The Role of Dopamine in Retinal Function
Abstract
Dopamine (DA) is the major catecholamine in all vertebrate retinas including man. All vertebrates have dopaminergic neurons identified as amacrine cells (ACs) and interplexiform cells (IPCs), with great variations among different species. DA neurons are comparatively rare with density about 10-100 per mm2, which means that they are less than 1% of all amacrine cells. In retinal circuitry DA serves principally as a neuromodulator, which reaches distant target cells by diffusion, and thus exerts a “volume transmission” mode of communication. Dopamine release appears to be circadian, with high release levels during the day in many species, and is a counterpart to the circadian rhythm in melatonin, which is released at night. Dopamine regulates voltage-gated ion channels and alters chemical and electrical synaptic transmission through five D1-like and D2-like G-protein-coupled receptors. DA is particularly associated with retinal circuitry reconfigurations with nighttime and daytime vision. DA actions are highly cell type, species and context dependent. DA acts through multiple intracellular pathways, in particular G-protein activated Adenylate Cyclase (AC) and Phospholipase C (PLC) pathways.
Introduction
In 1970 Julius Axelrod received the Nobel prize for Neuropharmacological studies of brain neurotransmitter pathways, among them biogenic amines such as dopamine (DA). His work was key in developing a treatment for Parkinsonism, a DA deficiency disorder (1). The discovery of enzymatic pathways of DA synthesis, an early histochemical DA marker involving formaldehyde-induced fluorescence for biogenic amines (2), and association with a prominent neurological disease gave incentive to study actions of DA circuitry in retina. DA is the major catecholamine in all vertebrate retinas including man. All vertebrates have dopaminergic neurons identified as amacrine cells (ACs) and interplexiform cells (IPCs) with great variations in neural types represented among different species. DA neurons are comparatively rare with density about 10-100 per mm2, which means that they are less than 1% of cells in the amacrine cell layer of the retinal inner nuclear layer. In retinal circuitry dopamine serves principally as a neuromodulator, which reaches distant target cells by diffusion and thus exerts a “volume” transmission mode of communication. DA regulates voltage-gated ion channels and alters chemical and electrical synaptic transmission through five D1-like and D2-like G-protein-coupled receptors. It is particularly associated with retinal circuitry reconfigurations undergone with nighttime and daytime vision. DA acts through multiple intracellular pathways, in particular, G-protein activated Adenylate Cyclase (AC) and Phospholipase C (PLC) pathways. Dopamine receptors also directly modulate voltage-gated membrane channels through protein-protein interactions as well as G-proteins. DA actions are highly cell type, species and context dependent. This article reviews five decades of DA research of actions on and within neurons of the retina.
Morphology and circuitry of Dopaminergic neurons in the retina
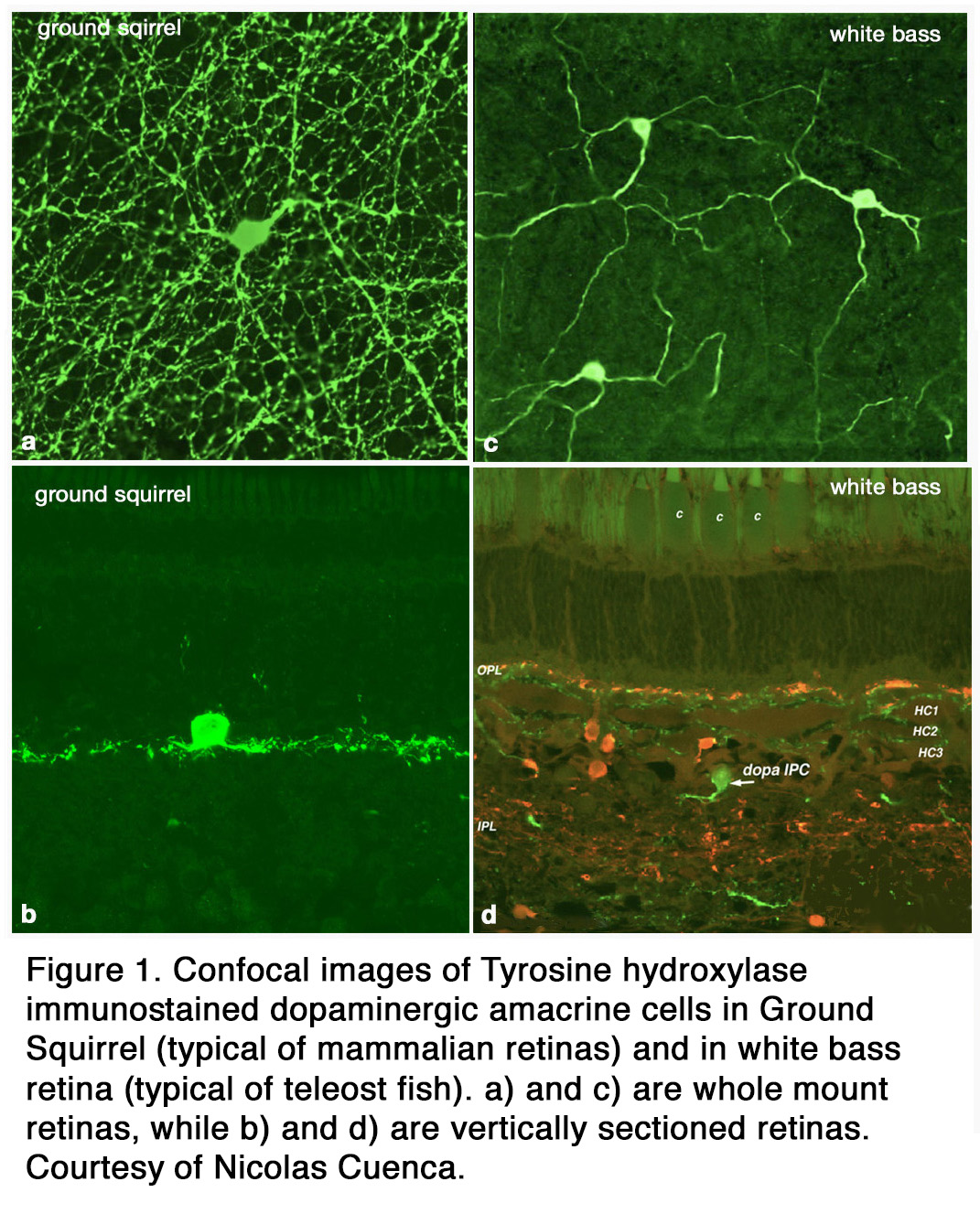
The distribution of DA among retinal cell types varies with species. Some species (amphibians) have both dopamine cell types (ACs and IPCs), while others (elasmobranch fishes, reptiles, birds ,cats, rabbits, monkeys, human) have only DA amacrine cells that spread over a wide field in stratum 1 of the inner plexiform layer (IPL) (Figure 1 a, b). Teleost fish have only DA IPCs (Figure 1 c, d) (3, 4). IPCs are wide field amacrine-like cells that have dendrites running through both the IPL and outer plexiform layer (OPL). Turtles appear to have DA cells with tristratified IPL dendritic trees but no dendrites to the OPL (5). In human, cat, and ground squirrel retinas dopaminergic ACs have some rare processes projecting towards and into the OPL (6-8) but their major dendritic plexus is in stratum 1 of the IPL (Figure 1 a, b; Figure 2 a, b). In cat the OPL-running DA dendrites make synaptic contacts upon a different IPC, which is GABAergic, and possibly upon B-type horizontal cells (7) (Figure 2 a, b). In primates, mouse, and rabbit retinas a second type of catecholamine amacrine (CA) is seen, called Type 2 CA (9-12) (see also Webvision chapter on Neurotransmitters in the Retina).
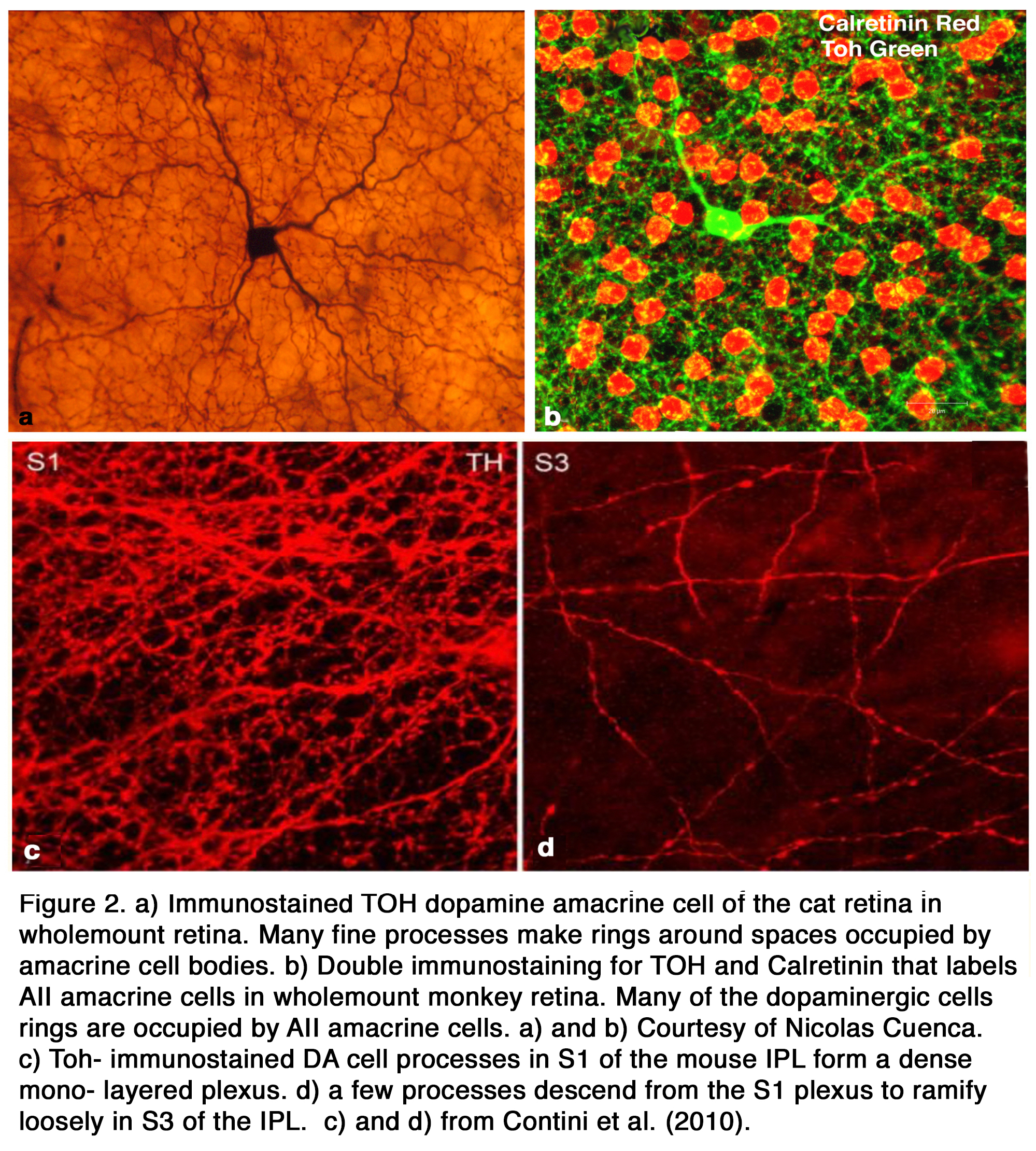
The primary Type 1 DA cell in mammals is very characteristic with a large cell body and a dense plexus of dendrites in stratum S1 of the inner plexiform layer (Figure 2 a, c). Holes or “rings” in the plexus of criss-crossing stained dendrites are sites of amacrine cell bodies or large amacrine dendrites (Figure 2 b), on which the processes of the DA cell plexus synapse. In this respect the processes of DA cells appear to be axon-like (Figure 2 a, b, c).
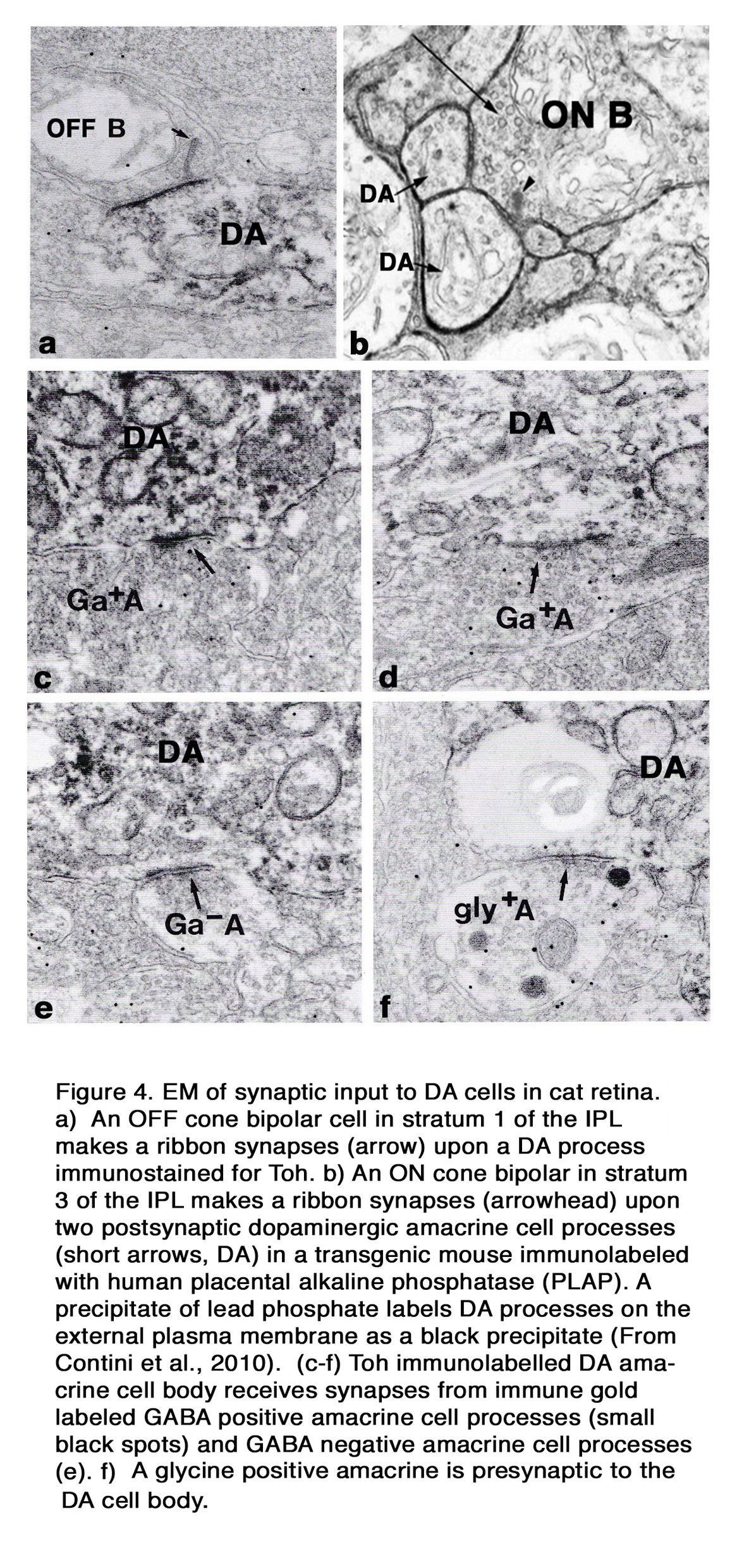
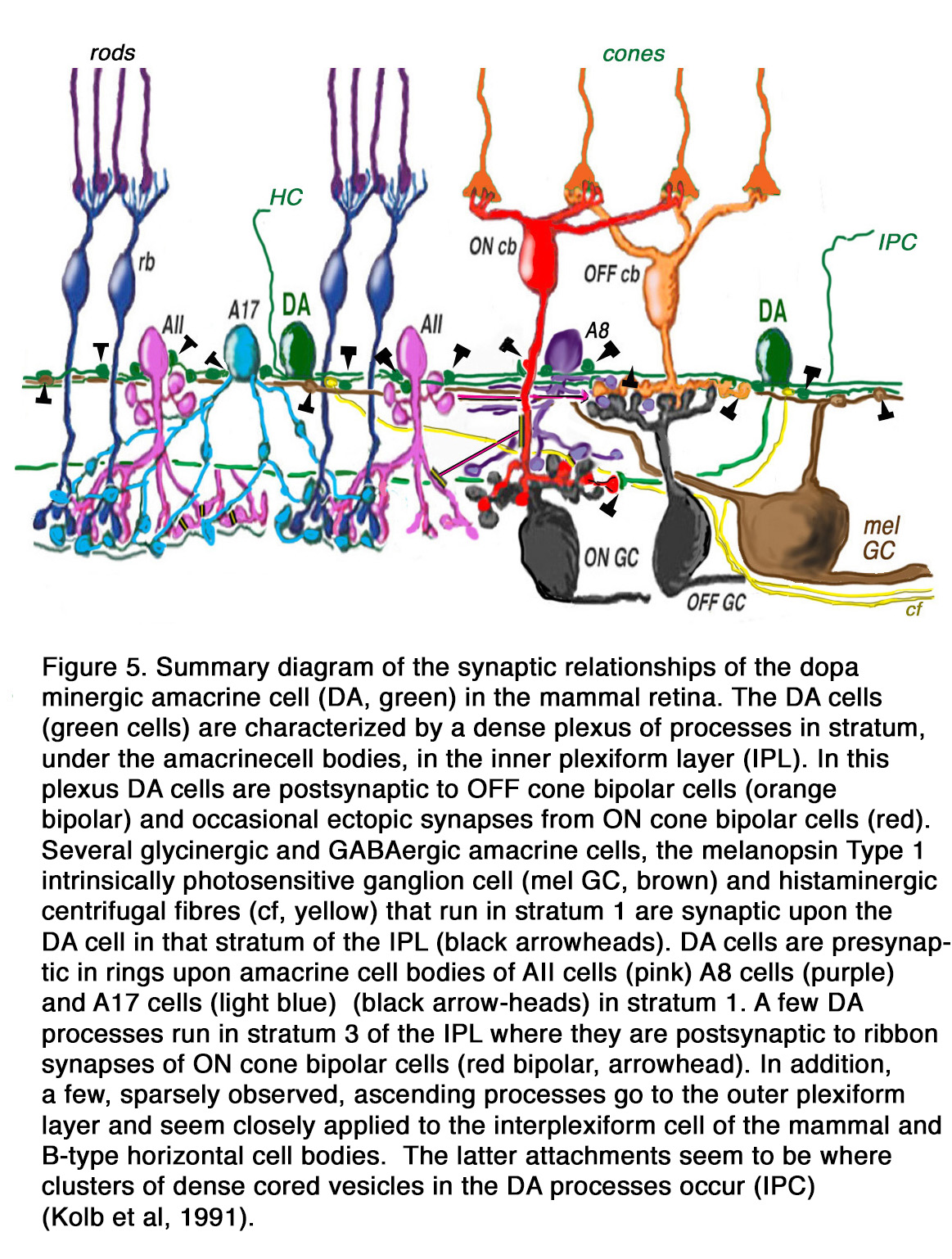
Electron microscopy of a cat Type 1 DA cell, in tyrosine hydroxylase (Toh) immunostained retinas (7), shows that Toh amacrine dendritic rings synapse upon the glycinergic AII rod amacrine cells (Figure 3 b), A8 glycinergic amacrine cells (Figure 3 a), and upon GABAergic A17 amacrine cells (not shown) (7, 13, 14). The type 1 DA cell is postsynaptic to OFF-bipolar cells in stratum 1 (Figure 4 a) and to occasional ectopic synapses from an ON-bipolar axon passing through the OFF layer (not shown) (15). The main DA plexus in S1 sends a few processes to the S3 level of the IPL (7, 11, 16) (Figure 2 d) where they are postsynaptic to ON-bipolar cells (Figure 4 b) (16). Glycinergic and GABAergic amacrine cells (Figure 4 c-f), melanopsin M1 ganglion cells (17-19) and histaminergic centrifugal fibers (20) make synapses upon the Type 1 DA cell as well (see chapters on Roles of Amacrine Cells and Melanopsin-expressing, Intrinsically Photosensitive Retinal Ganglion Cells (ipRGCs) in Webvision).
In cat the OPL-running DA dendrites make synaptic contacts upon a non-dopaminergic IPC, which is GABAergic, and possibly upon B-type horizontal cells (7) (Figure 3 c and d). These contacts are, however, probably not signs of synaptic vesicle release of transmitter because no synaptic vesicle proteins have been found at these attachment points. They are possibly sites of dopamine release and action through “volume transmission”.
The DA Type 1 cell exhibits glutamate, GABA and histamine H1 receptors (7, 19, 20). Glutamate (21), GABA and dopamine colocalize to the Type 1 DA cell (22, 23), and serotonin has also been found to co-exist in these same amacrine cells in cat retina (23). Thus, the dopamine amacrine cells of the vertebrate retina are a complex cell type, differing in branching patterns in different species, making different synaptic connections dependent on species, and synthesizing multiple neurotransmitters. A general summary diagram of the DA cell in mammalian retinas with the synaptic inputs and outputs is shown in Figure 5 (explanations are in the figure legend).
Physiology of Dopaminergic amacrine cells
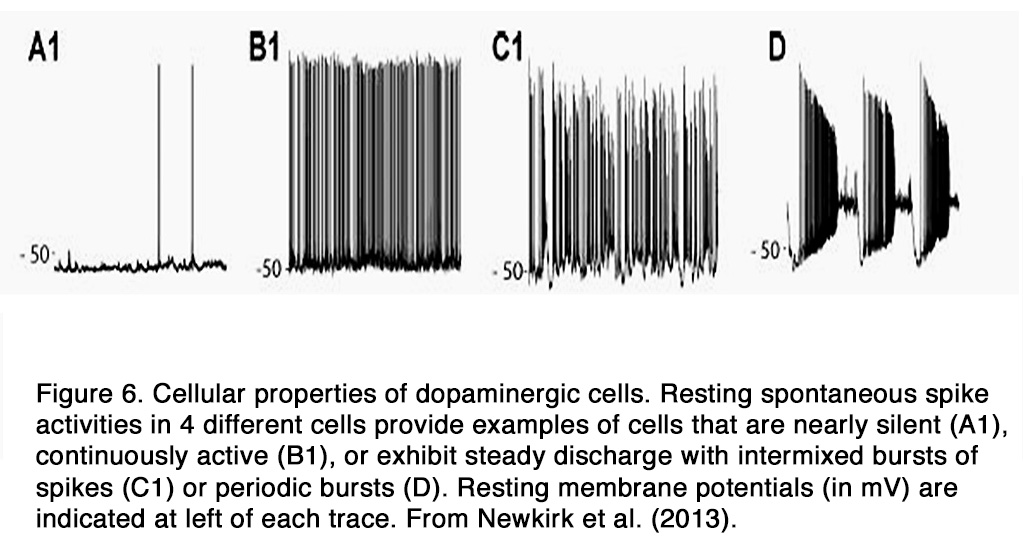
DA amacrine cells fire spontaneously at a modest rate (< 10 spikes/s) and the action potentials trigger dopamine release by exocytosis (22, 24). In the dark, the spontaneous spike activity differs markedly among DA cells. The characteristics of their spike activity falls into four categories in intact mouse retina: quiet cells that generated spikes infrequently at random intervals (Figure 6, A1), rhythmic cells that fire spikes at a maintained rate, and cells that generate bursts that are either mixed with single spikes (Figure 6 B1) or occur in discrete bursts at regular intervals (Figure 6, C1, D) (25). The bursting, but not the overall firing rate, increases during blockade of the GABAergic and glycinergic retinal synapses, indicating that an inhibitory synaptic input from amacrine cells in the dark modulates the bursting aspect of DA cell spontaneous activity (12). On the other hand, blockade of AMPA/kainate-type or NMDA-type glutamate receptors affects neither the firing rate nor the firing pattern of DA cells, indicating a lack of direct excitatory glutamate synaptic input from bipolar cells in the dark.
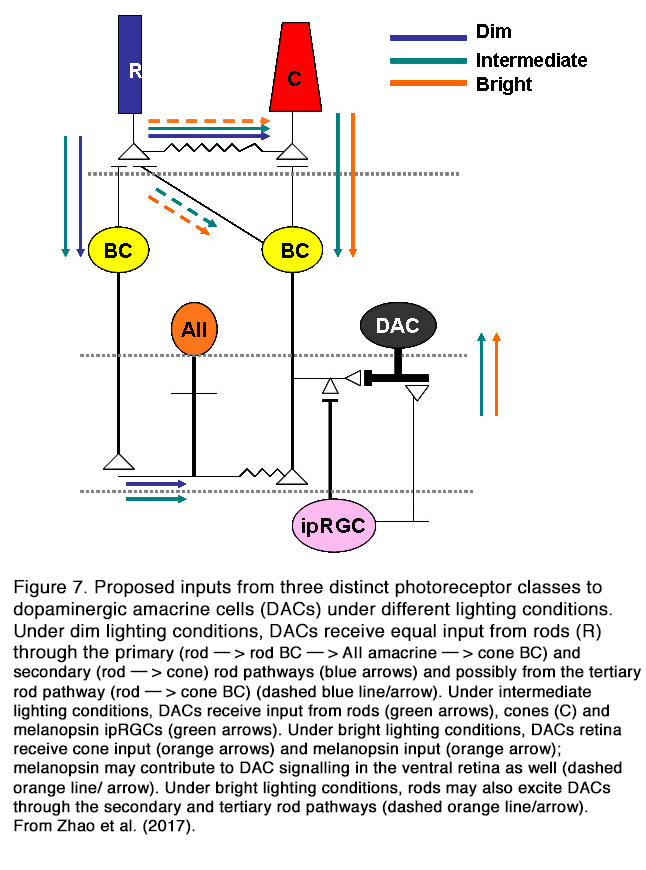
In contrast to spontaneous firing, the light-evoked activity of DA cells in mammalian retina is modulated by the excitatory glutamatergic input from the ON cone bipolar cells (CBCs) (16) and melanopsin-expressing intrinsically photosensitive retinal ganglion cells (ipRGCs) (19) (Figure 7) (for relationship between DA and ipRGCs see Melanopsin-expressing, Intrinsically Photosensitive Retinal Ganglion Cells (ipRGCs) in Webvision). All mammalian DA amacrine cells are ON-type, while amphibian DA amacrine cells showed excitatory ON and OFF responses (26). Inputs from ON-BCs probably account for the light-evoked ON-transient responses of mammalian DA cells, while inputs from ipRGCs likely account for ON-sustained DA cells.
Zhang et al. (12, 19, 27) found that all ON-transient, but not ON-sustained light responses of DA neurons were entirely eliminated in photoreceptor degenerate mouse retinas and under the influence of L-AP4, which blocked the transmission from rod/cones to ON bipolar cells. On the other hand, the ON-transient light responses persisted in melanopsin knockout mice, where all ON-sustained light responses were eliminated, supporting the dependence of the latter on the inputs only from ipRGCs. Other authors argued, however, that the nature of generated spike response (sustained or transient) of DA neurons depended only on stimulus intensity (25). Newkirk et al. (25) showed that the sustained spike trains were evoked by weak stimuli, whereas the transient trains were evoked by bright light, because the latter caused a depolarization block that limited the ON response to an initial transient burst of spikes. This was also the case in DA cells that generated exceptionally prolonged large depolarizing responses to strong stimuli, which they considered to be the result of direct excitatory input from ipRGCs. Newkirk et al. (25) suggested that a reduced light sensitivity of the retina in experiments of Zhang et al. (12, 19), due to differences in methodology, may account for the discrepancies between the results of the two groups. The reduced sensitivity may serve to reduce the amplitude of the depolarizing response evoked by excitatory input to a level that was not sufficient to cause a depolarizing spike block. This could explain the presence of sustained spike train in response to saturating light in experiments of Zhang et al. (12, 19). This suggestion could not explain, however, why all DA cells had transient responses in melanopsin knockout mice under the same conditions of stimulation and adaptation (27).
The relative contribution of different photoreceptor inputs to the light-evoked ON responses of DA cells was recently investigated in mouse retina (28). It was found that rods excited the dark-adapted DA amacrine cells across a wide range of stimulation intensities (6 log units) primarily (but not solely) through connexin-36-dependent rod pathways. This result is at odds with previous findings that DA cell threshold response to scotopic stimuli was generated only by inhibitory input at light onset from glycinergic ACs (25). The discrepancy between cited results is probably due to the different adaptation state of the retina. Zhao et al. (28) performed their recordings in dark-adapted retina, while Newkirk et al. (25) conducted their experiments under weak background illumination that could eliminate the ON-EPSPs evoked by dimmer light. Zhao et al. (28) found that the ON-responses of DA cells to prolonged adapting light were mediated by rods under dim lighting conditions, rods/M-cones/melanopsin under intermediate lighting conditions, and cones and melanopsin under bright lighting conditions (Figure 7). Thus, it appears that mammalian DA cells receive excitatory ON inputs from all photoreceptor types and can influence their target cells under different conditions of light stimulation and adaptation. It should be mentioned that not all DA cells have light-modulated responses. Some authors reported that 40% of mouse DA cells are unresponsive to light (12), while other authors (25) reported much smaller portion (1 out of 300 cells).
The light-evoked activity of DA cells is modulated also by inhibitory glycinergic and GABAergic AC synaptic inputs (25, 29). This inhibitory input can occur during light onset and/or at light offset. It appears that the ON inhibition is mediated by glycinergic ACs (25, 29), while the OFF inhibition is mediated by GABAergic ACs solely (25) or by GABAergic and glycinergic ACs (29). In addition to their intraretinal inputs, DA cells receive centrifugal influences from different brain structures. In fishes the activity of DA-IPCs cells was modulated by centrifugal fibers originating in the olfactory bulb and containing FMRFamide-like and luteinizing hormone releasing hormone (30, 31). In other species (guinea pig, mouse, rat, monkey) histaminergic centrifugal fibers originating in the tuberomamillary nucleus of hypothalamus innervated retina and they may also modulate the activity of DA cells (20).
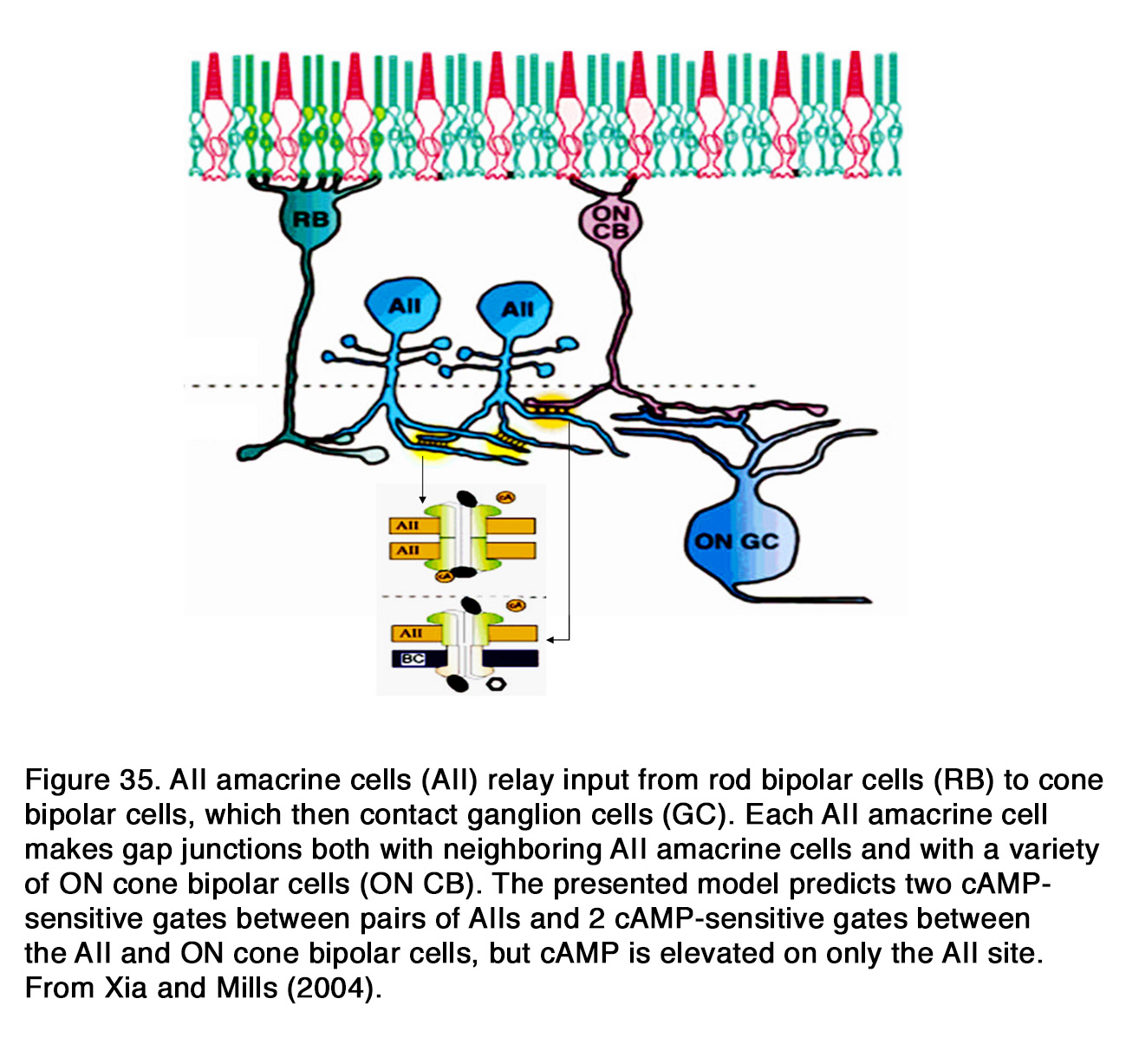
The output synapses of DA amacrine cells are in the IPL, while DA interplexiform cells transmit signals from inner to outer retina. The main output synapses of mammalian DA amacrine cells in the IPL are onto two types of amacrine cells – АII (glycinergic) and А17 (GABAergic), which belong to the rod pathway (see Webvision: AII Amacrine Cells and Roles of amacrine cells). In addition, DA cells make reciprocal, conventional GABAergic synapses onto the presynaptic ON CBCs (16). These synapses probably function to exert an inhibitory feedback onto the presynaptic bipolar cell. DA amacrine cells synapse also onto ipRGS and thus can modulate their activity (17, 18) (see Webvison: Melanopsin-expressing, Intrinsically Photosensitive Retinal Ganglion Cells (ipRGCs). In cold blooded animals, DA amacrine cells make output synapses onto other amacrine cells, ganglion cells and axon terminals of bipolar cells (32, 33). The well-known synaptic outputs of DA IPCs in the OPL are on the GABAergic IPC as seen in cat (7, 8), horizontal cells of cat (7, 8), Cebus monkey and goldfish (34), human (6), and on bipolar cells of Cebus monkey and goldfish (34). In rodent retina, DA IPC distal processes have multiple varicosities that terminate throughout the INL, the OPL, and, rarely, in the layer of photoreceptor cells without making morphologically defined synapses (35). Dopamine is packed into membrane-bound compartments that are not conventional synaptic vesicles. These reside in the dendritic varicosities and can be released from them at multiple horizontal levels of the outer retina. Dopamine reaches its target cells by diffusion over distances of up to tens of microns giving rise to the concept of ‘‘volume transmission’’ (36, 37).
It is known that DA amacrine cells co-localize GABA, in cat (23, 38), turtle, chick, mouse (39) and rat (38, 40, 41). It was shown that a proportion of the secretory organelles in the cytoplasm of mouse DA cell bodies contained both GABA and dopamine and that both transmitters were released simultaneously by exocytosis upon depolarization of the cell membrane (22). The synapses made by rodent DA cells onto AII amacrine cells appeared to be GABAergic, since GABAA but not DA receptors were clustered in the postsynaptic active zone (42). A hypothesis exists that GABA, released by the DA cell, acts on the ionotropic GABA receptors clustered at the postsynaptic active zone of AII amacrine cell, whereas dopamine diffuses to more distant, slower-acting metabotropic receptors (22, 42). This hypothesis is probably not true for rabbit retina, where synapses made by DA amacrine cells on AII cells were not GABAergic (43). Lee et al. (43) suggested that AII synaptic input from DACs in the rabbit retina may be mediated only by dopamine. This is consistent with earlier results showing that a very few rabbit DACs contained very low levels of GABA (38). GABA co-localization was not observed in DA cells of some lower vertebrates including amphibians and fishes (39).
Photic and circadian regulation of dopamine release
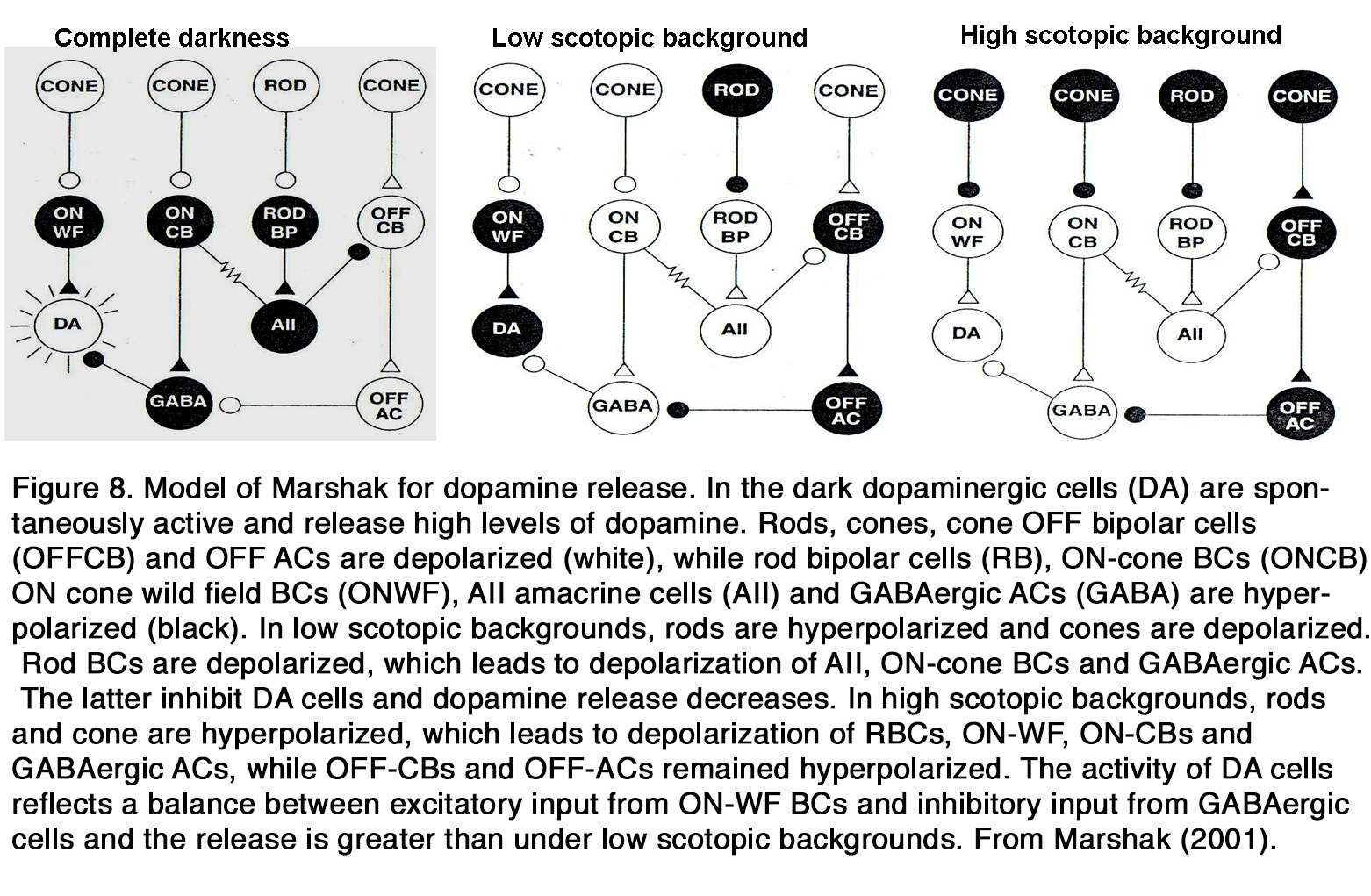
Much data indicates that endogenous dopamine release is low in darkness but increases during retinal exposure to constant or flickering light, as seen in frog (37, 44, 45), turtle (46), pigeon (47), chick (48, 49), duck (50), rodents (29, 51, 52), rabbit (53-56), cat (57), and monkey (58). These results led to the suggestion that dopamine functions as a chemical signal for light (59-62). However, there are some opposite results, indicating that the dopamine release was high in darkness and decreased during retinal illumination as seen in cat (63, 64). According to some authors the latter effect was seen only when the experiments were performed at night, not at day (65). There is some suggestion that in mammalian retina there is a U-shaped relationship between dopamine release and light intensity (66, 67). In total darkness, when DA cells receive no input, they fire spontaneously and release dopamine. The release of dopamine diminishes at the low scotopic range, because the DA cells are inhibited by GABAergic amacrine cells driven through the OFF system (67) or through rod BCs via AII amacrine cells (66). There is no consensus among the authors cited, however, at what intensity range the DA release increases again. According to Marshak (66) it occurs in the higher scotopic range, when the DA cells receive direct excitatory input from ON cone bipolar cells driven from the less sensitive rod pathway via rod-cone junctions (Figure 8).
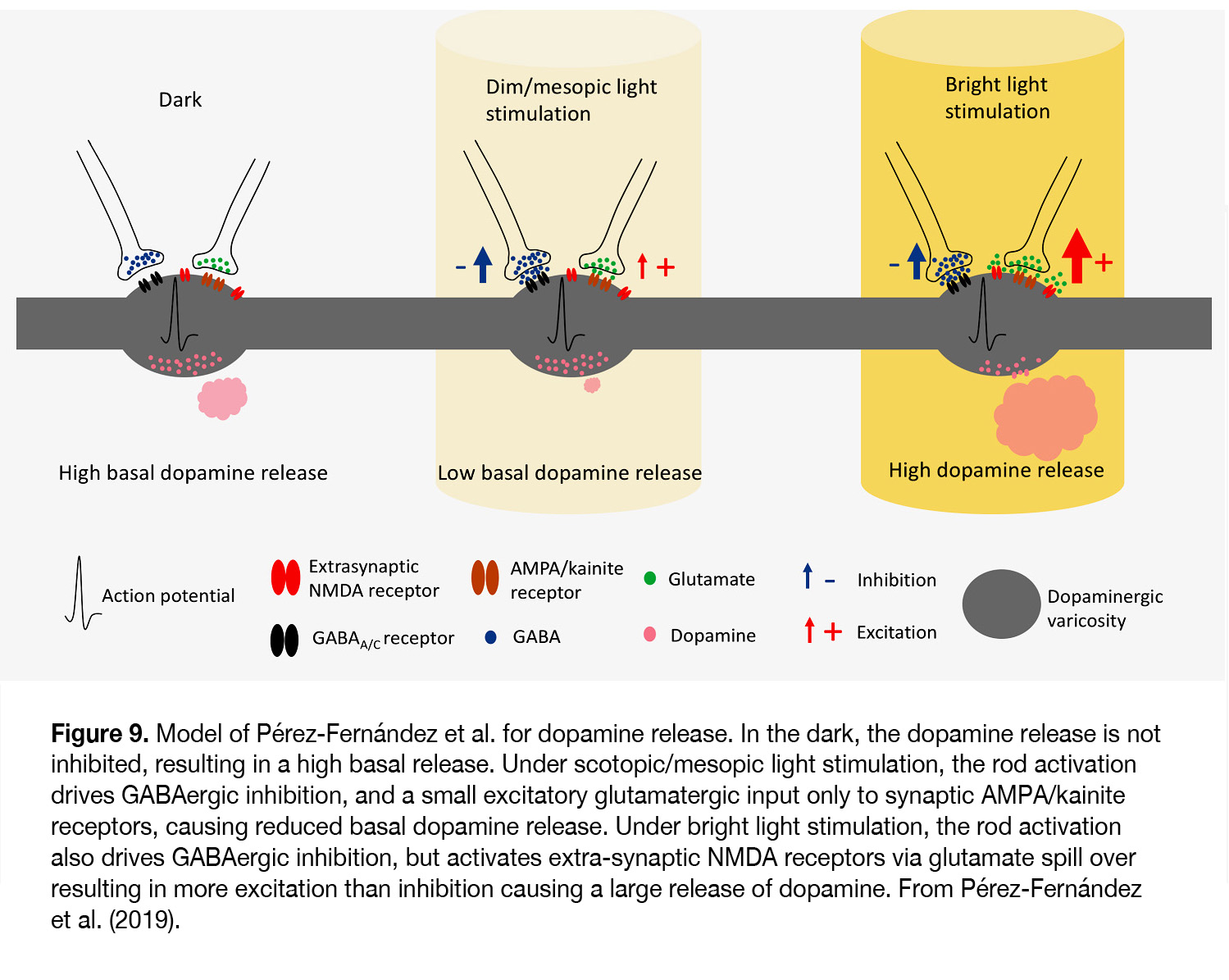
According to Pérez-Fernández et al. (67) it occurs at light intensities when transition from mesopic to photopic vision takes place (> 6 log units above ERG threshold). The latter authors suggest that under scotopic and mesopic light stimuli, although the glutamate release increases and stimulates synaptic AMPA/kainite receptors, it does not outweigh the inhibition caused by the GABAergic system and DA release is low (Figure 9). Under bright light conditions both inhibition and excitation are increased, but due to glutamate spill over to extra-synaptic NMDA receptors the level of excitation tips the balance and allows a large release of dopamine.
Pérez-Fernández et al. (67) presented evidence that DA release in mouse retina depended neither on cone photoreceptors nor on ipRGC function and they insist that it entirely depended on the rod pathway function. This suggestion is not supported by results of Cameron et al. (51), who reported that light-induced dopamine release was retained in mice lacking rod phototransduction, although the magnitude of this response was substantially reduced compared with wild-types. By contrast, light-induced dopamine release was not evident in mice lacking both rods and cones, indicating that light regulation of retinal dopamine release relies upon both rod and cone function.
Another hypothesis is proposed by Dowling (68), who suggested that different types of dopaminergic cells in mammalian retina, as described by Zhang (12), can account for the dopamine release under different conditions of light stimulation. The spontaneously active dopaminergic cells, which are not light-driven, would release dopamine in the dark. The ON-transient cells would release dopamine best when exposed to flickering light and the ON-sustained dopaminergic cells would release dopamine best in steady light. The amount of dopamine released, however, may be determined not only by spike frequency, but also by the pattern of spike activity (single spike, random, or bursting). It was shown that bursting stimulations were twice as potent as regularly spaced ones having the same average frequency in evoking dopamine release from brain dopaminergic neurons (69). In this regard, Cameron et al. (51) suggested that the slow sustained activation, typically associated with ipRGCs, could account for their inability to release measurable amounts of DA from the dopaminergic cells upon light stimulation, although ipRGCs send excitatory outputs onto the dopaminergic cells.
In some non-mammalian species (fishes, reptiles, birds), dopamine synthesis and release are controlled by a circadian clock so that dopamine release is highest during the daytime in light and lowest at night in darkness, as seen in fish (70-72), iguana (73, 74), pigeon (47), turkey (75), quail (76), chick (48, 49), and duck (50). In fish retina, the circadian rhythm of dopamine release was eliminated during the continuous presence of melatonin and melatonin receptor antagonists, indicating that the circadian rhythm of dopamine release was primarily driven by the circadian rhythm of melatonin (71). Similar results were obtained in reptiles, where the circadian rhythm of retinal dopamine content and metabolism was abolished in melatonin depleted eyes (73). Whether dopamine release is predominantly light-driven or controlled by a circadian rhythm in birds is a subject of dispute. It was shown that retinal DA content oscillated rhythmically in pigeons kept for 4 days under continuous dim light illumination, indicating the dominant role of circadian oscillator for retinal dopamine release (47). In these cases, the dopamine rhythms were not just driven by melatonin rhythms, because inhibition of melatonin release by quinpirole did not always change dopamine level (77). However, in retinas of chick (49, 61), quail (76) and turkey (75), a progressive decline of rhythmic oscillations of DA, its synthesis and degradation products was observed in constant darkness, emphasizing the important role of light as a stimulatory signal for the retinal dopaminergic system, in addition to the circadian pacemaker. The authors cited proposed that the small circadian increase in dopamine synthesis, release, and metabolism resulted from disinhibition of dopaminergic neurons from the suppressive action of melatonin during the light phase of the cycle.
A consensus is lacking regarding the circadian regulation of dopamine metabolism in mammalian retina. Although the concentration and release of dopamine was higher during the day and lower at night in mouse(78, 79), rat (80-84), rabbit (55), human (85), some authors reported no circadian rhythm in dopamine synthesis and utilization in rats (81), rabbits (55), mice (79) kept in constant darkness. The latter authors suggested that the diurnal changes of dopamine synthesis and utilization are entirely dependent on the presence or absence of light and not driven by a circadian clock. Other authors, however, demonstrated circadian rhythm of dopamine content and turnover that persisted in constant darkness (78, 80, 84). The circadian rhythm of dopamine content and metabolism was evident in mice that synthesize melatonin, but not in mice that were genetically incapable of synthesizing melatonin. Daily injections of melatonin induced circadian rhythms of dopamine in retinas of mice that were unable to synthesize melatonin (78). These observations suggest that the circadian rhythm of dopamine synthesis in the mouse retina, like fishes and reptiles, is controlled by rhythmic release of melatonin from the photoreceptors. It is tempting to speculate that melatonin released from photoreceptor cells is required to entrain the circadian oscillators in dopamine ACs to the daily light-dark cycle. However, when photoreceptors degenerate (as in Royal College of Surgeons rats), the retinal dopamine content, its metabolites and melatonin synthesis still displayed circadian rhythms (80, 86),thus suggesting the presence of a circadian clock outside the photoreceptors. It was shown that the mouse dopaminergic neurons expressed core circadian clock genes (87-90), which supports the hypothesis that dopaminergic amacrine cells may contain an autonomous circadian clock.
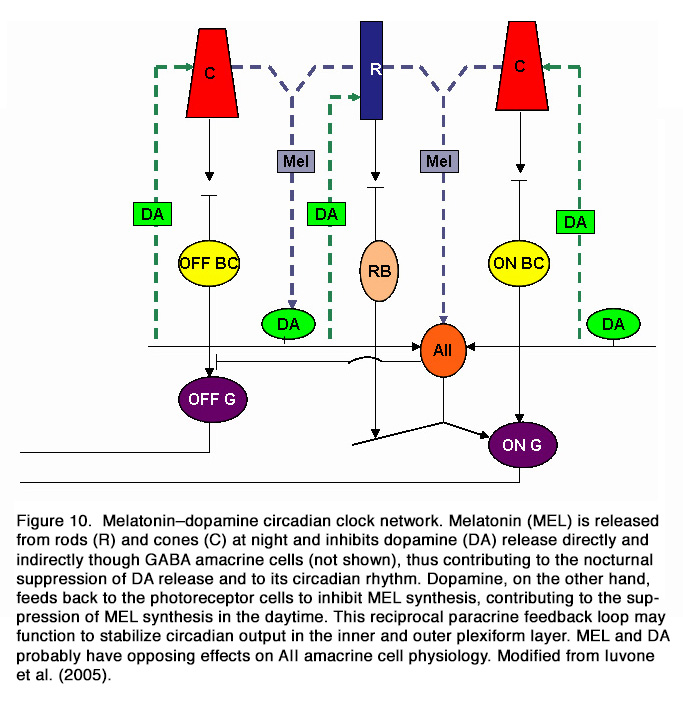
It appears that at least two different circadian pacemakers are present in the mammalian retina. The first pacemaker drives the circadian rhythm of melatonin synthesis and is likely to be located in the photoreceptor cells, while the second pacemaker drives the circadian rhythm of dopamine and is located in neurons of the inner retina (91). It was postulated that the melatonin secreting photoreceptors and dopaminergic amacrine/interplexiform cells are components of a mutually interplaying system (in a negative manner) and act as chemical analogs of light and darkness, respectively (62, 92). Melatonin appears to contribute to the nocturnal suppression of DA release and to its circadian rhythm. Dopamine, on the other hand, feeds back to the photoreceptor cells to inhibit melatonin synthesis, contributing to the suppression of melatonin synthesis in the daytime. Dopamine also serves as a zeitgeber that together with light entrains the photoreceptor clock (Figure 10).
Some data suggest that the circadian regulation of dopamine synthesis in mouse retina depended also on melanopsin ipRGCs. It was shown that lack of melanopsin (in melanopsin-/-knockout mouse retinas) prevented the light-dependent increase of tyrosine hydroxylase mRNA and of dopamine and, in constant darkness, led to comparatively high levels of both components (93).
Dopamine receptors in the retina
Dopamine actions are mediated by five G-protein-coupled receptors named D1 to D5.
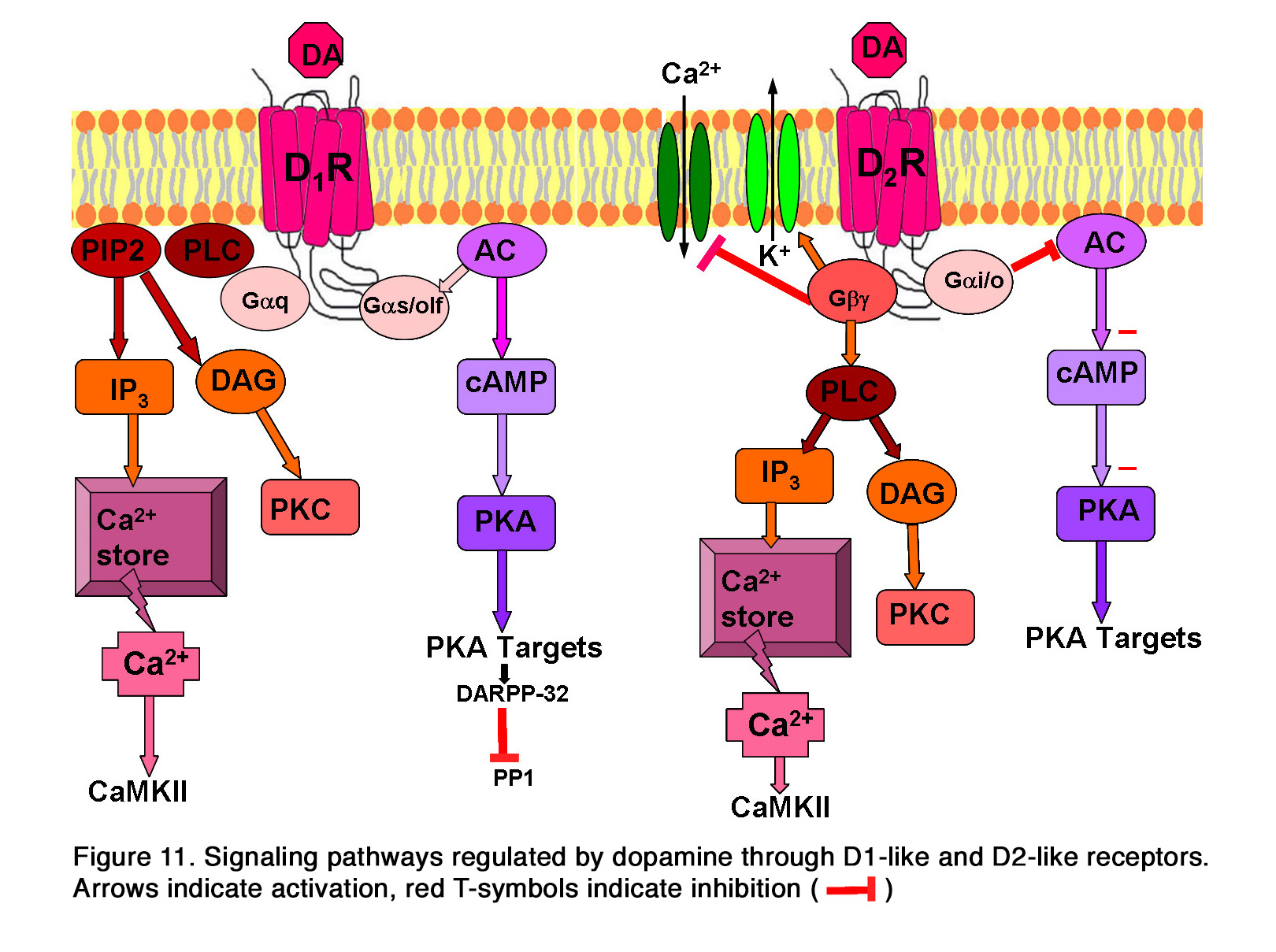
These receptors are allocated to two groups: 1. The D1-like (including D1 and D5 subtypes), which are generally coupled to Gas/olf and stimulate the production of cyclic Adenosine Monophosphate (cAMP) and the activity of Protein Kinase A (PKA). 2. The D2-like (including D2, D3 and D4 subtypes), which are coupled to Gai/o and negatively regulate the production of cAMP, resulting in a decrease in PKA activity. This is reviewed in Beaulieu and Gainetdinov (94). Activation of PKA by D1-like receptors also results in the phosphorylation of the cyclic AMP-regulated phosphoprotein, 32 kDa (DARPP-32), which is an inhibitor of protein phosphatase 1 and thus prevents dephosphorylation of activated proteins (Figure 11). In addition to this main pathway for intracellular signaling, dopamine receptors can also utilize other pathways. Some data indicate that the D5dopamine receptor can be coupled to Gaq to regulate PLC activity and intracellular calcium signaling, as reviewed in articles by Beaulieu (94, 95) (Figure 11). The participation of the D1 receptor in the same signaling pathway is uncertain. The D2-like receptors acting through Gbg subunits of heterotrimeric G- proteins can increase the activity of inwardly rectifying potassium channels (94, 96) and reduce the activity of L-type and N-type calcium channels (97) (Figure 11). Similar to D1-like receptors, D2-like receptors can also modulate intracellular calcium levels (98, 99). Some of these actions may be mediated by Gbg subunits of heterotrimeric G-proteins that could activate PLC and thus lead to release of Ca2+ from intracellular stores (94, 100). Other data suggest, however, that the calcium signal from D2-like receptor activation may originate from activation of D1-D2 or D5-D2 receptor hetero-oligomeric complexes, reviewed in Hasbi et al (101, 102).
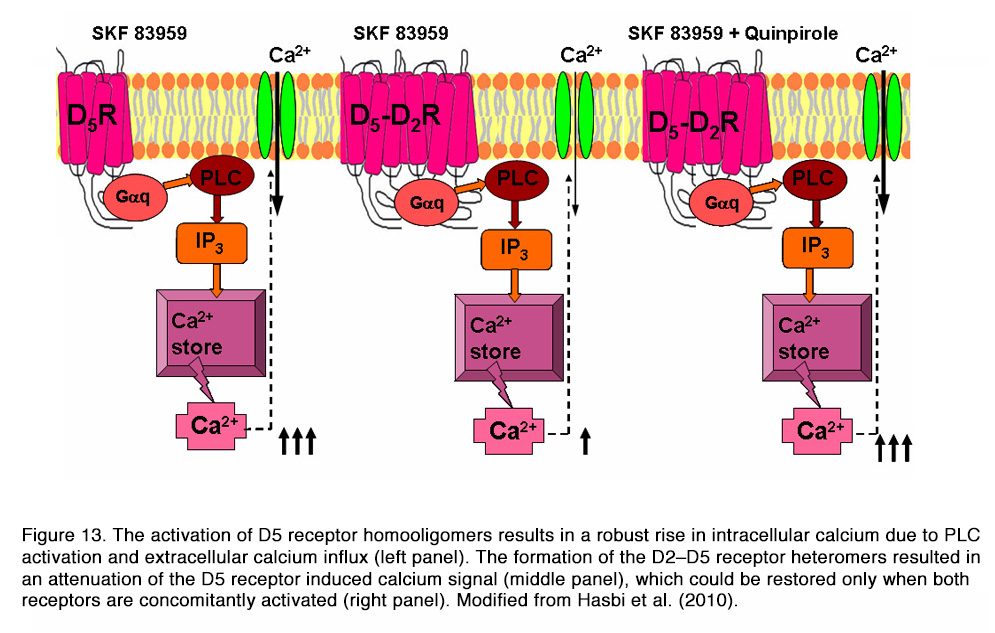
It was shown that D1–D2 receptor heteromer activation (by SKF 83959) led to a rapid, transient, intracellular calcium mobilization (via Gq, PLC, PKC, IP3), independent of extracellular calcium influx (Figure 12), that was not observed with either D1R or D2R activation alone. The activation of the D5 receptor had similar effects, with the exception that the calcium signal depended on calcium influx. The formation of the D2–D5 receptor heteromers resulted in an attenuation of the D5 receptor induced calcium signal, which could be restored only when both receptors are concomitantly activated (Figure 13).
Some authors question these results because SKF 83959 may not exhibit selective activation of D1-D2heteromers, and also may show cross-reactivity to other receptors, as reviewed in Lee et al (103). One might conclude that current evidence does not allow us to determine the detailed mechanism by which D1, D5 or D1–D2 dopamine receptors participate in calcium signaling in target cells. In addition to their G-protein dependent action, the D1 and D2 receptors could directly alter the activity of the ion channels in the cell membrane. It was shown that both D1 and D2 receptors inhibited the activity of N-type calcium channels through a direct protein-protein interaction (104). Direct interaction of D1 and D2 receptors and Na+-K+-ATPase was also demonstrated (105, 106). Finally, there is strong evidence that dopamine receptors can signal in vivo by activating cAMP-independent mechanisms involving the multifunctional adaptor protein β-arrestin 2 and transactivation of receptor tyrosine kinases in different experimental systems, as reviewed by Beaulieu et al (95).
The D1-like receptors are found exclusively postsynaptically on dopamine target cells, whereas D2-like receptors are expressed both postsynaptically and presynaptically on dopaminergic neurons (107, 108). The presynaptic autoreceptors generally provide an important negative feedback mechanism, which regulates the synthesis and release of the neurotransmitter in response to changes in its extracellular concentration (109, 110). The D2-like receptors, including those in the retina, are 2-3 times more sensitive to dopamine than D1-like receptors (62, 98, 111-113).
All dopamine receptors undergo desensitization as a result of prolonged exposure to DA or its agonists (114-117). This is a characteristic feature of G-protein-coupled receptors. On the other hand, the receptors can undergo sensitization when an agonist does not activate them for an extended period. Some data indicated that retinal dopamine receptors in rat developed a state of hypersensitivity during period of prolonged light deprivation (118). The hypersensitivity of D1-like receptors developed earlier (within 6 hours) in comparison with that of the D2-like receptors (after 2 or, even better, 4 days). Hypersensitivity of D2-like receptors regulating serotonin N-acetyltransferase activity was found in kainic acid treated chick retinas, where retinal levels of dopamine were markedly reduced (119).
The activity of all neurons in the retina might be modulated by dopamine because all express D1– or D2-like receptors. Rod and cone photoreceptors have D2-like receptors in all species studied. This includes frog (120, 121), salamander (120), turtle (121), fish (121), chick (121, 122), rat (36, 121, 123-126), bovine (121, 127),rabbit (121, 123), and human (128). Some D1R-immunoreactivity was observed on the axons of cones (129)and in the outer segments of the photoreceptor layer (36). It was shown that dopamine receptors on photoreceptors were of the D4 type in the retinas of rodents, birds and fishes (120, 130-134). Horizontal cells (HC), bipolar cells (BC), amacrine and ganglion cells (GC) are thought to express mainly D1-like receptors. The species studied were frog (135), turtle (135), fish (129, 136), chick (137), rat (124, 138, 139), and mouse(140-142). D1-like receptors were expressed in a type-dependent manner on the ON-CBCs and OFF-CBCs, but not rod BCs (RBC) in mouse retina (140). In ON CBCs, they were expressed in transient, but not sustained cells, while in OFF CBCs they were expressed in sustained, but not transient cells. In addition to the photoreceptors, D2-like receptors were localized on horizontal cells in fish (136), amacrine cells in frog (120),salamander (120), or rat (131), and in cells resembling amacrines and bipolars in the chick (121, 122), rat (36, 121, 125), bovine (121), rabbit (121), and human (128), and in the ganglion cell layer of chick (121, 122), rat(36, 121, 125), bovine (121), and rabbit (121) retinas. The dopaminergic neurons themselves express D2-like autoreceptors, which function to inhibit dopamine release (125, 131, 143). The Műller glial cells also express dopamine receptors in frog (120, 135), salamander (120), turtle (135), chick (144), mouse (144), rat (129, 145)and guinea pig (145).
Effects of dopamine on photoreceptors
Dopamine has numerous effects on photoreceptors. It inhibits the synthesis of melatonin (119, 146), influences retinomotor movements, as reviewed by Popova (147), inhibits hyperpolarization-activated currents as seen in frog (148, 149) and human (149), modulates light-evoked responses, changes the coupling between photoreceptors, etc. Most of these effects are mediated through D2-like receptors expressed on the photoreceptors. It was shown that activation of dopamine D4 receptors in photoreceptors suppressed the light-sensitive pool of cAMP (130, 150-152), reduced the PKA activity and down-regulated the type1 Adenylyl cyclase (AC1) expression (153). In addition, the regulation of cAMP level in photoreceptors by dopamine may occur indirectly, via regulation of the intracellular calcium concentration (154). It was shown that dopamine, quinpirole and the selective D4 receptor agonist PD 168,077 significantly suppressed K+-stimulated uptake of Ca2+ and decreased [Ca2+]i in chicken cones. The effects of the agonists were blocked by dopamine D2/D4receptor antagonists or by pertussis toxin. Because quinpirole inhibited the increase in cAMP level elicited by K+, which requires Ca2+ influx through voltage-gated Ca2+ channels, but not that induced by the calcium ionophore A23187, the authors concluded that the decrease of cAMP elicited by dopamine D4 receptor stimulation may be secondary to decreased [Ca2+]i. The latter was probably evoked by a direct action of dopamine on photoreceptor calcium permeability.
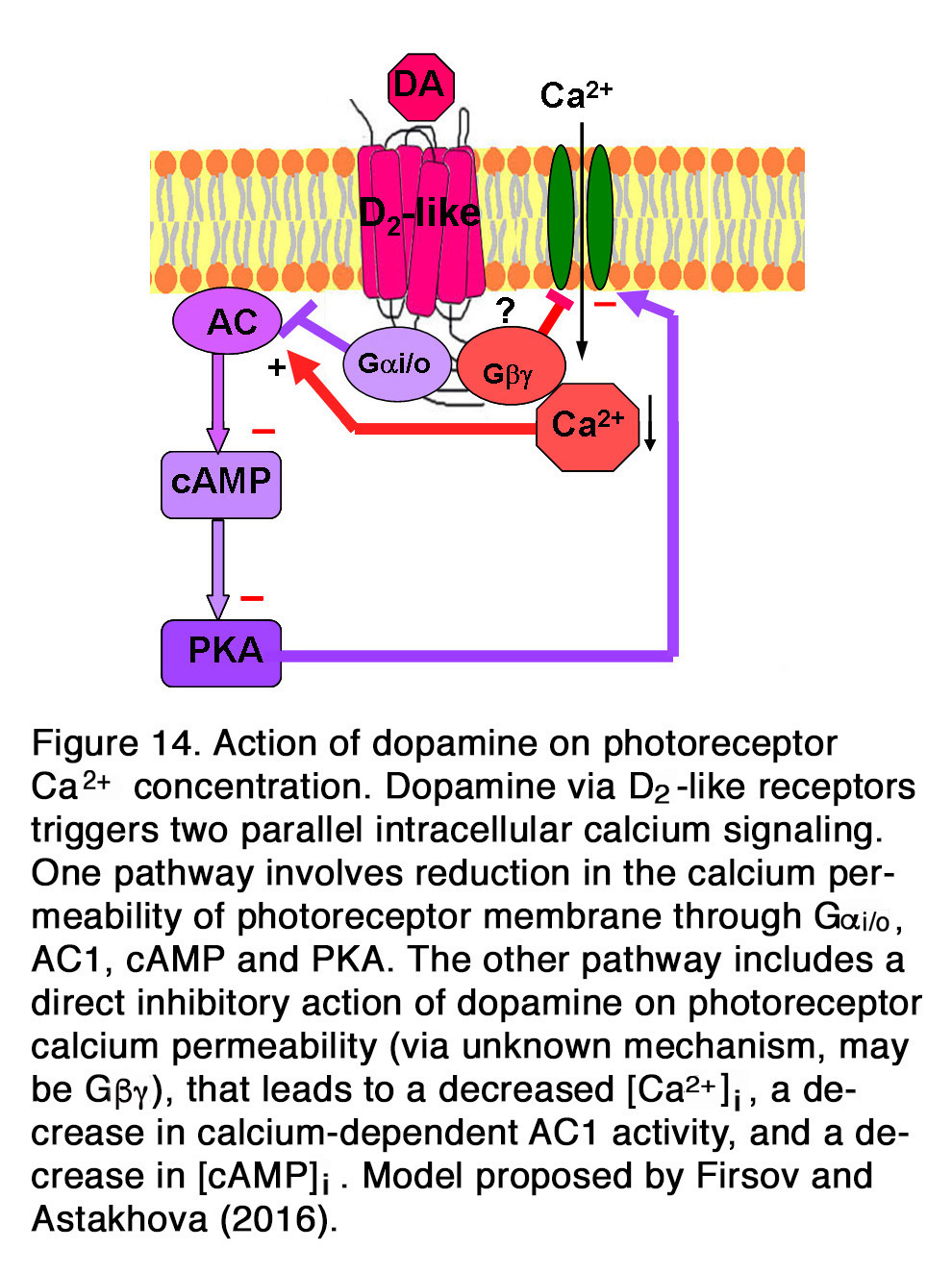
Other authors showed, however, that the suppressive action of dopamine and quinpirole on the influx of Ca2+through L-type channels in salamander large cones depended on the activation of D2-like receptors acting through Gi/o, AC1, cAMP and PKA pathway (155). A suggestion was proposed that dopamine via D2-like receptors triggered two parallel intracellular calcium signaling pathways in photoreceptors (156). One pathway involved inhibition of AC1 (via Gi/o), leading to a decrease in [cAMP]i, a decrease in PKA activity and to a reduction in the calcium permeability of photoreceptor plasma membrane channels. The other pathway included a direct action of dopamine on photoreceptor calcium permeability, leading to a decreased Ca2+influx, a decrease in [Ca2+]i, a decrease in calcium-dependent AC1 activity, and a decrease in [cAMP]i (Figure 14). It was shown, however, that the dopamine effect on calcium influx current was not one and the same in all photoreceptors, but it strongly depended on the photoreceptor type (155). Dopamine reduced ICa in salamander large single cones, but enhanced ICa in rods and in all spectral subtypes of small single cones. Surprisingly, Stella and Thoreson (155) found that the intracellular mechanism of those opposite dopamine effects on ICa was one and the same – via Gi/o, AC1, cAMP and PKA. They proposed that differences among calcium channel subtypes may account for differences among photoreceptors in their responses to activation of the D2-like receptors. Because of dopamine-induced changes in ICa, rods would have an augmented release of neurotransmitter, while the large cones would have a diminished release of neurotransmitter. Thoreson et al. (157) showed, however, that although the rod calcium currents were elevated, rod input to second order retinal neurons was diminished. They provided evidence that dopamine and D2R agonists activated calcium-dependent chloride channels, leading to efflux of Cl– from rods, resulting in subsequent fall of [Ca2+]i. Thus, the transmitter released by rods diminished without changing the membrane potential. In mammalian (rat) retina, however, it appeared that dopamine had no modulatory effect on the photoreceptor Ca2+ signals (evoked by high K+ stimulus), because neither D2R antagonist spiperone nor D1R agonist SKF38393 had effect on them (158).
The effect of dopamine on the photoreceptor light responses is not well established. In goldfish dopamine did not affect the amplitude of light-evoked responses in green- and red-sensitive cones (159). The only effect of dopamine on the cone responses was to abolish the initial transient phase of membrane relaxation that occurred after the hyperpolarization peak (Figure 15). Similar results were obtained in turtles, where neither the rods, long-wavelength-sensitive, or medium-wavelength-sensitive cones exhibited any apparent change in responsiveness to light under the influence of dopamine (160). No dopamine-induced changes of rod light-evoked responses were seen in Xenopus retina (161) (Figure 16 A). In contrast to the above cited results, Hare and Owen (162) demonstrated that dopamine decreased the light-evoked responses of rods over the whole intensity range tested in tiger salamander retina (Figure 16 B). In mammalian retina, the effects of dopamine on the rod light responses depended critically on the time of the day (163). Jin et al. (163)demonstrated that application of spiperone during the subjective day in mice decreased the amplitude and slowed the kinetics of rod responses, so that they resembled the responses typically observed at night. Application of the D2R agonist quinpirole during the subjective night had opposite effects – enhancing the amplitude and speeding up the kinetics of rod responses. The same pharmacologic agents had no significant effect on the rod light responses, when they were applied during the opposite phase of the diurnal cycle. Jin et al. (163) concluded that a circadian clock in mouse retina controlled the light-evoked responses of rod photoreceptors and that the pathway of this control included dopamine and D2-like receptors.
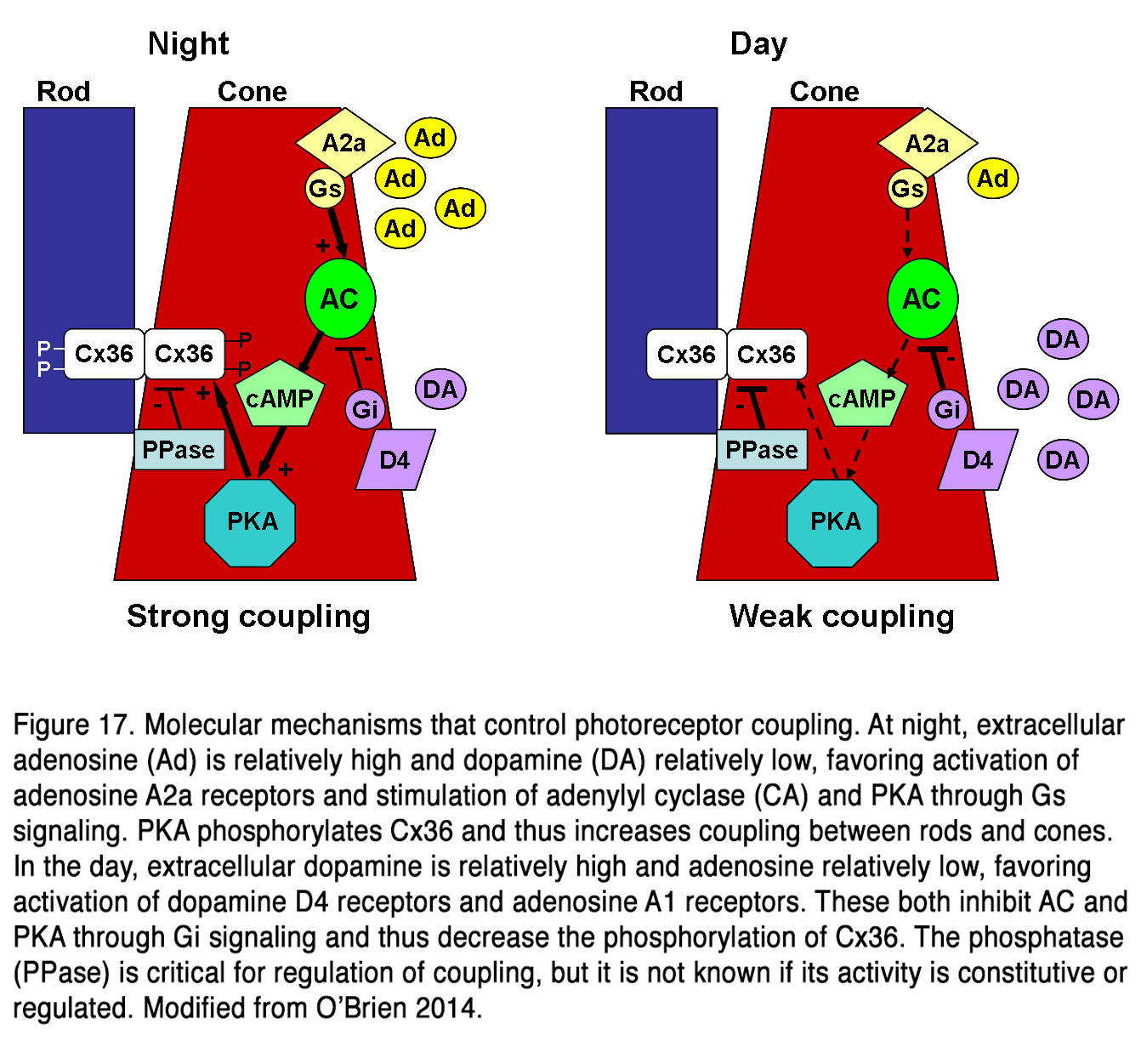
Much data indicates that dopamine influences the electrical coupling between rods and cones, but the effects described are contradictory. Some authors presented evidence that dopamine, acting through D2-like receptors, increased the electrical coupling between rods and cones in amphibian retina (164-166). Krizaj (164) suggests that the effect is most important in the mesopic state, when both rods and cones are light responsive, and the electrotonic rod-cone bridges would permit one photoreceptor class to serve as a conduit for signals arising in the other class. At night or following prolonged dark adaptation, when dopamine levels are low, the rod-cone coupling is minimal, and this would prevent the shunting of the rod signal into the cones. Thus, the greater sensitivity to light of the rods would be preserved in postsynaptic cells. Neither light nor dopamine modulated rod-rod coupling in amphibian retina (165, 167), indicating that the modulation of electrical synapses between photoreceptors is specifically related to light adaptation. Other authors suggest, however, that the electrical communication between rods and cones in fish and mammalian retinas (rabbit, mouse) is weak during the day, when dopamine levels are high and robust at night, when dopamine levels are low (168-171). It was found that cones and cone-connected second order neurons could respond to very dim light stimuli in the scotopic range at night, but not during the day, indicating that rods could shunt currents to cones only during the night (170, 171). The authors suggest that the retinal circadian clock decreases both rod-cone tracer coupling and rod input to second order retinal neurons in the day by increasing dopamine D2R, but not D1R activation in the outer retina. The changes in photoreceptor coupling were driven by PKA-dependent phosphorylation of gap junctions (168, 169). There was a direct correlation between connexin 36/35 phosphorylation and rod-cone coupling such that both were high in darkness at night, and low under daytime illumination (168, 169, 172, 173). Pharmacological blockade of D4 receptors under photopic light exposure in daytime resulted in significantly increased rod-cone coupling (169). This effect was also observed by the Li et al. (169) during adenosine A2a receptor activation under the same conditions of light stimulation. Conversely, inhibiting A2a or activating D4 receptors in daytime dark-adapted retina decreased Cx36 phosphorylation with similar PKA dependence. Li et al. (169) concluded that adenosine and dopamine co-regulated photoreceptor coupling through opposite action on the PKA pathway and Cx36 phosphorylation (Figure 17).
This allows the photoreceptors to respond to extracellular cues that have opposite phases (dopamine: high in the day, low at night; adenosine: high at night, low in the day). Dopamine acting through D2-like receptors during daytime not only suppressed rod-cone coupling in mouse retina, but it reduced the rod-rod coupling as well (163). The authors presented evidence that rod-rod coupling was weak during daytime (or subjective day) and stronger during nighttime (or subjective night). Application of quinpirole during the subjective night mimicked the daytime state, while application of spiperone during the subjective day mimicked the night-time state. It appears that a circadian clock in mammalian retina uses dopamine to control both rod-rod and rod-cone electrical coupling. It remains to be determined why dopamine, acting on D2-like receptors, has no effect on rod-rod coupling in amphibian retina, but strongly modulates rod-rod coupling in mouse retina and why it has opposite effects on rod-cone coupling in amphibian vs fish, mouse and rabbit retinas. A possible explanation for the latter observation is that the decreased cAMP levels and suppressed PKA activity, due to D2R activation, may have different effect on gap junction conductance in different species. It should be noted that electrophysiological studies on monkey photoreceptors failed to find any modulation of rod-cone coupling by dim backgrounds, dopamine or flupenthixol, a D1/D2 dopamine receptor blocker (174).
Effects of dopamine on horizontal cells
Horizontals cells are one of major targets of dopamine actions in the distal retina. Dopamine reduced HC calcium currents through L-type channels via D1R activation (175), affected spinule formation in HC dendrites, as reviewed in (147), strongly influenced the electrical coupling between adjacent HCs, changed HC responsiveness to light, and modulated the balance between HC rod and cone inputs, etc.
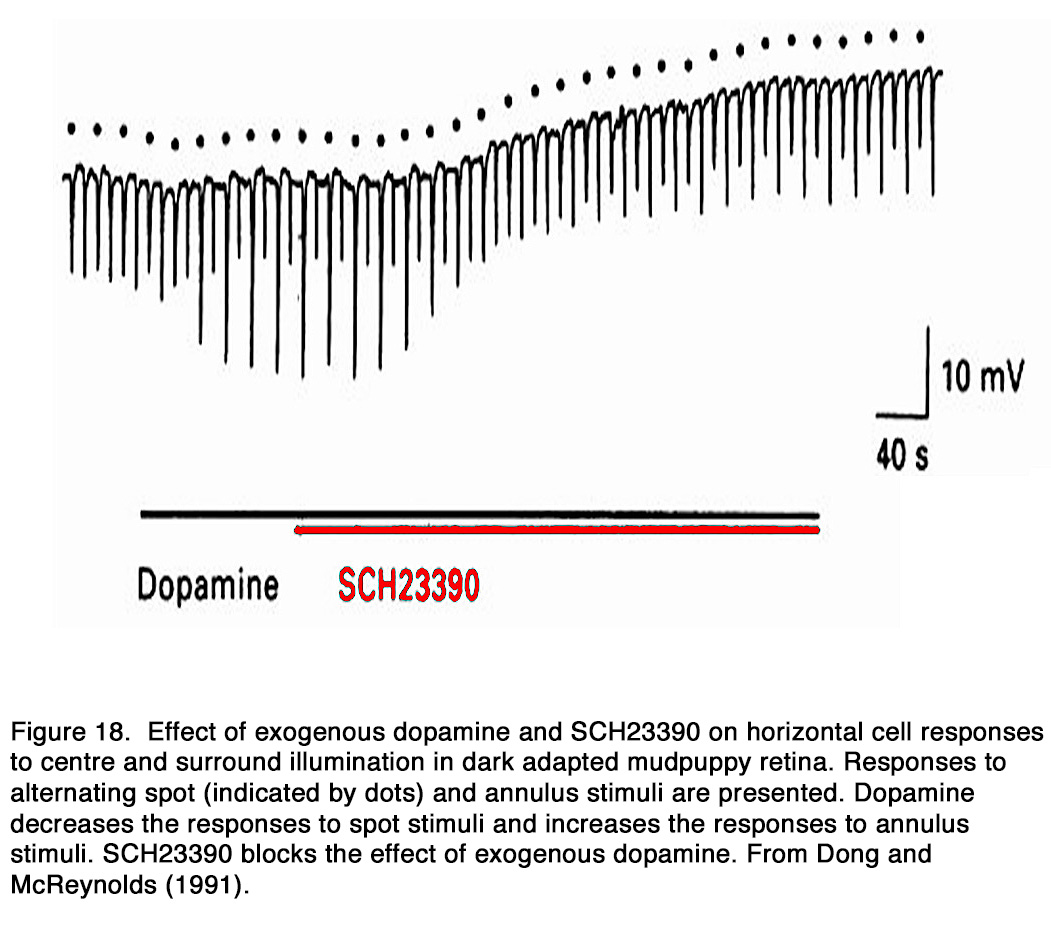
The dopamine action on the electrical coupling between adjacent horizontal cells was studied in a number of species. Exogenous dopamine uncoupled horizontal cells in non-mammalian species: amphibia (162, 176), reptiles (177, 178), fishes (143, 179-187) and mammalian species: rodents (188-190), rabbit (191, 192). As a result, the response amplitude to a centered light spot increased, while that to an eccentric light spot or annulus decreased (64, 143, 177, 178, 180, 186-188, 193-197). According to Tornqvist et al. (64) this effect was seen in the first 5-7 minutes of dopamine application, while application of dopamine for longer periods of time (17–20 minutes) reduced the responses to both small and large spot stimuli. They suggested that the earlier effect was due to the uncoupling of HCs and the latter effect was due to suppressed responsiveness of HCs caused by dopamine (see below). Dopamine-induced uncoupling caused a marked reduction of the HC receptive field size (160, 177, 180, 186, 194, 195, 198, 199). Destruction of retinal dopaminergic neurons with 6-hydroxydopamine, on the other hand, broadened the receptive field profile of HCs (180, 186, 187, 199, 200). According to some authors this effect in fishes was seen only in cone-, but not rod-driven HCs (201, 202), while other authors observed it in rod-driven HCs as well (185, 203). The uncoupling effect of dopamine was mediated by D1-like receptors, because D1-, but not D2-R agonists mimicked the uncoupling effect of exogenous dopamine and the effect was antagonized by D1R, but not D2R antagonists (162, 176, 178, 184, 188, 191, 192, 204) (Figure 18).
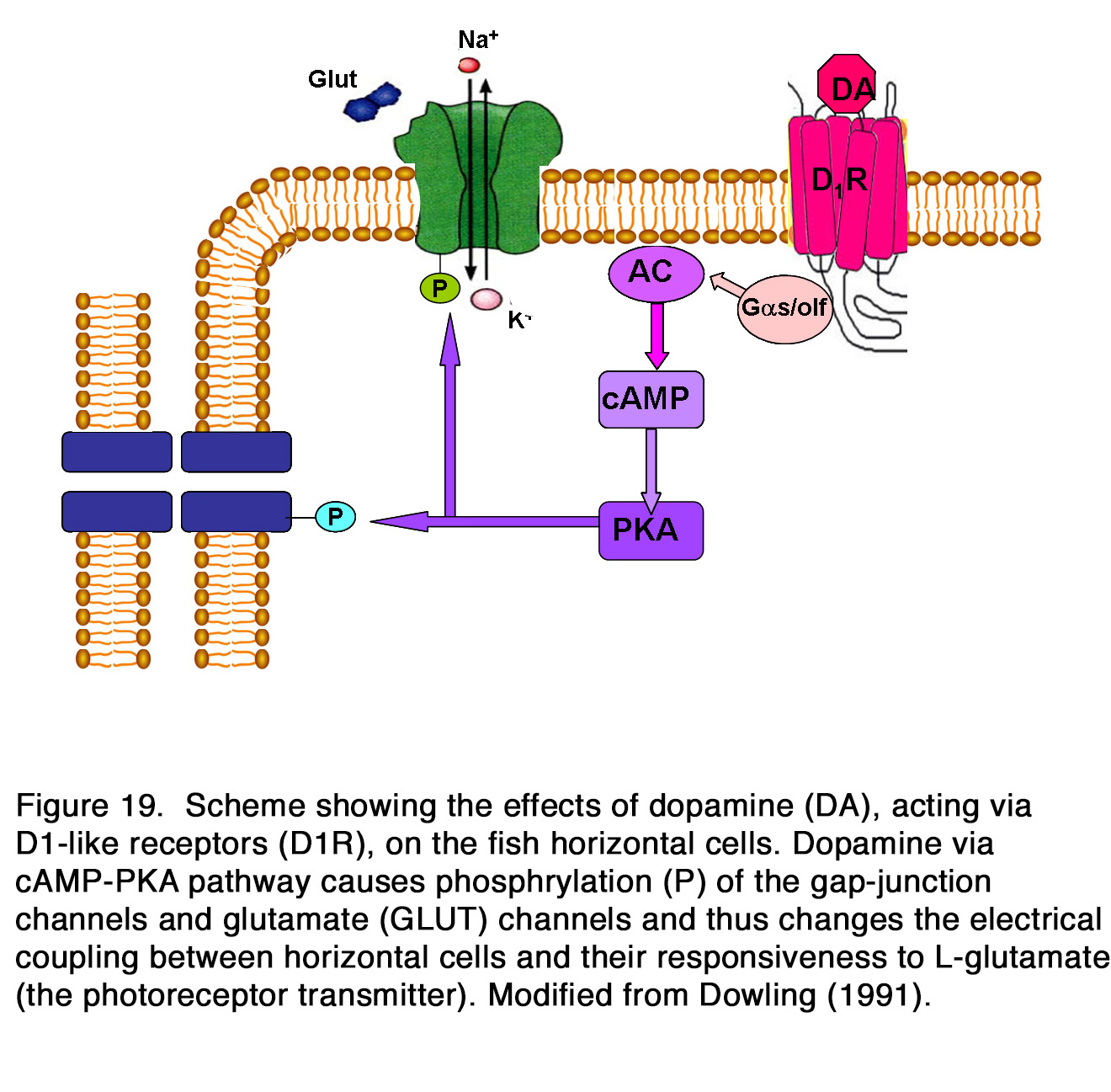
Whether the release of endogenous dopamine uncouples HCs via action on D1R is a matter of debate. Some authors reported that application of D1R antagonists alone had no effect on the coupling between HCs (184, 185, 192, 205), while other authors reported an increase of coupling between HCs and therefore insisted that HC coupling was tonically modulated by the endogenous dopamine (176, 188-191, 204). Activation of D1R in HCs led to cAMP production, the latter likely modified the gap junction conduction between HCs by alteration in protein phosphorylation (Figure 19).
It was reported that elevated levels of cAMP in HCs decreased the electrical coupling between horizontal cells (178, 179, 181, 186, 189-192, 206). The effect in fishes and mice was independent of changes in [Ca2+]i or pH (179, 190), while in rabbit retina, it was pH-gated and thus, may be mediated by a different mechanism (192). Dopamine reduced the open probability of gap-junction channels by reducing both the duration and frequency of channel opening (182, 183). The electrical coupling of HCs may be modulated also by dopamine acting on D2-like receptors. Some authors found that D2R agonists increased the HC coupling and D2R antagonists decreased it (143, 176, 199, 207). The coupling effect of D2R agonists was blocked completely by prior application of D1R agonists or treatment with 6-OHDA. These results supported the suggestion that activation of D2 autoreceptors on dopaminmergic IPCs inhibited dopamine release onto HCs so that the electrical coupling between fish horizontal cells increased (143, 199). In turtle retina, however, the effect of D2 agonists was preserved in the presence of a D1R antagonist, indicating that D2R played a direct role in electrical coupling between HCs (207).
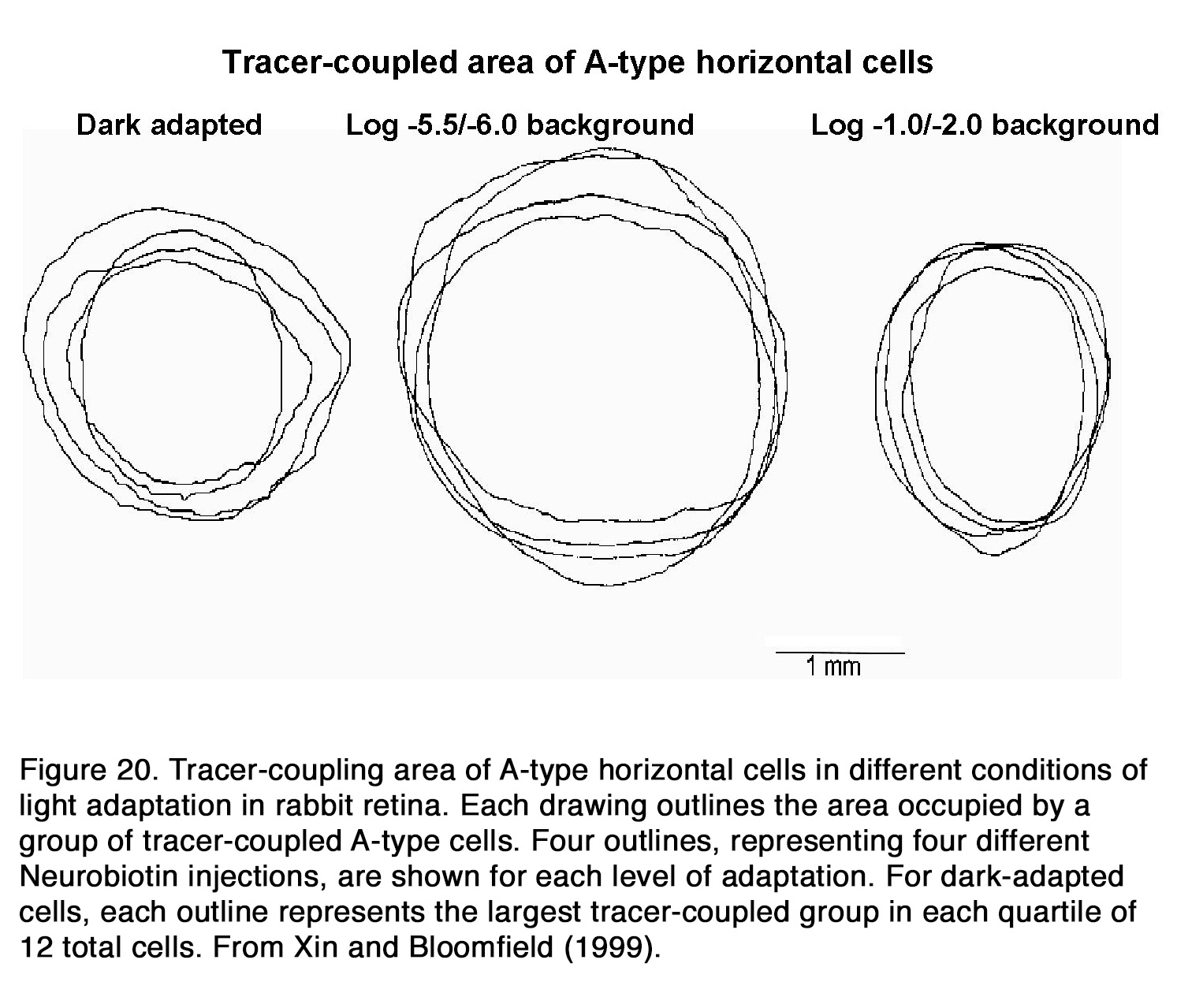
There is no consensus among authors how the electrical coupling and receptive field size of horizontal cells change with retinal adaptation. Some authors insisted that background illumination had no effect on the HC coupling and receptive field size (177, 208), while other authors stated that background light significantly decreased electrical coupling among HCs (176, 184, 204, 209-212). Still other authors reported that electrical coupling between horizontal cells increased during light adaptation (213) and had minimal values during prolong periods in darkness (64, 194, 195, 214). An interesting relationship between retinal adaptation state and HC coupling was suggested for fish and rabbit retina. Baldridge (209) proposed a triphasic model for adaptation changes of teleost H1 cells. He suggested that the HC responsiveness and receptive field sizes were minimal during prolonged darkness but increased considerably when moderate illumination was applied after darkness. Coupling decreased again following exposure to bright illumination. Similar triphasic adaptation was suggested to exist in rabbit HCs (215). It was shown that the coupling between HCs was relatively weak in darkness as well as under background illumination with intensity higher than 3 log units above the rod threshold (Figure 20). The coupling between cells increased only under dim background illumination with intensity from one-quarter to 1.5 log units above the rod threshold (215).
Most authors related the changes of electrical coupling between HCs during dark or light adaptation to changes of endogenous dopamine release. According to some, dopamine mimicked the effect of a prolonged period of darkness, but not continuous light background (64, 195, 214), while others suggested that dopamine was responsible for the uncoupling effect of the adapting light (176, 212). The latter suggestion was supported by the fact that the uncoupling effect of both flickering and steady adapting light was blocked by nonselective DR antagonist fluphenazine (212) and selective D1R antagonist SCH 23390 (176). Some authors argued that dopamine is not the signal of HC adaptation to steady illumination in teleosts, because DA depletion or application of DR antagonists did not alter the effect of background illumination upon fish horizontal cell coupling and receptive field size (209, 211). In the case of flickering light adaptation, however, dopamine did seem to be involved, because haloperidol blocked the reduction of HC receptive field size induced by a flickering light (211). In primate retina, SCH 23390 only partially blocked the effect of background light on the HC receptive field size, suggesting that other mechanisms, in addition to the dopaminergic system, may be involved in background-induced reduction of HC receptive field size (204).
Dopamine has an additional effect on the horizontal cells that is independent of its action on the electrical coupling between adjacent horizontal cells. It decreased the response of fish cone-HCs to full-field stimuli and enhanced glutamate- and kainate-induced currents (64, 143, 159, 194, 195, 199, 216). Thus, dopamine, by enhancing the potency of the photoreceptor transmitter, rendered the light-induced reduction of transmitter release from the photoreceptors less effective, leading to decreased amplitude of the HC response to light. Some authors suggested that dopamine was responsible for the strongly suppressed light responsiveness of cone HCs in prolonged darkness (64, 194, 195, 202).
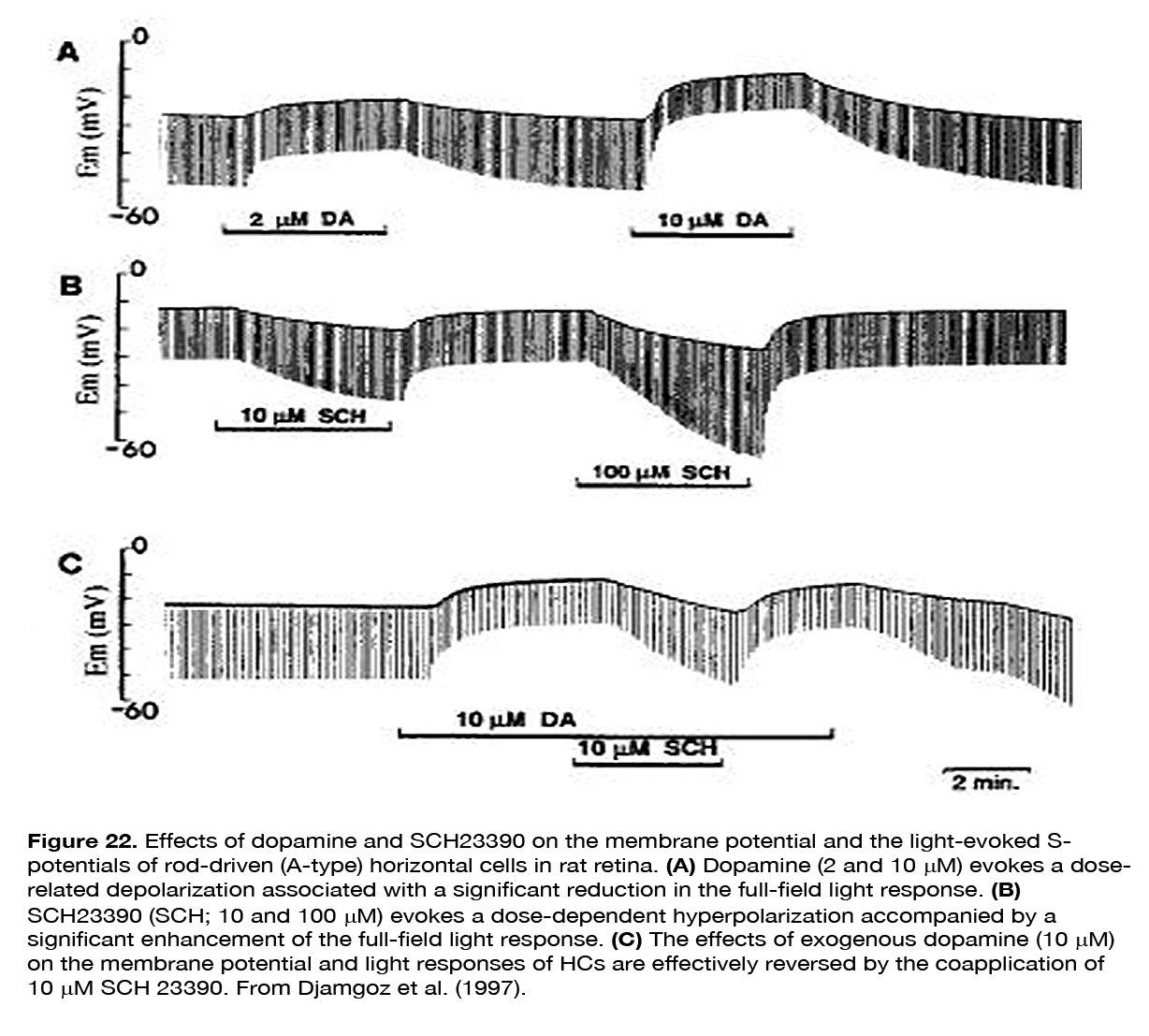
Yang et al. (202) demonstrated that HCs became highly responsive to illumination in dopamine depleted retinas maintained in prolonged darkness (Figure 21). Furthermore, application of DA to dopamine-depleted or light-sensitized retinas strongly suppressed the responsiveness of cone HCs, mimicking the effects of prolonged darkness. The effect was probably mediated by D1-like receptors, because SCH 23390 (a specific DlR antagonist), enhanced the responsiveness of cone HCs in prolonged darkness, mimicking the effect of background illumination. It was suggested that DA was released in the dark by the IPCs and enhanced the potency of the photoreceptor transmitter (202, 217). Dowling (217) proposed a hypothesis that dopamine increased cAMP which activated kinases that phosphorylated both glutamate-gated channels and gap-junction channels (Figure 19). Destruction of dopaminergic neurons significantly decreased, but not fully eliminated the suppressive effect of prolonged darkness on responsiveness of fish cone HCs in vivo (218). The latter result suggests that a substance or substances other than dopamine may contribute to the dark suppression effect. In turtle retina, dopamine reduced the responsiveness of all except L1-type horizontal cells (160). A reduction of the response amplitude to full field stimuli caused by dopamine was observed in mammalian rod-driven HCs (188, 219). The effect was mediated by D1-like receptors, because it was reversed effectively by the co-application of the D1R antagonist SCH 23390, but not by the D2R antagonist L-sulpiride (219) (Figure 22).
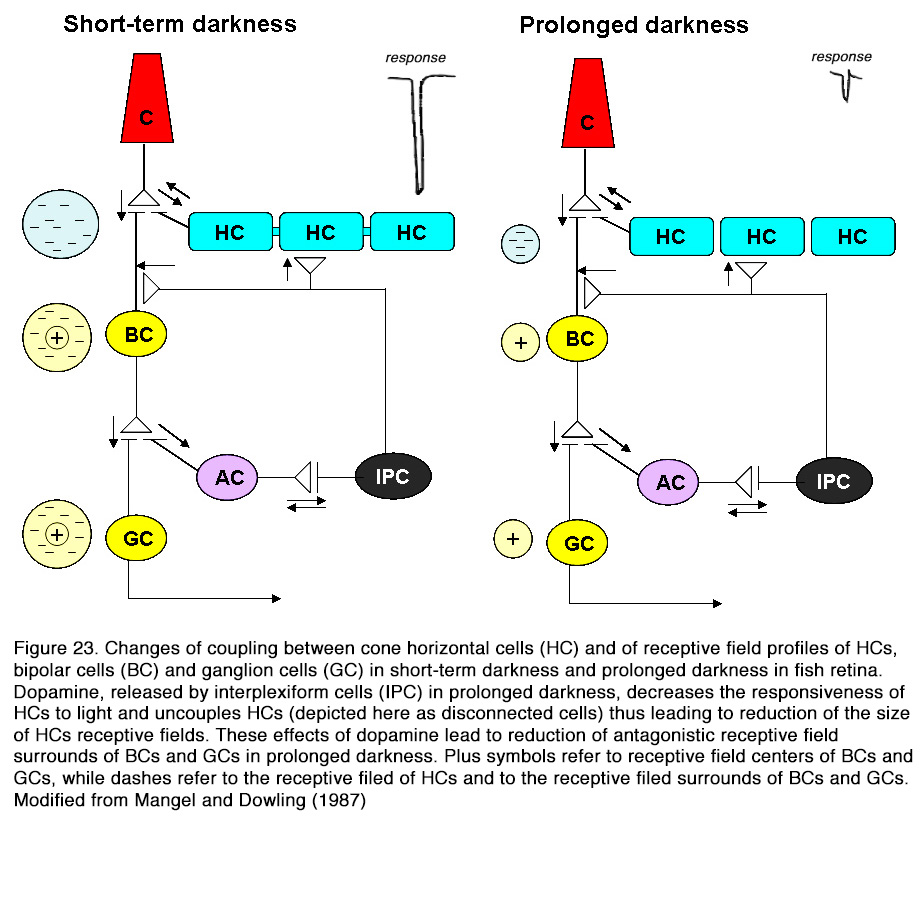
SCH 23390 alone, but not sulpiride, enhanced the responses to full-field light stimuli, revealing an active endogenous DA input to HCs. Because the DA effect on HCs was not seen in preparations with blocked synaptic transmission, Hankins and Ikeda (188) suggested that dopamine action resulted from modulation of the L-glutamate activated currents of these cells. The described two effects of dopamine on horizontal cells – decreasing light responsiveness and shrinking receptive field size – are effective ways to lessen HC influence upon the other retinal neurons. It was suggested (159, 194, 195) that these effects of dopamine may account for the reduced or fully eliminated antagonistic surrounds of ganglion cells with prolonged time in the dark (Figure 23). Baldridge (209) proposed that the reduction of HC receptive field size following light adaptation would narrow the receptive filed surround of GCs and therefore permit spatial contrast analysis to be done more locally.
Much data indicate that dopamine effects on the HC light responses depend on the photoreceptor input. Separate horizontal cells in fishes receive inputs from cones or rods. It was shown that dopamine had apparent effects on light responses of cone-driven HCs, while contradictory results exist concerning its effects on the light responses of rod-driven HCs. Some authors found that dopamine effects on the rod-driven HCs were similar to those on cone-driven HCs (196), other authors obtained much smaller effects on rod- than cone-driven HCs, as in perch (64, 195, 202) and still other authors found no dopamine effects on the rod-driven HCs in skate (208), dogfish (220) or goldfish (159). In amphibian retina, where horizontal cells receive converging synaptic inputs from both rods and cones, dopamine enhanced the amplitude of the cone-mediated component of the HC response, but diminished the amplitude of the rod-mediated one (161, 162, 164, 221). The authors cited suggested that dopamine decreased the gain of the rod-HC synapse and increased the gain of the cone-HC synapse. Dopamine action was probably mediated by postsynaptic D1-like receptors, because D1 activation eliminated the rod component of the flash response and caused the HCs to display all the signs characteristic of the photopic state (164). The effect of injecting cAMP was identical to that obtained by the application of the D1R agonist or by adapting the retina with strong background light. Krizaj (164) concluded that dopamine via D1R acted as a postsynaptic switch modulating the balance between the rod and cone pathways, and that intracellular cAMP could play an important role in regulating the relative weight of rod and cone information in retinal cells. Other authors argued, however, that the dopamine effect on the rod-cone balance in amphibian HCs was mediated synergistically by both D1– and D2-like receptors (162, 221). How dopamine influences the balance between rod and cone inputs to mammalian HCs is not well evaluated. Ribelayga and Mangel (170) reported that the blockade of D2R, but not D1R during the day increased the absolute and relative light sensitivity of rabbit A-type HCs, so that their responses resembled those typically recorded at night. The authors proposed that the retinal circadian clock increased the dopamine levels and D2R activation in the outer retina during the day, so that rod input (via rod-cone coupling) to A-type HCs was greatly reduced. On the other hand, Pflug et al. (222) found that D1R agonists depolarized somata of both A- and B-type HCs in rabbit retina and enhanced the overall and flicker amplitudes of their scotopic responses, but almost completely suppressed the cone flicker amplitudes of the photopic responses, without changing the overall response amplitude (222). The authors proposed that the dopamine-induced HC depolarization caused a shift of the cone ICa activation curve to more positive values and thus reduced the transmission of the most hyperpolarizing cone signal component, i.e. the cone flickering part of the light response. Pflug et al. (222)imply that shifting the operating range of cone synapses to more depolarized levels increases the gain for low amplitude rod signals which enter cones through rod-cone gap junctions but did not elaborate on the mechanism, whether at the rod-cone gap junctions, or at the tuning of the cone synapse itself. More studies are needed to fully understand how the dopamine action upon mammalian HCs depends on the photoreceptor input.
Dopamine may participate in the control of spectral responses of cone luminosity (H1) and chromaticity (C-type) horizontal cells in fish and turtle retina. Mangel and Dowling (195) reported that dopamine reduced the responses to long wavelength full-field light stimuli more than the responses to short wavelength stimuli in one third of carp H1 cells. Contrary to this observation, other authors found that dopamine increased the responses of carp H1 cells to long wavelengths (223, 224), but had no effect (224) or decreased (223) responses to short wavelengths. In the latter case, dopamine decreased the blue/red response amplitude ratio in a way similar to that induced by red-background adaptation (223). The effect of light adaptation, however, was also recorded in dopamine depleted retinas and in the presence of haloperidol, indicating that dopamine was not responsible for the effect of light adaptation on the spectral response characteristics of H1 cells. Djamgoz et al. (223)suggested that spectral responses of H1 cells were controlled independently by light-adaptation (primarily by long wavelengths) and dopamine. The effects of dopamine on C-type HCs are not well understood. No differential spectral response effects of dopamine on C-type HCs were observed in turtle (160) or carp retina (195). In goldfish retina, dopamine increased the hyperpolarizing component evoked with white light, but decreased the depolarizing component evoked with red light in half of C-type HCs, while in the other half it had no effect irrespective of the cell subtype (159). In roach retina, haloperidol increased the hyperpolarizing component (to blue-green light), but decreased the depolarizing component (to red light) of biphasic (green/red) H2 horizontal cells (225). In addition, haloperidol prevented the enhancement of the depolarizing component induced by light adaptation. Destruction of the DA cells with 6-OHDA also prevented the enhancing effect of red flickering light on the depolarizing response of R/G HCs in carp retina (226). The effect was probably mediated by D2R, because application of D2R, but not D1R antagonists was similar to that of 6-OHDA. Liu et al. (226) suggested that the activation of D2R in the OPL might be related to the synaptic plasticity of the GABAergic pathway between HCs and cones, which is responsible for the change in red flickering induced responsiveness in the R/G HCs.
Effects of dopamine on bipolar cells
There are not many studies undertaken to evaluate dopamine action on retinal bipolar cells; however, the effects of DA on the ionic currents (Ca2+, Na+, K+), membrane potential, light-evoked responses and responses to other neurotransmitter of retinal BCs have been reported. It was shown that dopamine and D1R agonist ADTN potentiated the voltage dependent Ca2+ currents in presynaptic terminals of all bipolar cells in fish retina (227, 228). The effects of dopamine antagonists on the same currents, however, depended on the BC type (ON or OFF) (227). The D1R antagonist SCH 23390 strongly suppressed Ca2+ currents at all stimulus intensities in OFF BCs but in ON BCs, SCH 23390 augmented Ca2+ currents at lower stimulus intensities but did not change them at higher intensities. The D2R antagonist sulpiride had similar effect as SCH 23390 upon the ON BCs, while it enhanced the Ca2+ currents at lower stimulus intensities and diminished them at higher intensities in the OFF BCs. The mechanism for the differential effects of dopamine antagonists on the ON and OFF pathways is still not clear. In amphibian retina, dopamine had no apparent effect on Ca2+ entry into the bipolar terminals, but it blocked the GABAergic inhibition on this entry and thus increased the transmitter release (229). The effect was similar for the ON and OFF BCs. In addition to Ca2+ currents, dopamine, via D1R, modulated the voltage-gated Na+ currents in amphibian transient ON BCs (230). The dopamine receptor agonist ADTN markedly reduced the Na+ currents in dim light conditions, while SCH 23390 prevented the depressing effect of light adaptation on these currents. Dopamine modulated also the K+ currents in retinal BCs. Dopamine acting via D1R-coupled G-protein pathways decreased the voltage-dependent K+ currents in ON BCs in zebrafish retina (231). In goldfish retina, dopamine depressed K+ currents but only in high concentrations, while in low concentrations it enhanced these currents in mixed rod-cone ON bipolar cells (Mb) (232, 233). Both the enhancement and suppressive effects were blocked by SCH23390, indicating involvement of D1-like receptors. Fan and Yazulla (233) proposed that D1R activation should make the ON response of Mb BCs more phasic by enhancing K+ currents. Depletion of retinal dopamine reduced by about half the amplitude of voltage-dependent K+ currents in Mb BCs (234).
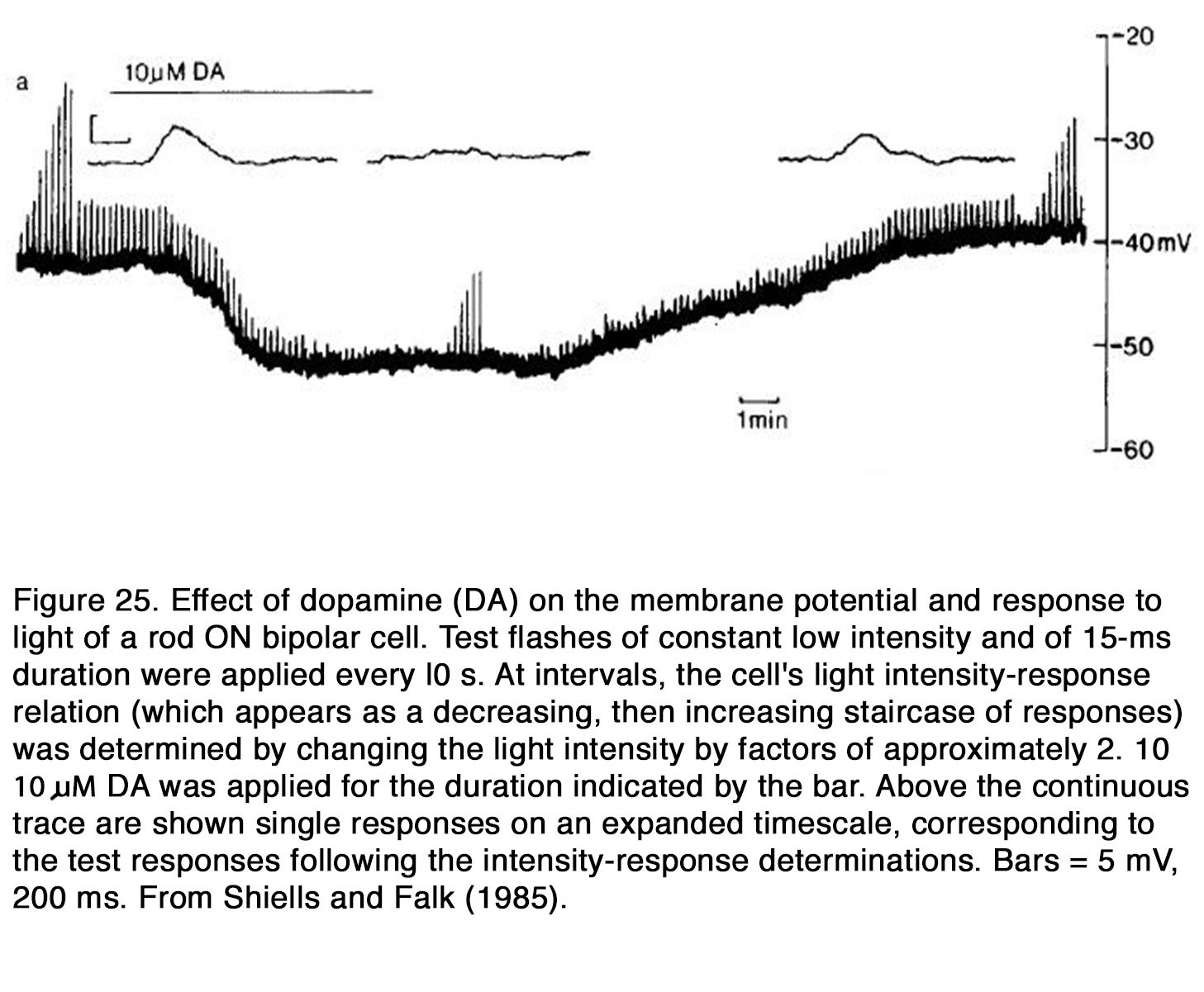
The actions of dopamine on membrane potential and light-evoked responses of retinal BCs were investigated mainly in fish and amphibian retina. In fish retina dopamine produced a hyperpolarization of the membrane potential of cone BCs and rod BCs (159, 220, 235). Dopamine enhanced the center light responses, but it reduced the antagonistic surround responses of both ON and OFF BCs under conditions in which the responses were mediated by cones (159, 235) (Figure 24). This effect is consistent with the suppressive action of dopamine on HC responsiveness, because the latter cells are thought to mediate the antagonistic surround of BC receptive field (68). In the all-rod retina of dogfish, dopamine reduced the responses to light flashes of ON RBCs (220) (Figure 25).
The suppressive effect of dopamine on RBCs was not due to its effect on HCs, because neither the membrane potential nor the light responses of rod HCs were influenced by dopamine. Thus, it appears that dopamine enhanced the responses of fish cone BCs but suppressed the responses of rod BCs.
In amphibian retina, where bipolar cells receive mixed rod and cone inputs, dopamine produced variable effects upon the dark membrane potential of OFF BCs, but upmodulated their glutamate-gated currents through D1R and cAMP-PKA pathway (236). Dopamine caused a significant reduction in the amplitudes of rod-driven responses, while it enhanced the cone-driven responses elicited from the receptive field center (162). It did not change the balance between center and surround of the BC receptive field, when the responses were rod-driven, but it favored the center over surround, when the responses were cone-driven. Opposite results were reported for the action of dopamine on the light responses of cone-driven transient ON BCs in the same species (230). It was shown that D1 agonist SKF38393 reduced the L-EPSPs in dim light conditions and thus mimicked the effect of bright light. On the other hand, D1 antagonist SCH23390 blocked the suppressive effect of bright light on L-EPSPs. The effect was seen in transient, but not sustained ON BCs. The authors concluded that dopamine released in bright light, attenuated the sodium channel-dependent amplification of L-EPSPs in transient cone-driven ON BCs and thus prevented response saturation. It remains to be determined if the opposite, depressing effects of D1R activation on the cone-mediated responses of ON BCs (230) and enhancing effects on OFF BCs (162) are species specific or they represent general ON/OFF asymmetry in dopamine action on BCs. The effects of dopamine on the mammalian bipolar cells are largely unknown. It was shown that dopamine induced changes of receptive field organization of rabbit ON-CBCs in the dark similar to those observed during light adaptation (237). During dopamine treatment, the ON-CBCs exhibited center and surround responses similar to those of bright-light-adapted ON-CBCs in intact retinal preparations. The dopamine effect was probably mediated by D1-like receptors, because the surround responses were not observed during D1R blockade but were preserved during D2R blockade.
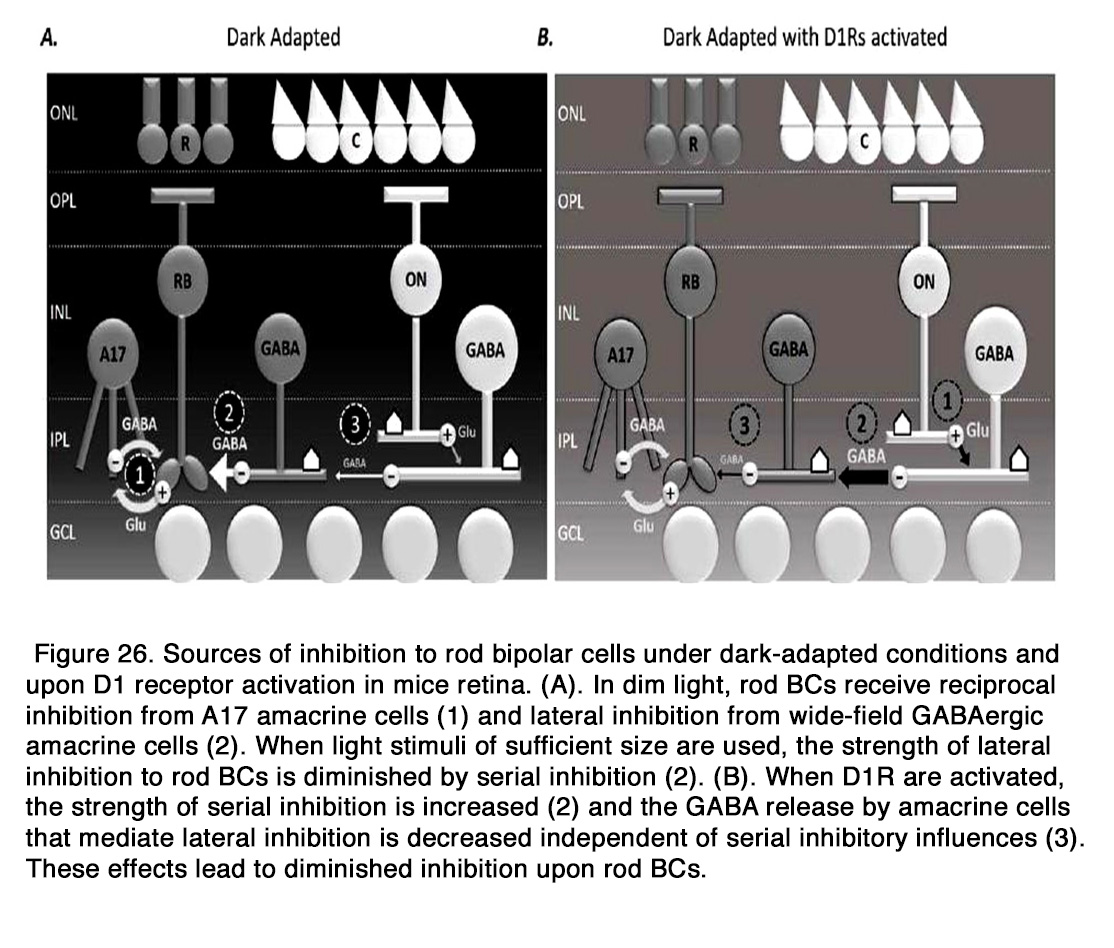
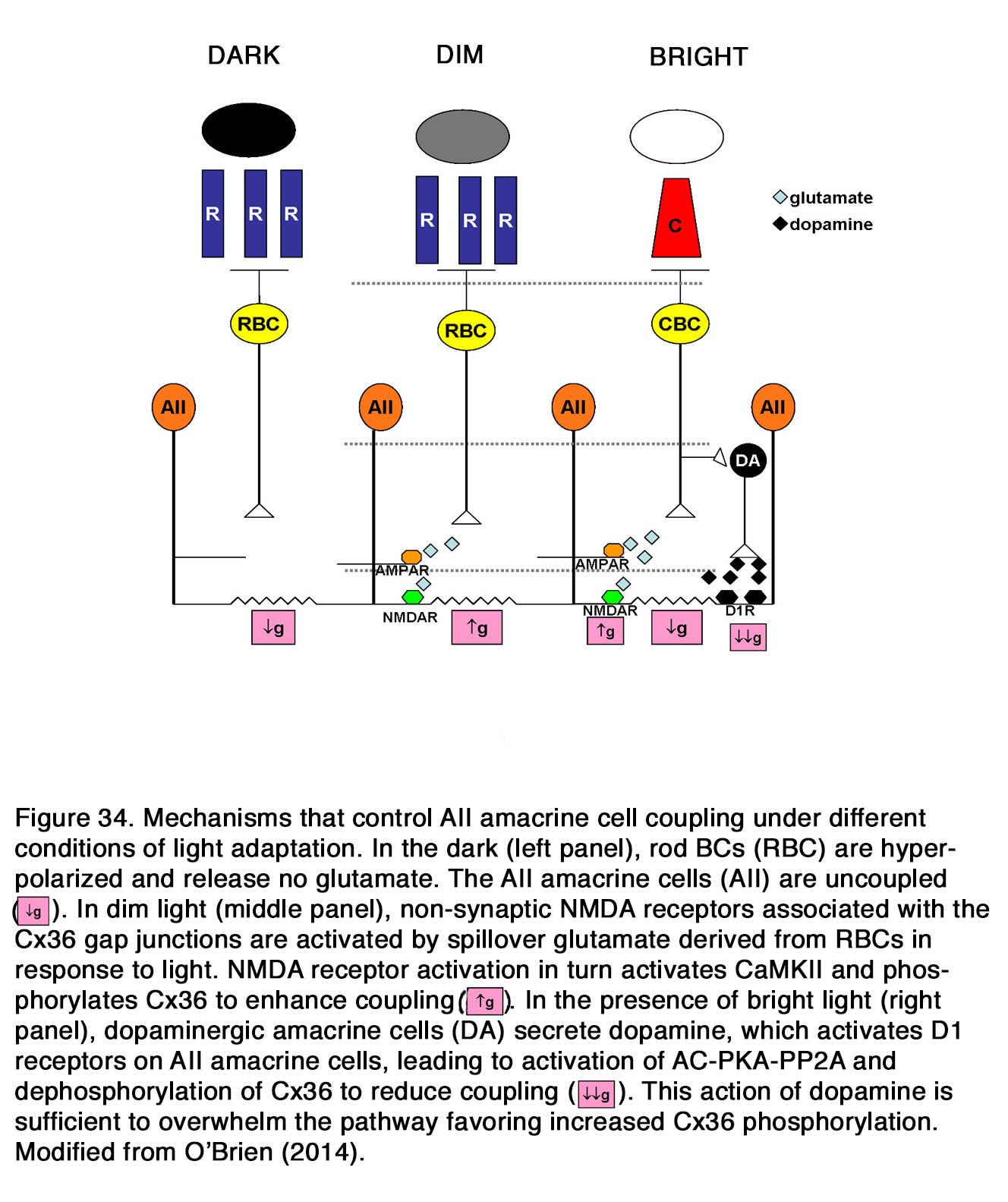
Dopamine may modulate the responses of bipolar cells to other neurotransmitters such as glutamate and GABA. Dopamine enhanced the response to glutamate in amphibian OFF BCs (236). The effect was mediated by D1-like receptors, because it was blocked by D1R antagonist SCH 23390, but not the D2R antagonist spiperone. Activation of D1 receptors in the dendrites of bipolar cells led to stimulation of Adenyl cyclase, which through cAMP and PKA enhanced a glutamate-gated current in BC dendrites. Dopamine plays a very important role in modulating the GABAergic inhibition onto BCs as a part of the light adaptation process. It was shown that activation of D1R significantly decreased the frequency and amplitude of spontaneous inhibitory postsynaptic currents (S-IPSCs) and the amplitudes of light-evoked inhibitory postsynaptic currents (L-IPSCs) evoked by full-field stimuli in dark adapted mouse rod BCs, the effect being similar to light adaptation (238, 239). Flood et al. (238) showed also that D1R activation directly diminished the inhibitory output of non-reciprocal ACs onto rod BCs, in the absence of serial inhibition. They proposed the following model for the inhibition of rod BCs under dark adapted conditions and D1R activation. In dim light, rod BCs receive reciprocal inhibition from A17 amacrine cells and lateral inhibition from wide-field GABAergic ACs, as well as narrow-field glycinergic ACs. When light stimuli of sufficient size are used, the strength of lateral inhibition to rod BCs is diminished by serial inhibition. When D1Rs are activated, the inhibition to rod BCs is greatly decreased, because the strength of serial inhibition is increased and the GABA release by ACs that mediate lateral inhibition is decreased (Figure 26).
However, unlike light adaptation, D1R activation did not shorten L-IPSC decays in rod BCs and its effect on the amplitude of L-IPSCs was smaller than that of light adaptation, indicating that some D1R independent component also played a role in the modulation of rod BC inhibition with light adaptation (238). Dopamine modulated also the GABAergic inhibition on the cone BCs. It was recently shown that bright-light-induced activation of D1Rs located on the ON CBC dendrites in rabbit retina increased the expression and activity of GABAA receptors on the dendrites of the cell (237). Endogenous activation of these receptors participated in the generation of the surround light responses of CBCs, which were diminished in maintained darkness and during D1R blockade (with SCH 23390). Dopamine via D1R modulated also the local glycinergic, but not GABAergic inhibition to mouse OFF cone bipolar cells (239). D1R activation significantly decreased the frequency and amplitude of S-IPSCs and L-IPSCs in OFF CBCs, evoked by local light stimuli in the dark (238, 239). The effect of D1R activation on the glycinergic L-IPSCs was similar, but not identical to the effect of light adaptation. D1R activation had no significant effect on the time course of the glycinergic L-IPSCs, while those currents become more transient with light adaptation. Mazade et al. (239) concluded that D1 receptors were not the only neuromodulator influencing light adapted changes in local inhibition of OFF BCs.
The overall activity of BCs in living animals could be monitored by recording the electroretinogram (ERG), because the b-wave (in response to stimulus onset) and the d-wave (in response to stimulus offset) are thought to depend mainly on the activity of ON and OFF bipolar cells, respectively, as reviewed by Frishman (240), see also Webvision: The Electroretinogram. Thus, the significance of dopaminergic neurotransmission for bipolar cell function could be investigated by exploring the effects of dopamine and DR agonists and antagonists on the ERG b- and d-waves.
Effects of dopamine on the diffuse electroretinogram
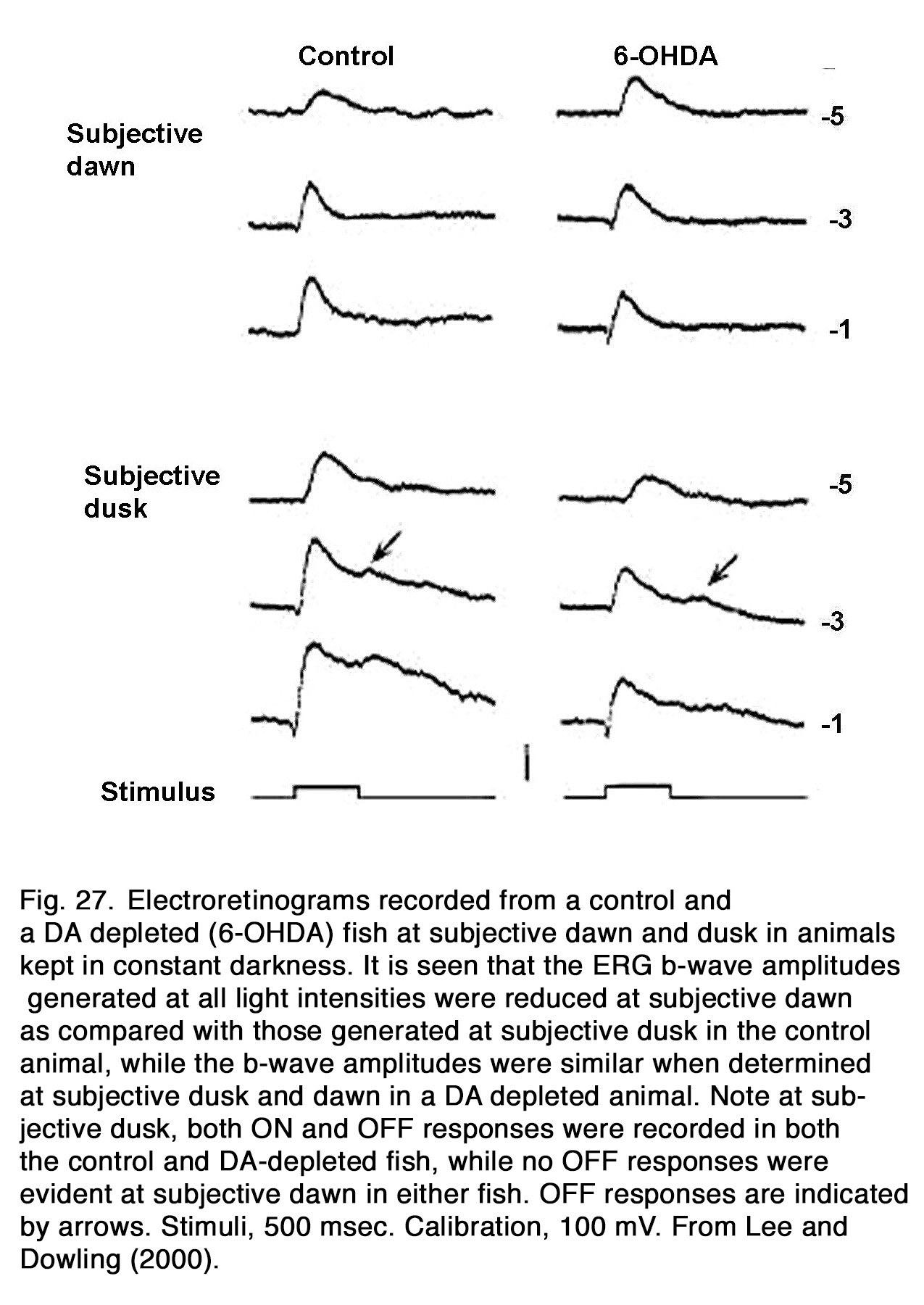
The ERG b-wave amplitude displayed a circadian rhythm in constant conditions in fishes (241), birds (76, 242-244), reptiles (245, 246), and mammals: rabbit (247, 248), mouse (249-252). Generally, the b-wave amplitude was highest during the subjective day and lowest during the subjective night. In dopamine depleted nonmammalian animals, no circadian rhythm of the b-wave amplitude was seen (Figure 27), indicating that retinal endogenous dopaminergic system is essential for the circadian rhythm of the b-wave amplitude as seen in zebrafish (241), iguana (74) and quail (76). The circadian rhythm was markedly changed during D2R, but not D1R manipulation in quail, indicating involvement of D2R mechanisms in its generation. Li and Dowling (241) found, however, that not all circadian effects were absent in dopamine-depleted fishes. In the latter animals, an ERG OFF-response was observed at subjective dusk, but not at subjective dawn, as also found in the control fishes (Figure 27). In mice, the cone-, but not rod-mediated ERG was under circadian control (249-251, 253). The role played by endogenous dopamine in this control is an object of debate. Sengupta et al. (251) observed that the circadian regulation in the photopic ERG was absent in mice lacking melatonin receptor type 1, although the circadian regulation of dopamine turnover was preserved. These results led to the suggestion that the circadian regulation of dopamine turnover did not drive the mouse cone-mediated ERG rhythm. Jackson et al. (253) reported, however, that the circadian rhythm of the light-adapted b-wave amplitude was lost in mice lacking retinal dopamine (with disrupted tyrosine hydroxylase), indicating that endogenous dopamine was essential for the circadian rhythm of the mouse ERG. This action of dopamine was probably mediated by D4 receptors, because the circadian rhythm was abolished in D4R-KO mice, but not in D1R-KO mice. However, D4Rs did not maintain circadian rhythm at DD1 (18–30 h in dark), since a substantial b-wave decrease was observed at midnight relative to midday in D4R-KO mice. Smith et al. (254) presented evidence that the robust circadian rhythm seen after 6–18 h in dark (DD1) was controlled predominantly by D1R and Nav channels. They proposed that circadian modulation of cone pathway function in mice involved an early mechanism based on D1Rs and Nav channels on ON-CBCs that acted in the short-term time scale (DD1, 6–18 h) and a longer-term modulation taken over by a mechanism based on D4Rs affecting persistent circadian rhythms after 42 h (DD2). Melanopsin-containing retinal ganglion cells probably are also involved in the circadian regulation of mouse cone-mediated b-wave, because genetic ablation of melanopsin blunted the circadian rhythm of b-wave amplitude and speed (249). Barnard et al. (249) suggested that melanopsin-containing retinal ganglion cells could influence dopaminergic amacrine cells via gap junctions and thus entrain a local retinal clock in inner retina.
There is no consensus among the authors about the role played by the dopaminergic system for the non-circadian regulation of ERG b-wave amplitude in nonmammalian retinas. In fish retina, some authors observed no apparent changes of the b-wave amplitude during blockade of retinal dopaminergic transmission in either dark or light adapted eyes (241, 255, 256), while other authors reported that the D1R agonist SKF 38393 as well as exogenous dopamine increased, while D1R antagonist SCH 23390 either decreased the cone-mediated b-wave amplitude (257), or led to a separation of the signal into transient peaks, emphasizing those components, and sharpening the peaks (258). Manipulation of D2R had opposite effects to those described for the manipulation of D1R (257, 258) (Figure 28).
The conflicting results obtained in fish retina may be due in part to differences in light stimulation used by the authors. While Kim and Jung (257) used a small spot light, other authors (241, 255, 256) used full field light stimuli. This suggestion is supported by the fact that meclofenamic acid (a non-specific gap junction blocker), which had similar action to that of dopamine on the fish ERG, had 5 times greater effect on the b-wave obtained with small spot than large spot (257).
In amphibian retina, a reduction of the suprathreshold b- and d-wave amplitudes was reported during blockade of the dopaminergic transmission (259-261). The isolated blockade of D1R with SCH 23390 and D2R with sulpiride had differential effects on the ERG waves depending on photoreceptor input (262-264). Both blockers enhanced the rod-mediated suprathreshold b- and d-waves, while the action on the cone-mediated responses was in an opposite direction. The D1R blocker enhanced the amplitude of the cone-dominated b- and d-waves, while D2R blocker diminished them (Figure 29).
These results suggested that activation of D1R by endogenous dopamine inhibited frog ERG ON and OFF responses, which was opposite to its action on fish photopic ON response (257). The inhibitory effect of endogenous dopamine on the frog b- and d-wave amplitudes may be due to D1R-induced GABA release, because this effect was prevented during ionotropic GABA receptor blockade (265).
In chicken retina, exogenous dopamine had no effect (266) or it had diverse effects on the b- and d-wave amplitudes depending on light stimulation conditions (267). The blockade of dopaminergic transmission (with haloperidol) caused a reduction of the b-wave amplitude at all stimulus intensities, indicating that endogenous dopamine had an enhancing effect on the chicken ERG ON response recorded under conditions of dim ambient illumination (268). In quail, the b-wave amplitude was reduced by the D2R agonist quinpirole injected during the subjective night and increased by D2R antagonist eticlopride injected during the subjective day, while manipulation of D1R had no effect (76). These results suggest that endogenous dopamine via D2R decreased the b-wave amplitude during the day in quail. In iguana, the manipulation of D1R had no effect on the dark-adapted b-wave, while D2R manipulation had opposite effects to those observed in quail (74). Quinpirole increased the amplitude of the b-wave, when it was injected during the subjective night and eticlopride reduced the b-wave amplitude, when it is injected during the subjective day. Miranda-Anaya et al. (74) suggested that endogenous dopamine increased the b-wave amplitude during the subjective day directly via D2R and indirectly by inhibiting melatonin synthesis. Because the phases of the rhythm of dopamine synthesis were similar in quail and iguana (74, 76), whereas the phase of the rhythm of b-wave amplitude and the effect of quinpirole and eticlopride were opposite, it seemed likely that dopamine exerted its effects on the ERGs of the two species by different mechanisms.
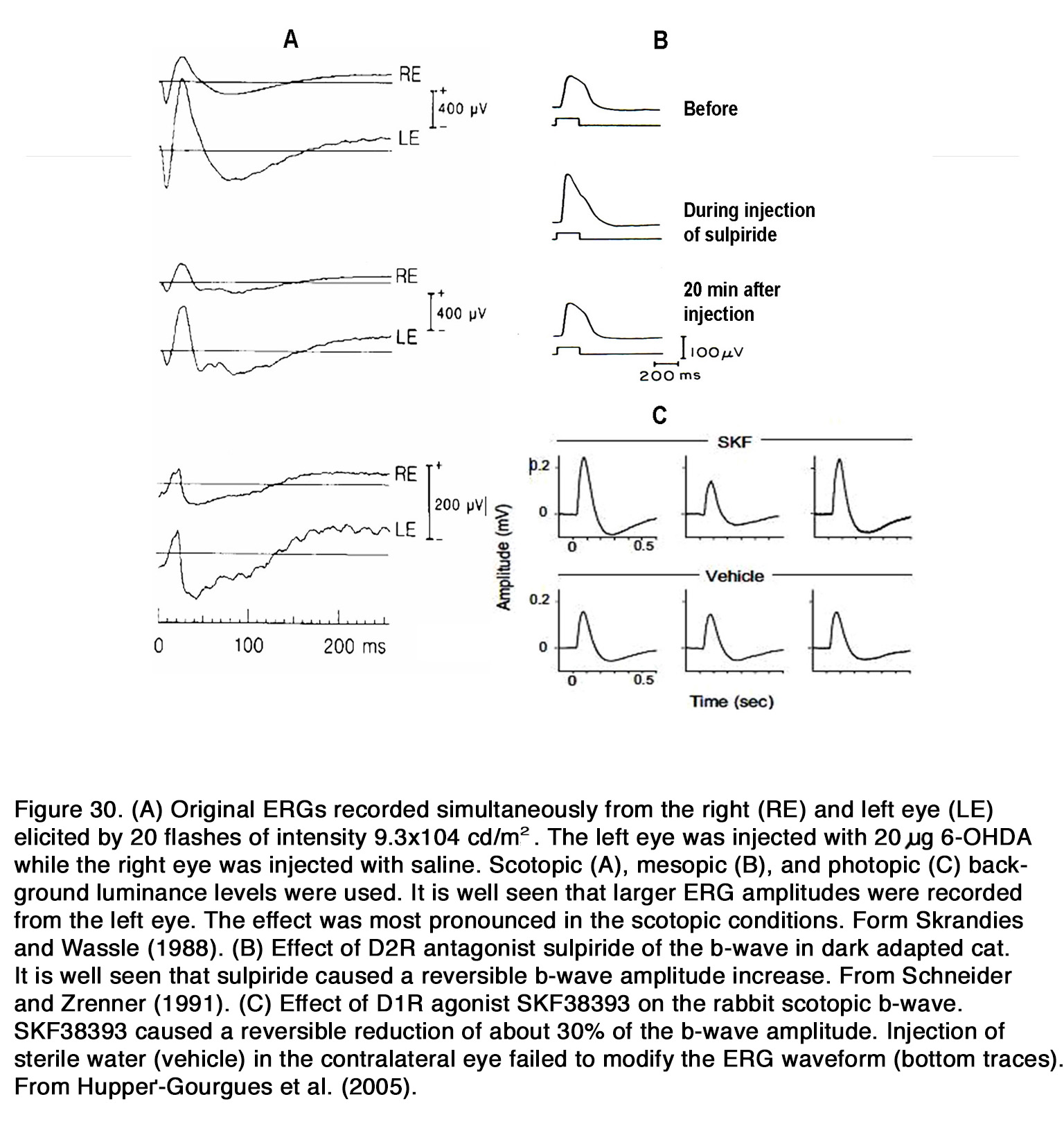
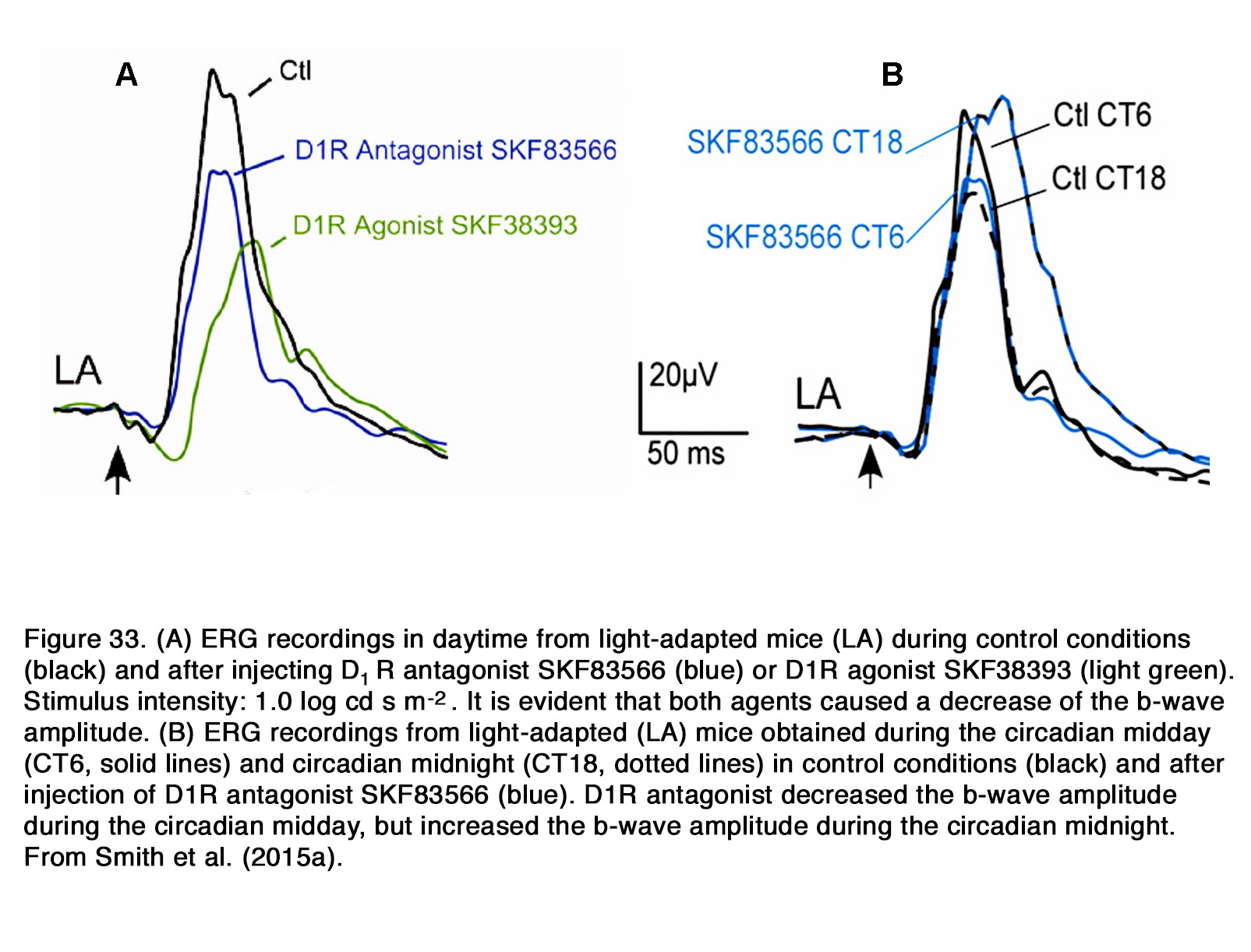
The dopaminergic modulation of mammalian ERG was investigated in many studies. Most of the authors reported that application of exogenous dopamine, its nonselective agonist apomorphine and the dopamineuptake blocker nomifensine decreased the amplitude of the b-wave as seen in rabbit (269-274) and cat (275, 276).The blockade of dopaminergic transmission, on the other hand, caused an increase of the suprathreshold b-wave amplitude as seen in cat (275, 277, 278), rabbit (272, 279) and monkey (280) without altering the dark adapted threshold ERG (275). The b-wave V – log I curve was shifted to the left along the intensity axis, indicating an increased relative sensitivity of the response during dopaminergic blockade (277). The effects of dopaminergic manipulation were maximal under scotopic conditions but were also seen in mesopic and photopic conditions (270, 278) (Figure 30 A).
All these results suggested that endogenous dopamine had a general suppressive influence on retinal ON channel activity in rabbits, cats and monkeys and that this influence did not depend critically on conditions of light adaptation. The DA receptors involved in this suppressive action appear to differ among species. In cats, the inhibitory effect of endogenous dopamine on the ERG b-wave was probably mediated through D2-like receptors, because sulpiride, similar to 6-OHDA, caused an increase of the scotopic b-wave amplitude (277, 278) (Figure 30 B). In contrast to cats, in rabbits the inhibitory action of exogenous dopamine and apomorphine upon the b-wave amplitude was probably mediated by D1-like receptors, because D1R agonists (SKF38393, A77636), but not a D2R agonist (NPA) decreased the b-wave amplitude in all conditions of illumination (281) (Figure 30 C). Contrary to these species, in patients with Parkinson’s disease, who had lower than normal retinal dopamine concentration, a reduction of both the scotopic and photopic b-wave amplitude was reported (282-284). Treatment with L-DOPA in these patients increased the amplitude of the scotopic and photopic b-wave (285), while treatment with L-DOPA in healthy humans increased the scotopic, but not the photopic b-wave amplitude (286-288). It appears that endogenous dopamine has an overall stimulating action on the ON-BC activity (reflected in ERG b-wave) in human retina opposite to its suppressive action in monkey, rabbit and cat retina. What types of dopamine receptors mediate this stimulating action in human retina is not determined yet.
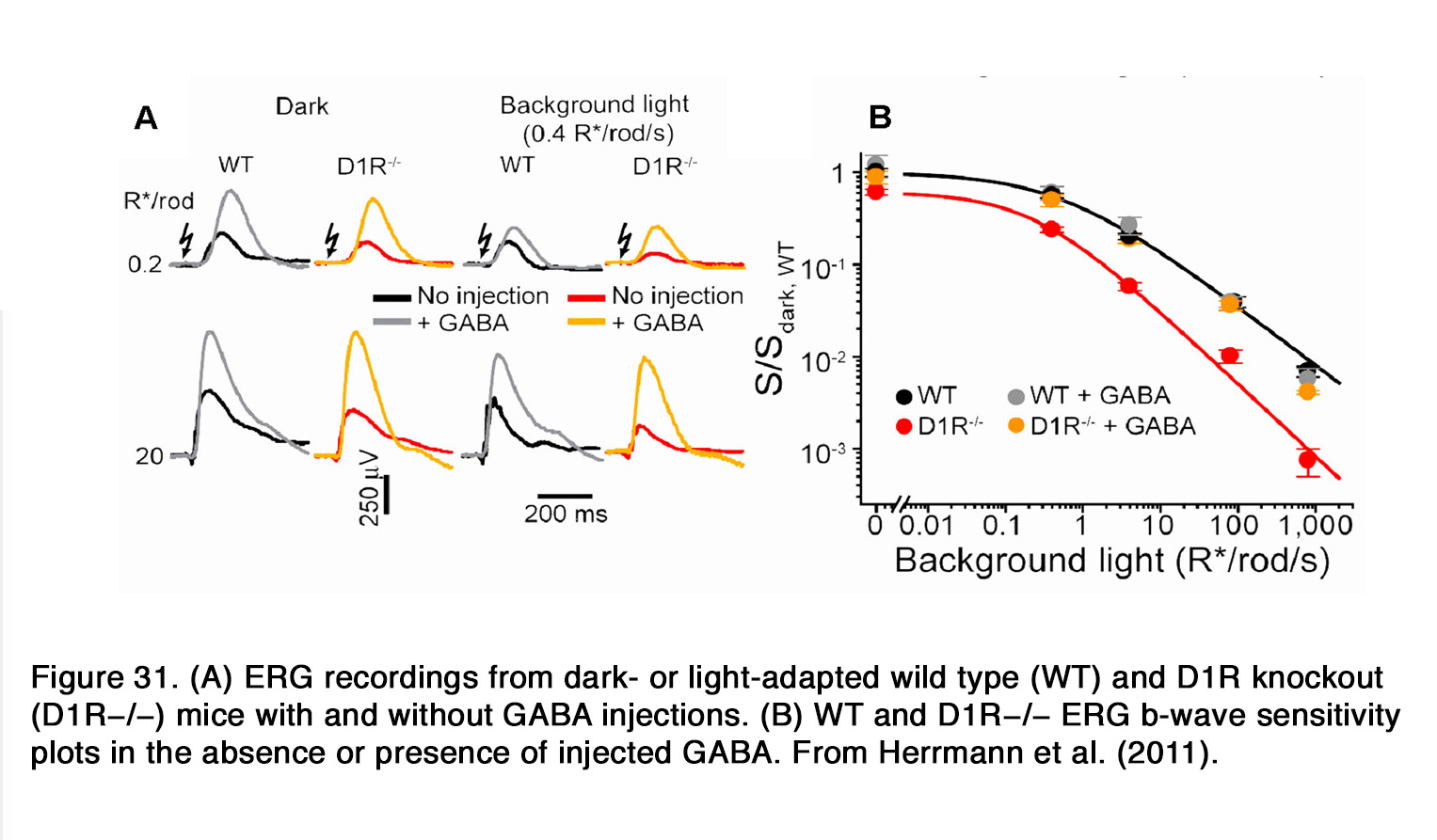
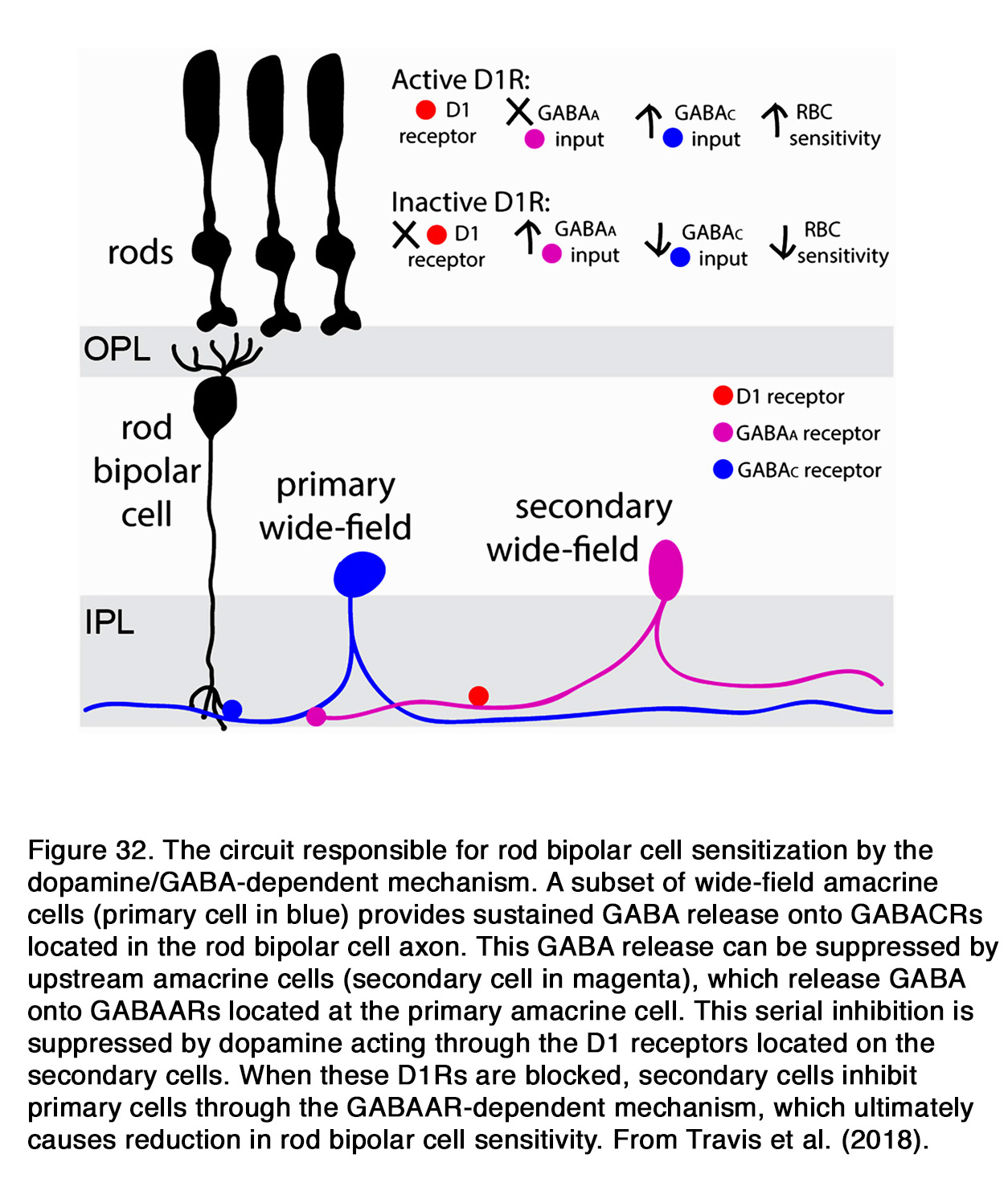
Similar to humans, a decrease of the b-wave amplitude was reported in mouse retina when dopaminergic transmission was compromised (253, 289, 290). This effect probably involved D1R, because SCH 23390 also diminished the b-wave amplitude (291). The action of SCH 23390 on the rod-mediated b-wave amplitude resembled the ERG phenotype of the D1R knockout mouse. In both cases a substantial reduction in dark sensitivity and compression of the operational range of the b-wave was observed. The authors reported that similar changes were seen when retinal GABACR, but not GABAAR signaling was prevented. In addition, Herrmann et al. (291) demonstrated that the lack of D1R-mediated signaling could be completely masked by exogenous GABA, consistent with a role for D1R in modulating a GABACR input onto rod-driven ON BC (Figure 31).They proposed that dopamine via D1R caused a release of GABA (possibly by HCs), which acted on GABACR on rod BCs and thus increased their sensitivity and operational range.
Travis et al. (141) eliminated the horizontal cells as a potential source of GABA release in this mechanism, because rod BC sensitivity was unaffected by horizontal cell-specific knockouts of either D1R or VGAT. The wide-field amacrine cells, whose activity is regulated by GABAAR-dependent serial inhibition, probably had an important role in GABA-induced rod BC sensitization (141, 292). Smith et al. (292) demonstrated that blocking of D1R did not suppress rod-driven ON-BC sensitivity when GABAA-mediated serial inhibition was blocked by gabazine, suggesting that the reduction in rod-driven BC sensitivity in the absence of D1R receptors was due to disinhibition of serial inhibitory GABAergic circuitry rather than a direct facilitatory effect on GABA release, as proposed by Herrmann et al. (291). Smith et al. (292) suggested that D1Rs may be expressed in all wide-field amacrine cells in this circuit, while Travis et al. (141) concluded that D1R-mediated regulation took place at the secondary GABAergic cell, responsible for the serial inhibition of the primary cell (Figure 32). This suggestion is supported by the fact that neither D1R full agonist SKF81297 not D1R antagonist SKF83566 changed the rod bipolar cell sensitivity when serial inhibition was blocked. Regardless of the exact mechanism of D1R-mediated action, it is clear that endogenous dopamine acting via D1-like receptors caused a sensitization of mouse rod BCs. Confirming this, a reduction of the b-wave amplitude was obtained by other authors working with D1R knockout mice (253, 293, 294). Lavoie et al. (294) reported that the b-wave amplitude decreased over the entire intensity range in scotopic and photopic conditions, while He et al. (293)observed reduced b-wave amplitude only for some stimulus intensities mostly towards the higher light intensities. All cited results obtained in mouse retina indicate that activation of D1R by endogenous dopamine has an enhancing effect on the ERG b-wave amplitude in both scotopic and photopic conditions.
Smith et al. (254) reported, however, that the D1R agonist SKF38393 reduced the mouse photopic b-wave amplitude at all stimulus intensities during daytime (Figure 33 A). They presented evidence that this suppressive effect was due to a direct inhibition of Nav channels in ON-cone BCs. The D1R antagonist SKF83566 increased the light-adapted b-wave amplitude during circadian midnight, but surprisingly it reduced the light-adapted b-wave amplitude during circadian midday (Figure 33 B). The authors proposed that at night, dopamine released during light adaptation was able to strongly suppress Nav channels via D1R, while at midday the light adaptation was unable to cause enough dopamine release to strongly suppress Nav channels on the ON-cone BCs. The D2-like receptors appeared to have a smaller role in dopamine actions on mouseERG than D1-like receptors. In D2R knockout mice no significant changes of the b-wave amplitude were observed in either scotopic or photopic conditions (291, 294). The b-wave amplitude was decreased, however, in D4R-KO mice in the first 10 minutes of dark adaptation after turning off a rod desensitizing ganzfeld background (151). Nir et al. (151) suggested that both rod and cone photoreceptors of the D4R-KO mouse had limited capacity to adapt to prolonged bright light exposure, although dopamine did not appreciably impact the phototransduction response to a flash stimulus. A decrease of the b-wave amplitude was obtained under photopic conditions of illumination, but not in the dark in D4R knockout mice (151, 253). Because the effect was similar to that obtained in D1R-KO mice, Jackson et al. (253) concluded that “both D1 and D4 receptors act to increase the amplitude of the light adapted ERG b-wave, with D4 receptors acting through circadian regulation of the ERG and D1 receptors acting on baseline amplitude in a non-circadian manner”. Thus, it appears that dopamine receptors belonging to the two main classes (D1 and D2) may have similar action on mouse cone-mediated b-wave amplitude (253), but opposite action on cone-mediated b-wave amplitude in fishes (257, 258) and frogs (262, 264).
Effect of dopamine on amacrine cells
One of the most studied effects of dopamine on the amacrine cell network was the effect it had on the electrical coupling of AII ACs, which are critical elements in the primary rod pathway of the mammalian retina (see Webvision chapter on AII Amacrine Cells, see also Webvision chapter Myriad roles for gap junctions in retinal circuits). It is well known that these cells receive excitatory input from ON-rod BCs (RBC) and they in turn form sign-conserving electrical synapses with the axon terminals of ON-cone BCs (CBC), and sign-inverting glycinergic chemical synapses with the axon terminals of OFF-CBCs (see chapter on AII ACs in Webvision). AII amacrine cells form homotypic gap junctions with neighboring AII amacrine cells, involving connexin 36 (Cx36) as seen in rat (295), rabbit (296) and mouse (297-299). Exogenous dopamine reduced the tracer coupling between adjacent AII amacrine cells in rabbit (300-302) (see Webvision chapter on AII ACs). The effect was mediated through activation of D1R, because it was mimicked by the specific D1R agonist SKF38393 and blocked by selective D1R antagonist SCH23390, an action seen in both rabbit (300, 303) and mouse (142). The dopamine antagonist SCH 23390, on its own, in low concentration (10 mM) did not increase significantly the extent of tracer coupling (142, 299, 300), while in higher concentration (100 mM) it increased the coupling (303). The exact mechanism underlying the D1R mediated uncoupling of AII ACs is a matter of debate. Some authors (299-302) suggested that uncoupling of gap junctions involved phosphorylation via protein kinase A of Cx36. Other authors, however, presented evidence that D1R-driven uncoupling of the AIInetwork resulted from protein kinase A activation of protein phosphatase 2A and subsequent dephosphorylation of Cx36 (303). The AII amacrine cells undergo well-known and prominent changes in electrical coupling driven by light adaptation that contribute to rewiring retinal circuitry from rod-pathway-dominated during dim light conditions to cone-pathway-dominated during bright light conditions. In rabbitretina, changes in light adaptation affected the coupling between AII amacrine cells in a triphasic manner (215, 304). Dark-adapted AII cells were coupled in relatively small groups and showed relatively small receptive fields. Exposure to dim background light increased both parameters approximately sevenfold allowing for summation of coincident signals and improved signal to noise ratio. Further light adaptation brought about a decrease in coupling to levels similar to those seen in dark-adapted retinas, which may act to prevent contamination of cone photoreceptor signals in cone bipolar cells by saturated rod signals in AII amacrine cells (See Webvision chapter on AII ACs). It is apparent that modulation of gap junctions between AII ACs is of physiological importance since it regulates the balance between sensitivity and spatial resolution in adjustment to the ambient light. Dopamine signaling alone cannot account, however, for the described ‘inverted U’ shaped AII coupling-background light relationship, because dopamine release increased steadily with increasing background illumination (54). It was shown that activation of extrasynaptic NMDA receptors on AII ACs led to increased phosphorylation of Cx36 and increased coupling between ACs (305). Kothmann et al. (305)proposed that the glutamate released from some rod BCs under scotopic conditions would depolarize AII ACs via postsynaptic AMPA receptors and would drive opening of nonsynaptic NMDA receptors on depolarized AIIdendrites, thus leading to increased local coupling between ACs. The coupling between ACs would increase with increased background illumination in the scotopic range, because the latter would stimulate more strongly a larger pool of rod BCs. This glutamate-driven increase in Cx36 phosphorylation and AII coupling is opposed by dopamine-driven decrease in Cx36 phosphorylation via D1R (305). Retinal dopamine secretion would be increased by bright light adaptation and it would be sufficient to overwhelm the pathway favoring increased Cx36 phosphorylation (Figure 34). This push-pull organization would result in the ‘U-shaped’ relationship between AII coupling and background illumination.
Modulation of the heterocellular gap junctions between AII amacrine cells and ON-cone bipolar cells by dopamine and cAMP is a matter of debate. In older studies it was reported that AII/CBC coupling, in contrast to AII/AII coupling, was modulated by NO through increases in cGMP concentration, but not by dopamine and cAMP (300, 301). In more recent studies evidence was presented that the AII/ON-CBC coupling was reduced by dopamine, D1R agonists and intracellular cAMP, and enhanced by D1R antagonist SCH23390 and thus resembled modulation of AII/AII coupling, as seen in both mouse (142, 299) and rabbit (302). Xia and Mills (302) showed, however, that dopamine reduced the AII/AII coupling more than AII/ON-CBC coupling, while cAMP affected them equally. The authors proposed that cAMP modulated gating in both hemichannels, while dopamine modulated gating on only the AII side of the channel, because dopamine-induced cAMP elevations normally occurred on only the AII side (Figure 35).
Nitric oxide, on the other hand, modulated the AII/ON-CBC hemichannel, but not AII/AII hemichannel and it acted independently of dopamine. Xia and Mills (302) suggested that the functional role of dopamine is to reduce both the scotopic flow via the AII pathway into cone pathway as well as the photopic flow from ON-CBCs into AII ACs, when light level (and dopamine) increases. Further reduction might occur with independent rises in cGMP in ON cone BCs possibly arising from nearby nitric oxide generators. There is no evidence, however, that the AII/ON-CBC coupling is modulated by background light as it was shown for the AII/AIIcoupling. Bloomfield et al. (306) could not find any light-induced modulation of AII/ON-CBC gap junctions under the 1000-fold range of background light intensities studied. No effect on Cx36 phosphorylation of AII/AIIand AII/ON-BC gap junctions was found, when dark adapted retina cultures were submitted to day lighting conditions (299). Urschel et al. (299) proposed that the dopamine released upon light stimulation was diluted too fast into the surround culture medium and therefore it could not reach threshold concentration to affect Cx36 phosphorylation.
Dopamine modulates the gap junctions formed by other types of ACs and between ACs and GCs. It was shown that dopamine decreased homotypic coupling between AC1 (GABAergic) cells via D1R and PKA-induced phosphorylation, while DA acting via D2R inhibited PKA and decreased phosphorylation of homotypic gap junctions between OFF a GCs in rabbit retina (54). A surprising inference is that, in contrast to AC1cells, ganglion cell hemichannels were closed by a decrease in phosphorylation. The heterotypic gap junctional channels between AC1 and OFF α GCs likely involved differential modulation of the two different types of hemichannels. One hemichannel was closed by phosphorylation on the side of AC1 cells, while the other hemichannel was opened by phosphorylation on the GC side. This differential modulation of the two hemichannels was accomplished by employing different dopamine receptors and opposite responses of two different hemichannels to PKA phosphorylation. Mills et al. (54) proposed that in the dark, where dopamine levels are minimal, both types of hemichannels may be relatively open and neighboring GCs will fire synchronously. As dopamine increases with background light, D2R will be activated first and will cause decrease in phosphorylation and closure of the GC channels. Ganglion-amacrine gap junctions would also be closed, although to a lesser extent, because there is only a single D2-sensitive hemichannel on the GC side. As dopamine levels increase with higher background intensities, activation of D1R accelerates the closure of the ganglion-amacrine cell channels and begins uncoupling the amacrine cells as well (Figure 36).
Thus, dopamine might regulate both the strength and spatial extent of inhibition mediated by the coupled AC1 GABAaergic amacrine cells as well as the degree of synchronous firing of the ganglion cells. It was shown, however, that light adaptation resulted in a dramatic increase instead of decrease in the coupling of α-GCs to both neighboring ganglion cells and amacrine cells in mouse and rabbit retina (307), which did not support the model of Mills et al. (54).
In contrast to the AII cells, dopamine, the D1R agonist SKF38393 and D1R antagonist SCH23390 did not modulate the homotypic and heterotypic gap junctions made by mouse A8 ACs (glycinergic), although the latter cells expressed D1 receptors and formed Cx36-containing junctions just like AII cells (142). A8 cells formed gap junctions with OFF bipolar cells, whereas AII cells only contacted ON CBCs via electrical synapses. It appears that dopaminergic regulation of amacrine cell gap junctions depends both on type and functional role. Exogenous dopamine did not influence the electrical coupling between ACs in carp retina, although it decreased coupling between horizontal cells (308).
How dopamine modulates the light-evoked responses of ACs is largely unknown. In goldfish it was shown that exogenous dopamine had no effect on the sustained ACs, while it depolarized the transient ACs and reduced their responsiveness to light stimuli (159).
Effect of dopamine on ganglion cells
Dopamine can modulate homotypic coupling among retinal GCs of the same subtype, which underlies the coherent firing of neighboring cells. This concerted firing is thought to form a separate stream of information to the brain, in addition to the asynchronous signals from individual ganglion cells (309, 310) (see also Webvision chapter Myriad roles for gap junctions in retinal circuits). Hu et al. (307) found that the D1R agonist SKF38393 and the D1R antagonist SCH23390 produced no significant change in the coupling of dark-adapted OFF α-GCs, while D2R antagonists (eticlopride and spiperone) produced a dramatic increase of homotypic (GC/GC) and heterotypic (GC/AC) coupling in OFF α-GCs and increased the concerted activity of α-GC neighbors in rabbit and mouse retina. These effects were like the effects obtained by the authors during light adaptation. Application of D2R agonists on light adapted retinas had no effect on coupling, while the D1R antagonist SCH23390 reduced both the coupling and the number of cells showing correlated light-evoked spike activity. Inhibition of adenylate cyclase and PKA in light adapted retinas had similar effect to those obtained with SCH23390. The authors proposed that the basal levels of dopamine in dark-adapted retinas activated high-affinity D2R resulting in an inhibition of adenylate cyclase, a dephosphorylation of connexins and a reduction in the permeability of α-GC gap junctions. Under mesopic/photopic conditions, dopamine levels increased and activated low-affinity D1R which, via a cAMP-mediated activation of PKA, increased connexin phosphorylation, and as a consequence, increased the coupling of the α-GC network (Figure 37). Thus, light-induced enhancement of GC coupling was due to activation of D1R, while uncoupling of dark-adapted GCs was due to activation of D2R. It is evident that the model of Hu et al. (307) differs markedly from the model of Mills et al(54), although they concern one and the same type of GC in rabbit retina.
Dopamine can modulate coupling among GCs in amphibian retina as well. Dopamine enhanced the synchronization of dimming detector (sustained OFF GCs) firing in bullfrog retina (311, 312). In addition, dopamine prevented the changes in synchronization and receptive field size induced by contrast adaptation. Li et al. (312) showed that single neuronal receptive field size was reduced during contrast adaptation, which was accompanied by weakening in synchronization strength between adjacent neurons’ activities. Dopamine increased the synchronization strength index and the mean receptive field size, indicating that dopamine caused a significant strengthening of the gap junctional connections among dimming detectors. Dopamine eliminated both the adaptation-related receptive field area shrinkage and the synchronization weakening. Application of the D2R antagonist sulpiride, but not D1R antagonist SCH23390, had effects similar to that obtained with dopamine. Li et al. (312) proposed that in early-adaptation, light-induced increase in dopamine release activated D1R and desensitized D2R. Thus, the gap junction conductance between ganglion cells was enhanced to a relatively high level, resulting in stronger synchronization. In late adaptation, as dopamine release declined, both the decrease in D1R activation and probable recovery of D2R from desensitized status in low dopamine concentration induced the decrease of gap junctional conductance. Bu et al. (311) found that the spatial correlation of dimming detector activity was enhanced, while temporal correlation in a single spike train was greatly decreased during dopamine treatment. The D2R antagonist sulpiride, but not D1R antagonist SCH23390 had similar effects to those of exogenous dopamine and Bu et al. (311) suggested that “endogenous dopamine influence on spatial and temporal correlation of firing activities of dimming detectorsmight be due to the activation of D1R”. Li and Liang (313) reported, however, that the exogenous dopamine did not alter the temporal structure of the firing sequences in single bullfrog neurons, although it decreased firing rates in response to three different stimulus patterns. Dopamine enhanced the synchronization of the GC population activity but decreased the rate of correct stimulus pattern discrimination and eliminated the linear correlation between correct rate and group size. Exogenous dopamine attenuated also the encoding of information about the stimulus duration carried by the response latency and not by the firing rate, although it increased the firing rate and shortened the latency of both the ON and OFF responses of bullfrog ON-OFF GCs (314).
Dopamine changes the sustained lateral inhibition of amphibian GCs during light adaptation (315). It was shown that both light adaptation and dopamine caused a decrease in the sustained lateral inhibition produced by distant windmill stimuli and an increase in the sustained lateral inhibition produced by nearby windmill stimuli. The authors suggested that the observed effects were due to a dopamine induced decrease of electrical coupling in the OPL and IPL and this might serve to increase contrast detection at higher spatial frequencies. Dopamine had no effect on the transient lateral inhibition of GCs, which was evident in light, but not in dark adapted amphibian retina. This type of inhibition was mediated by transient ACs and spread laterally via action potentials.
In cell culture dopamine may act directly on retinal ganglion cells to regulate excitability by affecting voltage-gated currents. It was shown that dopamine did not change the membrane potential or membrane resistance but reduced the excitability of dissociated GCs through activation of D1R in nonmammalian retina as seen in both turtle (316) and goldfish (317, 318). In turtles, the dopamine effect was attributed to changes in conductance of voltage-gated Ca2+ channels (316), while in goldfish the effect did not depend on voltage-gated Ca2+ currents (318) and it was probably due to a reduction of the voltage-gated Na+ current amplitude(317). Reduced excitability of dissociated GCs by the action of dopamine was obtained also in rat retina, but the underlying mechanism is a matter of debate (319-321). Chen and Yang (319) showed that exogenous dopamine, acting via D1R, induced a small depolarization of GC membrane potential, associated with decreased membrane resistance and diminished number of current-evoked action potentials (Figure 38).
The membrane potential effects of dopamine were prevented by the blockade of hyperpolarization-activated cation currents (Ih) with ZD7288, while dopamine alone increased the amplitude of Ih. Therefore Chen and Yang (319) suggested that the depressing effects of dopamine on rat GCs may be, at least in part, mediated by increasing the Ih amplitude through the cAMP- and PKA-dependent pathway. Hayashida et al. (320)suggested that dopamine and D1R agonists inhibited GC spiking primarily by reducing Na+ currents, because dopamine reduced the amplitude of both voltage-gated Na+ currents and current-evoked action potentials and it also slowed the rising and repolarizing sides of each spike. The reported effects were not affected by the blockade of Ih. Involvement of intracellular Ca2+ in the depressant effect of dopamine on current-evoked GC spiking was proposed by Ogata et al. (321). The authors demonstrated that dopamine and SKF 83959 (an agonist that activates heteromeric, but not homomeric DR) induced a Ca2+ influx in GCs, which was prevented by lowering of extracellular Ca2+, by SCH23390 and by eticlopride. The blockade of IP3-mediated Ca2+release also prevented the dopamine-induced rise in intracellular Ca2+. All these data indicated that rat GCs possessed heterodimeric D2-D5 receptors that mediated the dopamine-induced Ca2+ influx. Ogata et al. (321) showed that D1R and D2R antagonists blocked also light-induced rises in cAMP and activation of Ca2+/calmodulin-dependent protein kinase II, suggesting that dopamine may activate two pairs of signaling pathways in rat GCs: cAMP and PKA, and Ca2+ and CaMKII. Dopamine may modulate K+ currents of dissociated rat GCs as well. Li et al. (322) demonstrated that D1R agonist SKF81297 suppressed the GC outward K+ currents, mainly mediated by Gb-sensitive and 4-AP-sensitive components, through the intracellular PKA and CaMKII signaling pathways. Cui et al. (323) reported that D1R activation suppressed Kircurrents through cAMP/ PKA signaling pathway and thus leading to enhancement of the temporal summation of EPSPs in GC dendrites. They showed that D1R activation increased the spontaneous firing of GCs and induced cell depolarization (Figure 39), strongly suggesting that D1R activation increased the excitability of rat GCs, instead of decreasing it (319, 320). The cause for these opposite results obtained in rat retina, is unclear.
Dopamine acting via the D1R signaling pathway (cAMP/PKA) directly affected the intrinsically photosensitive retinal ganglion cells (ipRGCs) in rat retina (324). Dopamine and D1R agonist SKF38393 attenuated the ipRGC photocurrent and dramatically reduced the light-evoked cell spiking. D1R activation caused a modest depolarization and input resistance decrease of ipRGCs without significantly altering the number of spikes evoked by injection of depolarizing current. The authors suggested that the effects of D1R activation on membrane properties of ipRGCs were due to modulation of the Ih and not of voltage-gated Na+ channels, while the exact mechanism of the effects on melanopsin phototransduction cascade was unclear. Van Hook et al. (324) proposed that dopamine acting via D1R activation contributed to light-driven desensitization in ipRGCs and thus supported a form of light adaptation. On the other hand, Sakamoto et al. (83) found that dopamine via D2R modulated the photic and circadian regulation of melanopsin mRNA in rat ipRGCs. Thus, it appears that the two classes of dopamine receptors have distinct roles in regulating activity of rat ipRGCs.
Much data suggests that dopamine depresses retinal ganglion cell activity in intact mammalian retina. In catretina dopamine reduced the spontaneous and light-evoked firing of GCs irrespectively of their type (ON or OFF, transient or sustained) and these effects were essentially the same under scotopic, mesopic and photopic conditions (325-327) (Figure 40). Dopamine antagonists haloperidol (mixed D1/D2R antagonist), flupenthixol (selective D1R antagonist) or sulpiride (selective D2R antagonist) had no effect on spontaneous or light-induced activity of GCs, suggesting there was no endogenous dopamine present on the dopamine receptors of retinal GCs (325). Ikeda et al. (325) suggested that the retinal GCs may initially be innervated by dopaminergic cells, but the connection is eliminated during formation of GABA and glycine synapses. Other authors showed, however, that the impairment of dopaminergic transmission (with 6-OHDA or haloperidol) in adult cat retina led to a marked ON/OFF asymmetry in transient GC activity under photopic conditions of illumination (328, 329). The maintained activity of OFF-Y GCs was decreased, while that of ON-Y GCs was increased during either treatment (6-OHDA or haloperidol). Maguire and Smith (329) found that the proportion of OFF, but not ON GCs, which displayed nonlinear spatial summation was larger in the 6-OHDA treated than in the control eyes. The modulation of shift response (elicited with a peripheral square-wave grating) became larger in OFF-Y, but smaller in ON-Y GCs and the light-evoked responses of OFF-Y cells became even more transient in 6-OHDA treated eyes. The effect of haloperidol on the shift response of ON-Y GCs was like that of 6-OHDA, while its effect on the shift response of OFF-Y GCs showed great dynamics with time. The response disappeared at 25 min postinjection, but reappeared and increased to supranormal levels reaching a 50% increase in response modulation over control at 4 h 30 min (328). The peak firing rate of the center responses to flashing spots was greatly decreased at all stimulus intensities during haloperidol and SCH23390 treatment in the OFF Y GCs, while in the ON Y GCs the response diminution was much smaller (328). Whether the impairment of dopaminergic transmission caused a similar ON/OFF asymmetry in sustained (X) GC activity in cats is a matter of debate.
Maguire and Smith (329) found that the intensity-response function of center responses was unaffected for both ON and OFF X cells during 6-OHDA treatment, while Maguire and Hamasaki (328) found that the same function was shifted to the right for the OFF-X cells and not changed for the ON-X cells during haloperidol application. It was also shown that the blockade of D1R with SCH23390 altered the dark-adaptation function of OFF-Y and OFF-X GCs so that no rod-cone break was observed; rather the threshold of the cell fell quickly in a single exponential function to rod levels (328) (Figure 41). Therefore, the authors proposed that one of the operations of retinal dopamine may be to eliminate rod input to GCs during brief periods of decreased retinal illumination. Maguire and Hamasaki (328) suggested that without dopamine acting on D1-like receptors, “rapid changes from light to dark would allow the rod photoreceptors to activate GCs and lead to a noisy system with poor spatial and temporal resolution”.
An ON/OFF imbalance of ganglion cell activity, caused by dopamine, was described also in rabbit retina (330-333). It was shown that exogenous dopamine increased the spontaneous activity of OFF GCs, but reduced the spontaneous activity of ON GCs (330, 331). In accordance with these findings, dopamine nonselective antagonists (haloperidol, fluphenazine, cis-flupenthixol) caused a decrease in the spontaneous activity of OFF GCs and an increase of the spontaneous activity of ON GCs, indicating that there was a continuous dopamine release in light adapted rabbit retinas (331-333). These results are similar to those obtained in cat retina (328, 329). The involvement of the two DR types in the observed effects was studied with selective D1R and D2R agonists and antagonists. It was demonstrated that D2R agonist LY 141865 and D1R antagonist SCH 23390 decreased the spontaneous activity of OFF GCs and increased of the spontaneous activity of ON GCs, while D2R antagonist sulpiride decreased the spontaneous activity of both OFF GCs and ON transient GCs (331). The authors proposed that D1R mediated the effects of dopamine on spontaneous firing of GCs, while D2R may function at presynaptic autoreceptors that reduced the release of dopamine. It remained unclear why both activation and blockade of D2R decreased the spontaneous activity of OFF GCs. How exogenous dopamine affected the light-evoked responses of rabbit GCs is a matter of debate. Ames and Pollen (330) reported that the light-evoked responses of OFF GCs were usually unaffected by dopamine, while Jensen and Daw (331)demonstrated that dopamine reduced the light-evoked responses of these cells and shifted the intensity-response function of cell center and surround response to the right by an equal amount. Thus, exogenous dopamine reduced OFF GC sensitivity to light without altering the center-surround balance. Exogenous dopamine exerted a suppressive action also on the light-evoked responses of two thirds of ON and ON-OFF GCs (330). Dopamine suppressed the light responses of all ON-transient GCs and ON-OFF GCs, while it had no effect on the responses of the ON-sustained GCs (331). The blockade of dopamine receptors revealed effects on the GC light responses that were not seen during the action of exogenous dopamine. Application of haloperidol reduced and delayed the antagonistic surround responses in both OFF and ON brisk GCs and shifted their center-surround balance in favor of the center (333). Similar effects were found with D1R antagonist SCH23390, but not D2R antagonist sulpiride (Figure 42) with the exception of OFF large field units (331).
In the latter, sulpiride also caused a shift in center-surround balance in favor of the center. Thus, it appears that D1R mediated the effects of endogenous dopamine on the center-surround balance of ON-brisk GCs, while both D1R and D2R mediated those effects on the OFF large field units. An interesting observation was reported for ON brisk-sustained GCs, in which haloperidol and SCH23390, but not sulpiride, caused a reduction of sustained ON activity to spots of light and in some of the cells, in others the sustained excitation to spots of light actually turned into sustained inhibition (331, 333). The latter effect was not seen during application of dopamine. Endogenous dopamine, acting via D1R was probably involved also in the directional selectivity of rabbit GCs. It was shown that haloperidol and SCH23390, but not sulpiride, reduced the leading edge response, while either not affecting or increasing slightly the trailing edge response to a moving light stimulus of the ON-OFF directionally selective cells (331, 333). Exogenous dopamine had similar action in 3 out of 5 directionally selective cells.
The effects of dopamine on the activity of other mammalian GCs (except cat and rabbit) are not thoroughly investigated. It was shown that in light-adapted mouse retina, D1R antagonist (SCH23390) decreased, while D2R antagonist (spiperone) increased the light-evoked OFF responses of GCs, indicating that D1 and D2receptors played opposite roles in OFF response generation (334). Simultaneous inhibition of both receptor types (D1 and D2) increased the OFF responses, showing that the D2R-mediated effect was stronger and it dominated over that mediated by D1R. The authors gave no proposal for the mechanism underlying the OFF-response enhancement caused by D2R blockade. In rat retina, the D2/D3 receptor antagonists (sulpiride and eticlopride) had no significant effect on the spontaneous activity of either ON or OFF GCs in a dimly lighted environment (335). On the other hand, the specific D4R antagonist L-745,870 reduced the spontaneous activity of OFF GCs but had no significant effect on the spontaneous firing of ON GCs. In addition, L-745,870 decreased both the light sensitivity (by 0.32 log unit) and the maximum peak response of the ON and OFF GCs, while sulpiride and eticlopride reduced only light sensitivity (0.23 log unit) without changing the maximum peak response. Jensen (335) proposed that D4R, like D2R, functions to regulate dopamine release, but did not provide any suggestion why the effect of D4R blockade inhibited the GC spontaneous and light-evoked activity more strongly than D2R blockade. More studies are needed to fully understand the role of dopamine in ganglion cell function in both mammalian and nonmammalian retina.
Summary
Dopamine is synthesized and released by a group of cells in the retinal amacrine cell layer. This group represents less than 1% of cells in this layer. Dopaminergic cells are morphologically either interplexiform cells, with dendritic arbors in both inner and outer plexiform layers, or large-bodied amacrine cells. The latter typically have dense, wide-field dendritic arbors in stratum S1 of the inner plexiform layer, adjacent to the amacrine cell layer, but sparse branching in more proximal inner plexiform layer strata. Dopaminergic amacrine or interplexiform cells synapse directly on other retinal neurons, but much of the dopaminergic action is thought to be ‘paracrine’, exerted by diffusion of dopamine to different target cells. Dopaminergic cells release other neurotransmitters such as GABA. Dopamine release appears to be circadian, with high release levels during the day in many species, and is a counterpart to circadian rhythms in melatonin, which is released at night. Consistent with a role as a humoral neuromodulator, dopaminergic actions are found in every retinal cell type, mediated through both D1-like and D2 like G-protein coupled receptors. One particularly prominent action is diurnal modulation of gap-junction coupling between horizontal, amacrine, ganglion cells, clearly observed in the regulation of tracer molecule spread among like cell types. In mammals gap-junction modulation corresponds to a retinal circuitry reconfiguration in rod and cone pathways so as to optimize nocturnal and diurnal vision. Further actions are found in the modulation of voltage-gated channels and chemical neurotransmission. Nonetheless, there are no general rules concerning dopaminergic action, which vary considerably depending on species, neural cell type, and the experimental context.
Acknowledgements
The author thanks Helga Kolb (University of Utah) for giving the idea for writing this chapter and her valuable support during the whole process of chapter construction. She especially helped for writing the section concerning the morphology and circuity of retinal dopaminergic cells. The author thanks also Dr. Ralph Nelson (NIH) for chapter editing and helpful comments.
About the author
Dr. Elka Popova, MD, PhD, was born in Sofia, Bulgaria. She received a Medical Degree from Medical University of Sofia in 1979 and started her work as assistant professor in the Department of Physiology at the same university. Dr. Popova worked in the visual electrophysiological laboratory of the department and received her Ph.D. in 2001 and her associated professor position in 2003. Her work is in the field of retinal physiology and includes investigation of various excitatory and inhibitory neurotransmitters (GABA, glycine, dopamine, histamine, serotonin, purines, enkephalins) and ion channel (Nav, HCN) modulation on the electroretinogram and ganglion cell activity in cold blooded species. She has a special interest in revealing the differences between retinal ON and OFF channels and the interaction between the two channels at different retinal levels. Dr. Popova investigates how some of these differences and interactions depend on different neurotransmitter systems and ion channels.
References
-
- Goldstein, D.S., G. Eisenhofer, and I.J. Kopin, Clinical catecholamine neurochemistry: a legacy of Julius Axelrod. Cellular and molecular neurobiology. 2006; 26(4-6):693-700. [PubMed]
- Falck, B., N.-Å. Hillarp, G. Thieme, and A.a. Torp, Fluorescence of catechol amines and related compounds condensed with formaldehyde. Journal of Histochemistry & Cytochemistry. 1962; 10(3):348-354. [PubMed]
- Djamgoz, M. and H.-J. Wagner, Localization and function of dopamine in the adult vertebrate retina. Neurochemistry international. 1992; 20(2):139-191. [PubMed]
- Massey, S.C. and D.A. Redburn, Transmitter circuits in the vertebrate retina. Progress in neurobiology. 1987; 28(1):55-96. [PubMed]
- Kolb, H., C. Cline, H.H. Wang, and N. Brecha, Distribution and morphology of dopaminergic amacrine cells in the retina of the turtle (Pseudemys scripta elegans). Journal of neurocytology. 1987; 16(5):577-588. [PubMed]
- Frederick, J.M., M.E. Rayborn, A.M. Laties, D.M. Lam, and J.G. Hollyfield, Dopaminergic neurons in the human retina. Journal of Comparative Neurology. 1982; 210(1):65-79. [PubMed]
- Kolb, H., N. Cuenca, and L. Dekorver, Postembedding immunocytochemistry for GABA and glycine reveals the synaptic relationships of the dopaminergic amacrine cell of the cat retina. Journal of Comparative Neurology. 1991; 310(2):267-284. [PubMed]
- Kolb, H., N. Cuenca, H.-H. Wang, and L. Dekorver, The synaptic organization of the dopaminergic amacrine cell in the cat retina. Journal of neurocytology. 1990; 19(3):343-366. [PubMed]
- Crooks, J. and H. Kolb, Localization of GABA, glycine, glutamate and tyrosine hydroxylase in the human retina. Journal of Comparative Neurology. 1992; 315(3):287-302. [PubMed]
- Mariani, A.P. and J.N. Hokoc, Two types of tyrosine hydroxylase‐immunoreactive amacrine cell in the rhesus monkey retina. Journal of Comparative Neurology. 1988; 276(1):81-91. [PubMed]
- Tauchi, M., N.K. Madigan, and R.H. Masland, Shapes and distributions of the catecholamine‐accumulating neurons in the rabbit retina. Journal of Comparative Neurology. 1990; 293(2):178-189. [PubMed]
- Zhang, D.-Q., T.-R. Zhou, and D.G. McMahon, Functional heterogeneity of retinal dopaminergic neurons underlying their multiple roles in vision. Journal of Neuroscience. 2007; 27(3):692-699. [PubMed]
- Casini, G., D.W. Rickman, and N.C. Brecha, AII amacrine cell population in the rabbit retina: identification by parvalbumin immunoreactivity. Journal of Comparative Neurology. 1995; 356(1):132-142. [PubMed]
- Voigt, T. and H. Wassle, Dopaminergic innervation of A II amacrine cells in mammalian retina. Journal of Neuroscience. 1987; 7(12):4115-4128. [PubMed]
- Dumitrescu, O.N., F.G. Pucci, K.Y. Wong, and D.M. Berson, Ectopic retinal ON bipolar cell synapses in the OFF inner plexiform layer: contacts with dopaminergic amacrine cells and melanopsin ganglion cells. Journal of Comparative Neurology. 2009; 517(2):226-244. [PubMed]
- Contini, M., B. Lin, K. Kobayashi, H. Okano, R.H. Masland, and E. Raviola, Synaptic input of ON‐bipolar cells onto the dopaminergic neurons of the mouse retina. Journal of Comparative Neurology. 2010; 518(11):2035-2050. [PubMed]
- Viney, T.J., K. Balint, D. Hillier, S. Siegert, Z. Boldogkoi, L.W. Enquist, M. Meister, C.L. Cepko, and B. Roska, Local retinal circuits of melanopsin-containing ganglion cells identified by transsynaptic viral tracing. Current Biology. 2007; 17(11):981-988. [PubMed]
- Vugler, A.A., P. Redgrave, J. Lawrence, J. Greenwood, and P.J. Coffey, Dopamine neurones form a discrete plexus with melanopsin cells in normal and degenerating retina. Experimental neurology. 2007; 205(1):26-35. [PubMed]
- Zhang, D.-Q., K.Y. Wong, P.J. Sollars, D.M. Berson, G.E. Pickard, and D.G. McMahon, Intraretinal signaling by ganglion cell photoreceptors to dopaminergic amacrine neurons. Proceedings of the National Academy of Sciences. 2008; 105(37):14181-14186. [PubMed]
- Frazao, R., D.G. McMahon, W. Schunack, P. Datta, R. Heidelberger, and D.W. Marshak, Histamine elevates free intracellular calcium in mouse retinal dopaminergic cells via H1-receptors. Investigative ophthalmology & visual science. 2011; 52(6):3083-3088. [PubMed]
- Marc, R.E., B.W. Jones, C.B. Watt, J.R. Anderson, C. Sigulinsky, and S. Lauritzen, Retinal connectomics: towards complete, accurate networks. Progress in retinal and eye research. 2013; 37:141-162. [PubMed]
- Hirasawa, H., M. Contini, and E. Raviola, Extrasynaptic release of GABA and dopamine by retinal dopaminergic neurons. Philosophical Transactions of the Royal Society B: Biological Sciences. 2015; 370(1672):20140186. [PubMed]
- Wassle, H. and M. Chun, Dopaminergic and indoleamine-accumulating amacrine cells express GABA-like immunoreactivity in the cat retina. Journal of Neuroscience. 1988; 8(9):3383-3394. [PubMed]
- Puopolo, M., S.E. Hochstetler, S. Gustincich, R.M. Wightman, and E. Raviola, Extrasynaptic release of dopamine in a retinal neuron: activity dependence and transmitter modulation. Neuron. 2001; 30(1):211-225. [PubMed]
- Newkirk, G.S., M. Hoon, R. Wong, and P.B. Detwiler, Inhibitory inputs tune the light response properties of dopaminergic amacrine cells in mouse retina. Journal of neurophysiology. 2013; 110(2):536-552. [PubMed]
- Yang, C.Y., P. Lukasiewicz, G. Maguire, F.S. Werblin, and S. Yazulla, Amacrine cells in the tiger salamander retina: morphology, physiology, and neurotransmitter identification. Journal of Comparative Neurology. 1991; 312(1):19-32. [PubMed]
- Zhang, D.-Q., M.A. Belenky, P.J. Sollars, G.E. Pickard, and D.G. McMahon, Melanopsin mediates retrograde visual signaling in the retina. PloS one. 2012; 7(8). [PubMed]
- Zhao, X., K.Y. Wong, and D.-Q. Zhang, Mapping physiological inputs from multiple photoreceptor systems to dopaminergic amacrine cells in the mouse retina. Scientific reports. 2017; 7(1):1-14. [PubMed]
- Qiao, S.-N., Z. Zhang, C.P. Ribelayga, Y.-M. Zhong, and D.-Q. Zhang, Multiple cone pathways are involved in photic regulation of retinal dopamine. Scientific reports. 2016; 6:28916. [PubMed]
- Li, L. and J.E. Dowling, Disruption of the olfactoretinal centrifugal pathway may relate to the visual system defect in night blindness b mutant zebrafish. Journal of Neuroscience. 2000; 20(5):1883-1892. [PubMed]
- Zucker, C.L. and J.E. Dowling, Centrifugal fibres synapse on dopaminergic interplexiform cells in the teleost retina. Nature. 1987; 330(6144):166-168. [PubMed]
- Kolb, H., E. Netzer, and J. Ammermüller, Neural circuitry and light responses of the dopamine amacrine cell of the turtle retina. Molecular vision. 1997; 3(6). [PubMed]
- Yazulla, S. and C.L. Zucker, Synaptic organization of dopaminergic interplexiform cells in the goldfish retina. Visual neuroscience. 1988; 1(1):13-29. [PubMed]
- Dowling, J.E. and B. Ehinger, Synaptic organization of the amine-containing interplexiform cells of the goldfish and Cebus monkey retinas. Science. 1975; 188(4185):270-273. [PubMed]
- Witkovsky, P., R. Gábriel, and D. Križaj, Anatomical and neurochemical characterization of dopaminergic interplexiform processes in mouse and rat retinas. Journal of Comparative Neurology. 2008; 510(2):158-174. [PubMed]
- Bjelke, B., M. Goldstein, B. Tinner, C. Andersson, S.R. Sesack, H.W. Steinbusch, J.Y. Lew, X. He, S. Watson, B. Tengroth, and K Fuxe, Dopaminergic transmission in the rat retina: evidence for volume transmission. Journal of chemical neuroanatomy. 1996; 12(1):37-50. [PubMed]
- Witkovsky, P., C. Nicholson, M.E. Rice, K. Bohmaker, and E. Meller, Extracellular dopamine concentration in the retina of the clawed frog, Xenopus laevis. Proceedings of the National Academy of Sciences. 1993; 90(12):5667-5671. [PubMed]
- Young, H., Co-localization of GABA-and tyrosine hydroxylase-like immunoreactivities in amacrine cells of the rabbit retina. Vision research. 1994; 34(8):995-999. [PubMed]
- Wulle, I. and H.J. Wagner, GABA and tyrosine hydroxylase immunocytochemistry reveal different patterns of colocalization in retinal neurons of various vertebrates. Journal of Comparative Neurology. 1990; 296(1):173-178. [PubMed]
- Kosaka, T., K. Kosaka, Y. Hataguchi, I. Nagatsu, J.-Y. Wu, O. Ottersen, J.a. Storm-Mathisen, and K. Hama, Catecholaminergic neurons containing GABA-like and/or glutamic acid decarboxylase-like immunoreactivities in various brain regions of the rat.Experimental brain research. 1987; 66(1):191-210. [PubMed]
- Nguyen‐Legros, J., C. Versaux‐Botteri, and C. Savy, Dopaminergic and GABAergic retinal cell populations in mammals. Microscopy research and technique. 1997; 36(1):26-42. [PubMed]
- Contini, M. and E. Raviola, GABAergic synapses made by a retinal dopaminergic neuron. Proceedings of the National Academy of Sciences. 2003; 100(3):1358-1363. [PubMed]
- Lee, J.W., M.Y. Lim, Y.S. Park, S.J. Park, and I.-B. Kim, Reexamination of Dopaminergic Amacrine Cells in the Rabbit Retina: Confocal Analysis with Double-and Triple-labeling Immunohistochemistry. Experimental neurobiology. 2017; 26(6):329-338. [PubMed]
- Boatright, J.H., M.J. Hoel, and P.M. Iuvone, Stimulation of endogenous dopamine release and metabolism in amphibian retina by light-and K+-evoked depolarization. Brain research. 1989; 482(1):164-168. [PubMed]
- Boatright, J.H., N.M. Rubim, and P.M. Iuvone, Regulation of endogenous dopamine release in amphibian retina by melatonin: the role of GABA. Visual neuroscience. 1994; 11(5):1013-1018. [PubMed]
- Kolbinger, W. and R. Weiler, Modulation of endogenous dopamine release in the turtle retina: effects of light, calcium, and neurotransmitters. Visual neuroscience. 1993; 10(6):1035-1041. [PubMed]
- Adachi, A., T. Nogi, and S. Ebihara, Phase-relationship and mutual effects between circadian rhythms of ocular melatonin and dopamine in the pigeon. Brain research. 1998; 792(2):361-369. [PubMed]
- Megaw, P., I. Morgan, and M. Boelen, Vitreal dihydroxyphenylacetic acid (DOPAC) as an index of retinal dopamine release.Journal of neurochemistry. 2001; 76(6):1636-1644. [PubMed]
- Zawilska, J.B., A. Bednarek, M. Berezińska, and J.Z. Nowak, Rhythmic changes in metabolism of dopamine in the chick retina: the importance of light versus biological clock. Journal of neurochemistry. 2003; 84(4):717-724. [PubMed]
- Zawilska, J.B., M. Berezińska, J. Rosiak, B. Vivien-Roels, and J.Z. Nowak, The relationship between melatonin and dopamine rhythms in the duck retina. Neuroscience letters. 2003; 347(1):37-40. [PubMed]
- Cameron, M.A., N. Pozdeyev, A. Vugler, H. Cooper, P.M. Iuvone, and R.J. Lucas, Light regulation of retinal dopamine that is independent of melanopsin phototransduction. European Journal of Neuroscience. 2009; 29(4):761-767. [PubMed]
- Gibson, C.J., A simple perfusion system for measuring endogenous retinal dopamine release. Journal of neuroscience methods. 1990; 32(1):75-79. [PubMed]
- Godley, B.F. and R.J. Wurtman, Release of endogenous dopamine from the superfused rabbit retina in vitro: effect of light stimulation. Brain research. 1988; 452(1-2):393-395. [PubMed]
- Mills, S.L., X.-B. Xia, H. Hoshi, S.I. Firth, M.E. Rice, L.J. Frishman, and D.W. Marshak, Dopaminergic modulation of tracer coupling in a ganglion-amacrine cell network. Visual neuroscience. 2007; 24(4):593-608. [PubMed]
- Nowak, J. and E. Zurawska, Dopamine in the rabbit retina and striatum: diurnal rhythm and effect of light stimulation. Journal of neural transmission. 1989; 75(3):201-212. [PubMed]
- Parkinson, D. and R. Rando, Effect of light on dopamine turnover and metabolism in rabbit retina. Investigative ophthalmology & visual science. 1983; 24(3):384-388. [PubMed]
- Kramer, S.G., Dopamine: A retinal neurotransmitter: I. Retinal uptake, storage, and light-stimulated release of H3-dopamine in vivo. Investigative ophthalmology & visual science. 1971; 10(6):438-452. [PubMed]
- Boelen, M.K., M.G. Boelen, and D.W. Marshak, Light-stimulated release of dopamine from the primate retina is blocked by l-2-amino-4-phosphonobutyric acid (APB). Visual neuroscience. 1998; 15(1):97-103. [PubMed]
- Besharse, J.C. and P.M. Iuvone, Is dopamine a light-adaptive or a dark-adaptive modulator in retina? Neurochemistry international. 1992; 20(2):193-199. [PubMed]
- Luft, W., P. Iuvone, and W. Stell, Spatial, temporal, and intensive determinants of dopamine release in the chick retina. Visual neuroscience. 2004; 21(4):627-635. [PubMed]
- Megaw, P.L., M.G. Boelen, I.G. Morgan, and M.K. Boelen, Diurnal patterns of dopamine release in chicken retina. Neurochemistry international. 2006; 48(1):17-23. [PubMed]
- Witkovsky, P., Dopamine and retinal function. Documenta ophthalmologica. 2004; 108(1):17-39. [PubMed]
- Hamasaki, D., W.B. Trattler, and A.S. Hajek, Light on depresses and light off enhances the release of dopamine from the cat’s retina. Neuroscience letters. 1986; 68(1):112-116. [PubMed]
- Tornqvist, K., X. Yang, and J. Dowling, Modulation of cone horizontal cell activity in the teleost fish retina. III. Effects of prolonged darkness and dopamine on electrical coupling between horizontal cells. Journal of Neuroscience. 1988; 8(7):2279-2288. [PubMed]
- Weiler, R., W.H. Baldridge, S.C. Mangel, and J.E. Dowling, Modulation of endogenous dopamine release in the fish retina by light and prolonged darkness. Visual neuroscience. 1997; 14(2):351-356. [PubMed]
- Marshak, D., Synaptic input to dopaminergic neurons in mammalian retina, in Concepts and Challenges in retinal Physiology, H. Kolb, R. H, and W. S, Editors. 2001, Elsevier Sci B.V. : Netherlands.
- Pérez-Fernández, V., N. Milosavljevic, A.E. Allen, K.A. Vessey, A.I. Jobling, E.L. Fletcher, P.P. Breen, J.W. Morley, and M.A. Cameron, Rod photoreceptor activation alone defines the release of dopamine in the retina. Current Biology. 2019; 29(5):763-774. e5. [PubMed]
- Dowling, J., The retina: an approachable part of the brain, Revised edn. 2012, World: Belknap Press. 384. [Link]
- Gonon, F., Nonlinear relationship between impulse flow and dopamine released by rat midbrain dopaminergic neurons as studied by in vivo electrochemistry. Neuroscience. 1988; 24(1):19-28. [PubMed]
- McCormack, C.A. and B. Burnside, Light and circadian modulation of teleost retinal tyrosine hydroxylase activity. Investigative ophthalmology & visual science. 1993; 34(5):1853-1860. [PubMed]
- Ribelayga, C., Y. Wang, and S.C. Mangel, A circadian clock in the fish retina regulates dopamine release via activation of melatonin receptors. The Journal of physiology. 2004; 554(2):467-482. [PubMed]
- Wulle, I., M. Kirsch, and H.-J. Wagner, Cyclic changes in dopamine and DOPAC content, and tyrosine hydroxylase activity in the retina of a cichlid fish. Brain research. 1990; 515(1-2):163-167. [PubMed]
- Bartell, P.A., M. Miranda-Anaya, W. McIvor, and M. Menaker, Interactions between dopamine and melatonin organize circadian rhythmicity in the retina of the green iguana. Journal of biological rhythms. 2007; 22(6):515-523. [PubMed]
- Miranda-Anaya, M., P.A. Bartell, and M. Menaker, Circadian rhythm of iguana electroretinogram: the role of dopamine and melatonin. Journal of biological rhythms. 2002; 17(6):526-538. [PubMed]
- Lorenc-Duda, A., M. Berezińska, A. Urbańska, K. Gołembiowska, and J.B. Zawilska, Dopamine in the turkey retina—an impact of environmental light, circadian clock, and melatonin. Journal of molecular neuroscience. 2009; 38(1):12-18. [PubMed]
- Manglapus, M.K., P.M. Iuvone, H. Underwood, M.E. Pierce, and R.B. Barlow, Dopamine mediates circadian rhythms of rod–cone dominance in the Japanese quail retina. Journal of Neuroscience. 1999; 19(10):4132-4141. [PubMed]
- Adachi, A., Y. Suzuki, T. Nogi, and S. Ebihara, The relationship between ocular melatonin and dopamine rhythms in the pigeon: effects of melatonin inhibition on dopamine release. Brain research. 1999; 815(2):435-440. [PubMed]
- Doyle, S.E., M.S. Grace, W. McIvor, and M. Menaker, Circadian rhythms of dopamine in mouse retina: the role of melatonin. Visual neuroscience. 2002; 19(5):593-601. [PubMed]
- Nir, I., R. Haque, and P.M. Iuvone, Diurnal metabolism of dopamine in the mouse retina. Brain research. 2000; 870(1-2):118-125. [PubMed]
- Doyle, S.E., W.E. McIvor, and M. Menaker, Circadian rhythmicity in dopamine content of mammalian retina: role of the photoreceptors. Journal of neurochemistry. 2002; 83(1):211-219. [PubMed]
- Melamed, E., Y. Frucht, M. Lemor, A. Uzzan, and Y. Rosenthal, Dopamine turnover in rat retina: a 24-hour light-dependent rhythm. Brain research. 1984; 305(1):148-151. [PubMed]
- Pozdeyev, N. and E. Lavrikova, Diurnal changes of tyrosine, dopamine, and dopamine metabolites content in the retina of rats maintained at different lighting conditions. Journal of Molecular Neuroscience. 2000; 15(1):1-9. [PubMed]
- Sakamoto, K., C. Liu, M. Kasamatsu, N.V. Pozdeyev, P.M. Iuvone, and G. Tosini, Dopamine regulates melanopsin mRNA expression in intrinsically photosensitive retinal ganglion cells. European Journal of Neuroscience. 2005; 22(12):3129-3136. [PubMed]
- Wirz-Justice, A., M. Da Prada, and C. Remé, Circadian rhythm in rat retinal dopamine. Neuroscience letters. 1984; 45(1):21-25. [PubMed]
- Di Paolo, T., C. Harnois, and M. Daigle, Assay of dopamine and its metabolites in human and rat retina. Neuroscience letters. 1987; 74(2):250-254. [PubMed]
- Sakamoto, K., C. Liu, and G. Tosini, Classical photoreceptors regulate melanopsin mRNA levels in the rat retina. Journal of Neuroscience. 2004; 24(43):9693-9697. [PubMed]
- Dorenbos, R., M. Contini, H. Hirasawa, S. Gustincich, and E. Raviola, Expression of circadian clock genes in retinal dopaminergic cells. Visual neuroscience. 2007; 24(4):573-580. [PubMed]
- Gustincich, S., M. Contini, M. Gariboldi, M. Puopolo, K. Kadota, H. Bono, J. LeMieux, P. Walsh, P. Carninci, and Y. Hayashizaki, Gene discovery in genetically labeled single dopaminergic neurons of the retina. Proceedings of the National Academy of Sciences. 2004; 101(14):5069-5074. [PubMed]
- Ruan, G.-X., D.-Q. Zhang, T. Zhou, S. Yamazaki, and D.G. McMahon, Circadian organization of the mammalian retina. Proceedings of the National Academy of Sciences. 2006; 103(25):9703-9708. [PubMed]
- Witkovsky, P., E. Veisenberger, J. LeSauter, L. Yan, M. Johnson, D.-Q. Zhang, D. McMahon, and R. Silver, Cellular location and circadian rhythm of expression of the biological clock gene Period 1 in the mouse retina. Journal of Neuroscience. 2003; 23(20):7670-7676. [PubMed]
- Sakamoto, K., C. Liu, M. Kasamatsu, P.M. Iuvone, and G. Tosini, Intraocular injection of kainic acid does not abolish the circadian rhythm of arylalkylamine N-acetyltransferase mRNA in rat photoreceptors. Mol Vis. 2006; 12:117-24. [PubMed]
- Iuvone, P.M., G. Tosini, N. Pozdeyev, R. Haque, D.C. Klein, and S.S. Chaurasia, Circadian clocks, clock networks, arylalkylamine N-acetyltransferase, and melatonin in the retina. Progress in retinal and eye research. 2005; 24(4):433-456. [PubMed]
- Dkhissi-Benyahya, O., C. Coutanson, K. Knoblauch, H. Lahouaoui, V. Leviel, C. Rey, M. Bennis, and H.M. Cooper, The absence of melanopsin alters retinal clock function and dopamine regulation by light. Cellular and molecular life sciences. 2013; 70(18):3435-3447. [PubMed]
- Beaulieu, J.M., S. Espinoza, and R.R. Gainetdinov, Dopamine receptors–IUPHAR review 13. British journal of pharmacology. 2015; 172(1):1-23. [PubMed]
- Beaulieu, J.-M. and R.R. Gainetdinov, The physiology, signaling, and pharmacology of dopamine receptors. Pharmacological reviews. 2011; 63(1):182-217. [PubMed]
- Kuzhikandathil, E.V., W. Yu, and G.S. Oxford, Human dopamine D3 and D2L receptors couple to inward rectifier potassium channels in mammalian cell lines. Molecular and Cellular Neuroscience. 1998; 12(6):390-402. [PubMed]
- Yan, Z., W.-J. Song, and D.J. Surmeier, D2 dopamine receptors reduce N-type Ca2+ currents in rat neostriatal cholinergic interneurons through a membrane-delimited, protein-kinase-C-insensitive pathway. Journal of neurophysiology. 1997; 77(2):1003-1015. [PubMed]
- Missale, C., S.R. Nash, S.W. Robinson, M. Jaber, and M.G. Caron, Dopamine receptors: from structure to function. Physiological reviews. 1998; 78(1):189-225. [PubMed]
- Nishi, A., G.L. Snyder, and P. Greengard, Bidirectional regulation of DARPP-32 phosphorylation by dopamine. Journal of Neuroscience. 1997; 17(21):8147-8155. [PubMed]
- Hernandez-Lopez, S., T. Tkatch, E. Perez-Garci, E. Galarraga, J. Bargas, H. Hamm, and D.J. Surmeier, D2 dopamine receptors in striatal medium spiny neurons reduce L-type Ca2+ currents and excitability via a novel PLC [beta] 1-IP3-calcineurin-signaling cascade. The Journal of Neuroscience: the Official Journal of the Society for Neuroscience. 2000; 20(24):8987-8995. [PubMed]
- Hasbi, A., B.F. O’Dowd, and S.R. George, Dopamine D1-D2 receptor heteromer signaling pathway in the brain: emerging physiological relevance. Molecular brain. 2011; 4(1):26. [PubMed]
- Hasbi, A., B.F. O’Dowd, and S.R. George, Heteromerization of dopamine D2 receptors with dopamine D1 or D5 receptors generates intracellular calcium signaling by different mechanisms. Current opinion in pharmacology. 2010; 10(1):93-99. [PubMed]
- Lee, S.-M., Y. Yang, and R.B. Mailman, Dopamine D1 Receptor Signaling: Does GαQ–Phospholipase C Actually Play a Role? Journal of Pharmacology and Experimental Therapeutics. 2014; 351(1):9-17. [PubMed]
- Kisilevsky, A.E. and G.W. Zamponi, D2 dopamine receptors interact directly with N-type calcium channels and regulate channel surface expression levels. Channels. 2008; 2(4):269-277. [PubMed]
- Blom, H., D. RöNnlund, L. Scott, Z. Spicarova, V. Rantanen, J. Widengren, A. Aperia, and H. Brismar, Nearest neighbor analysis of dopamine D1 receptors and Na+‐K+‐ATPases in dendritic spines dissected by STED microscopy. Microscopy research and technique. 2012; 75(2):220-228. [PubMed]
- Hazelwood, L.A., R.B. Free, D.M. Cabrera, M. Skinbjerg, and D.R. Sibley, Reciprocal modulation of function between the D1 and D2 dopamine receptors and the Na+, K+-ATPase. Journal of Biological Chemistry. 2008; 283(52):36441-36453. [PubMed]
- Rankin, M.L., L.A. Hazelwood, R.B. Free, E. Rex, R. Roof, and D. Sibley, Molecular Pharmacology of the Dopamine Receptors, in Dopamine handbook, L. Iversen, et al., Editors. 2010, Oxford University Press: New York. p. 63–87.
- Rondou, P., G. Haegeman, and K. Van Craenenbroeck, The dopamine D4 receptor: biochemical and signalling properties. Cellular and molecular life sciences. 2010; 67(12):1971-1986. [PubMed]
- Sibley, D.R., New insights into dopaminergic receptor function using antisense and genetically altered animals. Annual review of pharmacology and toxicology. 1999; 39(1):313-341. [PubMed]
- Wolf, M.E. and R.H. Roth, Autoreceptor regulation of dopamine synthesis. Annals of the New York Academy of Sciences. 1990; 604(1):323-343. [PubMed]
- Civelli, O., J.R. Bunzow, and D.K. Grandy, Molecular diversity of the dopamine receptors. Annual review of pharmacology and toxicology. 1993; 33(1):281-307. [PubMed]
- Ribelayga, C., Y. Wang, and S.C. Mangel, Dopamine mediates circadian clock regulation of rod and cone input to fish retinal horizontal cells. The Journal of physiology. 2002; 544(3):801-816. [PubMed]
- Seeman, P. and H.H. Van Tol, Dopamine receptor pharmacology. Trends in pharmacological sciences. 1994; 15(7):264-270.
- Barton, A.C., L.E. Black, and D.R. Sibley, Agonist-induced desensitization of D2 dopamine receptors in human Y-79 retinoblastoma cells. Molecular pharmacology. 1991; 39(5):650-658. [PubMed]
- Gardner, B., Z.F. Liu, D. Jiang, and D.R. Sibley, The role of phosphorylation/dephosphorylation in agonist-induced desensitization of D1 dopamine receptor function: evidence for a novel pathway for receptor dephosphorylation. Molecular pharmacology. 2001; 59(2):310-321. [PubMed]
- Ito, K., T. Haga, J. Lameh, and W. Sadée, Sequestration of dopamine D2 receptors depends on coexpression of G‐protein‐coupled receptor kinases 2 or 5. European journal of biochemistry. 1999; 260(1):112-119. [PubMed]
- Ko, F., P. Seeman, W. Sun, and S. Kapur, Dopamine D2 receptors internalize in their low-affinity state. Neuroreport. 2002; 13(8):1017-1020. [PubMed]
- Nowak, J.Z., B. Sek, and M. Schorderet, Dark-induced supersensitivity of dopamine D-1 and D-2 receptors in rat retina. NeuroReport. 1991; 2(8):429-432. [PubMed]
- Zawilska, J.B. and P.M. Iuvone, Melatonin synthesis in chicken retina: effect of kainic acid-induced lesions on the diurnal rhythm and D2-dopamine receptor-mediated regulation of serotonin N-acetyltransferase activity. Neuroscience letters. 1992; 135(1):71-74. [PubMed]
- Muresan, Z. and J.C. Besharse, D2‐like dopamine receptors in amphibian retina: Localization with fluorescent ligands. Journal of Comparative Neurology. 1993; 331(2):149-160. [PubMed]
- Wagner, H.J., B.G. Luo, M.A. Ariano, D.R. Sibley, and W.K. Stell, Localization of D2 dopamine receptors in vertebrate retinae with anti‐peptide antibodies. Journal of Comparative Neurology. 1993; 331(4):469-481. [PubMed]
- Rohrer, B. and W.K. Stell, Localization of putative dopamine D2-like receptors in the chick retina, using in situ hybridization and immunocytochemistry. Brain research. 1995; 695(2):110-116. [PubMed]
- Elena, P., P. Denis, M. Kosina-Boix, and P. Lapalus, Dopamine receptors in rabbit and rat eye: characterization and localization of DA1 and DA2 binding sites. Current eye research. 1989; 8(1):75-83. [PubMed]
- Tran, V.T. and M. Dickman, Differential localization of dopamine D1 and D2 receptors in rat retina. Investigative ophthalmology & visual science. 1992; 33(5):1620-1626. [PubMed]
- Veruki, M.L., Dopaminergic neurons in the rat retina express dopamine D2/3 receptors. European Journal of Neuroscience. 1997; 9(5):1096-1100. [PubMed]
- Vuvan, T., M. Geffard, P. Denis, A. Simon, and J. Nguyen-Legros, Radioimmunoligand characterization and immunohistochemical localization of dopamine D2 receptors on rods in the rat retina. Brain research. 1993; 614(1-2):57-64. [PubMed]
- Brann, M.R. and W.S. Young III, Dopamine receptors are located on rods in bovine retina. Neuroscience letters. 1986; 69(3):221-226. [PubMed]
- Dearry, A., P. Falardeau, C. Shores, and M.G. Caron, D 2 dopamine receptors in the human retina: Cloning of cDNA and localization of mRNA. Cellular and molecular neurobiology. 1991; 11(5):437-453. [PubMed]
- Mora‐Ferrer, C., S. Yazulla, K.M. Studholme, and M. Haak‐Frendscho, Dopamine D1‐receptor immunolocalization in goldfish retina. Journal of Comparative Neurology. 1999; 411(4):705-714. [PubMed]
- Cohen, A.I., R.D. Todd, S. Harmon, and K.L. O’Malley, Photoreceptors of mouse retinas possess D4 receptors coupled to adenylate cyclase. Proceedings of the National Academy of Sciences. 1992; 89(24):12093-12097. [PubMed]
- Derouiche, A. and E. Asan, The dopamine D2 receptor subfamily in rat retina: ultrastructural immunogold and in situhybridization studies. European Journal of Neuroscience. 1999; 11(4):1391-1402. [PubMed]
- Hillman, D.W., D. Lin, and B. Burnside, Evidence for D4 receptor regulation of retinomotor movement in isolated teleost cone inner‐outer segments. Journal of neurochemistry. 1995; 64(3):1326-1335. [PubMed]
- Klitten, L.L., M.F. Rath, S.L. Coon, J.-S. Kim, D.C. Klein, and M. Møller, Localization and regulation of dopamine receptor D4 expression in the adult and developing rat retina. Experimental eye research. 2008; 87(5):471-477. [PubMed]
- Zawilska, J. and J. Nowak, Dopamine D4-like receptors in vertebrate retina: does the retina offer a model for the D4-receptor analysis? Polish journal of pharmacology. 1997; 49(4):201-211. [PubMed]
- Zhekova, D. and L. Vitanova, Tyrosine hydroxylase activity and dopamine receptors D1 and D5 in frog and turtle retina: An immunofluorescence study. Comptes rendus de l’Académie bulgare des Sciences. 2016; 69(2).
- Yazulla, S. and Z.-S. Lin, Differential effects of dopamine depletion on the distribution of [3H] SCH 23390 and [3H] spiperone binding sites in the goldfish retina. Vision Research. 1995; 35(17):2409-2414. [PubMed]
- Firth, S., I. Morgan, and M. Boelen, Localization of D, dopamine receptors in the chicken retina. Australian and New Zealand journal of ophthalmology. 1997; 25(4):64-66.
- Koulen, P., Postnatal development of dopamine D1 receptor immunoreactivity in the rat retina. Journal of neuroscience research. 1999; 56(4):397-404. [PubMed]
- Veruki, M.L. and H. Wässle, Immunohistochemical Localization of Dopamine D Receptors in Rat Retina. European Journal of Neuroscience. 1996; 8(11):2286-2297.
- Farshi, P., B. Fyk‐Kolodziej, D.M. Krolewski, P.D. Walker, and T. Ichinose, Dopamine D1 receptor expression is bipolar cell type‐specific in the mouse retina. Journal of Comparative Neurology. 2016; 524(10):2059-2079. [PubMed]
- Travis, A.M., S.J. Heflin, A.A. Hirano, N.C. Brecha, and V.Y. Arshavsky, Dopamine-dependent sensitization of rod bipolar cells by GABA is conveyed through wide-field amacrine cells. Journal of Neuroscience. 2018; 38(3):723-732. [PubMed]
- Yadav, S.C., S. Tetenborg, and K. Dedek, Gap junctions in A8 amacrine cells are made of connexin36 but are differently regulated than gap junctions in AII amacrine cells. Frontiers in molecular neuroscience. 2019; 12:99. [PubMed]
- Wang, Y., K. Harsanyi, and S.C. Mangel, Endogenous activation of dopamine D2 receptors regulates dopamine release in the fish retina. Journal of Neurophysiology. 1997; 78(1):439-449. [PubMed]
- Kubrusly, R.C., R. Panizzutti, P.F. Gardino, B. Stutz, R.A. Reis, A.L.M. Ventura, M.C.F. de Mello, and F.G. de Mello, Expression of functional dopaminergic phenotype in purified cultured Müller cells from vertebrate retina. Neurochemistry international. 2008; 53(3-4):63-70. [PubMed]
- Biedermann, B., E. Fröhlich, J. Grosche, H.-J. Wagner, and A. Reichenbach, Mammalian Müller (glial) cells express functional D2 dopamine receptors. Neuroreport. 1995; 6(4):609-612. [PubMed]
- Nguyen‐Legros, J., E. Chanut, C. Versaux‐Botteri, A. Simon, and J.H. Trouvin, Dopamine inhibits melatonin synthesis in photoreceptor cells through a D2‐like receptor subtype in the rat retina: biochemical and histochemical evidence. Journal of neurochemistry. 1996; 67(6):2514-2520. [PubMed]
- Popova, E., Role of dopamine in distal retina. Journal of Comparative Physiology A. 2014; 200(5):333-358. [PubMed]
- Akopian, A. and P. Witkovsky, D2 dopamine receptor-mediated inhibition of a hyperpolarization-activated current in rod photoreceptors. Journal of Neurophysiology. 1996; 76(3):1828-1835. [PubMed]
- Kawai, F., M. Horiguchi, and E.-i. Miyachi, Dopamine modulates the voltage response of human rod photoreceptors by inhibiting the h current. Investigative ophthalmology & visual science. 2011; 52(7):4113-4117. [PubMed]
- Cohen, A.I. and C. Blazynski, Dopamine and its agonists reduce a light-sensitive poor of cyclic AMP in mouse photoreceptors. Visual Neuroscience. 1990; 4(1):43-52. [PubMed]
- Nir, I., J.M. Harrison, R. Haque, M.J. Low, D.K. Grandy, M. Rubinstein, and P.M. Iuvone, Dysfunctional light-evoked regulation of cAMP in photoreceptors and abnormal retinal adaptation in mice lacking dopamine D4 receptors. Journal of Neuroscience. 2002; 22(6):2063-2073. [PubMed]
- Patel, S., K.L. Chapman, D. Marston, P.H. Hutson, and C.I. Ragan, Pharmacological and functional characterisation of dopamine D4 receptors in the rat retina. Neuropharmacology. 2003; 44(8):1038-1046. [PubMed]
- Jackson, C.R., S.S. Chaurasia, H. Zhou, R. Haque, D.R. Storm, and P.M. Iuvone, Essential roles of dopamine D4 receptors and the type 1 adenylyl cyclase in photic control of cyclic AMP in photoreceptor cells. Journal of neurochemistry. 2009; 109(1):148-157. [PubMed]
- Ivanova, T.N., A.L. Alonso-Gomez, and P.M. Iuvone, Dopamine D4 receptors regulate intracellular calcium concentration in cultured chicken cone photoreceptor cells: relationship to dopamine receptor-mediated inhibition of cAMP formation. Brain research. 2008; 1207:111-119. [PubMed]
- Stella Jr, S.L. and W.B. Thoreson, Differential modulation of rod and cone calcium currents in tiger salamander retina by D2 dopamine receptors and cAMP. European Journal of Neuroscience. 2000; 12(10):3537-3548. [PubMed]
- Firsov, M. and L. Astakhova, The role of dopamine in controlling retinal photoreceptor function in vertebrates. Neuroscience and Behavioral Physiology. 2016; 46(2):138-145.
- Thoreson, W.B., S.L. Stella, E.J. Bryson, J. Clements, and P. Witkovsky, D 2-like dopamine receptors promote interactions between calcium and chloride channels that diminish rod synaptic transfer in the salamander retina. Visual neuroscience. 2002; 19(3):235-247. [PubMed]
- Liu, Y., F.-J. Luo, and P.-J. Liang, Dopamine effect on the stimulus pattern related changes in response characteristics of R/G horizontal cells in carp retina. Brain research. 2003; 973(2):190-195. [PubMed]
- Hedden Jr, W. and J. Dowling, The interplexiform cell system II. Effects of dopamine on goldfish retinal neurones. Proceedings of the Royal Society of London. Series B. Biological Sciences. 1978; 201(1142):27-55. [PubMed]
- Ammermüller, J., R. Weiler, and I. Perlman, Short-term effects of dopamine on photoreceptors, luminosity-and chromaticity-horizontal cells in the turtle retina. Visual neuroscience. 1995; 12(3):403-412. [PubMed]
- Witkovsky, P., S. Stone, and J.C. Besharse, Dopamine modifies the balance of rod and cone inputs to horizontal cells of the Xenopus retina. Brain research. 1988; 449(1-2):332-336. [PubMed]
- Hare, W.A. and W.G. Owen, Similar effects of carbachol and dopamine on neurons in the distal retina of the tiger salamander. Visual neuroscience. 1995; 12(3):443-455. [PubMed]
- Jin, N.G., A.Z. Chuang, P.J. Masson, and C.P. Ribelayga, Rod electrical coupling is controlled by a circadian clock and dopamine in mouse retina. The Journal of physiology. 2015; 593(7):1597-1631. [PubMed]
- Krizaj, D., Mesopic state: Cellular mechanisms involved in pre‐and post‐synaptic mixing of rod and cone signals. Microscopy research and technique. 2000; 50(5):347-359. [PubMed]
- Krizaj, D., R. Gábriel, W.G. Owen, and P. Witkovsky, Dopamine D2 receptor‐mediated modulation of rod‐cone coupling in the Xenopus retina. Journal of Comparative Neurology. 1998; 398(4):529-538. [PubMed]
- Krizaj, D., T. Vu, and D. Copenhagen, On the shaping, modulation and synaptic transmission of rod and cone signals. Toyoda, J.; Murakami, M.; Kaneko, A. 1999:7-83.
- Yang, X.-L. and S.M. Wu, Modulation of rod-cone coupling by light. Science. 1989; 244(4902):352-354. [PubMed]
- Li, H., A.Z. Chuang, and J. O’Brien, Photoreceptor coupling is controlled by connexin 35 phosphorylation in zebrafish retina. Journal of Neuroscience. 2009; 29(48):15178-15186. [PubMed]
- Li, H., Z. Zhang, M.R. Blackburn, S.W. Wang, C.P. Ribelayga, and J. O’Brien, Adenosine and dopamine receptors coregulate photoreceptor coupling via gap junction phosphorylation in mouse retina. Journal of Neuroscience. 2013; 33(7):3135-3150. [PubMed]
- Ribelayga, C. and S.C. Mangel, Identification of a circadian clock-controlled neural pathway in the rabbit retina. PloS one. 2010; 5(6). [PubMed]
- Ribelayga, C., Y. Cao, and S.C. Mangel, The circadian clock in the retina controls rod-cone coupling. Neuron. 2008; 59(5):790-801. [PubMed]
- Li, H., J. O’Brien, E. Akutagawa, and K. Ozaki, Photoreceptors: physiology, types and abnormalities. 2012.
- Zhang, Z., H. Li, X. Liu, J. O’BRIEN, and C.P. Ribelayga, Circadian clock control of connexin36 phosphorylation in retinal photoreceptors of the CBA/CaJ mouse strain. Visual neuroscience. 2015; 32. [PubMed]
- Schneeweis, D.M. and J.L. Schnapf, The photovoltage of macaque cone photoreceptors: adaptation, noise, and kinetics. Journal of Neuroscience. 1999; 19(4):1203-1216. [PubMed]
- Liu, X., J.C. Grove, A.A. Hirano, N.C. Brecha, and S. Barnes, Dopamine D1 receptor modulation of calcium channel currents in horizontal cells of mouse retina. Journal of neurophysiology. 2016; 116(2):686-697. [PubMed]
- Dong, C. and J.S. McREYNOLDS, The relationship between light, dopamine release and horizontal cell coupling in the mudpuppy retina. The Journal of physiology. 1991; 440(1):291-309. [PubMed]
- Perlman, I. and J. Ammermuller, Receptive-field size of L1 horizontal cells in the turtle retina: effects of dopamine and background light. Journal of Neurophysiology. 1994; 72(6):2786-2795. [PubMed]
- Piccolino, M., J. Neyton, and H. Gerschenfeld, Decrease of gap junction permeability induced by dopamine and cyclic adenosine 3′: 5′-monophosphate in horizontal cells of turtle retina. Journal of Neuroscience. 1984; 4(10):2477-2488. [PubMed]
- DeVries, S. and E. Schwartz, Modulation of an electrical synapse between solitary pairs of catfish horizontal cells by dopamine and second messengers. The Journal of physiology. 1989; 414(1):351-375. [PubMed]
- Hida, E., K. Negishi, and K.I. Naka, Effects of dopamine on photopic L‐type s‐potentials in the catfish retina. Journal of neuroscience research. 1984; 11(4):373-382. [PubMed]
- Lasater, E.M. and J.E. Dowling, Dopamine decreases conductance of the electrical junctions between cultured retinal horizontal cells. Proceedings of the National Academy of Sciences. 1985; 82(9):3025-3029. [PubMed]
- McMahon, D.G. and D.R. Brown, Modulation of gap-junction channel gating at zebrafish retinal electrical synapses. Journal of neurophysiology. 1994; 72(5):2257-2268. [PubMed]
- McMahon, D.G. and M.P. Mattson, Horizontal cell electrical coupling in the giant danio: synaptic modulation by dopamine and synaptic maintenance by calcium. Brain research. 1996; 718(1-2):89-96. [PubMed]
- Ribelayga, C. and S.C. Mangel, Absence of circadian clock regulation of horizontal cell gap junctional coupling reveals two dopamine systems in the goldfish retina. Journal of Comparative Neurology. 2003; 467(2):243-253. [PubMed]
- Ribelayga, C. and S.C. Mangel, Tracer coupling between fish rod horizontal cells: modulation by light and dopamine but not the retinal circadian clock. Visual neuroscience. 2007; 24(3):333-344. [PubMed]
- Teranishi, T., K. Negishi, and S. Kato, Dopamine modulates S-potential amplitude and dye-coupling between external horizontal cells in carp retina. Nature. 1983; 301(5897):243-246. [PubMed]
- Teranishi, T., K. Negishi, and S. Kato, Regulatory effect of dopamine on spatial properties of horizontal cells in carp retina. Journal of Neuroscience. 1984; 4(5):1271-1280. [PubMed]
- Hankins, M. and H. Ikeda, Non-NMDA type excitatory amino acid receptors mediate rod input to horizontal cells in the isolated rat retina. Vision research. 1991; 31(4):609-617. [PubMed]
- He, S., R. Weiler, and D.I. Vaney, Endogenous dopaminergic regulation of horizontal cell coupling in the mammalian retina. Journal of Comparative Neurology. 2000; 418(1):33-40. [PubMed]
- Weiler, R., M. Pottek, S. He, and D.I. Vaney, Modulation of coupling between retinal horizontal cells by retinoic acid and endogenous dopamine. Brain research reviews. 2000; 32(1):121-129. [PubMed]
- Reitsamer, H.A., R. Pflug, M. Franz, and S. Huber, Dopaminergic modulation of horizontal-cell-axon-terminal receptive field size in the mammalian retina. Vision research. 2006; 46(4):467-474. [PubMed]
- Hampson, E., D.I. Vaney, and R. Weiler, Dopaminergic modulation of gap junction permeability between amacrine cells in mammalian retina. Journal of Neuroscience. 1992; 12(12):4911-4922. [PubMed]
- Drujan, B., K. Negishi, and M. Laufer, Studies on putative neurotransmitters in the distal retina, in Neurochemistry of the Retina. 1980, Elsevier. p. 143-150. [PubMed]
- Mangel, S.C. and J. Dowling, Responsiveness and receptive field size of carp horizontal cells are reduced by prolonged darkness and dopamine. Science. 1985; 229(4718):1107-1109. [PubMed]
- Mangel, S.C. and J. Dowling, The interplexiform–horizontal cell system of the fish retina: effects of dopamine, light stimulation and time in the dark. Proceedings of the Royal society of London. Series B. Biological sciences. 1987; 231(1262):91-121. [PubMed]
- Negishi, K. and B. Drujan, Effects of catecholamines on the horizontal cell membrane potential in the fish retina. Sensory Processes. 1978; 2(4):388-395. [PubMed]
- Negishi, K. and B. Drujan, Effects of catecholamines and related compounds on horizontal cells in the fish retina. Journal of Neuroscience Research. 1979; 4(5‐6):311-334. [PubMed]
- Bornstein, O., G. Twig, J. Benda, R. Weiler, and I. Perlman, Dynamic changes in the receptive fields of L1-type horizontal cells in the retina of the turtle Mauremys caspica. Visual neuroscience. 2002; 19(5):621-632. [PubMed]
- Harsanyi, K. and S.C. Mangel, Activation of a D2 receptor increases electrical coupling between retinal horizontal cells by inhibiting dopamine release. Proceedings of the National Academy of Sciences. 1992; 89(19):9220-9224. [PubMed]
- Cohen, J.L. and J.E. Dowling, The role of the retinal interplexiform cell: effects of 6-hydroxydopamine on the spatial properties of carp horizontal cells. Brain research. 1983; 264(2):307-310. [PubMed]
- Qian, H., R.P. Malchow, and H. Ripps, Gap-junctional properties of electrically coupled skate horizontal cells in culture. Visual neuroscience. 1993; 10(2):287-295. [PubMed]
- Yang, X., K. Tornqvist, and J. Dowling, Modulation of cone horizontal cell activity in the teleost fish retina. II. Role of interplexiform cells and dopamine in regulating light responsiveness. Journal of Neuroscience. 1988; 8(7):2269-2278. [PubMed]
- Yamada, M., Y. Shigematsu, Y. Umetani, and T. Saito, Dopamine decreases receptive field size of rod-driven horizontal cells in carp retina. Vision research. 1992; 32(10):1801-1807. [PubMed]
- Zhang, A.J., R. Jacoby, and S.M. Wu, Light‐and dopamine‐regulated receptive field plasticity in primate horizontal cells. Journal of Comparative Neurology. 2011; 519(11):2125-2134. [PubMed]
- Piccolino, M., P. Witkovsky, and C. Trimarchi, Dopaminergic mechanisms underlying the reduction of electrical coupling between horizontal cells of the turtle retina induced by d-amphetamine, bicuculline, and veratridine. Journal of Neuroscience. 1987; 7(8):2273-2284. [PubMed]
- Miyachi, E. and M. Murakami, Decoupling of horizontal cells in carp and turtle retinae by intracellular injection of cyclic AMP. The Journal of physiology. 1989; 419(1):213-224. [PubMed]
- Piccolino, M., G. Demontis, P. Witkovsky, E. Strettoi, G. Cappagli, M. Porceddu, M. De Montis, S. Pepitoni, G. Biggio, and E. Meller, Involvement of D1 and D2 dopamine receptors in the control of horizontal cell electrical coupling in the turtle retina. European Journal of Neuroscience. 1989; 1(3):247-257. [PubMed]
- Qian, H. and H. Ripps, Receptive field properties of rod-driven horizontal cells in the skate retina. The Journal of general physiology. 1992; 100(3):457-478. [PubMed]
- Baldridge, W.H., Triphasic adaptation of teleost horizontal cells, Volume 131, in Progress in brain research. 2001, Elsevier. p. 437-449. [PubMed]
- Shigematsu, Y. and M. Yamada, Effects of dopamine on spatial properties of horizontal cell responses in the carp retina. Neuroscience Research Supplements. 1988; 8:S69-S80. [PubMed]
- Umino, O., Y. Lee, and J.E. Dowling, Effects of light stimuli on the release of dopamine from interplexiform cells in the white perch retina. Visual neuroscience. 1991; 7(5):451-458. [PubMed]
- Weiler, R. and A. Akopian, Effects of background illuminations on the receptive field size of horizontal cells in the turtle retina are mediated by dopamine. Neuroscience letters. 1992; 140(1):121-124. [PubMed]
- Villa, P., M. Bedmar, and M. Barón, Studies on rod horizontal cell s-potential in dependence of the dark/light adapted state: a comparative study in Cyprinus carpio and Scyliorhinus canicula retinas. Vision research. 1991; 31(3):425-435. [PubMed]
- Yang, X.-L., Modulation of synaptic transmission in the retina. Documenta ophthalmologica. 1991; 76(4):377-387. [PubMed]
- Xin, D. and S.A. Bloomfield, Dark‐and light‐induced changes in coupling between horizontal cells in mammalian retina. Journal of Comparative Neurology. 1999; 405(1):75-87. [PubMed]
- Knapp, A.G. and J.E. Dowling, Dopamine enhances excitatory amino acid-gated conductances in cultured retinal horizontal cells. Nature. 1987; 325(6103):437-439. [PubMed]
- Dowling, J.E., Retinal neuromodulation: the role of dopamine. Visual neuroscience. 1991; 7(1-2):87-97. [PubMed]
- Yang, X.-L., T.-X. Fan, and W. Shen, Effects of prolonged darkness on light responsiveness and spectral sensitivity of cone horizontal cells in carp retina in vivo. Journal of Neuroscience. 1994; 14(1):326-334. [PubMed]
- Djamgoz, M., M. Hankins, J. Hirano, and S. Archer, Neurobiology of retinal dopamine in relation to degenerative states of the tissue. Vision research. 1997; 37(24):3509-3529. [PubMed]
- Shiells, R. and G. Falk, Dopamine hyperpolarizes and reduces the light responses of rod ON-centre bipolar cells in the retina of the dogfish, Scyliorhinus canicula. Neuroscience letters. 1985; 55(3):331-336. [PubMed]
- Witkovsky, P., S. Stone, and D. Tranchina, Photoreceptor to horizontal cell synaptic transfer in the Xenopus retina: modulation by dopamine ligands and a circuit model for interactions of rod and cone inputs. Journal of Neurophysiology. 1989; 62(4):864-881. [PubMed]
- Pflug, R., R. Nelson, S. Huber, and H. Reitsamer, Modulation of horizontal cell function by dopaminergic ligands in mammalian retina. Vision research. 2008; 48(12):1383-1390. [PubMed]
- Djamgoz, M.B., E.M. Fitzgerald, and M. Yamada, Spectral Plasticity of H1 Horizontal Cells in Carp Retina: Independent Modulation by Dopamine and Light‐adaptation. European Journal of Neuroscience. 1996; 8(8):1571-1579. [PubMed]
- Yasui, S., M. Yamada, and M. Djamgoz, Dopamine and 2-amino-4-phosphonobutyrate differentially modify spectral responses of H1 horizontal cells in carp retina. Experimental brain research. 1990; 83(1):79-84. [PubMed]
- Djamgoz, M., M. Kirsch, and H.-J. Wagner, Haloperidol suppresses light-induced spinule formation and biphasic responses of horizontal cells in fish (roach) retina. Neuroscience letters. 1989; 107(1-3):200-204. [PubMed]
- Liu, Y., F.-J. Luo, and P.-J. Liang, Dopamine effect on the stimulus pattern related changes in response characteristics of R/G horizontal cells in carp retina. Brain research. 2003; 973(2):190-195. [PubMed]
- Esposti, F., J. Johnston, J.M. Rosa, K.-M. Leung, and L. Lagnado, Olfactory stimulation selectively modulates the OFF pathway in the retina of zebrafish. Neuron. 2013; 79(1):97-110. [PubMed]
- Heidelberger, R. and G. Matthews, Dopamine enhances Ca2+ responses in synaptic terminals of retinal bipolar neurons. Neuroreport. 1994; 5(6):729-732. [PubMed]
- Wellis, D.P. and F.S. Werblin, Dopamine modulates GABAc receptors mediating inhibition of calcium entry into and transmitter release from bipolar cell terminals in tiger salamander retina. Journal of Neuroscience. 1995; 15(7):4748-4761. [PubMed]
- Ichinose, T. and P.D. Lukasiewicz, Ambient light regulates sodium channel activity to dynamically control retinal signaling. Journal of Neuroscience. 2007; 27(17):4756-4764. [PubMed]
- Yu, C.J. and L. Li, Dopamine modulates voltage‐activated potassium currents in zebrafish retinal on bipolar cells. Journal of neuroscience research. 2005; 82(3):368-376. [PubMed]
- Fan, S.-F. and S. YAZULLA, Modulation of voltage-dependent K+ currents (I K (V)) in retinal bipolar cells by ascorbate is mediated by dopamine D1 receptors. Visual neuroscience. 1999; 16(5):923-931. [PubMed]
- Fan, S.-F. and S. Yazulla, Reciprocal inhibition of voltage-gated potassium currents (I K (V)) by activation of cannabinoid CB 1 and dopamine D 1 receptors in ON bipolar cells of goldfish retina. Visual neuroscience. 2005; 22(1):55-63. [PubMed]
- Fan, S.-F. and S. YAZULLA, Dopamine depletion with 6-OHDA enhances dopamine D1-receptor modulation of potassium currents in retinal bipolar cells. Visual neuroscience. 2001; 18(2):327-337. [PubMed]
- Yamada, M. and T. Saito, Effects of dopamine on bipolar cells in the carp retina. BIOMEDICAL RESEARCH-TOKYO. 1988; 9:125-130.
- Maguire, G. and F. Werblin, Dopamine enhances a glutamate-gated ionic current in OFF bipolar cells of the tiger salamander retina. Journal of Neuroscience. 1994; 14(10):6094-6101. [PubMed]
- Chaffiol, A., M. Ishii, Y. Cao, and S.C. Mangel, Dopamine regulation of GABAA receptors contributes to light/dark modulation of the ON-cone bipolar cell receptive field surround in the retina. Current Biology. 2017; 27(17):2600-2609. e4. [PubMed]
- Flood, M.D., J.M. Moore-Dotson, and E.D. Eggers, Dopamine D1 receptor activation contributes to light-adapted changes in retinal inhibition to rod bipolar cells. Journal of neurophysiology. 2018; 120(2):867-879. [PubMed]
- Mazade, R.E., M.D. Flood, and E.D. Eggers, Dopamine D1 receptor activation reduces local inner retinal inhibition to light-adapted levels. Journal of neurophysiology. 2019; 121(4):1232-1243. [PubMed]
- Frishman, L.J., Origins of the electroretinogram, Volume 2, in Principles and practice of clinical electrophysiology of vision, J. Heckenlively and A. GB, Editors. 2006, MIT Press: London. p. 139-183.
- Li, L. and J.E. Dowling, Effects of dopamine depletion on visual sensitivity of zebrafish. Journal of Neuroscience. 2000; 20(5):1893-1903. [PubMed]
- Lu, J., M. Zoran, and V. Cassone, Daily and circadian variation in the electroretinogram of the domestic fowl: effects of melatonin. Journal of Comparative Physiology A. 1995; 177(3):299-306. [PubMed]
- McGoogan, J.M. and V.M. Cassone, Circadian regulation of chick electroretinogram: effects of pinealectomy and exogenous melatonin. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 1999; 277(5):R1418-R1427. [PubMed]
- Wu, W.Q., J.M. McGoogan, and V.M. Cassone, Circadian regulation of visually evoked potentials in the domestic pigeon, Columba livia. Journal of Biological Rhythms. 2000; 15(4):317-328. [PubMed]
- Miranda-Anaya, M., P.A. Bartell, S. Yamazaki, and M. Menaker, Circadian rhythm of ERG in Iguana iguana: role of the pineal. Journal of biological rhythms. 2000; 15(2):163-171. [PubMed]
- Shaw, A.P., C.R. Collazo, K. Easterling, C.D. Young, and C.J. Karwoski, Circadian rhythm in the visual system of the lizard Anolis carolinensis. Journal of biological rhythms. 1993; 8(2):107-124. [PubMed]
- Brandenburg, J., A. Bobbert, and F. Eggelmeyer, Circadian changes in the response of the rabbit’s retina to flashes. Behavioural brain research. 1983; 7(1):113-123. [PubMed]
- White, M.P. and P.A. Hock, Effects of continuous darkness on ERG correlates of disc shedding in rabbit retina. Experimental eye research. 1992; 54(2):173-180. [PubMed]
- Barnard, A.R., S. Hattar, M.W. Hankins, and R.J. Lucas, Melanopsin regulates visual processing in the mouse retina. Current biology. 2006; 16(4):389-395. [PubMed]
- Cameron, M.A., A.R. Barnard, R.A. Hut, X. Bonnefont, G.T. van der Horst, M.W. Hankins, and R.J. Lucas, Electroretinography of wild-type and Cry mutant mice reveals circadian tuning of photopic and mesopic retinal responses. Journal of biological rhythms. 2008; 23(6):489-501. [PubMed]
- Sengupta, A., K. Baba, F. Mazzoni, N.V. Pozdeyev, E. Strettoi, P.M. Iuvone, and G. Tosini, Localization of melatonin receptor 1 in mouse retina and its role in the circadian regulation of the electroretinogram and dopamine levels. PloS one. 2011; 6(9). [PubMed]
- Storch, K.-F., C. Paz, J. Signorovitch, E. Raviola, B. Pawlyk, T. Li, and C.J. Weitz, Intrinsic circadian clock of the mammalian retina: importance for retinal processing of visual information. Cell. 2007; 130(4):730-741. [PubMed]
- Jackson, C.R., G.-X. Ruan, F. Aseem, J. Abey, K. Gamble, G. Stanwood, R.D. Palmiter, P.M. Iuvone, and D.G. McMahon, Retinal dopamine mediates multiple dimensions of light-adapted vision. Journal of Neuroscience. 2012; 32(27):9359-9368. [PubMed]
- Smith, B.J., P.D. Côté, and F. Tremblay, D1 dopamine receptors modulate cone ON bipolar cell Nav channels to control daily rhythms in photopic vision. Chronobiology international. 2015; 32(1):48-58. [PubMed]
- Lin, Z.-S. and S. Yazulla, Depletion of retinal dopamine does not affect the ERG b-wave increment threshold function in goldfish in vivo. Visual neuroscience. 1994; 11(4):695-702. [PubMed]
- Yazulla, S., Z.-S. Lin, and K.M. Studholme, Doparninergic control of light-adaptive synaptic plasticity and role in goldfish visual behavior. Vision research. 1996; 36(24):4045-4057. [PubMed]
- Kim, D.-Y. and C.-S. Jung, Gap junction contributions to the goldfish electroretinogram at the photopic illumination level. The Korean Journal of Physiology & Pharmacology. 2012; 16(3):219-224. [PubMed]
- Mora-Ferrer, C. and K. Behrend, Dopaminergic modulation of photopic temporal transfer properties in goldfish retina investigated with the ERG. Vision research. 2004; 44(17):2067-2081. [PubMed]
- Citron, M.C., L. Erinoff, D.W. Rickman, and N.C. Brecha, Modification of electroretinograms in dopamine-depleted retinas. Brain research. 1985; 345(1):186-191. [PubMed]
- Kupenova, P. and S. Belcheva, Effects of haloperidol, methylergometrine and phentolamine on the frog ERG. Experientia. 1981; 37(8):852-854. [PubMed]
- Wachtmeister, L., Further studies of the chemical sensitivity of the oscillatory potentials of the electroretinogram (ERG) II. Glutamate‐aspartate‐and dopamine antagonists. Acta ophthalmologica. 1981; 59(2):247-258. [PubMed]
- Popova, E., Effects of dopamine receptor blockade on the intensity-response function of electroretinographic b-and d-waves in light-adapted eyes. Journal of Neural Transmission. 2014; 121(3):233-244. [PubMed]
- Popova, E. and P. Kupenova, Effects of dopamine D1 receptor blockade on the intensity-response function of ERG b-and d-waves under different conditions of light adaptation. Vision research. 2011; 51(14):1627-1636. [PubMed]
- Popova, E. and P. Kupenova, Effects of dopamine receptor blockade on the intensity-response function of ERG b-and d-waves in dark adapted eyes. Vision research. 2013; 88:22-29. [PubMed]
- Popova, E., M. Kostov, and P. Kupenova, Effects of dopamine D 1 receptor blockade on the ERG b-and d-waves during blockade of ionotropic GABA receptors. Eye and Vision. 2016; 3(1):32. [PubMed]
- Sato, T., T. Yoneyama, H. Kim, and T. Suzuki, Effect of dopamine and haloperidol on the c-wave and light peak of light-induced retinal responses in chick eye. Documenta ophthalmologica. 1987; 65(1):87-95. [PubMed]
- Fujikado, T., The effect of dopamine on the response to pattern stimulation–study of the chick ERG. Japanese journal of ophthalmology. 1994; 38(4):368-374. [PubMed]
- Wioland, N., G. Rudolf, and N. Bonaventure, Electrooculographic and electroretinographic study in the chicken after dopamine and haloperidol. Documenta ophthalmologica. 1990; 75(2):175-180. [PubMed]
- Gottvall, E. and O. Textorius, Concentration-dependent effects of dopamine on the direct current electroretinogram of pigmented rabbits during prolonged intermittent recording. Documenta ophthalmologica. 2003; 106(2):161-169. [PubMed]
- Jagadeesh, J. and R. Sanchez, Effects of apomorphine on the rabbit electroretinogram. Investigative ophthalmology & visual science. 1981; 21(4):620-624. [PubMed]
- Kobayashi, A., Y. Shirao, S. Tagawa, K. Katoh, and T. Tamura, Effects of retinal intrinsic dopamine on the in in vivo electroretinogram of rabbits. Nippon Ganka Gakkai zasshi. 1996; 100(2):111-117. [PubMed]
- Nakagawa, T., S. Kurasaki, T. Masuda, K. Ukai, S. Kubo, and H. Kadono, Effects of some psychotropic drugs on the b-wave of the electroretinogram in isolated rabbit retina. Jpn J Pharmacol 1988; 46:97-100. [PubMed]
- Starr, M.S., The effects of various amino acids, dopamine and some convulsants on the electroretinogram of the rabbit. Experimental eye research. 1975; 21(1):79-87. [PubMed]
- Textorius, O., S.E.G. Nilsson, and B.-E. Andersson, Effects of intravitreal perfusion with dopamine in different concentrations on the DC electroretinogram and the standing potential of the albino rabbit eye. Documenta ophthalmologica. 1989; 73(2):149-162. [PubMed]
- Naarendorp, F., P. Hitchock, and P.A. Sieving, Dopaminergic modulation of rod pathway signals does not affect the scotopic ERG of cat at dark-adapted threshold. Journal of neurophysiology. 1993; 70(4):1681-1691. [PubMed]
- Naarendorp, F. and P.A. Sieving, The scotopic threshold response of the cat ERG is suppressed selectively by GABA and glycine. 1991. [PubMed]
- Schneider, T. and E. Zrenner, Effects of D-1 and D-2 dopamine antagonists on ERG and optic nerve response of the cat. Experimental eye research. 1991; 52(4):425-430. [PubMed]
- Skrandies, W. and H. Wässle, Dopamine and serotonin in cat retina: electroretinography and histology. Experimental brain research. 1988; 71(2):231-240. [PubMed]
- Olivier, P., F. Jolicoeur, G. Lafond, A. Drumheller, and J. Brunette, Effects of retinal dopamine depletion on the rabbit electroretinogram. Documenta ophthalmologica. 1987; 66(4):359-371. [PubMed]
- Bodis-Wollner, I., M. Marx, and M. Ghilardi, Systemic haloperidol administration increases the amplitude of the light-and dark-adapted flash ERG in the monkey. Clinical vision sciences. 1989; 4(1):19-26.
- Huppé-Gourgues, F., G. Coudé, P. Lachapelle, and C. Casanova, Effects of the intravitreal administration of dopaminergic ligands on the b-wave amplitude of the rabbit electroretinogram. Vision research. 2005; 45(2):137-145. [PubMed]
- Burguera, J., C. Vilela, A. Traba, Y. Ameave, and M. Vallet, The electroretinogram and visual evoked potentials in patients with Parkinson’s disease. Archivos de neurobiologia. 1990; 53(1):1-7.
- Gottlob, I., E. Schneider, W. Heider, and W. Skrandies, Alteration of visual evoked potentials and electroretinograms in Parkinson’s disease. Electroencephalography and clinical neurophysiology. 1987; 66(4):349-357. [PubMed]
- Nightingale, S., K. Mitchell, and J. Howe, Visual evoked cortical potentials and pattern electroretinograms in Parkinson’s disease and control subjects. Journal of Neurology, Neurosurgery & Psychiatry. 1986; 49(11):1280-1287. [PubMed]
- Jaffe, M., G. Bruno, G. Campbell, R. Lavine, C. Karson, and D. Weinberger, Ganzfeld electroretinographic findings in parkinsonism: untreated patients and the effect of levodopa intravenous infusion. Journal of Neurology, Neurosurgery & Psychiatry. 1987; 50(7):847-852. [PubMed]
- Filipova, M., J. Balik, V. Filip, J. Rodný, and H. Krejcova, Electroretinographic changes in patients with parkinsonism treated with various classes of antiparkinsonian drugs. Activitas nervosa superior. 1979; 21(3):136-138. [PubMed]
- Fornaro, P., P. Castrogiovanni, M. Perossini, G. Placidi, and G. Cavallacci, Electroretinography (ERG) as a tool of investigation in human psychopharmacology. Electroretinographic changes induced by a combination of carbi-dopa and levo-dopa. Acta neurologica. 1980; 2(4):293-299. [PubMed]
- Gottlob, I., H. Weghaupt, and C. Vass, Effect of levodopa on the human luminance electroretinogram. Investigative ophthalmology & visual science. 1990; 31(7):1252-1258. [PubMed]
- Adachi-Usami, E., H. Ikeda, and H. Satoh, Haloperidol delays visually evoked cortical potentials but not electroretinograms in mice. Journal of Ocular Pharmacology and Therapeutics. 1990; 6(3):203-210. [PubMed]
- Mizota, A. and E. Adachi-Usami, Electroretinographic effects of haloperidol on the mouse retina. Documenta ophthalmologica. 1993; 85(2):151-160. [PubMed]
- Herrmann, R., S.J. Heflin, T. Hammond, B. Lee, J. Wang, R.R. Gainetdinov, M.G. Caron, E.D. Eggers, L.J. Frishman, and M.A. McCall, Rod vision is controlled by dopamine-dependent sensitization of rod bipolar cells by GABA. Neuron. 2011; 72(1):101-110. [PubMed]
- Smith, B.J., P.D. Côté, and F. Tremblay, Dopamine modulation of rod pathway signaling by suppression of GABAC feedback to rod‐driven depolarizing bipolar cells. European Journal of Neuroscience. 2015; 42(6):2258-2270. [PubMed]
- He, Q., H.-p. Xu, P. Wang, and N. Tian, Dopamine D1 receptors regulate the light dependent development of retinal synaptic responses. PloS one. 2013; 8(11). [PubMed]
- Lavoie, J., P. Illiano, T.D. Sotnikova, R.R. Gainetdinov, J.-M. Beaulieu, and M. Hébert, The electroretinogram as a biomarker of central dopamine and serotonin: potential relevance to psychiatric disorders. Biological psychiatry. 2014; 75(6):479-486. [PubMed]
- Feigenspan, A., B. Teubner, K. Willecke, and R. Weiler, Expression of neuronal connexin36 in AII amacrine cells of the mammalian retina. Journal of Neuroscience. 2001; 21(1):230-239. [PubMed]
- Mills, S.L., J.J. O’Brien, W. Li, J. O’Brien, and S.C. Massey, Rod pathways in the mammalian retina use connexin 36. Journal of Comparative Neurology. 2001; 436(3):336-350. [PubMed]
- Deans, M.R., B. Volgyi, D.A. Goodenough, S.A. Bloomfield, and D.L. Paul, Connexin36 is essential for transmission of rod-mediated visual signals in the mammalian retina. Neuron. 2002; 36(4):703-712. [PubMed]
- Meyer, A., G. Hilgen, B. Dorgau, E.M. Sammler, R. Weiler, H. Monyer, K. Dedek, and S.G. Hormuzdi, AII amacrine cells discriminate between heterocellular and homocellular locations when assembling connexin36-containing gap junctions. Journal of cell science. 2014; 127(6):1190-1202. [PubMed]
- Urschel, S., T. Höher, T. Schubert, C. Alev, G. Söhl, P. Wörsdörfer, T. Asahara, R. Dermietzel, R. Weiler, and K. Willecke, Protein kinase A-mediated phosphorylation of connexin36 in mouse retina results in decreased gap junctional communication between AII amacrine cells. Journal of biological chemistry. 2006; 281(44):33163-33171. [PubMed]
- Hampson, E.C., D.D.I. Vaney, and R. Weiler, Dopaminergic modulation of gap junction permeability between amacrine cells in mammalian retina. Journal of Neuroscience. 1992; 12(12):4911-4922. [PubMed]
- Mills, S.L. and S.C. Massey, Differential properties of two gap junctional pathways made by AII amacrine cells. Nature. 1995; 377(6551):734-737. [PubMed]
- Xia, X.-B. and S.L. Mills, Gap junctional regulatory mechanisms in the AII amacrine cell of the rabbit retina. Visual neuroscience. 2004; 21(5):791-805. [PubMed]
- Kothmann, W.W., S.C. Massey, and J. O’Brien, Dopamine-stimulated dephosphorylation of connexin 36 mediates AII amacrine cell uncoupling. Journal of Neuroscience. 2009; 29(47):14903-14911. [PubMed]
- Bloomfield, S.A. and B. Völgyi, Function and plasticity of homologous coupling between AII amacrine cells. Vision research. 2004; 44(28):3297-3306. [PubMed]
- Kothmann, W.W., E.B. Trexler, C.M. Whitaker, W. Li, S.C. Massey, and J. O’Brien, Nonsynaptic NMDA receptors mediate activity-dependent plasticity of gap junctional coupling in the AII amacrine cell network. Journal of Neuroscience. 2012; 32(20):6747-6759. [PubMed]
- Bloomfield, S.A., D. Xin, and T. Osborne, Light-induced modulation of coupling between AII amacrine cells in the rabbit retina. Visual neuroscience. 1997; 14(3):565-576. [PubMed]
- Hu, E.H., F. Pan, B. Völgyi, and S.A. Bloomfield, Light increases the gap junctional coupling of retinal ganglion cells. The Journal of physiology. 2010; 588(21):4145-4163. [PubMed]
- Teranishi, T. and K. Negishi, Double-staining of horizontal and amacrine cells by intracellular injection with lucifer yellow and biocytin in carp retina. Neuroscience. 1994; 59(1):217-226. [PubMed]
- Bloomfield, S.A. and B. Völgyi, The diverse functional roles and regulation of neuronal gap junctions in the retina. Nature Reviews Neuroscience. 2009; 10(7):495-506. [PubMed]
- Mastronarde, D.N., Correlated firing of cat retinal ganglion cells. I. Spontaneously active inputs to X-and Y-cells. Journal of Neurophysiology. 1983; 49(2):303-324. [PubMed]
- Bu, J.-Y., H. Li, H.-Q. Gong, P.-J. Liang, and P.-M. Zhang, Gap junction permeability modulated by dopamine exerts effects on spatial and temporal correlation of retinal ganglion cells’ firing activities. Journal of computational neuroscience. 2014; 36(1):67-79. [PubMed]
- Li, H., W.-Z. Liu, and P.-J. Liang, Adaptation-dependent synchronous activity contributes to receptive field size change of bullfrog retinal ganglion cell. PLoS One. 2012; 7(3). [PubMed]
- Li, H. and P.-J. Liang, Stimulus discrimination via responses of retinal ganglion cells and dopamine-dependent modulation. Neuroscience bulletin. 2013; 29(5):621-632. [PubMed]
- Xiao, L., P.-M. Zhang, H.-Q. Gong, and P.-J. Liang, Effects of dopamine on response properties of ON-OFF RGCs in encoding stimulus durations. Frontiers in neural circuits. 2014; 8:72. [PubMed]
- Cook, P.B. and J.S. McReynolds, Modulation of sustained and transient lateral inhibitory mechanisms in the mudpuppy retina during light adaptation. Journal of neurophysiology. 1998; 79(1):197-204. [PubMed]
- Liu, Y. and E.M. Lasater, Calcium currents in turtle retinal ganglion cells. II. Dopamine modulation via a cyclic AMP-dependent mechanism. Journal of Neurophysiology. 1994; 71(2):743-752. [PubMed]
- Hayashida, Y. and A.T. Ishida, Dopamine receptor activation can reduce voltage-gated Na+ current by modulating both entry into and recovery from inactivation. Journal of neurophysiology. 2004; 92(5):3134-3141. [PubMed]
- Vaquero, C.F., A. Pignatelli, G.J. Partida, and A.T. Ishida, A dopamine-and protein kinase A-dependent mechanism for network adaptation in retinal ganglion cells. Journal of Neuroscience. 2001; 21(21):8624-8635. [PubMed]
- Chen, L. and X.-L. Yang, Hyperpolarization-activated cation current is involved in modulation of the excitability of rat retinal ganglion cells by dopamine. Neuroscience. 2007; 150(2):299-308. [PubMed]
- Hayashida, Y., C.V. Rodríguez, G. Ogata, G.J. Partida, H. Oi, T.W. Stradleigh, S.C. Lee, A.F. Colado, and A.T. Ishida, Inhibition of adult rat retinal ganglion cells by D1-type dopamine receptor activation. Journal of Neuroscience. 2009; 29(47):15001-15016. [PubMed]
- Ogata, G., T.W. Stradleigh, G.J. Partida, and A.T. Ishida, Dopamine and full‐field illumination activate D1 and D2‐D5‐type receptors in adult rat retinal ganglion cells. Journal of Comparative Neurology. 2012; 520(17):4032-4049. [PubMed]
- Li, Q., N. Wu, P. Cui, F. Gao, W.-J. Qian, Y. Miao, X.-H. Sun, and Z. Wang, Suppression of outward K+ currents by activating dopamine D1 receptors in rat retinal ganglion cells through PKA and CaMKII signaling pathways. Brain research. 2016; 1635:95-104. [PubMed]
- Cui, P., X.-Y. Li, Y. Zhao, Q. Li, F. Gao, L.-Z. Li, N. Yin, X.-H. Sun, and Z. Wang, Activation of dopamine D1 receptors enhances the temporal summation and excitability of rat retinal ganglion cells. Neuroscience. 2017; 355:71-83. [PubMed]
- Van Hook, M.J., K.Y. Wong, and D.M. Berson, Dopaminergic modulation of ganglion‐cell photoreceptors in rat. European Journal of Neuroscience. 2012; 35(4):507-518. [PubMed]
- Ikeda, H., T. Priest, J. Robbins, and K. Wakakuwa, Silent dopaminergic synapse at feline retinal ganglion -cells. Clinical Vision Sciences. 1986; 1(1):25-38.
- Straschill, M. and J. Perwein, The inhibition of retinal ganglion cells by catecholeamines and γ-aminobutyric acid. Pflügers Archiv. 1969; 312(3):45-54. [PubMed]
- Thier, P. and V. Alder, Action of iontophoretically applied dopamine on cat retinal ganglion cells. Brain research. 1984; 292(1):109-121. [PubMed]
- Maguire, G. and D. Hamasaki, The retinal dopamine network alters the adaptational properties of retinal ganglion cells in the cat. Journal of Neurophysiology. 1994; 72(2):730-741. [PubMed]
- Maguire, G.W. and E. Smith 3rd, Cat retinal ganglion cell receptive-field alterations after 6-hydroxydopamine induced dopaminergic amacrine cell lesions. Journal of neurophysiology. 1985; 53(6):1431-1443. [PubMed]
- Ames 3rd, A. and D. Pollen, Neurotransmission in central nervous tissue: a study of isolated rabbit retina. Journal of Neurophysiology. 1969; 32(3):424-442. [PubMed]
- Jensen, R. and N. Daw, Effects of dopamine and its agonists and antagonists on the receptive field properties of ganglion cells in the rabbit retina. Neuroscience. 1986; 17(3):837-855. [PubMed]
- Jensen, R.J. and N.W. Daw, Towards an understanding of the role of dopamine in the mammalian retina. Vision research. 1983; 23(11):1293-1298. [PubMed]
- Jensen, R.J. and N.W. Daw, Effects of dopamine antagonists on receptive fields of brisk cells and directionally selective cells in the rabbit retina. Journal of Neuroscience. 1984; 4(12):2972-2985. [PubMed]
- Yang, J., J. Pahng, and G.Y. Wang, Dopamine modulates the off pathway in light‐adapted mouse retina. Journal of neuroscience research. 2013; 91(1):138-150. [PubMed]
- Jensen, R., Effects of dopamine D2-like receptor antagonists on light responses of ganglion cells in wild-type and P23H rat retinas. PloS one. 2015; 10(12). [PubMed]
- O’Brien, J., The ever-changing electrical synapse. Current opinion in neurobiology. 2014; 29:64-72. [PubMed]