G., et al., Activation of visual pigments by light and heat. Science, 2011. 332: p. 1307-3012. [PubMed]
169. Ala-Laurila, P., K. Donner, and A. Koskelainen, Thermal activation and photoactivation of visual pigments. Biophys J, 2004. 86(6): p. 3653-3662. [PubMed]
170. Gozem, S., et al., The Molecular Mechanism of Thermal Noise in Rod Photoreceptors. Science, 2012. 337: p. 1225-1228. [PubMed]
171. Ma, J., et al., A visual pigment expressed in both rod and cone photoreceptors. Neuron, 2001. 32(3): p. 451-61. [PubMed]
172. Matthews, G., Photocurrents of single retinal rods from Rana pipiens tadpoles. Vision Res, 1984. 24(8): p. 821-5. [PubMed]
173. Lamb, T.D. and E.J. Simon, Analysis of electrical noise in turtle cones. J Physiol, 1977. 272(2): p. 435-68. [PubMed]
174. Lamb, T.D. and E.J. Simon, The relation between intercellular coupling and electrical noise in turtle photoreceptors. J Physiol, 1976. 263(2): p. 257-86. [PubMed]
175. Schneeweis, D.M. and J.L. Schnapf, The photovoltage of macaque cone photoreceptors: adaptation, noise, and kinetics. J Neurosci, 1999. 19(4): p. 1203-16. [PubMed]
176. Baylor, D.A., G. Matthews, and K.W. Yau, Two components of electrical dark noise in toad retinal rod outer segments. J Physiol, 1980. 309: p. 591-621. [Library of Medicine]
177. Whitlock, G.G. and T.D. Lamb, Variability in the time course of single photon responses from toad rods: termination of rhodopsin’s activity. Neuron, 1999. 23(2): p. 337-51. [PubMed]
178. Hamer, R.D., et al., Toward a unified model of vertebrate rod phototransduction. Vis Neurosci, 2005. 22(4): p. 417-36. [PubMed]
179. Hamer, R.D., et al., Multiple steps of phosphorylation of activated rhodopsin can account for the reproducibility of vertebrate rod single-photon responses. J Gen Physiol, 2003. 122(4): p. 419-44. [PubMed]
180. Gross, O.P., E.N. Pugh, Jr., and M.E. Burns, Calcium feedback to cGMP synthesis strongly attenuates single-photon responses driven by long rhodopsin lifetimes. Neuron, 2012. 76(2): p. 370-82. [PubMed]
181. Cangiano, L., et al., The photovoltage of rods and cones in the dark-adapted mouse retina. J Physiol, 2012. 590(Pt 16): p. 3841-55. [PubMed]
182. Lamb, T.D., P.A. McNaughton, and K.W. Yau, Spatial spread of activation and background desensitization in toad rod outer segments. J Physiol, 1981. 319: p. 463-96. [PubMed]
183. Walls, G.L., The reptilian retina. Am. J. Ophthalmol, 1934. 17: p. 892-915.
184. Walls, G.L., The vertebrate eye and its adaptive radiation. Cranbrook Institute of Science. 1942, Bloomfield Hills, Michigan: Cranbrook Institute of Science. 785 p.
185. Kleinschmidt, J. and J.E. Dowling, Intracellular recordings from gecko photoreceptors during light and dark adaptation. J Gen Physiol, 1975. 66(5): p. 617-48. [PubMed]
186. Rispoli, G., W.A. Sather, and P.B. Detwiler, Visual transduction in dialysed detached rod outer segments from lizard retina. J Physiol, 1993. 465: p. 513-37. [PubMed]
187. Roll, B., Gecko vision-visual cells, evolution, and ecological constraints. J Neurocytol, 2000. 29(7): p. 471-84. [PubMed]
188. Yoshida, M., Some observations on the patency in the outer segments of photoreceptors of the nocturnal gecko. Vision Res, 1978. 18(2): p. 137-43. [PubMed]
189. Kojima, D., et al., Cone visual pigments are present in gecko rod cells. Proc Natl Acad Sci U S A, 1992. 89(15): p. 6841-5. [PubMed]
190. Zhang, X., T.G. Wensel, and C. Yuan, Tokay gecko photoreceptors achieve rod-like physiology with cone-like proteins. Photochem Photobiol, 2006. 82(6): p. 1452-60. [PubMed]
191. Ripps, H. and J.E. Dowling, Structural features and adaptive properties of photoreceptors in the skate retina. J Exp Zool Suppl, 1990. 5: p. 46-54. [PubMed]
192. Dowling, J.E. and H. Ripps, On the duplex nature of the skate retina. J Exp Zool Suppl, 1990. 5: p. 55-65. [PubMed]
193. Dowling, J.E. and H. Ripps, Adaptation in skate photoreceptors. J Gen Physiol, 1972. 60(6): p. 698-719. [PubMed]
194. Cornwall, M.C., et al., Membrane current responses of skate photoreceptors. J Gen Physiol, 1989. 94(4): p. 633-47. [PubMed]
195. Wang, J.-S. and V.J. Kefalov, The cone-specific visual cycle. Prog Retin Eye Res, 2011. 30(2): p. 115-128. [PubMed]
196. Saari, J.C., Vitamin A metabolism in rod and cone visual cycles. Annu Rev Nutr, 2012. 32: p. 125-45. [PubMed]
197. Kaylor, J.J., et al., Identification of DES1 as a vitamin A isomerase in Muller glial cells of the retina. Nat Chem Biol, 2013. 9(1): p. 30-6.
198. Albalat, R., Evolution of the genetic machinery of the visual cycle: a novelty of the vertebrate eye? Mol Biol Evol, 2012. 29(5): p. 1461-9. [PubMed]
199. Poliakov, E., et al., Origin and evolution of retinoid isomerization machinery in vertebrate visual cycle: hint from jawless vertebrates. PLoS One, 2012. 7(11): p. e49975. [PubMed]
200. Takimoto, N., et al., Origin of the vertebrate visual cycle: III. Distinct distribution of RPE65 and beta-carotene 15,15′-monooxygenase homologues in Ciona intestinalis. Photochem Photobiol, 2006. 82(6): p. 1468-74. [PubMed]
201. Stavenga, D.G., Dark regeneration of invertebrate visual pigments, in Photoreceptor Optics, A.W. Synder and R. Menzel, Editors. 1975, Springer. p. 290-295.
202. Wang, X., et al., Requirement for an enzymatic visual cycle in Drosophila. Curr Biol, 2010. 20(2): p. 93-102. [PubMed]
203. Baer, K.v., Entwicklungsgeschichte der thiere: Beobachtung und reflexion. Bornträger, Königsberg, 1828. 1837. {Internet Archive]
204. Brauckmann, S., Karl Ernst von Baer (1792-1876) and evolution. Int J Dev Biol, 2012. 56(9): p. 653-60. [PubMed]
205. Haeckel, E., Natürliche schöpfungsgeschichte. Gemeinverständliche wissenschaftliche vorträge über die entwickelungslehre im allgemeinen und diejenige von Darwin, Goethe und Lamarck im besonderen. 5. verb. aufl. Mit dem porträt des verfassers (nach einer photographie) und mit 16 tafeln, 19 holzschnitten, 18 stammbäumen und 19 systematischen tabellen. ed. 1874, Berlin,: G. Reimer. xlvi, 2 , 688 p. [Google Books]
206. Eakin, R.M., A Third Eye: A century-old zoological enigma yields its secrets to electron-microscopist and neurophysiologist. American Scientist, 1970. 58(1): p. 73-79.
207. Vigh, B., et al., The pineal organ as a folded retina: immunocytochemical localization of opsins. Biol Cell, 1998. 90(9): p. 653-9. [PubMed]
208. Reichenbach, A. and S.R. Robinson, Phylogenetic constraints on retinal organisation and development. Prog Retin Eye Res, 1995. 15: p. 139-171.
209. Vigh, B., et al., Nonvisual photoreceptors of the deep brain, pineal organs and retina. Histol Histopathol, 2002. 17(2): p. 555-90. [PubMed]
210. Ekstrom, P. and H. Meissl, Evolution of photosensory pineal organs in new light: the fate of neuroendocrine photoreceptors. Philos Trans R Soc Lond B Biol Sci, 2003. 358(1438): p. 1679-700.
211. Klein, D.C., Evolution of the vertebrate pineal gland: the AANAT hypothesis. Chronobiol Int, 2006. 23(1-2): p. 5-20. [Pubmed]
212. Mano, H. and Y. Fukada, A median third eye: pineal gland retraces evolution of vertebrate photoreceptive organs. Photochem Photobiol, 2007. 83(1): p. 11-8. [PubMed]
213. Eakin, R.M. and J.A. Westfall, Further observations on the fine structure of the parietal eye of lizards. J Biophys Biochem Cytol, 1960. 8: p. 483-99. [PubMed]
214. Martinez-Morales, J.R. and J. Wittbrodt, Shaping the vertebrate eye. Curr Opin Genet Dev, 2009. 19(5): p. 511-7. [PubMed]
215. Valleix, S., et al., Homozygous nonsense mutation in the FOXE3 gene as a cause of congenital primary aphakia in humans. Am J Hum Genet, 2006. 79(2): p. 358-64. [PubMed]
216. Eiraku, M., et al., Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature, 2011. 472(7341): p. 51-6. [PubMed]
217. Bharti, K., et al., A regulatory loop involving PAX6, MITF, and WNT signaling controls retinal pigment epithelium development. PLoS Genet, 2012. 8(7): p. e1002757. [PubMed]
218. England, S.J., et al., A dynamic fate map of the forebrain shows how vertebrate eyes form and explains two causes of cyclopia. Development, 2006. 133(23): p. 4613-7. [PubMed]
219. Rembold, M., et al., Individual cell migration serves as the driving force for optic vesicle evagination. Science, 2006. 313: p. 1130-4. [PubMed]
220. Kwan, K.M., et al., A complex choreography of cell movements shapes the vertebrate eye. Development, 2012. 139(2): p. 359-72. [PubMed]
221. Rubinson, K. and H. Cain, Neural differentiation in the retina of the larval sea lamprey (Petromyzon marinus). Vis Neurosci, 1989. 3(3): p. 241-8. [PubMed]
222. Rubinson, K., The developing visual system and metamorphosis in the lamprey. J Neurobiol, 1990. 21(7): p. 1123-35. [PubMed]
223. Meyer-Rochow, V.B. and D. Stewart, Review of larval and postlarval eye ultrastructure in the lamprey (Cyclostomata) with special emphasis on Geotria australis (Gray). Microsc Res Tech, 1996. 35(6): p. 431-44. [PubMed]
224. Dickson, D.H., D.A. Graves, and M.R. Moyles, Corneal splitting in the developing lamprey Petromyzon marinus L. eye. Am J Anat, 1982. 165(1): p. 83-98. [PubMed]
225. Price, G.C., Some points in the development of a myxinoid (Bdellostoma stouti Lockington). Verhandlungen der Anatomischen Gesellschaft, Anatomischer Anzeiger, 1896. 6: p. 81-86. [Google Books]
226. Dean, B., On the development of the Californian hag-fish, Bdellostoma stouti, Lockington. Q J Microsc Sci, 1897. 40: p. 269-280. http://jcs.biologists.org/content/s2-40/158/269
227. Dean, B., On the embryology of Bdellostoma stouti: A general account of myxinoid development from the egg and segmentation to hatching, in Festschrift zum siebensigsten Geburtstag von Carl von Kupffer. 1899, Gustav Fisher: Jena. [Google Books]
228. von Kupffer, C., Zur Kopfentwicklung von Bdellostoma. Studien zur vergleichenden Entwicklungsgeschichte des Kopfes der Kranioten, Heft 4. 1900, München: Lehman. www.worldcat.org/title/zur-kopfentwicklung-von-bdellostoma/oclc/250304157
229. Stockard, C.R., The embryonic history of the lens in Bdellostoma stouti in relation to recent experiments. Am J Anat, 1906. 6(1): p. 511-515. [Wiley]
230. Ota, K.G., S. Kuraku, and S. Kuratani, Hagfish embryology with reference to the evolution of the neural crest. Nature, 2007. 446(7136): p. 672-5. [PubMed]
231. Arendt, D., Evolution of eyes and photoreceptor cell types. Int J Dev Biol, 2003. 47(7-8): p. 563-71. [PubMed]
232. Sernagor, E., et al., Retinal development. 2006, Cambridge: Cambridge University Press. 383 p.
233. Agathocleous, M. and W.A. Harris, From progenitors to differentiated cells in the vertebrate retina. Annu Rev Cell Dev Biol, 2009. 25: p. 45-69. [PubMed]
234. Swaroop, A., D. Kim, and D. Forrest, Transcriptional regulation of photoreceptor development and homeostasis in the mammalian retina. Nat Rev Neurosci, 2010. 11(8): p. 563-76. [PubMed]
235. Reese, B.E., Development of the retina and optic pathway. Vision Res, 2011. 51(7): p. 613-32. [PubMed]
236. Rapaport, D.H., Retinal neurogenesis, in Retinal development, E. Sernagor, et al., Editors. 2006, Cambridge University Press: Cambridge. p. 30-58.
237. Rapaport, D.H., et al., Timing and topography of cell genesis in the rat retina. J Comp Neurol, 2004. 474(2): p. 304-24. [PubMed]
238. Wong, L.L. and D.H. Rapaport, Defining retinal progenitor cell competence in Xenopus laevis by clonal analysis. Development, 2009. 136(10): p. 1707-15. [PubMed]
239. Gomes, F.L., et al., Reconstruction of rat retinal progenitor cell lineages in vitro reveals a surprising degree of stochasticity in cell fate decisions. Development, 2011. 138(2): p. 227-35. [PubMed]
240. He, J., et al., How variable clones build an invariant retina. Neuron, 2012. 75(5): p. 786-98. [PubMed]
241. Godinho, L., et al., Nonapical symmetric divisions underlie horizontal cell layer formation in the developing retina in vivo. Neuron, 2007. 56(4): p. 597-603. [PubMed]
242. Dowling, J.E., The retina : an approachable part of the brain. Rev. ed. 2012, Cambridge, Massachusetts: Belknap Press of Harvard University Press. xvi, 355 p.
243. Manns, M. and B. Fritzsch, The eye in the brain: retinoic acid effects morphogenesis of the eye and pathway selection of axons but not the differentiation of the retina in Xenopus laevis. Neurosci Lett, 1991. 127(2): p. 150-4. [PubMed]
244. Johnson, P.T., M.A. Raven, and B.E. Reese, Disruption of transient photoreceptor targeting within the inner plexiform layer following early ablation of cholinergic amacrine cells in the ferret. Vis Neurosci, 2001. 18(5): p. 741-51. [Pubmed]
245. Johnson, P.T., et al., Rods and cones project to the inner plexiform layer during development. J Comp Neurol, 1999. 414(1): p. 1-12. [Pubmed]
246. Dodt, E., The parietal eye (pineal and parietal organs) of lower vertebrates, in Visual Centers in the Brain (Handbook of Sensory Physiology), R. Jung, Editor. 1973, Springer. p. 113-140.
247. Ekstrom, P. and H. Meissl, Intracellular staining of physiologically identified photoreceptor cells and hyperpolarizing interneurons in the teleost pineal organ. Neuroscience, 1988. 25(3): p. 1061-70. [PubMed]
248. Uchida, K., T. Nakamura, and Y. Morita, Signal transmission from pineal photoreceptors to luminosity-type ganglion cells in the lamprey, Lampetra japonica. Neuroscience, 1992. 47(1): p. 241-7. [PubMed]
249. Meves, A., [Electron microscopical investigation on the cytoarchitecture of the brain of Branchiostoma lanceolatum]. Z Zellforsch Mikrosk Anat, 1973. 139(4): p. 511-32. [PubMed]
250. Ruiz, S. and R. Anadon, The fine structure of lamellate cells in the brain of amphioxus (Branchiostoma lanceolatum, Cephalochordata). Cell Tissue Res, 1991. 263(3): p. 597-600. [PubMed]
251. Hendrickson, A., Landolt’s club in the amphibian retina: a Golgi and electron microscope study. Invest Ophthalmol, 1966. 5(5): p. 484-96. [IOVS]
252. Locket, N.A., Landolt’s club in the retina of the African lungfish, Protopterus aethiopicus, Heckel. Vision Res, 1970. 10(4): p. 299-306. [PubMed]
253. Vigh, B., et al., Cerebrospinal fluid-contacting neurons, sensory pinealocytes and Landolt’s clubs of the retina as revealed by means of an electron-microscopic immunoreaction against opsin. Cell Tissue Res, 1983. 233(3): p. 539-48. [PubMed]
254. Quesada, A. and J.M. Genis-Galvez, Morphological and structural study of Landolt’s club in the chick retina. J Morphol, 1985. 184(2): p. 205-14. [PubMed]
255. Vigh, B. and I. Vigh-Teichmann, Cytochemistry of CSF-contacting neurons and pinealocytes. Prog Brain Res, 1992. 91: p. 299-306. [PubMed]
256. David, C., et al., Cerebrospinal fluid contacting neurons in the reduced brain ventricular system of the atlantic hagfish, Myxine glutinosa. Acta Biol Hung, 2003. 54(1): p. 35-44. [PubMed]
257. García-Fernández, J.M. and R.G. Foster, Immunocytochemical identification of photoreceptor proteins in hypothalamic cerebrospinal fluid-contacting neurons of the larval lamprey (Petromyzon marinus). Cell Tissue Res, 1994. 275(2): p. 319-326.
258. Kolb, H. and E.V. Famiglietti, Rod and cone pathways in the inner plexiform layer of cat retina. Science, 1974. 186: p. 47-9. [PubMed]
259. Nelson, R., AII amacrine cells quicken time course of rod signals in the cat retina. J Neurophysiol, 1982. 47(5): p. 928-47. [PubMed]
260. Kolb, H. and R. Nelson, Neural architecture of the cat retina. Prog Retin Eye Res, 1984. 3: p. 21-60.
261. Strettoi, E., R.F. Dacheux, and E. Raviola, Synaptic connections of rod bipolar cells in the inner plexiform layer of the rabbit retina. J Comp Neurol, 1990. 295(3): p. 449-66. [PubMed]
262. Vaney, D.I., H.M. Young, and I.C. Gynther, The rod circuit in the rabbit retina. Vis Neurosci, 1991. 7(1-2): p. 141-54. [PubMed]
263. Strettoi, E., E. Raviola, and R.F. Dacheux, Synaptic connections of the narrow-field, bistratified rod amacrine cell (AII) in the rabbit retina. J Comp Neurol, 1992. 325(2): p. 152-68. [PubMed]
264. Ashmore, J.F. and G. Falk, Responses of rod bipolar cells in the dark-adapted retina of the dogfish, Scyliorhinus canicula. J Physiol, 1980. 300: p. 115-50. [PubMed]
265. Nilsson, D.E. and S. Pelger, A pessimistic estimate of the time required for an eye to evolve. Proc Roy Soc B, 1994. 256: p. 53-8. [PubMed]
266. D’Aniello, S., et al., The ascidian homolog of the vertebrate homeobox gene Rx is essential for ocellus development and function. Differentiation, 2006. 74(5): p. 222-34. [PubMed]
267. Zeiss, C.J., et al., Comparative retinal morphology of the platypus. J Morphol, 2011. 272(8): p. 949-57. [PubMed]
268. Burns, M.E. and T.D. Lamb, Visual transduction by rod and cone photoreceptors, in The visual neurosciences, J.S. Werner and L.M. Chalupa, Editors. 2004, MIT Press: Cambridge, Mass. p. 215-233.
269. Raviola, E. and N.B. Gilula, Intramembrane organization of specialized contacts in the outer plexiform layer of the retina. A freeze-fracture study in monkeys and rabbits. J Cell Biol, 1975. 65(1): p. 192-222. [PubMed]
270. Rieke, F. and D.A. Baylor, Origin and functional impact of dark noise in retinal cones. Neuron, 2000. 26(1): p. 181-6. [PubMed]
271. Brzezinski, J. and T. Reh, Retinal Histogenesis, in Encyclopedia of the Eye. Elsevier Ltd. 2010. p. 73-80.
272. Gomes, F.L. and M. Cayouette, Retinal development: Cell type specification, in Encyclopedia of neuroscience, L.R. Squire, Editor. 2009, Elsevier/Academic Press: Amsterdam ; Boston. p. 203-209.
273. Chen, Z., X. Li, and C. Desplan, Deterministic or stochastic choices in retinal neuron specification. Neuron, 2012. 75(5): p. 739-42. [PubMed]
274. Reese, B.E., Developmental plasticity of photoreceptors. Prog Brain Res, 2004. 144: p. 1-19. [PubMed]
275. Lamb, T.D., E.N. Pugh Jr, and S.P. Collin, The origin of the vertebrate eye. Evolution: Education and Outreach, 2008. 1(4): p. 415-426.
276. Wässle, H., Parallel processing in the mammalian retina. Nat Rev Neurosci, 2004. 5(10): p. 747-57. [PubMed]
The author
Trevor D. Lamb received a degree in Electronic Engineering from Melbourne University in 1971 and then moved to Cambridge, UK, to do his graduate studies under the guidance of Sir Alan Hodgkin, working with Denis Baylor on retinal horizontal cells and photoreceptors. He received his PhD in 1975. Then as a post-doc at Stanford, USA, Denis Baylor, King-Wai Yau and Trevor developed the suction pipette technique for recording from photoreceptors. For the following 25 years he again worked in Cambridge, UK, on phototransduction and light- and dark-adaptation. He was elected a Fellow of the Royal Society in 1993. With Ed Pugh in the USA he developed a mathematical description of the molecular reactions underlying the photoreceptor light response, as well as a model of dark adaptation and the retinoid cycle. In 2003, Trevor returned to Australia, as a Federation Fellow at the Australian National University (ANU), in Canberra. Since 2011 he has been an Emeritus Professor at ANU, where he pursues his current interest in the evolutionary origin of photoreceptors and the retina. e-mail Trevor at Trevor.Lamb@anu.edu.au