Ralph Nelson and Victoria Connaughton
1. Introduction.
Retinal ganglion cells are typically only two synapses distant from retinal photoreceptors, yet ganglion cell responses are far more diverse than those of photoreceptors. The most direct pathway from photoreceptors to ganglion cells is through retinal bipolar cells. Thus, it is of great interest to understand how bipolar cells transform visual signals.
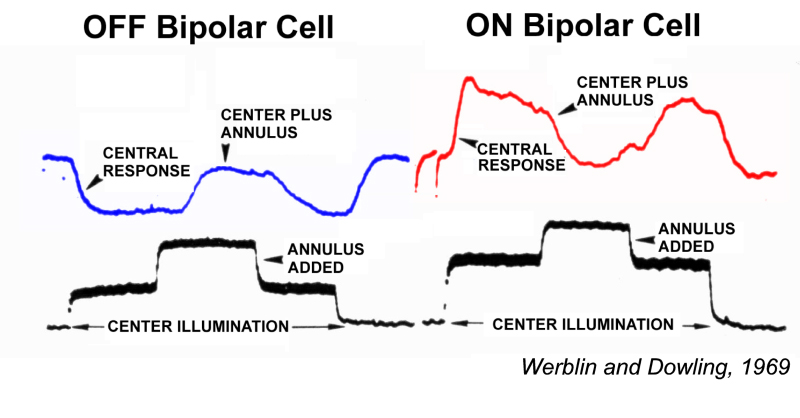
Werblin and Dowling (1) were among the first to investigate light-evoked responses of retinal bipolar cells. Based on these studies using penetrating microelectrodes, they proposed that retinal bipolar cells lacked impulse activity, and that they processed visual signals through integration of analogue signals, that is synaptic currents and non-spike-generating voltage-gated membrane currents.
Werblin and Dowing also proposed that retinal bipolar cells come in two fundamental varieties: ON-center and OFF-center (Fig. 1). Both types displayed a surround region in their receptive field that opposed the center, similar to the classic, antagonistic center-surround organization earlier described for ganglion-cell receptive fields (2). Ganglion cell receptive field organization is further reviewed in the Webvision chapter on ganglion cells. ON-center bipolar cells are depolarized by small spot stimuli positioned in the receptive field center. OFF-center bipolar cells are hyperpolarized by the same stimuli. Both types are repolarized by light stimulation of the peripheral receptive field outside the center (Fig. 1). Bipolar cells with ON-OFF responses were not encountered (1). ON-OFF responses, excitation at both stimulus onset and offset, first occur among amacrine cells, neurons postsynaptic to bipolar cells.
The Werblin and Dowling characterization of bipolar-cell physiology has proved quite durable over many decades. The notion that bipolar cells do not spike has found exception for some bipolar types. Dark-adapted Mb1 (rod bipolar cells) of goldfish generate light-evoked calcium spikes. These spikes originate in bipolar-cell axon terminals (3, 4). Through genetic imaging techniques this finding has been extended to the axon terminals of many zebrafish bipolar-cell types. In these studies bipolar terminals were labeled transgenically with the Ca2+ reporter protein SyGCaMP2 and light-induced fluctuations in Ca2+ were followed by 2-photon photometry. Fully 65% of the terminals delivered a spiking Ca2+ signal (4). In the cb5b bipolar-cell type of ground squirrel retina Na+ action potentials are driven by light. Other bipolar types in this retina do not exhibit spiking (5). These results suggest that bipolar cells are responsible for significantly more of the encoding of visual signals than had been previously supposed, and that axon-terminal spiking is actively involved. Impulse generation in bipolar cells is further discussed in the section on Voltage-gated currents.
Morphology and connectivity
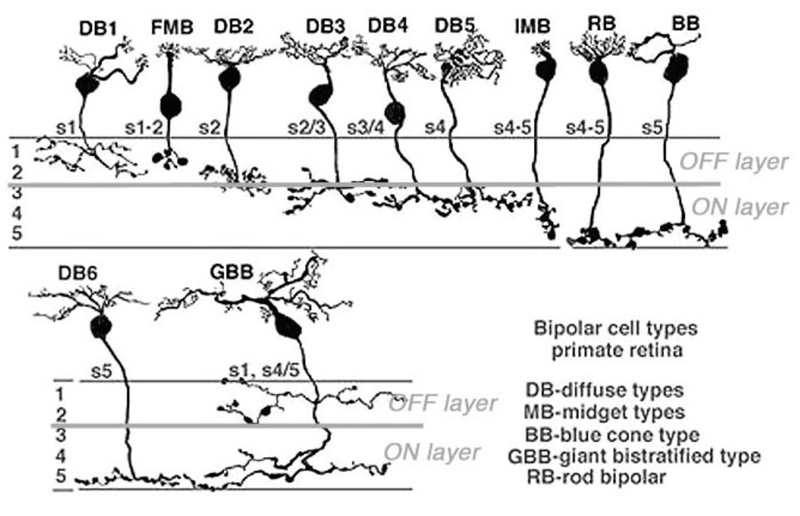
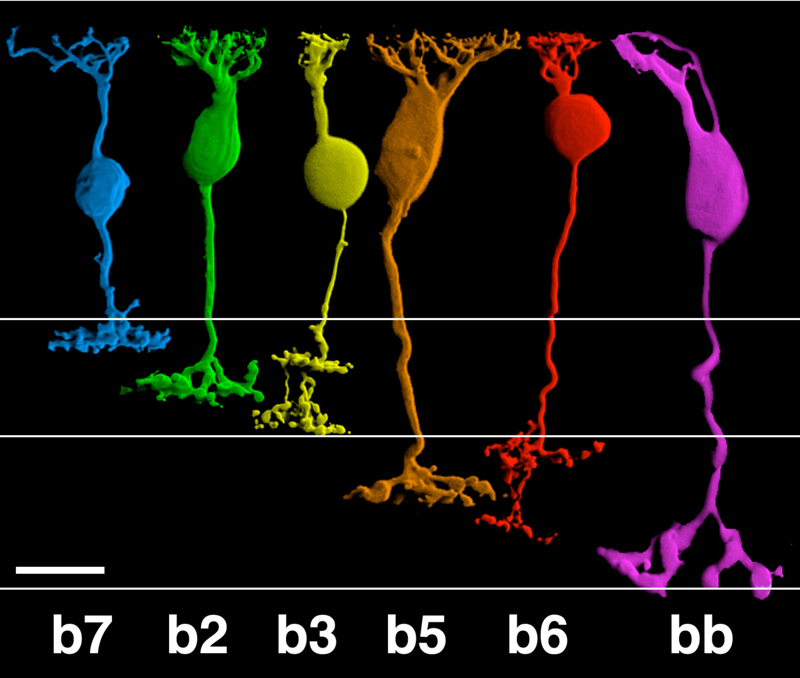
Anatomical investigations of bipolar cells reveal a multiplicity (4-22 depending on species) of different morphological types (6-12), significantly more than the just two types that early physiology implied. The diversity of human retinal bipolar types is illustrated in Fig. 2. Nonetheless all of these are either ON- or OFF-types and their diversity results from other factors, such as differing connectivity with photoreceptors and differing postsynaptic targets, as evidenced in the diversity of dendritic and axon-terminal ramification patterns. Some bipolar cells are postsynaptic only to rods, others only to cones (Fig. 2), and still others receive mixed rod-cone input. Among cone-selective bipolar cells, some innervate only red, green, or blue cones, while others are ‘diffuse’, that is, not selective (13-19). Different bipolar types express different glutamate receptors at subsynaptic contacts with cones.
Bipolar cell axon terminals are either mono- or multistratified, depending on the location of axonal boutons and branches in the inner plexiform layer (IPL). Differing terminal position and branching morphology within the IPL suggests that different morphological types selectively innervate different types of amacrine and ganglion cell (Fig 2). In primate retinas, bipolar cells are described as diffuse or midget types, based on the extent of the dendritic arbor. Midgets contact only a single cone, while diffuse types contact multiple cones. Bipolar cells are also termed ‘flat’ or ‘invaginating’ (20) depending on the placement of dendritic tips, either on the surface of (flat), or penetrating within photoreceptor synaptic terminals to approach presynaptic ribbons (invaginating). Fig. 2 illustrates 11 morphological types of bipolar cell seen in Golgi-stained human retinas.
Bipolar cell axon terminals are either mono- or multistratified, depending on the location of axonal boutons and branches in the inner plexiform layer (IPL). Differing terminal position and branching morphology within the IPL suggests that different morphological types selectively innervate different types of amacrine and ganglion cell (Fig 2). In primate retinas, bipolar cells are described as diffuse or midget types, based on the extent of the dendritic arbor. Midgets contact only a single cone, while diffuse types contact multiple cones. Bipolar cells are also termed ‘flat’ or ‘invaginating’ (20) depending on the placement of dendritic tips, either on the surface of (flat), or penetrating within photoreceptor synaptic terminals to approach presynaptic ribbons (invaginating). Fig. 2 illustrates 11 morphological types of bipolar cell seen in Golgi-stained human retinas.
2. Different glutamate receptor types for ON and OFF bipolar cells.
Light responses in bipolar cells are initiated by synapses with photoreceptors. Photoreceptors release only one neurotransmitter, glutamate (21); yet bipolar cells react to this stimulus with two different responses, ON-center (glutamate hyperpolarization) and OFF-center (glutamate depolarization). Different postsynaptic glutamate receptor proteins mediate these different membrane polarizing mechanisms. The different glutamate-gated responses are associated with the differential expression of either ionotropic (iGluR) glutamate receptors (OFF bipolar cells), metabotropic (mGluR) glutamate receptor types (ON bipolar cells) or glutamate transporters (ON bipolar cells). As a result, signal transduction at the photoreceptor-to-bipolar synapse has a range of properties. The process of splitting images into multiple components tuned to selective visual features begins with differentiation of different photoreceptor types but is then greatly elaborated at the synapses between photoreceptors and bipolar cells.
Metabotropic responses of ON bipolar cells: mGluR6, Go, TRPM1, Nyctalopin
The conductance of ON bipolar cells increases in the light, whereas OFF bipolar cell conductance decreases (22, 23). The decrease in OFF bipolar cell conductance is easily explained as a loss of excitation by glutamate, as light inhibits glutamate release from photoreceptors (24). The positive reversal potential of the ON bipolar cell light response, coupled with a conductance increase (22, 25), implies that glutamate blocks a cation-permeable channel. Originally a puzzle, this was the first evidence of what we now understand as the action of metabotropic glutamate receptors (mGluRs). These receptors do not form ion channels themselves, but act as isolated antennae on the cell surface sensing glutamate and activating intracellular pathways, ultimately affecting membrane potential through mechanisms several steps removed from the binding site for glutamate. Metabotropic receptors have been identified on the axon terminals of both photoreceptors (26) and bipolar cells (27) where they serve as autoreceptors regulating glutamate release. However, the expression of one specific mGluR in the subsynaptic membrane of ON bipolar cell dendrites, the APB receptor, is unique to retina, where it is used in the direct signal transmission pathway from photoreceptors to ON bipolar cells.
The mGluR6 receptor
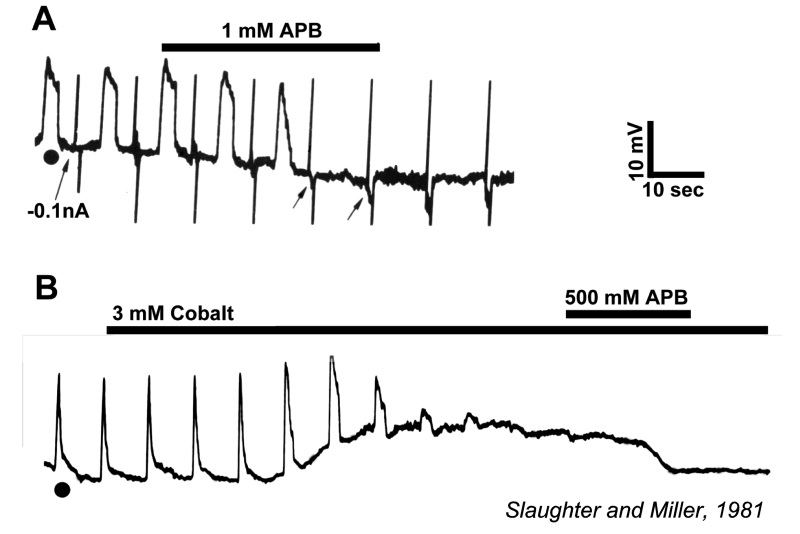
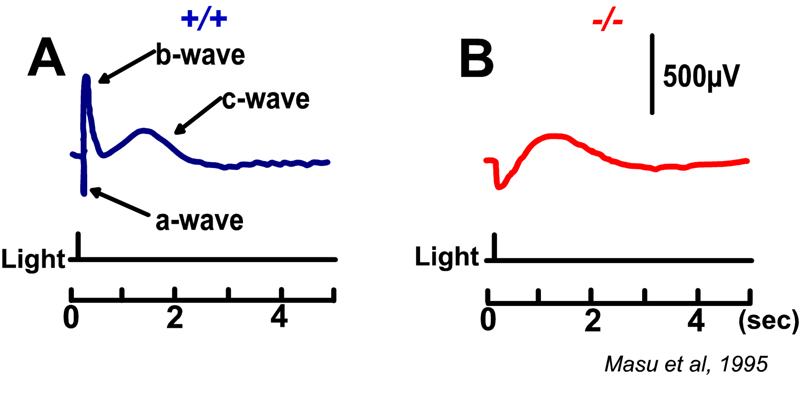
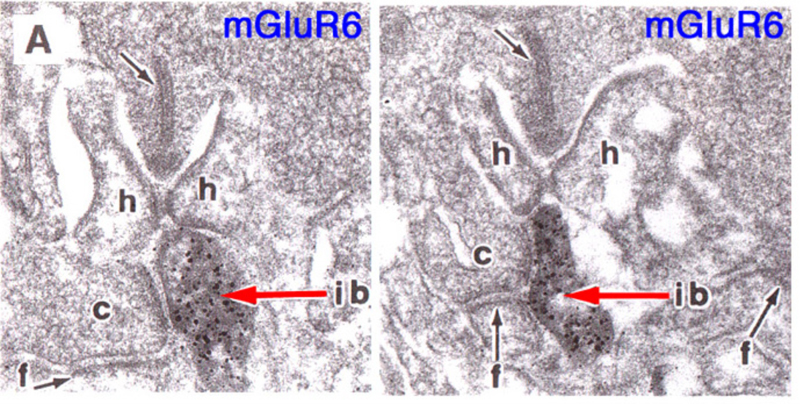
Slaughter and Miller (28) were the first to observe that the metabotropic glutamate agonist 2-amino-4-phosphonobutric acid (APB or DL-AP4, with the L enantiomer being effective) completely blocks the light responses of ON bipolar cells. In these neurons, APB acts as a substitute for photoreceptor-released glutamate (Fig. 3AB). Thus, ON bipolar cells utilize a metabotropic pathway to sense light-induced variations in release of photoreceptor glutamate. The metabotropic receptor has been identified as mGluR6 (29, 30). Transgenic knockout mice lacking the mGluR6 gene lack the electroretinographic b-wave (Fig. 4AB), an evoked-potential component associated with ON bipolar activity (31). The relation of electroretinogram components to cellular electrophysiology is further discussed in the Webvision chapter ‘The Electroretinogram: ERG’. Immunocytochemical localization for mGluR6 shows staining in the invaginating dendritic tips of monkey bipolar cells (Fig. 5) (32). Invaginating bipolar cells are thought to be mainly ON types in primate retina. Some foveal flat contacts also stained for mGluR6 (32).
The G-protein Go
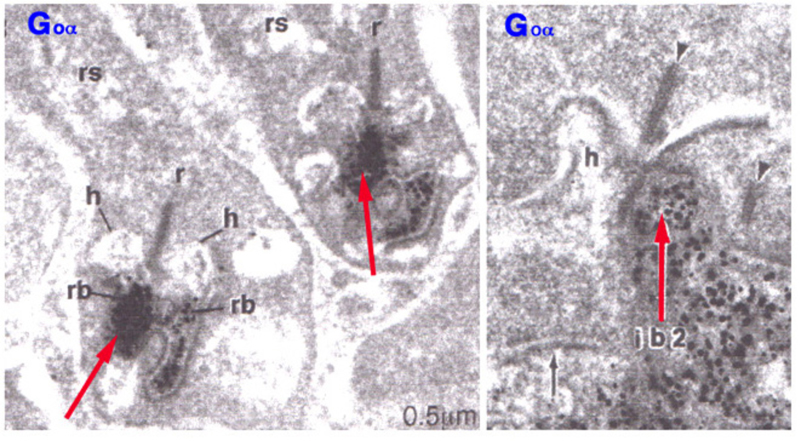
In addition to mGluR6, the G-protein Go is cytoplasmically localized in the dendritic tips of ON bipolar cells (Fig. 6) (33). Removal of the alpha subunit (Gαo) by knockout results in b-wave loss (34), similar to the mGluR6 knockout. Go was originally localized in rod bipolar cells, known to be ON-type, in a screen of potential G-protein second messengers for the metabotropic light response (35). This suggests that Go is directly involved in the intracellular pathway following mGluR6 activation.
The ion channel coupled to the APB receptor was originally thought to be cGMP-modulated (36).The closure of ion channels following APB binding onto mGluR6 seemed to require GTP and phosphodiesterase similar to phototransduction (36). However, the exact cascade by which this happened was less clear, as blocking phosphodiesterase (PDE) activity, or adding non-hydrolyzable cGMP analogs, did not inhibit the glutamate responses generated through APB-receptors (37). Further, it was Go that suppressed glutamate-gated current in ON bipolar cells, not transducin, the G-protein of the phototransduction cascade (37). Thus, removal of cGMP appears not to be required for channel closure (37).
The TRPM1 channel
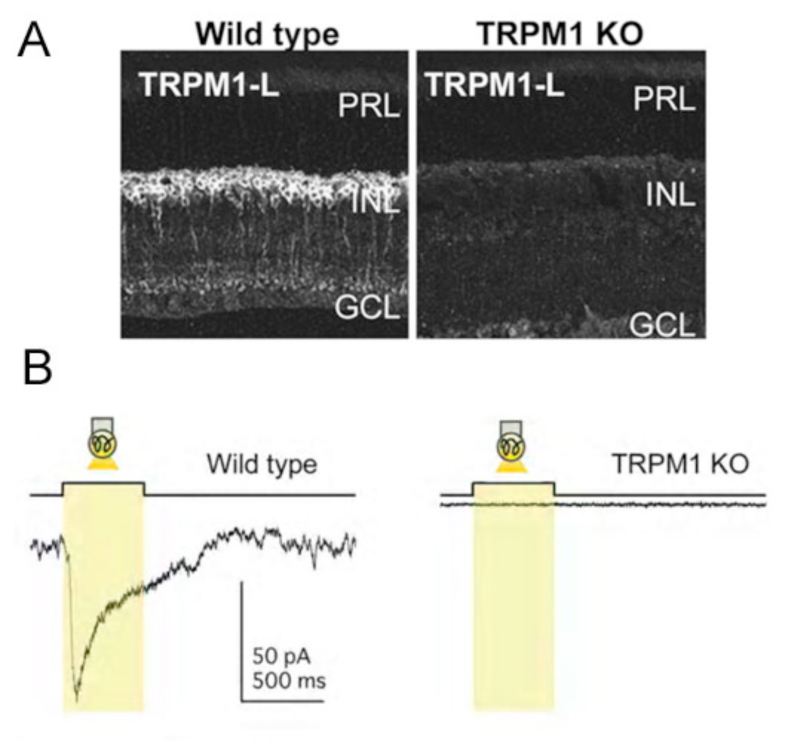
In agreement with these findings, recent work suggests the ON-bipolar-cell ion channel downstream of the mGluR6 receptor is not cGMP-gated (38). Rather, this non-selective cation channel – identified as a TRPM1-L channel – appears to be regulated by Gαo (38-40) in conjunction with Gβγ (41). The activity of the TRPM1 channel requires the presence of mGluR6, as the channel, though present, can not be activated in mGluR6 knockout mice (42).
TRP channels, or transient receptor potential channels, first identified in Drosophila photoreceptors (43), are present in all animal groups, including vertebrates (44), and as many as 28 channel subtypes have been identified. The TRP superfamily includes 7 subfamilies separated into two groups: TRPC, TRPV, TRPM, TRPN, and TRPA channels form Group 1; TRPP and TRPML channels form Group 2. TRPM1-L or melastatin, a melanoma related TRP channel, belongs to Group 1, and is found in ON bipolar cells. All channels share structural similarities and are permeable to cations; however, there is great functional diversity among the different channel subtypes. TRP channels are involved in many sensory systems including vision, hearing, taste, temperature-sensitivity, and osmoregulation, and are also involved in human disease (44-48).
In retina, TRP channels have been identified on photoreceptors (49), amacrine cells (50, 51), and ON-type bipolar cells. ON-bipolars (Fig. 7A), specifically, are antigenic for TRPM1 channels (52, 53) or TRPM1-L (38, 39, 54). Immunocytochemical and/or in situ hybridization studies have localized TRPM1 expression to the dendritic tips of ON-bipolar cells (38, 39, 52), though labeling is seen in cell bodies and axons as well (Fig. 7A). TRP channels are absent in OFF-type bipolar cells. TRPM1-L channel currents have a reversal potential ~0mV (38) similar to the reversal potential of glutamate-gated currents in these cells. TRPM1-L has been shown to co-localize with and/or be functionally coupled to mGluR6 (38, 40, 42, 52). In transfected CHO cells that express mGluR6, Gαo, and TRPM1-L, Koike and colleagues (38) showed that all three of these components must be present for glutamate-evoked whole-cell currents to be recorded. Cells expressing only mGluR6 and Gαo, or only Gαo and TRPM1-L, did not respond to glutamate application (38, 39). These findings suggest TRPM1 channels are downstream of the mGluR6 receptor and are necessary for glutamate-elicited responses in these cells. Further, TRPM1 -/- knockout mice (Fig. 7B) do not have light-evoked ON-bipolar-cell responses and there is no ERG b-wave (38, 39, 55). The loss of response is similar to that reported for mGluR6 -/- mice (Fig. 4) (31, 56), again suggesting that both mGluR6 and TRPM1 channels are required for ON-bipolar-cell photic responses. While all cone bipolar cells in mouse appear to use an mGluR6 synapse with cones, there is evidence that some of these cells may modulate a cation channel in addition to TRPM1-L. In the TRPM1 -/- mouse, the mGluR6 antagonist CPPG still blocks a minor APB-induced membrane current (52).
The proteoglycan nyctalopin
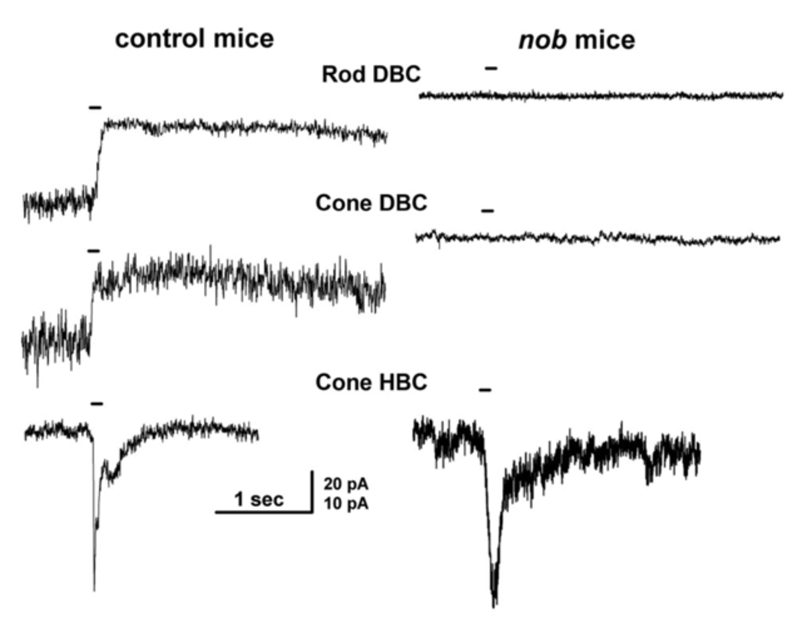
Nyctalopin is another protein expressed on the dendritic tips of ON-bipolar cells (Fig. 8a). It is encoded by the NYX gene. NYX is required for light- and glutamate-elicited responses in ON bipolar cells (57). Mutant nob mice (58) lack an ERG b-wave and are not responsive to focal applications of glutamate onto the bipolar cell dendritic arbor (57). In wild type mice ON bipolar cells respond with outward currents to this treatment, but in nob mice they do not (Fig. 9). The nob strain is an NYX -/- mutant (59). Generation of transgenic nob mice selectively expressing EYFP-nyctalopin fusion protein in bipolar cells completely rescued the mutant phenotype. Cellular expression was restricted to bipolar cells using a regulatory sequence for GABAcρ1, a GABA receptor subunit selectively produced by bipolar cells. In the EYFP-NYX line, the fusion protein expression was localized to the tips of ON-bipolar cells (Fig. 8a), the b-wave was restored, and inner retinal function was similar to controls (57).
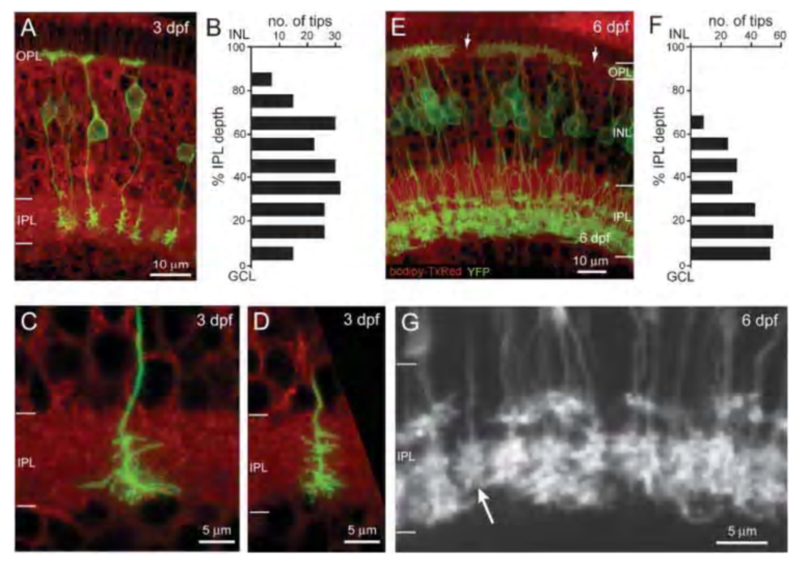
In zebrafish a membrane-targeted yellow fluorescent protein (MYFP) reporter strain has been generated using the upstream regulatory sequences for the NYX gene to express MYFP. This reporter marks a subset of ON-type bipolar cells with characteristic long axons and terminal boutons restricted to the inner half of the inner plexiform layer. Many of these also express the ON-bipolar marker protein kinase C (PKC) (60). This genetic reporter shows the complete morphology of the cells expressing the nyctalopin gene. This transgenic tool was used to follow embryonic refinement and development of axonal projection patterns for nyctalopin-expressing ON bipolars (60) (Fig. 8b).
Subsequent studies have reported that nyctalopin complexes with both mGluR6 and TRPM1 channels in ON-bipolar cells, serving a structural role that allows proper assembly and organization of the receptor and the channel (61). In addition, nyctalopin is able to modulate TRPM1 channels, as is mGluR6 (42, 62). Thus, glutamate binding onto mGluR6 activates a G-protein (Gαo and/or Gβγ) leading to the closure of TRPM1 channels. The receptor and the channel are held in close proximity by nyctalopin. Alteration or mutation of any of these components – mGluR6, nyctalopin, TRPM1, and/or Go – can lead to a loss of response by ON-bipolar cells. In agreement with this, individuals with congenital stationary night blindness (CSNB – discussed below) display a loss of ON-bipolar cell responses as evidenced in an absent ERG b-wave, and mutations in the genes encoding mGluR6, nyctalpin, and TRPM1 are associated with at least 75% of CSNB cases (62).
Modulators and subtypes
Calcium ions are a modulator of the ON bipolar metabotropic ion channel. Calcium ions, entering through the TRPM1 ion channel (63, 64) affect channel function, either by directly down regulating the channel (63) or by activating calcium-dependent enzymes, such as CaMKII (65-67), which modulate ion channel conductance. cGMP has been shown to selectively enhance ON bipolar cell responses to dim light, and may play a modulatory role for the TRPM1 channel (68).
Metabotropic receptors for ON-center bipolar cells have sustained and transient subtypes (69). The molecular basis is not yet known. However, it appears that the sustained and transient responses of ON-center ganglion cells, such as the classic X- and Y-types (70), may have their origin, at least in part, in the type of glutamate receptor expressed on the bipolar cells which innervate them (71).
Glutamate transporter mediated responses of ON bipolar cells
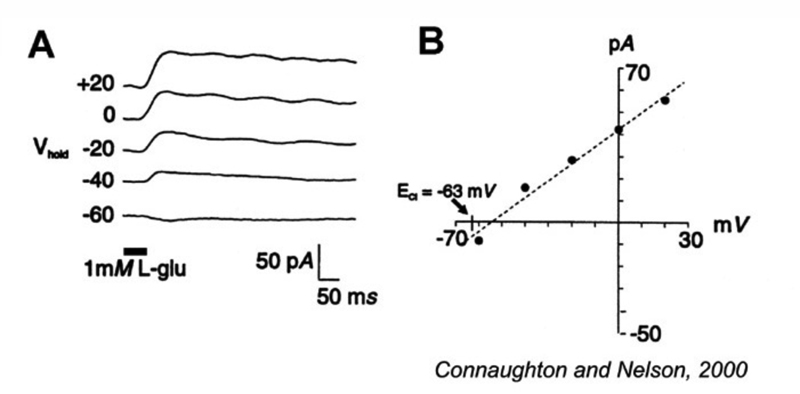
Ionotropic glutamate receptors with transporter-like properties are also present on some ON-center bipolar-cell dendrites. When photoreceptor glutamate binds to these transporters, a Cl− conductance forms and hyperpolarizes the cells in the dark (Fig. 10). Release from this Cl− inhibition occurs in the light with the decrease in glutamate released from photoreceptors. This allows the bipolar cells to depolarize (Fig. 10). Like transporters, this glutamate-gated Cl− mechanism requires [Na+]o in order to function. Thus far this mechanism has been found as a dendritic glutamate ‘receptor’ only in cyprinid ON bipolar cells (72-74), though it is reported in turtle, salamander and mouse photoreceptors (75-78) and is also present in mammalian central nervous system (79). Interestingly it occurs on the axon terminals of mouse rod and cone bipolar cells, where it acts to regulate glutamate release through inhibitory feedback (78).
Some non-mammalian bipolar cells contain both the APB and the ionotropic (transporter-like) receptor on their dendrites, while other ON-cells express either the metabotropic or the ionotropic receptor but not both (72, 73). EAAT5 has been identified as the chloride-channel-forming glutamate transporter (80). The ionotropic mechanism is used for sustained transmission between cones and bipolar cells (81, 82), and is likely to be a fast mechanism as compared to metabotropic pathways, which involve multi-step intracellular pathways and are often relatively slow (22).
The classic Mb rod bipolar cell of fish makes synapses with both rods and cones. The rod synapse mediates a conductance increase with reversal potential positive to resting potential. The cone synapse mediates a conductance decrease with reversal potential negative to the resting potential (82). Both mechanisms provide ON-type photic responses. In retrospect it would appear that the rod synapse is metabotropic, while the cone synapse is transporter-like, two different, selectively directed post-synaptic glutamate mechanisms on the same neuron.
AMPA kainate receptor expression in ON bipolar cells
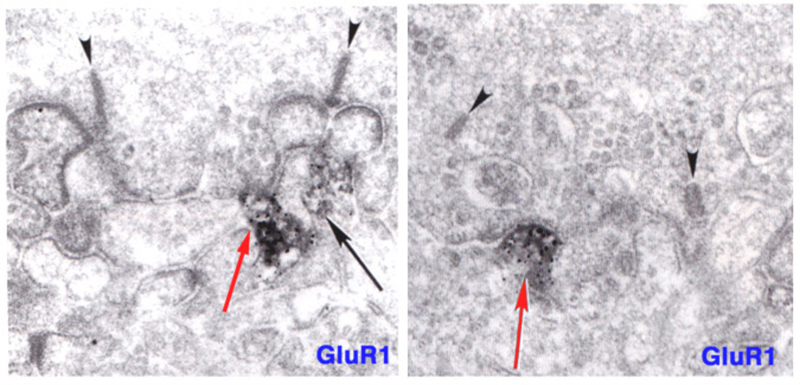
ON-center bipolar cells of mammals are immunoreactive for ionotropic AMPA receptors as well as metabotropic mGluR6 receptors (83-85). In figure 11 (right panel) immunoreactivity for GluR2/3, an ionotropic AMPA subunit, appears at an invaginating, ON-type ribbon contact in cat. Similarly in teleost retinas, ON-center bipolar cells are immunoreactive for ionotropic kainate receptors (86, 87). Particularly in mammals, no physiological role has been suggested for these conventional ionotropic receptors, usually associated with OFF bipolar cells, but also seen in ON-center bipolar cells. In giant danio Wong and Dowling find that bistratified cone bipolar cells mix ON-type and OFF-type glutamate receptor mechanisms, and utilize both transporter-like receptors and AMPA/kainate receptors in generating ON and OFF color responses respectively to different spectral stimuli (88).
Ionotropic glutamate responses of OFF bipolar cells
Like ON bipolar cells, OFF bipolar cells express more than one type of glutamate receptor, though all are ionotropic. There are three principal types of ionotropic glutamate receptors (AMPA, kainate, and NMDA) as originally defined by agonist selectivity. Though immunocytochemical studies (84, 89, 90) and in situ hybridization (91) have identified specific NMDA receptor subunits in the outer retina, OFF bipolar cells have never been observed to utilize NMDA receptors in the generation of light responses. OFF bipolar cells selectively express either AMPA or kainate receptors (92, 93). These receptors resensitize at different rates after exposure to glutamate (Fig. 13), and as a result, emphasize different temporal characteristics of the light signal. Kainate-type glutamate receptors transfer the sustained characteristics of the visual stimulus. AMPA receptors are more selective for the transient components of the signal (92). In ground squirrel retina bipolar cells are selective for one or the other (93). The situation is interesting in so far as neurons using kainate receptors exclusively are rare in the central nervous system. Nonetheless, AMPA and kainate receptors on retinal bipolar cells are pharmacologically well-behaved. Bipolar-cell AMPA-type responses can be selectively suppressed by the lipophilic AMPA receptor antagonist GYKI 52466 (94). Conversely, bipolar-cell kainate-type responses are blocked by the desensitizing kainate receptor agonist SYM 2081 (95).
While all retinas contain ON and OFF bipolar cell pathways, it is easy to imagine that among these pathways natural selection might cause a divergence in the expression of dendritic glutamate receptor types depending on the visual requirements of the species. In agreement with this hypothesis, species-specific differences between ON and OFF bipolar cell dendritic glutamate responses have been found. For example, ionotropic glutamate channels with transporter-like pharmacology occur exclusively in ON type bipolar cells in fish retinas. Conversely in salamander, OFF bipolar cells utilize only AMPA receptors (96). This may also be the case in zebrafish retina where dissociated cells fail to respond to the kainate agonist SYM 2081 (86) and electroretinographic OFF responses (d-waves) are blocked by the AMPA antagonist GYKI 52466 (97). One might expect also that even within the broad classes of AMPA and kainate receptors, subforms may have evolved to fit particular visual niches. In salamander retina indeed, there are separate classes of AMPA receptors postsynaptic to rods and to cones (96, 98).
3 Bipolar-cell axons: ON and OFF lamination in the inner plexiform layer
In work performed at the National Institutes of Health in the mid 1970s (99, 100), it was noted that the ON or OFF property of cat retinal ganglion cells was related to the level of stratification of dendrites within the retinal inner plexiform layer. This led to a general scheme for ON and OFF layering illustrated in figure 14. The dendrites of OFF-center ganglion cells always arborize distal to the dendrites of ON-center ganglion cells. The zone of OFF-center dendritic arborization is called sublamina a, while the zone of ON-center dendritic arborization is called sublamina b (Fig. 14). Within each sublamina ganglion cells make selective contacts with ON- or OFF-type bipolar cells. The pattern of ON and OFF layering of bipolar cell synaptic terminals and ganglion cell dendrites has proved to be a consistent pattern among all vertebrate retinas examined (101, 102). ON and OFF layering is particularly pronounced in retinas where ganglion cell types are predominantly monostratified. However, in more anatomically complex retinas, (i.e., turtle) with multistratified and/or diffusely stratified ganglion cell types, the ON vs. OFF layering pattern applies to monostratified cells only. The physiology of cells with processes ramifying throughout the IPL is more difficult to predict based on morphology alone (103).
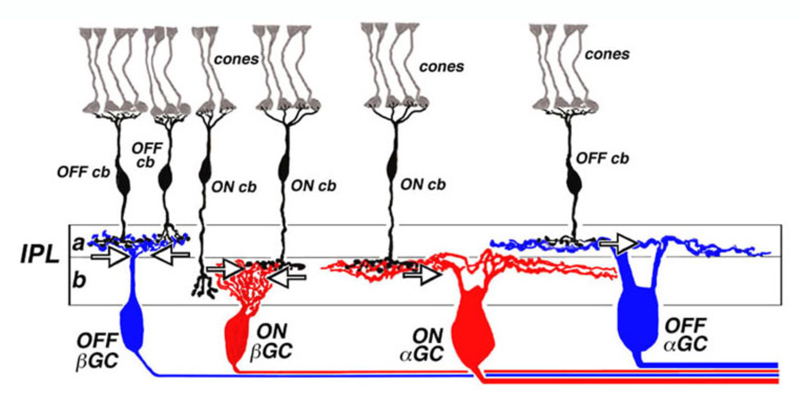
Stratification of cone bipolar cell axon terminals
The axon terminals of ON and OFF bipolar cells ramify in distinct IPL layers, where they are presynaptic to ON- and OFF-type ganglion cells, respectively. The axon terminal arborizations of OFF-type cone-contacting bipolar cells lie in sublamina a, where they synapse with the dendrites of OFF-type ganglion cells, while the axon terminal arborizations of ON-type cone contacting bipolar cells lie in sublamina b, where they contact the dendrites of ON-center ganglion cells. Synapses between OFF-type bipolar terminals and ganglion cell dendrites in sublamina a, and ON-type cells in sublamina b, have been observed electron-microscopically (104-109). Synapses between pairs of bipolar and ganglion cells with mismatched response polarity were never observed, even where processes come in close proximity (105). Melanopsin ganglion cells may provide an exception to this rule. The outer stratifying melanopsin ganglion cells of mouse and primate receive synapses from ON-cone bipolar cell axons en passant (110, 111).
Awatramani and Slaughter (69) proposed a further refinement of the bipolar-cell stratification scheme: cells with a glutamate receptor physiology emphasizing transients in the visual pattern are layered more toward the center of the IPL, while those with glutamate receptor patterns emphasizing sustained contrast are layered more towards IPL inner and outer edges. Although based in salamander retina, such a pattern would be appropriate to the layering of the sustained X (IPL inner and outer edge) and transient Y type ganglion cells of the cat retina (central IPL), as well as P and M cells of the primate retina. Wu et al (11) find a similar pattern in salamander, but attribute it to an elaboration of other circuitry elements, as bipolar cells with arbors in the mid-IPL tend to be cone dominated, emphasizing speed and transient response, whereas bipolar cells with axon terminals stratified at the inner and outer edges of the IPL process rod signals, which are relatively more lethargic in wave form (11).
Exhaustive correlations of ON or OFF bipolar cell physiology with axonal stratification patterns are now available for several species. Light responses or, as a surrogate for light responses, either dendritic glutamate responses, or responses to electrical stimulation of individual cones, can be measured in patch microelectrode recordings in retinal slice. Electrophysiological findings are correlated with axonal and dendritic morphology through microelectrode staining.
Salamander
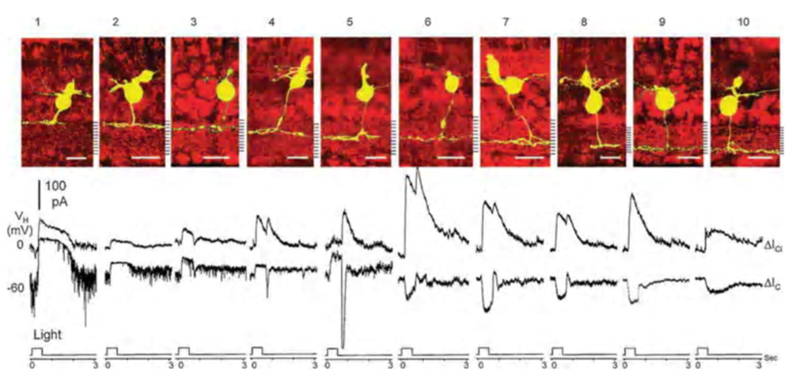
In salamander retina Pang, Gao and Wu (2004) (112) separated bipolar-cell light-evoked responses into cationic currents originating from glutamatergic synapses with photoreceptors, and chloride currents originating with GABAergic or glycinergic synapses with amacrine cells. The polarity of light-evoked cationic currents (ΔIC) correlated perfectly with axonal branching levels in the inner plexiform layer. Salamander bipolar cell types 1-5, with axonal stratification in the outer half of the inner plexiform layer (sublamina a), were OFF types, giving outward ΔIC. Types 6-10, with axonal stratification in the inner half of the inner plexiform layer (sublamina b), were ON-types, giving inward ΔIC (Fig. 15). The cationic synaptic currents were isolated from inhibitory chloride currents by clamping the cells at chloride reversal potential (Vm = −60 mV). Light evoked inhibitory chloride currents (ΔICl ) were examined by clamping the cells at cationic reversal potential (Vm = 0 mV). Both ON and OFF bipolar cells were inhibited by chloride currents, most likely generated by glycinergic and GABAergic synapses from amacrine cells onto bipolar cell axon terminals. One inhibitory pattern was ON-sustained, while the other was ON/OFF. In addition to bipolar cells with highly stratified axon terminals (Fig. 15) others with broader stratification or even bistratification were encountered, giving a total of 22 morphological types (12). These latter types also followed the ON-OFF stratification rule, except for the types that were bistratified in both sublaminae, the a/b types. These could be either ON or OFF, but often a dual ON and OFF dendritic input could be dissected with pharmacological treatments (112).
Rod-dominated mammals
In rat retina, bipolar cell axon terminal arborization appears to obey perfectly the rule of ON and OFF layering (7). As judged by the level of axonal stratification, there are 9 bipolar types. The axons of cone bipolar cells 1-5 branch in the outer half of rat IPL (sublamina a). These cells responded with inward currents when stimulated with kainate, an agonist for AMPA/kainate receptors on the dendrites of OFF bipolar cells. Of a combined 15 recordings from types 1-5, only one cell responded to APB (DL AP-4), a glutamate agonist selective for ON center bipolar cells. Four cone bipolar cell types (types 6-9) with axons branching in the inner half of rat IPL (sublamina b) were identified. In net 12 of 14 of cells of these types responded with outward currents to APB, whereas only one responded to kainate (7). This is a better than 90% compliance with the ON and OFF stratification pattern for cone bipolar cell axon terminals in rat retina. Bipolar cell branching patterns in mouse retina appear similar to rat (113). A more limited set of recordings in cat and monkey retinas suggest also that there may be good adherence to ON and OFF stratification of bipolar terminals (109, 114).
Ground squirrel
Li and DeVries (15) have provided a significant technical advance in the mapping of form onto function for biplolar cells of the all-cone retina of ground squirrel. In these studies one patch electrode was used to electrically stimulate an individual cone within the dendritic arbor of a bipolar cell simultaneously patched and stained by a second microelectrode. In this way both the cone contacts and the nature of these contacts onto bipolar cells are directly verified. Six cone bipolar types have been mapped by this technique, one ON type contacting only blue cones, an ON and an OFF type contacting both blue and green cones, and remaining types contacting only green cones (Fig. 16). No color opponent cone synaptic inputs using different glutamate receptors were detected in ground squirrel bipolar cells. The lamination patterns for ON and OFF types follows that proposed for rod-dominated mammalian retinas (100, 115).
Cyprinids
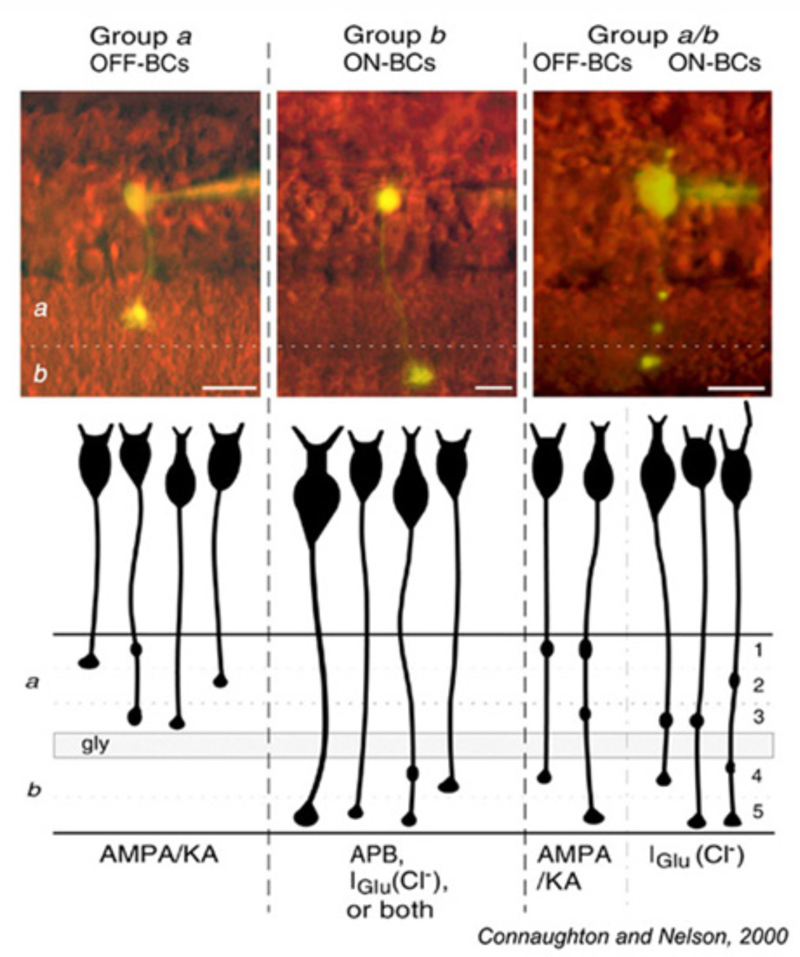
In anatomical studies using a technique called ‘diolistic labeling’, Connaughton et al (2004) (6) distinguished 17 bipolar-cell types in zebrafish based on axonal stratification patterns. In work still in progress in the Dowling laboratory, cone contact patterns have been added to axonal stratification patterns (116, 117), resulting in perhaps 20 or more bipolar types that can be anatomically distinguished. However, in the latter studies 90% of the examples are accounted for by as few as 9 of these types. There are 5 exclusively cone-contacting groups that are distinguished by the pattern of cones contacted and by axonal stratification. There are 4 types that contact rods in addition to various cone groupings (Mb types). The most selective cone-contacting type contacts only green-cones. It is very wide in dendritic and axonal field and is OFF-stratified. The most diffuse pattern of photoreceptor contacts is an OFF stratified type that contacts all photoreceptors, rods, red, blue, green, and UV cones. In mammals it appears that all the dendritic contacts emanating from a single bipolar cell express the same glutamate receptor (15). This appears not to be the case in fish or amphibian bipolar cells, where the same cell may express different glutamate receptors at different dendritic tips (72, 82, 88, 112), with each receptor type segregating according to the photoreceptor type contacted (82, 88).
Glutamate responses of zebrafish bipolar cells with axonal boutons in sublamina a (Group a) and sublamina b (Group b) are summarized in Fig. 17. Bipolar cells with 1 or 2 terminal boutons restricted either to sublamina a or to sublamina b obey the ON and OFF stratification rule. OFF cells branching in sublamina a express AMPA/KA type receptors. ON cells branching in sublamina b express either or both of two inhibitory glutamate mechanisms, ionotropic (Iglu, transporter-like chloride current) or metabotropic (APB receptor).
Carp retina (115, 118) also exhibits a bisublaminar organization of bipolar-cell terminals.
Bistratified axon terminals
Bisublaminar orderliness breaks down to some extent in species where multistratified bipolar cell axons are common. While multistratified cells exist in mammals like monkeys, cats, and squirrels (10, 15, 119, 120) (Fig. 2-GBB; Fig. 16 b3), these types are much more common in birds, reptiles, amphibians and fishes. Multistratified types selectively contact cones (19, 120) and so may be characteristic of cone-dominated species. If broad or multistratified bipolar terminals are restricted to either the ON or the OFF sublaminae (i.e. all branches and boutons within the same sublamina), the ON and OFF stratification rules apply. This is seen for type b3 bipolar cells in ground squirrel (Fig. 16). However, if the axonal stratification pattern crosses the ON-OFF boundary, the cells may be either ON-type or OFF-type (11, 72, 103). In some cases such multistratified bipolar cells may even express both ON and OFF physiologies (11, 88, 112), with the OFF physiology mediated by AMPA receptors, and the ON physiology mediated by either mGluR6, or EAAT. In giant danio such cells are color opponent (88). In zebrafish multistratified bipolar cells with boutons in both sublaminae were found to be either ON or OFF types Fig. 17. Connaughton and Nelson concluded that multistratified ON types use only Iglu, the transporter-like chloride current (72), however in the related giant danio, Wong et al find a bistratified type that is both ON and OFF, expressing both Iglu and AMPA/kainate receptors (88). The pattern for other giant danio ON bipolar cells is similar to zebrafish where the Iglu, or excitatory amino acid transporter (EAAT), mechanism is found particularly in ON-type cone contacting bipolar cells, while the mixed Mb rod and cone contacting types utilize both EAAT and MGluR6 mechanisms (121). The glutamate transporter blockers TBOA was found effective in blocking EAAT transmission (121).
Stratification of rod bipolar cell axon terminals and rod pathways
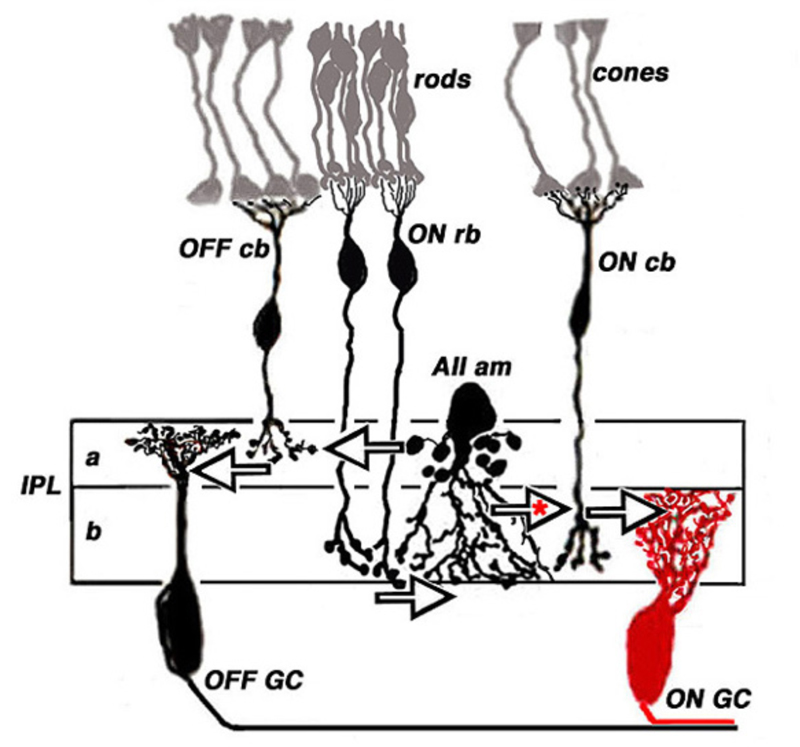
Rod bipolar cells are unique. These cells are easily identified in both mammals and fish (122). In most mammals, judged by axonal stratification pattern and contacts with photoreceptors, there is only a single class of rod bipolar cell (20, 122). The axon descends deep into ON sublamina b of the IPL, where it arborizes in a bulbous terminal just adjacent to ganglion cells (Fig. 18, rb). The mammalian rod bipolar cell is an ON-type cell (123) utilizing metabotropic APB-sensitive, glutamate receptors (31, 32, 64, 124). In fish, the comparable cell type is the Mb bipolar cell (125). In fish retinas, rod-contacting bipolar cells also contact cones. Five such types are found in goldfish retina, including multiple ON types with axon terminals branching in sublamina b, and likely OFF types with axon terminals branching in sublamina a (14). Interestingly in mouse retina there also appear to be multiple ON type rod bipolar cells. While axon-terminal morphology differs only subtly, light-evoked signals in DBCR1 are completely rod dominated, whereas DBCR2, similar to fish, receives substantial cone signal input in addition to rod input. The cone signals in DBCR2 types disappear in connexin 36 knockout mice (126, 127). Regardless of species, classic ON-center rod bipolar cells are all universally recognized by high immunoreactivity for protein kinase C (PKC) (64, 113, 126, 128-130).
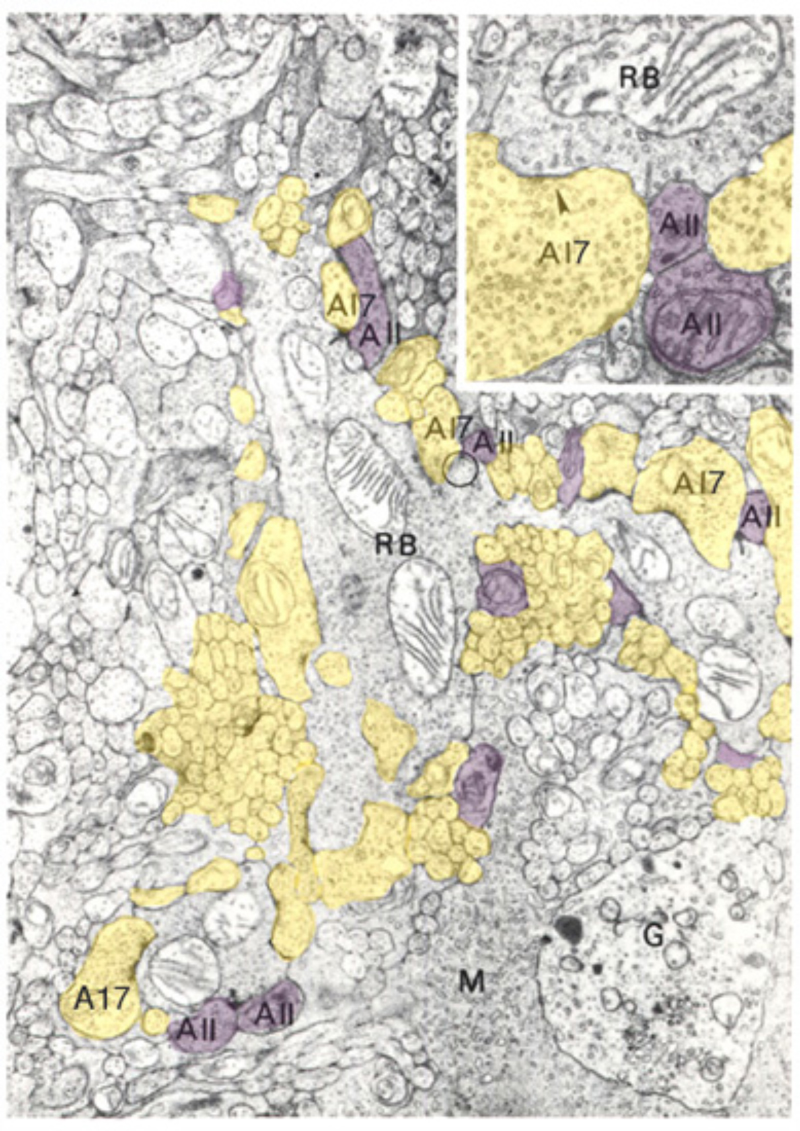
Although the axon terminals of rod bipolar cells are directly adjacent to ganglion cells, they do not contact them directly. In the mammalian retina, the AII amacrine cell (105, 131, 132) is the first intermediary in transferring rod bipolar signals to ganglion cells. The AII cell achieves this through direct innervations of cone bipolar processes within the IPL, either by means of chemical synapses with OFF bipolar cells (large arrow in sublamina a, Fig. 18) or through gap junctions between AII dendrites and ON cone bipolar axons (large arrow with red asterisk, Fig. 18). The cone bipolar axon terminals are the second intermediary in transferring rod bipolar signals to ganglion cells. The AII-amacrine-to-cone-bipolar gap junction is down-regulated by the humeral factor nitric oxide acting through an intracellular cGMP pathway (133) and also is susceptible to pharmacological blockade by meclofenamic acid (134).
Cone bipolar cells participate in transferring ON and OFF rod signals from distal retina to ganglion cells even without AII amacrine intermediation. This occurs through two pathways. In the first, signals flow through gap junctions between rods and cones (135-139), and by this pathway into cone bipolar cells. In the second, cone-contacting bipolar cells make direct dendritic contact with rods, as commonly occurs in non-mammals (14, 117, 140, 141). In mammals this type of cone bipolar pathway is usually restricted to OFF cone bipolar cell dendritic contacts with rods (139, 142, 143). This pathway bypasses the slower, albeit higher gain and more sensitive, metabotropic pathway of rod bipolar cells using the faster ionotropic pathways in the OFF cone bipolar cells (143). In contrast to the typical mammalian pattern, mouse retina shows an analogy to fish retina in having ON cone bipolar cells that directly contact rods (144). Separate rod signaling pathways can be distinguished in humans, both behaviorally, and in evoked potential recordings (145). A sensitive scotopic pathway may represent the rod-bipolar-to-AII pathway, whereas a less sensitive and faster mesopic pathway may represent a rod-to-cone gap-junction pathway, or perhaps additionally, an OFF cone bipolar pathway with direct rod input.
4 Electrical properties, lateral inhibition, and synaptic release
Voltage-gated currents
Voltage-gated channels permeate the membranes of retinal bipolar cells and contribute to voltage responses. In general, these channels carry calcium (Ca2+) and/or potassium (K+) ionic currents. Intracellular recordings show bipolar cells produce graded potentials in response to a light stimulus, but not action potentials (1). This finding is supported by several studies in which voltage-gated Na+ currents were not identified in bipolar neurons (146-148). However, some other studies do report voltage-gated Na+ currents in some cone bipolar cells in rat (149-151) and in goldfish (152). In general such Na+ currents may not produce action potentials, except sometimes under special conditions where K+ channels are blocked. It seems rather that these currents may typically serve to amplify synaptic events and shape photoresponse waveforms.
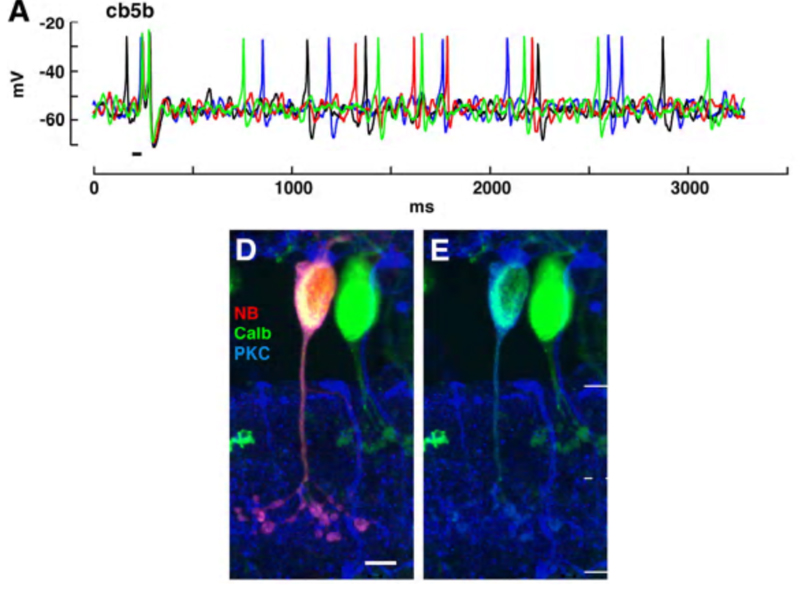
The cb5b bipolar cell of ground squirrel retina is an exception. Spontaneous and light-evoked Na+ spikes can be recorded from these bipolar cells using perforated patch recordings (Fig. 19) (5). The light-evoked bipolar spikes appear to trigger impulse generation in post-synaptic ganglion cells.
Na+ spikes have not been seen in rod bipolar cells. However in fish retinas, Ca2+ spikes have been identified in the rod bipolar axon terminals (3, 153, 154). In zebrafish with bipolar cells transgenically labeled with the Ca2+ fluorescent indicator SyGCaMP2, 65% of bipolar terminals produced at least 1 Ca2+ spike every second (4). This suggests that Ca+ spikes occur in the axon terminals of both rod and cone bipolar cells. As yet light-evoked Ca2+ spiking is only reported in cyprinid retinas.
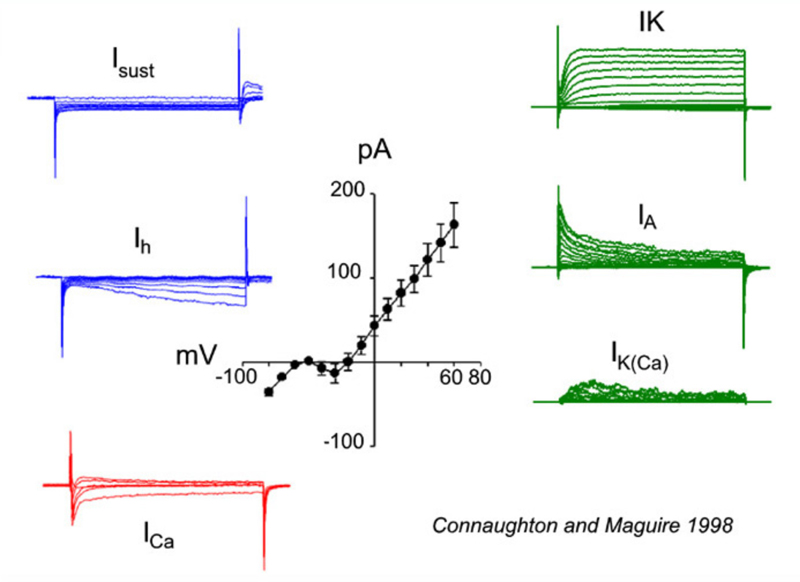
All bipolar cells examined to date express inward Ca2+ currents in response to membrane depolarizations. The Ca2+ currents are either transient (T-type/low voltage activated) and/or sustained (L-type/high voltage activated). The expression of these different Ca2+ current types varies among vertebrate species. For example, in goldfish, L-type Ca2+ currents are present (155, 156); whereas, mouse bipolar cells express only a transient T-type current (156, 157). In salamander (158) and zebrafish (146), however, both T- and L-type Ca2+ channels are present, with the L-type localized to the axon terminal. Recordings of a zebrafish bipolar cell expressing both L-type and T-type Ca2+ currents appear in figure 20, ICa.
A number of potassium (K+) currents have been identified in bipolar cells. Membrane depolarization typically elicits a combination of outward K+ currents. In goldfish (159) and tiger salamander retina (25), a slowly activating, delayed rectifying (IK) potassium current, modulated by dopamine (160), is activated. In contrast, axolotl bipolar neurons (161) express a rapidly activating, slowly inactivating IA current in response to depolarizing membrane potentials. Bipolar cells in fish, such as white bass and zebrafish, express one or the other of these two types of K+ currents, suggesting that these neurons can be differentiated into distinct populations based on the voltage-gated currents they express (146, 148). Membrane depolarizations also elicit a calcium-dependent K+ current (IK(Ca)) that contributes to the overall outward current amplitude observed (146, 148, 159). These outward currents tend to hyperpolarize the cell, restoring membrane potential after depolarization. On the other hand membrane hyperpolarizations elicit the slowly activating, inward rectifying (Ih) current (146-148, 159). This also tends to restore membrane potential. Examples of K+ currents evoked by membrane depolarization (IK, IA, IK(Ca)), or hyperpolarization (Isust, Ih) appear in figure 20. These were recorded in zebrafish retinal bipolar cells.
Inhibitory GABA-gated currents
As described above, light stimulation of the bipolar cell receptive field reveals characteristic center-surround antagonism (Fig. 1), where the response of the center is of opposite polarity to the response of the surround. It is believed that the center component of the bipolar cell light response arises from direct glutamatergic inputs from photoreceptors, while the surround response is generated indirectly by horizontal-cell suppression of glutamate release from cones (162). Horizontal-cell feedback to cones is further discussed in the Webvision chapter S-potentials and horizontal cells.
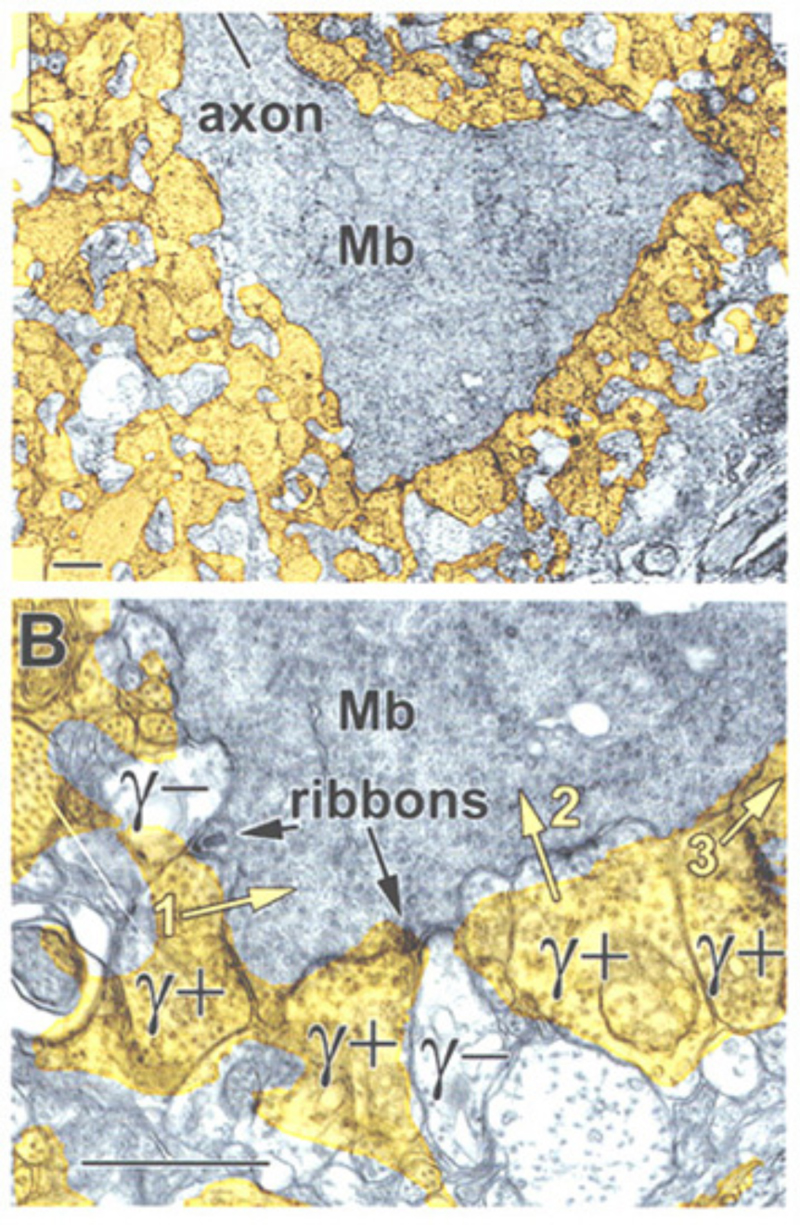
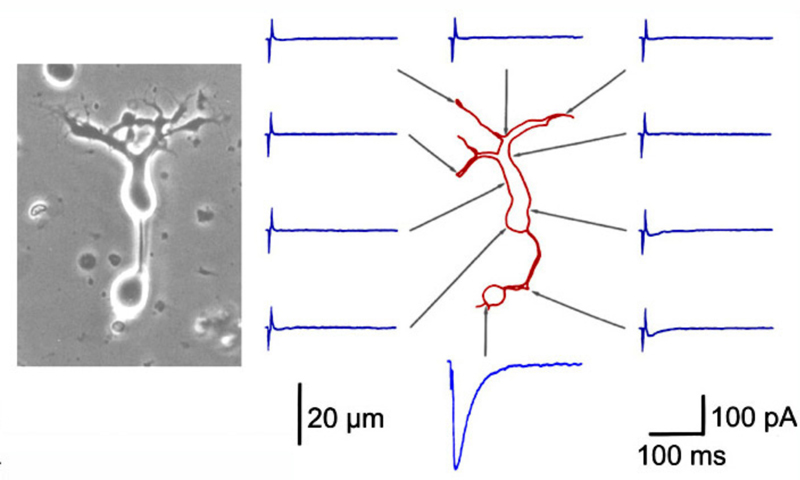
A further potential surround mechanism is through direct inhibitory GABAergic and/or glycinergic inputs from horizontal cells or amacrine cells. These neurotransmitters open chloride channels and generate signals of either hyperpolarizing or depolarizing polarity, depending on the magnitude of the chloride (Cl−) gradient across the synaptic membranes (163). Chloride-mediated responses at light onset and offset have been observed in ON bipolar cells of tiger salamander (12, 25, 112). These components appear to originate with AMPA excitation of GABAergic and glycinergic inhibitory interneurons, rather than with photoreceptors directly (25, 164). The waveforms of these ON or OFF inhibitions depend on whether the excitatory ON responses are rod or cone dominated. Rod dominated cells tend to have sustained ON inhibition without offset inhibitory transient, whereas cone dominated cells are transiently inhibited at both onset and offset of the stimulus (112). Immunocytochemical studies show that bipolar-cell dendrites and terminals are surrounded by GABAergic processes. In the OPL, these processes belong to horizontal cells; whereas, in the IPL, GABAergic amacrine cell processes surround bipolar terminals (165-170). Illustrations of the GABAergic inhibitory synaptic arrangements surrounding bipolar cell axon terminals appear in figure 21 for a cyprinid Mb (rod bipolar) terminal and in figure 22 for a cat rod bipolar cell axon terminal.
Bipolar cell processes are selectively sensitive to external GABA application (Fig. 23). GABA-evoked currents are typically greatest at the axon terminals (171-174), though smaller amplitude currents can be elicited from the soma and/or dendrites (Fig. 23). This suggests a major inhibitory feedback circuit from neighboring amacrine cells (171-175) occurs through direct GABAergic input onto bipolar-cell terminals. GABAergic inputs to bipolar dendrites may also occur indirectly, through a feedback synapse involving photoreceptors (176-179).
GABA application elicits a chloride (Cl−) current. Depending on the Cl− gradient either depolarization or hyperpolarization may result, though the most common result is hyperpolarization. GABA-elicited currents have both transient and sustained components. The transient response is mediated by GABAA receptors; while the sustained component results from the activation of GABAC receptors (173, 180-187). The sustained components of GABAC responses last many minutes and the hyperpolarizing action is readily seen in voltage probe studies of dissociated bipolar cells using oxonol, a slow distributive probe, as voltage reporter (171, 188). The different time courses of GABAA and GABAC currents and the different sensitivity to selective antagonists are illustrated for ferret bipolar cells in figure 24. The molecular properties of retinal GABAC receptors are explained in the Webvision chapter GABAc Receptors in the Vertebrate Retina. Interestingly, though most bipolar cells appear to express both GABAA and GABAC receptors, it is the GABAC receptor that underlies 70-80% of GABA-elicited responses. With light stimuli these receptors transfer more net charge to bipolar cell terminals than either GABAA or glycine receptors (189). Expression of both GABAA and GABAC receptors allows bipolar axon terminals to respond to a range of GABA concentrations and time courses within the synaptic cleft, as these different receptor types display collectively high sensitivity to long duration applications of low GABA doses (GABAC), and short duration applications of high GABA doses (GABAA) (181, 183).
GABAB is the ‘metabotopic’ GABA receptor. These receptors have been identified on the axon terminals of salamander and goldfish bipolar cells, where they reduce calcium influx (158, 190), modulating synaptic release of bipolar-cell glutamate.
Functional consequences of GABA inhibitory circuitry
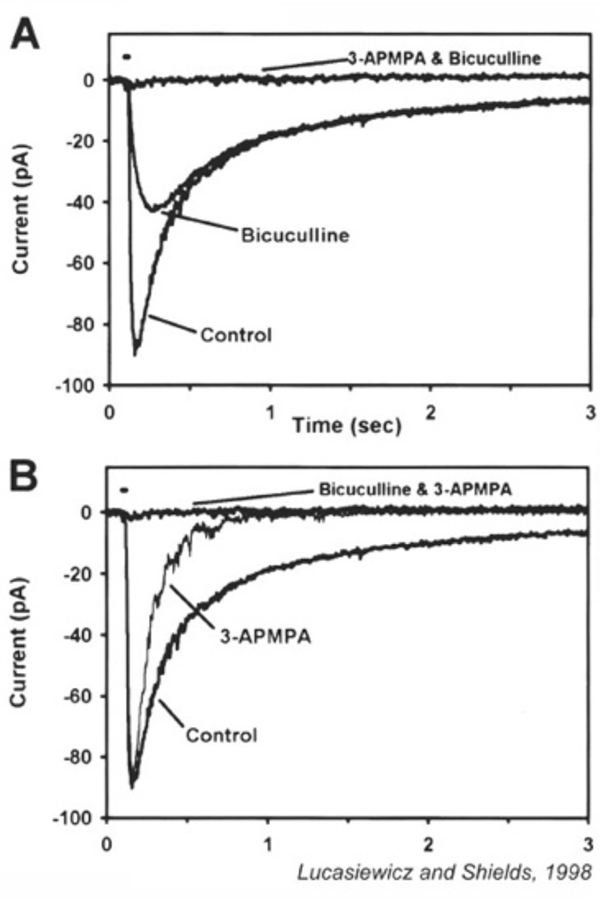
GABA receptors affect the dynamics of retinal light responses (191-194). GABAA antagonists cause ganglion cell ON discharges to become more transient (193, 195), an effect seen in ON bipolar cells (195). Zhang et al suggest a serial synaptic pathway to explain this counterintuitive result. On this model, the native action of GABA, not GABA antagonists, is to make light responses more transient, through delayed inhibition. Amacrine cells synapsing on bipolar cells with GABAC synapses, however, are themselves inhibited by other amacrine cells utilizing GABAA synapses. Blockade of the GABAA input causes more robust inhibition of bipolar terminals by GABAC and further transience of light responses. In this model a counterintuitive action is explained by a polysnaptic pathway. GABAC currents measured in bipolar cells actually do increase in the presence of the GABAA antagonist bicuculline (189). GABA release from amacrine cells activates receptors on bipolar cell terminals, causing the suppression of a depolarization-elicited calcium current (158, 196) and associated synaptic release (197) presumably modulating or reducing neurotransmitter release from these cells (198). Measurement of the light evoked excitatory currents in amacrine cells postsynaptic to bipolar cells suggests that the kinetically slower GABAC inhibition limits the duration of excitatory post-synaptic events, whereas the faster glycine and GABAA inhibition limits the peak amplitude of postsynaptic excitatory events (191).
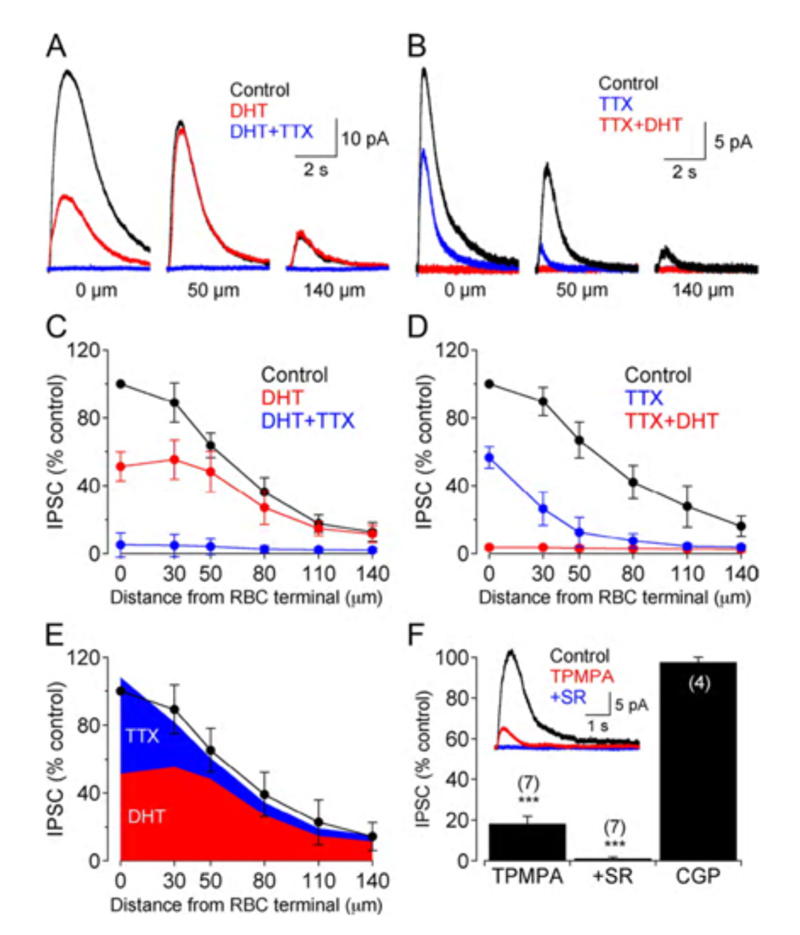
GABAergic synapses between A17 amacrine cells and rod bipolar cells (Fig. 22) activate GABAA receptors (199). While being wide in dendritic field, the action of A17 amacrine cells on rod-bipolar axon terminals is local (200, 201) and depends on calcium entry through local A17 AMPA channels excited by the rod-bipolar-cell ribbon synapse (201), not impulses propagating in the A17 dendrites (200). GABAC inhibition of rod bipolar terminals is long range and depends on impulse activity (200). These two inhibitory systems have been dissected in the Diamond laboratory. The short range reciprocal GABAA feedback is eliminated by ablating A17 amacrine cells with 5,7 dihydroxytryptamine (DHT), a toxic serotonin analogue that is selectively taken up by A17. The long range GABAC feedback is abolished by tetrodotoxin (TTX). This abolishes nerve spikes that allow signal propagation along amacrine cell dendrites other than A17. Interestingly, though wide in dendritic field, the regulatory actions of A17 appear to be independent local-circuit reciprocal interactions with rod bipolar cells (Fig. 25).
Inhibitory glycine-gated currents
Glycine applied to bipolar cells elicits a strychnine-sensitive, hyperpolarizing chloride current. As with GABA, different bipolar-cell regions are differentially sensitive to glycine. In mouse (156, 202) and carp (177) the axon terminal shows the greatest sensitivity; while in rat (147) and salamander (203) the dendrites are more sensitive. There is a tendency for glycinergic inhibitory circuitry to impinge selectively on OFF cone bipolar cells (177, 204); the outstanding example is AII amacrine innervation of the mammalian OFF cone bipolar cells (105, 205-207). In this case glycine acts as a neurotransmitter intermediary in the pathway to dark adapted ganglion-cell center responses (Fig. 18). In general the inhibitory pathway onto cone-bipolar-cell axon terminals uses glycine, whereas the rod bipolar axon terminal receives GABAergic inhibition (208). Glycine, however, is a component of inhibition on rod bipolar terminals. This pathway is innervated by both ON and OFF bipolar cells through a preferentially NMDA glutamate receptor mechanism impinging on amacrine cells (209). Dendritic impulse propagation plays a role in the transmission of glycinergic inhibition at this synapse. Noise analysis performed on glycine-elicited currents from dendrites and axon terminal suggests that each region may express a different subtype of glycine receptor (210). In fish retinas glycinergic inputs to bipolar-cell dendrites and axon terminals are believed to arise directly from populations of amacrine and glycinergic interplexiform cells (203). Glycine-containing interplexiform cells have only been seen so far in fish retinas; glycine receptors can be found on processes postsynaptic to photoreceptors, including bipolar cell dendritic processes (211).
Glycine is believed to modulate the surround light responses of bipolar cells, though the reported effects of glycine are not consistent. Stone and Schutte (1991) (212), working in Xenopus, report that glycine application eliminated surround responses in both ON- and OFF-type bipolar cells. In contrast, surround responses in salamander are not blocked by glycine (213). While GABA application elicits responses in all bipolar cells examined, glycine elicits responses from only a subset of bipolar cells, such as OFF bipolar cells in carp (177) and the small-field bipolar cells in skate (214), suggesting glycine may have a selective role in retina. Glycinergic feedback connections between amacrine and bipolar cells decrease light-evoked glutamate release onto ganglion cell dendrites (198). Glycinergic feed-forward synapses transfer rod bipolar signals from AII amacrine cells to OFF cone bipolar terminals in mammalian retinas (105, 215). Thus, glycinergic synapses onto bipolar cells may be important in mediating the transfer of information among neurons in both the proximal and distal retina.
Glutamate release from vesicles in goldfish bipolar cell axon terminals
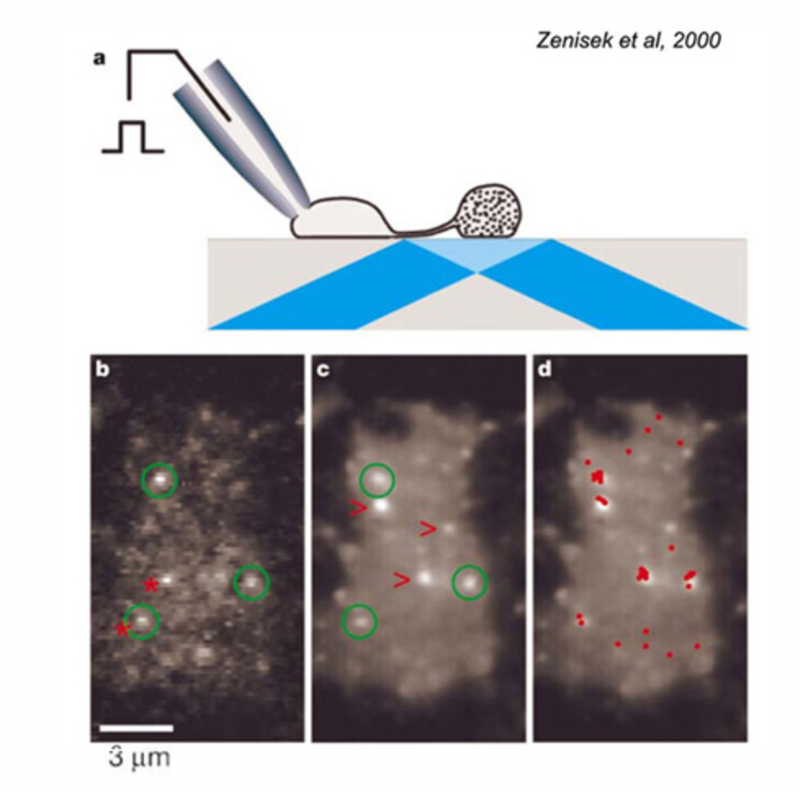
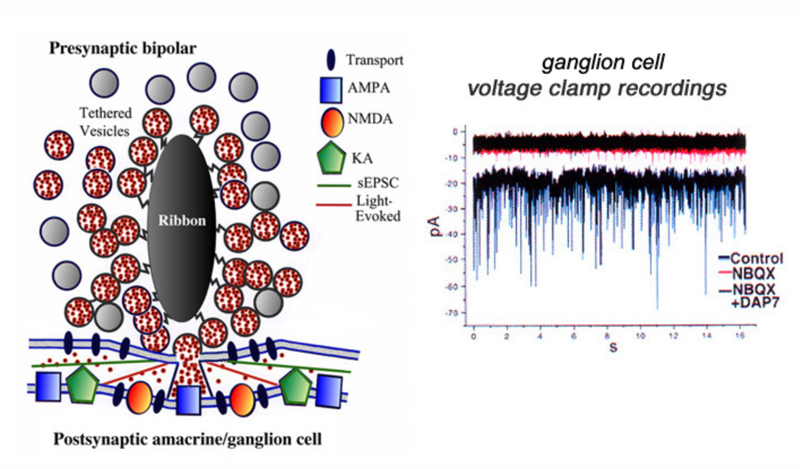
Due to their large size, the axon terminals of goldfish Mb1-type ON-bipolar cells are used as a model system in which to examine neurotransmitter release. The axon terminal can be directly recorded in patch clamp studies (216). Changes in internal calcium (217) and both exocytosis and endocytosis activity (217, 218) can be detected using fluorescent calcium probes and observing capacitance changes as vesicle membrane is added or removed from this presynaptic terminal. Images of releasing vesicles at Mb1 terminals are seen by evanescent fluorescence microscopy in figure 26.
Neurotransmitter release from bipolar terminals occurs at ribbon synapses (219, 220); conventional synapses are rare in bipolar axon terminals although they have been seen in some species (221, 222). Each ribbon is an electron dense structure oriented perpendicular to the plasma membrane. One ribbon may contain as many as ~110 tethered vesicles along the sides. Vesicles along the base of the ribbon, or “docked vesicles”, are in contact with the presynaptic membrane (216). The arrangement of the ribbon synapse at the bipolar cell axon terminal and associated post-synaptic events are shown in figure 27. All vesicles associated with the ribbon constitute the readily releasable pool of vesicles. Since one axon terminal averages 55 active zones in the goldfish, each with an associated ribbon (218), there are ~6000 tethered or rapidly releasable vesicles per terminal (von Gersdorff et al., 1996). Combining these values with capacitance measurements indicates that maximal release per active zone occurs at a rate of ~500 vesicles/sec (216). Species with smaller rod or cone bipolar axon terminals (amphibians, reptiles and mammals) have smaller numbers of ribbons and smaller volumes to contain synaptic vesicles so the release rates may be different compared with the model goldfish system.
The ribbon synapse, in the absence of light, releases vesicles spontaneously. The single spontaneous excitatory postsynaptic synaptic currents (sEPSCs) can be studied by voltage clamp techniques in ganglion cells (221). It is thought that normal spontaneous release activates AMPA receptors located immediately below the active release zone (Fig. 27). Light stimulation of ON bipolar cells induces release of many vesicles along a single ribbon site and glutamate spillover activates both AMPA and NMDA receptors (Fig. 27). The patch recordings of sEPSCs, under conditions of hyperosmotic Ringer to enhance rates of spontaneous events, reveal large and small as well as fast and slow events (Fig. 27, control). The sEPSCs are mostly eliminated by NBQX, a highly selective antagonist for AMPA and kainate receptors (Fig. 27, NBQX). But small amplitude events still are seen (Fig. 27, NBQX) until the NMDA antagonist D-AP7 is added (Fig. 27, NBQX+DAP7). This indicates that both AMPA and NMDA receptors are active in post-synaptic ganglion cells (223). Further, a maintained inward current is also blocked by both these antagonists, suggesting bipolar-cell ribbon synapses transfer information across a broad temporal spectrum.
Vesicular release at the bipolar ribbon synapse occurs in a calcium-dependent manner (224-226), though there does not appear to be a selective requirement for calcium, as other divalent ions, such as strontium and barium, can stimulate exocytosis, though to a lesser degree (227). Calcium entry occurs through L-type channels in goldfish (155) and/or T-type channels in mouse (228). These channels are located on the axon terminal membrane. Internal calcium concentrations of 10-20 μM stimulate exocytosis (155) of the readily releasable pool (216, 218, 229). Exocytosis occurs in two phases. Initially, membrane depolarization elicits an increase in capacitance (time constant ~1.5 ms) corresponding to the rapid release of docked vesicles. This is followed by a second capacitance increase with a slower time constant (~250-300ms), believed to represent the movement of tethered vesicles to the active zone and their subsequent release (218, 230). Estimates indicate that 20% or ~1100 vesicles (of the 6000 in the readily releasable pool) are released during rapid exocytosis (229, 230), with the remainder released during the slow component. The depleted pool is restocked with a time constant of about 8s (231). Both the fast and slow phases are calcium-dependent, though they display differential sensitivities to calcium buffers (230).
Following neurotransmitter release, the vesicular membrane is recovered rapidly (226) and continuously (217, 232). The continual cycling of vesicles through the processes of exocytosis and endocytosis, is compatible with tonic release of neurotransmitter (217). This is suspected as bipolar cells generate sustained responses to light, and at least in some cases, transmit sustained signals to postsynaptic neurons (1). Though neurotransmitter is released following continuous or paired-pulse stimulation of the terminals, release decreases with time (229, 230). This synaptic depression is believed to be due to depletion of the readily releasable pool and a decrease in exocytosis of this pool (229).
Vesicle release at the mammalian rod-bipolar-to-AII-amacrine synapse
The pharmacology and kinetics of the mammalian ribbon synapse between rod bipolar cells and AII amacrine cells has been studied by simultaneous patch recording of presynaptic and postsynaptic cells (199). This glutamatergic synapse uses exclusively AMPA receptors (233). Within the retinal inner plexiform layer there is a transformation from the largely sustained light response of bipolar cells to the largely transient responses of amacrine cells (1). The mechanism of this transformation is a major topic for retinal neural circuitry. Rod bipolar cells respond to maintained light stimuli with maintained depolarizations (123), whereas the AII amacrine response to light is a transient depolarization (123, 132). Singer and Diamond (2003) (199) argue that vesicle depletion, at least a higher stimulus levels, is a major contributor to the sustained-transient transformation (123, 132). The rod-bipolar-to-AII synapse is fast, rising to peak in about 1 ms following depolarization of the rod bipolar. It is also transient, responding to a depolarizing rectangular voltage step in the presynaptic rod bipolar with a saw-toothed waveform decaying to a lesser steady-state release level within 20 msec. Presynaptic calcium currents that lead to vesicle release are L-type and sustained and do not explain the decay (199). The sustained-to-transient transformation occurs even in the presence of inhibitory synaptic blockers that block reciprocal GABAA feedback. GABA feedback could be observed as a delayed transient outward current in the rod bipolar recording, but did not measurably alter synaptic release.
After the decaying phase of the response completes, a residual train of small synaptic events persists. The transient waveform slows, but is evident, even at stimulation threshold (199). Singer and Diamond (2003) conclude that presynaptic vesicle depletion within a ‘releasable pool’ accounts for the transient waveform of the synapse. Decline in the number of available vesicles reduces the number of vesicles released over time, even though for an individual vesicle this probability may remain constant. This is typical of synaptic transmission. One unique feature of this synapse however is synchronous release of vesicle pairs (234). Singer and Diamond (2006) (235) estimate the readily releasable pool in the rat rod-bipolar-cell terminal is in net about 70 vesicles, which amounts to about 7 vesicles per ribbon. The synapse appears able to replace vesicles at about 0.5 vesicles/ ms, giving each ribbon a steady-state release rate of about 50/s. Release is easily depressed in paired pulse experiments and recovers with a time constant only of about 4 s, but sustained exocytosis appears to accelerate vesicle recovery. These properties may confer the ability for synaptically-based neural adaptation (235).
5 Behavioral and clinical implications of bipolar-cell abnormalities
ON bipolar cells appear uniquely vulnerable to insult. The mGluR6 receptor, for example, can be selectively removed (i.e., knocked out) without major effect on other neural pathways. This can be accomplished through a variety of techniques, such as use of selective pharmacological blocking agents (i.e., APB) or site directed mutagenisis techniques designed to eliminate the mGluR6 receptors themselves (Fig. 4). Further, naturally occurring mutations and disease processes related to mGluR6 selectively target ON bipolar cells. These include compromise of the TRPM1 channel (Fig. 7), and the associated glycoprotein nyctalopin (Fig. 9). Other disease processes also single out ON bipolar cells. Surprisingly humans or animal models with ON-bipolar deficit(s) perform visual tasks relatively normally. The major problem is loss of nocturnal vision, which appears dependent on the physiological integrity of a single ON bipolar-cell type, the mammalian rod bipolar cell.
Mice with ON bipolar knockout perform visual tasks
The electrophysiological consequences of site-directed mutagenisis directed at the ON pathway are seen in figure 4 for mGluR6 deficient mice. The electroretinographic b-wave component, which arises from the activity of ON bipolar cells, is abolished (31). These authors further demonstrate that light-evoked field potentials from the superior colliculus, which in mouse is the main termination site for ganglion cell axons, lack ON responses, though OFF-type waves appear. In wild type mice, transient collicular waves are seen at both onset and cessation of light stimuli. The altered mGluR6 physiology would seem to imply a behavioral deficit.
Interestingly some tests do suggest deficits in behavior while others do not. In a shuttle box avoidance learning analysis, both mutant and wild type mice performed equally well (31). Since the original site-directed mutant line was created, a forward-genetic mGluR6-mutant line, nob4, has been found (236). Circadian clocks, characterized by wheel running activity in the dark phase, are normal in both mutants. In each mutant light stops this activity (237, 238). In the site-directed mutant this action is phase delayed (237); in nob4 this action is sensitized, a seemingly paradoxical result (238). In both mutants pupillary responses were observed only at high light levels (238, 239). In the site-directed mutant optokinetic responses were seen only at high contrasts (239), suggesting a general loss of sensitivity. These studies indicate that the OFF bipolar cells alone can mediate vision, even if somewhat impaired. Interestingly the ON and OFF layering of the inner plexiform layer persists in the site-directed mutant (240).
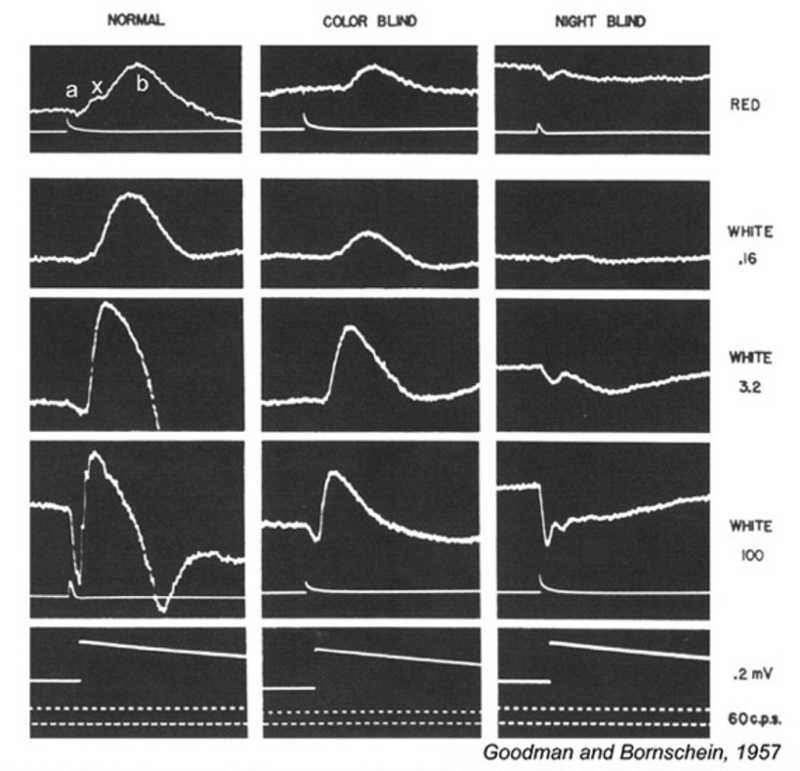
Melanoma-associated retinopathy (MAR)
Some patients with malignant melanomas lose night vision. They may further report hallucinations consisting of shimmering blobs of white light (239, 240). Visual processing in daylight appears otherwise normal. Color vision is not affected, nor is visual acuity dramatically worsened. Electroretinography reveals ON bipolar cell deficits for these patients. As with mGluR6 deficient mice, the b-wave is selectively absent in both rod and cone driven responses (241) and the patients may have reduced contrast sensitivity (242). Although originally thought to be a side effect of chemotherapy (241), it became clear that the syndrome might arise in cancer patients prior to chemotherapy (242, 243). The IgG serum fraction of MAR patients induces reversible electroretinographic disturbances similar to MAR when injected into the eyes of monkeys (244). Electroretinographic a-waves and d-waves remain, the b-wave is lost from both rod and cone responses (Fig. 28). MAR appears to be caused by an autoimmune attack against retinal ON bipolar cells for both rod and cone vision. Antibodies induced by the melanoma cause the visual deficit. One of the epitopes appears to be the ON-bipolar-cell TRPM1 channel (245, 246), perhaps not surprisingly as the channel name derives from ‘melanoma related’. MAR is one of several paraneoplastic retinopathies (PR) or cancer-associated retinopathies (CAR).
Congenital stationary night blindness (CSNB)
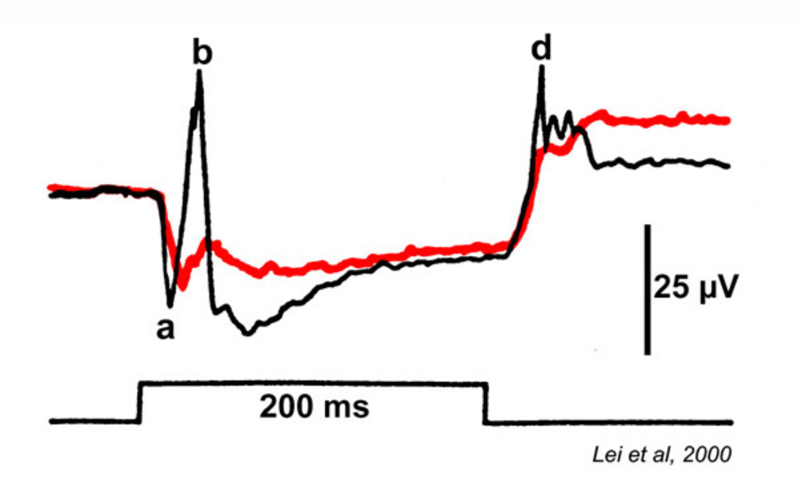
This visual dysfunction is an inherited retinal disease very similar in symptoms to MAR. In the Schubert-Bornschein, or complete, type (248), there is loss of nocturnal or rod vision (Fig. 29), but in daylight, cone-mediated color vision and visual acuity are relatively normal (249, 250), though the sensitivity of cone vision is somewhat reduced (250, 251). The disease is X-linked, affecting primarily males (250, 251).
Electroretinograms (Fig. 29) reveal a loss of b-waves for both the rod and cone systems. ON bipolar cell signals appear greatly depressed or absent, OFF cone bipolar signals, as represented by the electroretinographic d-wave, are spared (241), as is the rod a-wave (249). The presence of a rod a-wave but not b-wave provided the first suggestion that the retina could have genetic diseases of the post receptor, neural processing machinery (249).
Early on it became apparent that CSNB was a heterogeneous disease. Miyake et al (250) classified CSNB as ‘complete’ and ‘incomplete’. ‘Complete’ or ‘Shubert-Bornshein’ type lacked all rod function and had no b-wave. ‘Incomplete’ or ‘Riggs’ type had reduced b-wave and rod function. Both ’complete’ and ‘incomplete’ forms occurred nearly exclusively in males (50 of 55 examples) (250), and so both were X-linked, but multiple examples within a family tree were only of one type. Genetic analysis reveals that the complete form of CSNB involves defects in the glycoprotein nyctalopin (252). Animal models of this gene defect appear in the section Metabotropic responses of ON bipolar cells. Mutant proteins disrupt neurotransmission in ON bipolar cells. The genetic defect in incomplete CSNB has been localized to a gene uniquely expressed in retina and similar to the L-type calcium channel alpha subunit (253). Defects in this gene, CACNA1F, provide an example of a human retinal channelopathy.
There are also examples of human mutations in the GRM6 gene, the gene that encodes mGluR6. This defect also results in CSNB, a type called ‘arCSNB’, ‘ar’ for ‘autosomal recessive’. In this type an abnormal 15Hz flicker response near rod threshold distinguishes the electrical phenotype (254). The authors interpret this phase-reversed scotopic threshold response as the survival of an alternative rod pathway, perhaps an element of the 2 rod pathway model (145). More recently another autosomal recessive form of ‘Complete CSNB’ has been identified. This is a particularly common in females. The ON-bipolar-cell TRPM1 cation channel is the genetic defect (62). In this case the 15Hz rod flicker is similar to X-linked (NYX -/-) CSNB. Animal models of these defects are discussed above (see Metabotropic responses of ON bipolar cells).
6 Visual processing under mGluR6 blockade of the ON bipolar cells
Lateral geniculate and cortical neurons.
The responses of visual neurons in the central nervous system of primates have been investigated under conditions of ON-bipolar blockade using the metabotropic glutamate receptor agonist APB (DL AP-4). Recordings from the dorsal lateral geniculate nucleus (LGNd) indicate that light responses of ON-center geniculate neurons are completely blocked by APB (255, 256). ON-center geniculate cells are also blocked selectively in rabbit (257). Projection of retinal ganglion cells onto geniculate relay cells is selective for physiological types (258) so that ON and OFF pathways are preserved and can be selectively affected by retinal blockers.
Recordings of light responses of individual neurons in visual cortex suggest this is the site of final integration for ON and OFF bipolar-cell signals and their associated retinal neurons. Complex cortical cells continue to respond under APB blockade of ON bipolar cells, but leading edge responses to bright squares drifting through the receptive field are lost (255). The directional selectivity and orientation selectivity of these cortical cells appears unperturbed by the blockade (255). In a parallel study, monkeys under APB blockade were found to have much poorer perception of light increments than light decrements, but relatively unimpaired perception of simultaneous spatial contrast (259). Elimination of synaptic function for all ON bipolar types in mammals leaves a surprisingly functional visual system.
Dim light responses require ON bipolar cells
One common feature of rod-dominated mammalian retinas is the emergence of a single, ON-type bipolar cell for rods (64, 123, 124) dominated by the metabotropic, mGluR6, glutamate receptor and associated molecules. Pharmacological blockade of this pathway leads to physiological deficits in night vision. This is true behaviorally for both light increments and decrements (260), as well as for dim light responses in ON or OFF ganglion cells (205). Knockout of ON bipolar cell signals in mGluR6-deficient mice leads to loss of light sensitivity evident in the electroretinogram (31). As noted above ON bipolar cell diseases in humans are always accompanied by a loss of nocturnal vision. The secondary rod pathways that utilize either cone-system OFF bipolar cells or gap-junction pathways between rods and cones appear not to be involved in rod-mediated nocturnal vision, but rather it is rod-bipolar-cell metabotropic receptors that mediate dim light responses.
7 Summary and conclusions
Bipolar cells are functionally crucial neurons that comprise the middle component of the vertical transduction pathway through the retina. ON- and OFF-type bipolar cells are presynaptic to similarly polarized ganglion cells in the retinal inner plexiform layer and can be distinguished by morphology, by light response, and by different types of dendritic glutamate receptor expression. These cells serve as models in which to examine different aspects of neurobiology, including embryological development, postsynaptic receptor systems, neurotransmitter release mechanisms, neural circuitry interactions, and visual system defects. ON and OFF bipolar cells initiate two functionally independent pathways, the ON and the OFF, each with center-surround organization, and each with a splitting into both transient and sustained signal types postsynaptic to bipolar cells. Each bipolar pathway is capable of independent image processing. The signals of ON and OFF bipolar cells are relayed separately to higher brain centers. ON bipolar cells appear selectively vulnerable to diseases, genetic defects, or pharmacological assault. Loss of ON bipolar cells results in a loss of nocturnal vision.
References
1. Werblin, F.S. and J.E. Dowling, Organization of the retina of the mudpuppy, Necturus maculosus. II. Intracellular recording. J Neurophysiol, 1969. 32(3): p. 339-55. [PubMed]
2. Kuffler, S.W., Discharge patterns and functional organization of mammalian retina. J Neurophysiol, 1953. 16(1): p. 37-68.
3. Protti, D.A., N. Flores-Herr, and H. von Gersdorff, Light evokes Ca2+ spikes in the axon terminal of a retinal bipolar cell. Neuron, 2000. 25(1): p. 215-27.
4. Dreosti, E., et al., In vivo evidence that retinal bipolar cells generate spikes modulated by light. Nat Neurosci, 2011. 14(8): p. 951-2.
5. Saszik, S. and S.H. DeVries, A Mammalian Retinal Bipolar Cell Uses Both Graded Changes in Membrane Voltage and All-or-Nothing Na+ Spikes to Encode Light J Neurosci, 2012. 32(1): p. 297-307.
6. Connaughton, V.P., D. Graham, and R. Nelson, Identification and morphological classification of horizontal, bipolar, and amacrine cells within the zebrafish retina. Journal of Comparative Neurology, 2004. 477: p. 371-385.
7. Euler, T., H. Schneider, and H. Wässle, Glutamate responses of bipolar cells in a slice preparation of the rat retina. J Neurosci, 1996. 16(9): p. 2934-44. [PubMed]
8. Kolb, H., R. Nelson, and A. Mariani, Amacrine cells, bipolar cells and ganglion cells of the cat retina: a Golgi study. Vision Res, 1981. 21(7): p. 1081-1114. [PubMed]
9. Wässle, H. and B.B. Boycott, Functional architecture of the mammalian retina. Physiol Rev, 1991. 71(2): p. 447-80.
10. West, R.W., Light and electron microscopy of the ground squirrel retina: functional considerations. J Comp Neurol, 1976. 168(3): p. 355-77.
11. Wu, S.M., F. Gao, and B.R. Maple, Functional architecture of synapses in the inner retina: segregation of visual signals by stratification of bipolar cell axon terminals. J Neurosci, 2000. 20(12): p. 4462-70. [PubMed]
12. Wu, S.M., Synaptic organization of the vertebrate retina: general principles and species-specific variations: the Friedenwald lecture. Invest Ophthalmol Vis Sci, 2010. 51(3): p. 1263-74.
13. Boycott, B.B. and H. Wässle, Morphological Classification of Bipolar Cells of the Primate Retina. Eur J Neurosci, 1991. 3(11): p. 1069-1088.
14. Ishida, A.T., W.K. Stell, and D.O. Lightfoot, Rod and cone inputs to bipolar cells in goldfish retina. J Comp Neurol, 1980. 191(3): p. 315-35. [PubMed]
15. Li, W. and S.H. DeVries, Bipolar cell pathways for color and luminance vision in a dichromatic mammalian retina. Nat Neurosci, 2006. 9(5): p. 669-75.
16. MacNeil, M.A. and P.A. Gaul, Biocytin wide-field bipolar cells in rabbit retina selectively contact blue cones. J Comp Neurol, 2008. 506(1): p. 6-15.
17. Mariani, A.P., The neuronal organization of the outer plexiform layer of the primate retina. Int Rev Cytol, 1984. 86: p. 285-320.
18. Marshak, D.W., et al., Localization of immunoreactive cholecystokinin precursor to amacrine cells and bipolar cells of the macaque monkey retina. J Neurosci, 1990. 10(9): p. 3045-55.
19. Scholes, J.H., Colour receptors, and their synaptic connexions, in the retina of a cyprinid fish. Philos Trans R Soc Lond B Biol Sci, 1975. 270(902): p. 61-118. [PubMed]
20. Kolb, H., Organization of the outer plexiform layer of the primate retina: electron microscopy of Golgi-impregnated cells. Philosophical Transactions of the Royal Society, London, B, 1970. 258: p. 261-283.
21. Ayoub, G.S. and D.R. Copenhagen, Application of a fluorometric method to measure glutamate release from single retinal photoreceptors. J Neurosci Methods, 1991. 37(1): p. 7-14. [PubMed]
22. Nelson, R., A comparison of electrical properties of neurons in Necturus retina. J Neurophysiol, 1973. 36(3): p. 519-535. [PubMed]
23. Toyoda, J., Membrane resistance changes underlying the bipolar cell response in the carp retina. Vision Res, 1973. 13(2): p. 283-94. [PubMed]
24. Dacheux, R.F. and R.F. Miller, Photoreceptor-bipolar cell transmission in the perfused retina eyecup of the mudpuppy. Science, 1976. 191(4230): p. 963-4. [PubMed]
25. Lasansky, A., Properties of depolarizing bipolar cell responses to central illumination in salamander retinal slices. Brain Res, 1992. 576(2): p. 181-96. [PubMed]
26. Hirasawa, H., R. Shiells, and M. Yamada, A metabotropic glutamate receptor regulates transmitter release from cone presynaptic terminals in carp retinal slices. J Gen Physiol, 2002. 119(1): p. 55-68. [PubMed]
27. Awatramani, G.B. and M.M. Slaughter, Intensity-dependent, rapid activation of presynaptic metabotropic glutamate receptors at a central synapse. J Neurosci, 2001. 21(2): p. 741-9. [PubMed]
28. Slaughter, M.M. and R.F. Miller, 2-amino-4-phosphonobutyric acid: a new pharmacological tool for retina research. Science, 1981. 211(4478): p. 182-5. [PubMed]
29. Nakajima, Y., et al., Molecular characterization of a novel retinal metabotropic glutamate receptor mGluR6 with a high agonist selectivity for L-2-amino-4-phosphonobutyrate. J Biol Chem, 1993. 268(16): p. 11868-73. [PubMed]
30. Nomura, A., et al., Developmentally regulated postsynaptic localization of a metabotropic glutamate receptor in rat rod bipolar cells. Cell, 1994. 77(3): p. 361-9. [PubMed]
31. Masu, M., et al., Specific deficit of the ON response in visual transmission by targeted disruption of the mGluR6 gene. Cell, 1995. 80(5): p. 757-65. [PubMed]
32. Vardi, N., et al., Localization of mGluR6 to dendrites of ON bipolar cells in primate retina. J Comp Neurol, 2000. 423(3): p. 402-412.
33. Vardi, N., Alpha subunit of Go localizes in the dendritic tips of ON bipolar cells. J Comp Neurol, 1998. 395(1): p. 43-52. [PubMed]
34. Dhingra, A., et al., The light response of ON bipolar neurons requires Gαo. J Neurosci, 2000. 20(24): p. 9053-8. [PubMed]
35. Vardi, N., et al., Identification of a G-protein in depolarizing rod bipolar cells. Vis Neurosci, 1993. 10(3): p. 473-8. [PubMed]
36. Nawy, S. and C.E. Jahr, Suppression by glutamate of cGMP-activated conductance in retinal bipolar cells. Nature, 1990. 346(6281): p. 269-71. [PubMed]
37. Nawy, S., The metabotropic receptor mGluR6 may signal through Go, but not phosphodiesterase, in retinal bipolar cells. J Neurosci, 1999. 19(8): p. 2938-44. [PubMed]
38. Koike, C., et al., TRPM1 is a component of the retinal ON bipolar cell transduction channel in the mGluR6 cascade. Proc Natl Acad Sci U S A, 2010. 107(1): p. 332-7.
39. Koike, C., et al., TRPM1: a vertebrate TRP channel responsible for retinal ON bipolar function. Cell Calcium, 2010. 48(2-3): p. 95-101.
40. Nakamura, M., et al., TRPM1 mutations are associated with the complete form of congenital stationary night blindness. Mol Vis, 2010. 16: p. 425-37.
41. Xu, Y. and N. Vardi, Modulation of the Light-Activated Cation Channel in Retinal ON Bipolar Cells by G-Protein Subunits. ARVO Meeting Abstracts, 2010. 51(5): p. 4797.
42. Xu, Y., et al., mGluR6 Deletion Renders the TRPM1 Channel in Retina Inactive. J Neurophysiol, 2011.
43. Montell, C. and G.M. Rubin, Molecular characterization of the Drosophila trp locus: a putative integral membrane protein required for phototransduction. Neuron, 1989. 2(4): p. 1313-23.
44. Myers, B.R., et al., Multiple unbiased prospective screens identify TRP channels and their conserved gating elements. J Gen Physiol, 2008. 132(5): p. 481-6.
45. Abramowitz, J. and L. Birnbaumer, Physiology and pathophysiology of canonical transient receptor potential channels. FASEB J, 2009. 23(2): p. 297-328.
46. Montell, C., The TRP superfamily of cation channels. Sci STKE, 2005. 2005(272): p. re3.
47. Montell, C., L. Birnbaumer, and V. Flockerzi, The TRP channels, a remarkably functional family. Cell, 2002. 108(5): p. 595-8.
48. Nishida, M. and H. Kurose, Roles of TRP channels in the development of cardiac hypertrophy. Naunyn Schmiedebergs Arch Pharmacol, 2008. 378(4): p. 395-406.
49. Krizaj, D., Compartmentalization of calcium entry pathways in mouse rods. Eur J Neurosci, 2005. 22(12): p. 3292-6.
50. Ke, J.B., et al., Characterization of spontaneous inhibitory postsynaptic currents in cultured rat retinal amacrine cells. Neuroscience, 2010. 165(2): p. 395-407.
51. Sosa, R., et al., Metabotropic glutamate receptor 5 and calcium signaling in retinal amacrine cells. J Neurochem, 2002. 81(5): p. 973-83.
52. Morgans, C.W., et al., TRPM1 is required for the depolarizing light response in retinal ON-bipolar cells. Proc Natl Acad Sci U S A, 2009. 106(45): p. 19174-8.
53. Shen, Y., et al., A transient receptor potential-like channel mediates synaptic transmission in rod bipolar cells. J Neurosci, 2009. 29(19): p. 6088-93.
54. Koike, C., et al., The functional analysis of TRPM1 in retinal bipolar cells. Neurosci Res, 2007. 58S: p. S41.
55. Morgans, C.W., R.L. Brown, and R.M. Duvoisin, TRPM!: the endpoint of the mGluR6 signal transduction cascade in retinal ON-bipolar cells. Bioessays, 2010. 32: p. 609-614.
56. Koyasu, T., et al., Photopic electroretinograms of mGluR6-deficient mice. Curr Eye Res, 2008. 33(1): p. 91-9.
57. Gregg, R.G., et al., Nyctalopin expression in retinal bipolar cells restores visual function in a mouse model of complete X-linked congenital stationary night blindness. J Neurophysiol, 2007. 98(5): p. 3023-33.
58. Pardue, M.T., et al., A naturally occurring mouse model of X-linked congenital stationary night blindness. Invest Ophthalmol Vis Sci, 1998. 39(12): p. 2443-9.
59. Gregg, R.G., et al., Identification of the gene and the mutation responsible for the mouse nob phenotype. Invest Ophthalmol Vis Sci, 2003. 44(1): p. 378-84.
60. Schroeter, E.H., R.O. Wong, and R.G. Gregg, In vivo development of retinal ON-bipolar cell axonal terminals visualized in nyx::MYFP transgenic zebrafish. Vis Neurosci, 2006. 23(5): p. 833-43.
61. Cao, Y., E. Posokhova, and K.A. Martemyanov, TRPM1 forms complexes with nyctalopin in vivo and accumulates in postsynaptic compartment of ON-bipolar neurons in mGluR6-dependent manner. J Neurosci, 2011. 31(32): p. 11521-6.
62. van Genderen, M.M., et al., Mutations in TRPM1 are a common cause of complete congenital stationary night blindness. Am J Hum Genet, 2009. 85(5): p. 730-6.
63. Nawy, S., Regulation of the on bipolar cell mGluR6 pathway by Ca2+. J Neurosci, 2000. 20(12): p. 4471-9. [PubMed]
64. Yamashita, M. and H. Wässle, Responses of rod bipolar cells isolated from the rat retina to the glutamate agonist 2-amino-4-phosphonobutyric acid (APB). J Neurosci, 1991. 11(8): p. 2372-82. [PubMed]
65. Shiells, R.A. and G. Falk, A rise in intracellular Ca2+ underlies light adaptation in dogfish retinal ‘on’ bipolar cells. J Physiol (Lond), 1999. 514(Pt 2): p. 343-50.
66. Shiells, R.A. and G. Falk, Activation of Ca2+–calmodulin kinase II induces desensitization by background light in dogfish retinal ‘on’ bipolar cells. J Physiol, 2000. 528 Pt 2: p. 327-38. [PubMed]
67. Walters, R.J., R.H. Kramer, and S. Nawy, Regulation of cGMP-dependent current in On bipolar cells by calcium/calmodulin-dependent kinase. Vis Neurosci, 1998. 15(2): p. 257-61. [PubMed]
68. Snellman, J. and S. Nawy, cGMP-dependent kinase regulates response sensitivity of the mouse on bipolar cell. J Neurosci, 2004. 24(29): p. 6621-8.
69. Awatramani, G.B. and M.M. Slaughter, Origin of transient and sustained responses in ganglion cells of the retina. J Neurosci, 2000. 20(18): p. 7087-95. [PubMed]
70. Enroth-Cugell, C. and J.G. Robson, The contrast sensitivity of retinal ganglion cells of the cat. J Physiol, 1966. 187(3): p. 517-52. [PubMed] [Free Full text in PMC]
71. Demb, J.B., et al., Bipolar cells contribute to nonlinear spatial summation in the brisk-transient (Y) ganglion cell in mammalian retina. J Neurosci, 2001. 21(19): p. 7447-54. [PubMed]
72. Connaughton, V.P. and R. Nelson, Axonal stratification patterns and glutamate-gated conductance mechanisms in zebrafish retinal bipolar cells. J Physiol, 2000. 524(Pt 1): p. 135-146. [PubMed]
73. Grant, G.B. and J.E. Dowling, A glutamate-activated chloride current in cone-driven ON bipolar cells of the white perch retina. J Neurosci, 1995. 15(5): p. 3852-62. [PubMed]
74. Grant, G.B. and J.E. Dowling, On bipolar cell responses in the teleost retina are generated by two distinct mechanisms. J Neurophysiol, 1996. 76(6): p. 3842-9. [PubMed]
75. Grant, G.B. and F.S. Werblin, A glutamate-elicited chloride current with transporter-like properties in rod photoreceptors of the tiger salamander. Vis Neurosci, 1996. 13(1): p. 135-44. [PubMed]
76. Picaud, S.A., et al., Glutamate-gated chloride channel with glutamate-transporter-like properties in cone photoreceptors of the tiger salamander. J Neurophysiol, 1995. 74(4): p. 1760-71. [PubMed]
77. Tachibana, M. and A. Kaneko, L-glutamate-induced depolarization in solitary photoreceptors: a process that may contribute to the interaction between photoreceptors in situ. Proc Natl Acad Sci U S A, 1988. 85(14): p. 5315-9. [PubMed] [Free Full text in PMC]
78. Wersinger, E., et al., The glutamate transporter EAAT5 works as a presynaptic receptor in mouse rod bipolar cells. J Physiol, 2006. 577(Pt 1): p. 221-34.
79. Otis, T.S. and C.E. Jahr, Anion currents and predicted glutamate flux through a neuronal glutamate transporter. J Neurosci, 1998. 18(18): p. 7099-110. [PubMed]
80. Arriza, J.L., et al., Excitatory amino acid transporter 5, a retinal glutamate transporter coupled to a chloride conductance. Proc Natl Acad Sci U S A, 1997. 94(8): p. 4155-60.
81. Saito, T., H. Kondo, and J. Toyoda, Ionic mechanisms of two types of on-center bipolar cells in the carp retina. II. The responses to annular illumination. J Gen Physiol, 1981. 78(5): p. 569-89. [PubMed]
82. Saito, T., H. Kondo, and J.I. Toyoda, Ionic mechanisms of two types of on-center bipolar cells in the carp retina. I. The responses to central illumination. J Gen Physiol, 1979. 73(1): p. 73-90.
83. Vardi, N., et al., Neurochemistry of the mammalian cone ‘synaptic complex’. Vision Res, 1998. 38(10): p. 1359-69. [PubMed]
84. Hughes, T.E., Are there ionotropic glutamate receptors on the rod bipolar cell of the mouse retina? Vis Neurosci, 1997. 14(1): p. 103-9. [PubMed]
85. Hughes, T.E., I. Hermans-Borgmeyer, and S. Heinemann, Differential expression of glutamate receptor genes (GluR1-5) in the rat retina. Vis Neurosci, 1992. 8(1): p. 49-55.
86. Nelson, R., et al., Physiological responses associated with kainate receptor immunoreactivity in dissociated zebrafish retinal neurons: a voltage probe study. Prog Brain Res, 2001. 131: p. 255-65. [PubMed]
87. Peng, Y.W., et al., Distribution of glutamate receptor subtypes in the vertebrate retina. Neuroscience, 1995. 66(2): p. 483-97. [PubMed]
88. Wong, K.Y. and J.E. Dowling, Retinal Bipolar Cell Input Mechanisms in Giant Danio: III. ON-OFF Bipolar Cells and Their Color-Opponent Mechanisms. J Neurophysiol, 2005. 94(1): p. 265-272.
89. Pourcho, R.G., P. Qin, and D.J. Goebel, Cellular and subcellular distribution of NMDA receptor subunit NR2B in the retina. J Comp Neurol, 2001. 433(1): p. 75-85. [PubMed]
90. Wenzel, A., et al., N-methyl-D-aspartate receptors containing the NR2D subunit in the retina are selectively expressed in rod bipolar cells. Neuroscience, 1997. 78(4): p. 1105-12. [PubMed]
91. Brandstatter, J.H., et al., Expression of NMDA and high-affinity kainate receptor subunit mRNAs in the adult rat retina. Eur J Neurosci, 1994. 6(7): p. 1100-12.
92. DeVries, S.H., Bipolar cells use kainate and AMPA receptors to filter visual information into separate channels. Neuron, 2000. 28(3): p. 847-56. [PubMed]
93. DeVries, S.H. and E.A. Schwartz, Kainate receptors mediate synaptic transmission between cones and ‘Off’ bipolar cells in a mammalian retina. Nature, 1999. 397(6715): p. 157-60. [PubMed]
94. Paternain, A.V., M. Morales, and J. Lerma, Selective antagonism of AMPA receptors unmasks kainate receptor-mediated responses in hippocampal neurons. Neuron, 1995. 14(1): p. 185-9. [PubMed]
95. Jones, K.A., et al., Desensitization of kainate receptors by kainate, glutamate and diastereomers of 4-methylglutamate. Neuropharmacology, 1997. 36(6): p. 853-63. [PubMed]
96. Maple, B.R., F. Gao, and S.M. Wu, Glutamate receptors differ in rod- and cone-dominated off-center bipolar cells. Neuroreport, 1999. 10(17): p. 3605-10. [PubMed]
97. Wesolowska, A., R. Nelson, and V. Connaughton, Glutamate Mechanisms Involved in the OFF Pathway of Zebrafish Retina. Invest. Ophthalmol. Vis. Sci., 2002. 43(12): p. E-abstract 1826.
98. Kim, H.G. and R.F. Miller, Properties of synaptic transmission from photoreceptors to bipolar cells in the mudpuppy retina. J Neurophysiol, 1993. 69(2): p. 352-60. [PubMed]
99. Famiglietti, E.V., Jr. and H. Kolb, Structural basis for ON-and OFF-center responses in retinal ganglion cells. Science, 1976. 194(4261): p. 193-5. [PubMed]
100. Nelson, R., E.V. Famiglietti Jr., and H. Kolb, Intracellular staining reveals different levels of stratification for on- and off-center ganglion cells in the cat retina. J. Neurophysiol., 1978. 41(2): p. 472-483. [PubMed]
101. Amthor, F.R., E.S. Takahashi, and C.W. Oyster, Morphologies of rabbit retinal ganglion cells with concentric receptive fields. J Comp Neurol, 1989. 280(1): p. 72-96. [PubMed]
102. Bloomfield, S.A. and R.F. Miller, A functional organization of ON and OFF pathways in the rabbit retina. J Neurosci, 1986. 6(1): p. 1-13. [PubMed]
103. Ammermüller, J. and H. Kolb, The organization of the turtle inner retina. I. ON- and OFF-center pathways. J Comp Neurol, 1995. 358(1): p. 1-34. [PubMed]
104. Cohen, E. and P. Sterling, Microcircuitry related to the receptive field center of the on-beta ganglion cell. J Neurophysiol, 1991. 65(2): p. 352-9. [PubMed]
105. Kolb, H., The inner plexiform layer in the retina of the cat: electron microscopic observations. J Neurocytol, 1979. 8(3): p. 295-329. [PubMed]
106. Kolb, H. and L. Dekorver, Midget ganglion cells of the parafovea of the human retina: a study by electron microscopy and serial section reconstructions. J Comp Neurol, 1991. 303(4): p. 617-36. [PubMed]
107. Kolb, H. and R. Nelson, OFF-alpha and OFF-beta ganglion cells in cat retina: II. Neural circuitry as revealed by electron microscopy of HRP stains. J Comp Neurol, 1993. 329(1): p. 85-110. [PubMed]
108. McGuire, B.A., J.K. Stevens, and P. Sterling, Microcircuitry of bipolar cells in cat retina. J Neurosci, 1984. 4(12): p. 2920-38. [PubMed]
109. Nelson, R. and H. Kolb, Synaptic patterns and response properties of bipolar and ganglion cells in the cat retina. Vision Res, 1983. 23(10): p. 1183-95. [PubMed]
110. Dumitrescu, O.N., et al., Ectopic retinal ON bipolar cell synapses in the OFF inner plexiform layer: contacts with dopaminergic amacrine cells and melanopsin ganglion cells. J Comp Neurol, 2009. 517(2): p. 226-44.
111. Grunert, U., et al., Bipolar input to melanopsin containing ganglion cells in primate retina. Vis Neurosci, 2011. 28(1): p. 39-50.
112. Pang, J.J., F. Gao, and S.M. Wu, Stratum-by-stratum projection of light response attributes by retinal bipolar cells of Ambystoma. J Physiol, 2004. 558(Pt 1): p. 249-62.
113. Ghosh, K.K., et al., Types of bipolar cells in the mouse retina. J Comp Neurol, 2004. 469(1): p. 70-82.
114. Dacey, D.M. and B.B. Lee, The ‘blue-on’ opponent pathway in primate retina originates from a distinct bistratified ganglion cell type. Nature, 1994. 367(6465): p. 731-5. [PubMed]
115. Famiglietti, E.V., Jr., A. Kaneko, and M. Tachibana, Neuronal architecture of on and off pathways to ganglion cells in carp retina. Science, 1977. 198(4323): p. 1267-9. [PubMed]
116. Li, Y.N. and J.E. Dowling, Specificity in the Bipolar Cell-Photoreceptor Connections in the Zebrafish Retina. ARVO Meeting Abstracts, 2010. 51(5): p. 4125.
117. Li, Y.N., et al., Bipolar Cell-Photoreceptor Connections in the Zebrafish Retina. ARVO Meeting Abstracts, 2011. 52(6): p. 2573.
118. Saito, T., et al., Reexamination of photoreceptor-bipolar connectivity patterns in carp retina: HRP-EM and Golgi-EM studies. J Comp Neurol, 1985. 236(2): p. 141-60. [PubMed]
119. Kolb, H. and R. Nelson, Amacrine cells of the cat retina. Vision Res, 1981. 21(11): p. 1625-33.
120. Mariani, A., Giant Bistratified Bipolar Cells in Monkey Retina. Anatomical Record, 1983. 206(2): p. 215-220.
121. Wong, K.Y., E.D. Cohen, and J.E. Dowling, Retinal bipolar cell input mechanisms in giant danio. II. Patch-clamp analysis of on bipolar cells. J Neurophysiol, 2005. 93(1): p. 94-107.
122. Cajal, S., The Structure of the retina. In: Thorpe SA, Glickstein M, translators (1972). 1892, Springfield: Thomas.
123. Dacheux, R.F. and E. Raviola, The rod pathway in the rabbit retina: a depolarizing bipolar and amacrine cell. J Neurosci, 1986. 6(2): p. 331-45. [PubMed]
124. de la Villa, P., T. Kurahashi, and A. Kaneko, L-glutamate-induced responses and cGMP-activated channels in three subtypes of retinal bipolar cells dissociated from the cat. J Neurosci, 1995. 15(5 Pt 1): p. 3571-82. [PubMed]
125. Sherry, D.M. and S. Yazulla, Goldfish bipolar cells and axon terminal patterns: a Golgi study. J Comp Neurol, 1993. 329(2): p. 188-200. [PubMed]
126. Pang, J.J., et al., Direct rod input to cone BCs and direct cone input to rod BCs challenge the traditional view of mammalian BC circuitry. Proc Natl Acad Sci U S A, 2010. 107(1): p. 395-400.
127. Pang, J.J., F. Gao, and S.M. Wu, Light-evoked current responses in rod bipolar cells, cone depolarizing bipolar cells and AII amacrine cells in dark-adapted mouse retina. J Physiol, 2004. 558(Pt 3): p. 897-912.
128. Greferath, U., U. Grunert, and H. Wässle, Rod bipolar cells in the mammalian retina show protein kinase C-like immunoreactivity. J Comp Neurol, 1990. 301(3): p. 433-42. [PubMed]
129. Kolb, H., L. Zhang, and L. Dekorver, Differential staining of neurons in the human retina with antibodies to protein kinase C isozymes. Vis Neurosci, 1993. 10(2): p. 341-51. [PubMed]
130. Negishi, K., S. Kato, and T. Teranishi, Dopamine cells and rod bipolar cells contain protein kinase C-like immunoreactivity in some vertebrate retinas. Neurosci Lett, 1988. 94(3): p. 247-52. [PubMed]
131. Kolb, H. and E.V. Famiglietti, Rod and cone pathways in the inner plexiform layer of cat retina. Science, 1974. 186(4158): p. 47-9. [PubMed]
132. Nelson, R., AII amacrine cells quicken time course of rod signals in the cat retina. J Neurophysiol, 1982. 47(5): p. 928-47. [PubMed]
133. Mills, S.L. and S.C. Massey, Differential properties of two gap junctional pathways made by AII amacrine cells [see comments]. Nature, 1995. 377(6551): p. 734-7.
134. Veruki, M.L. and E. Hartveit, Meclofenamic acid blocks electrical synapses of retinal AII amacrine and on-cone bipolar cells. J Neurophysiol, 2009. 101(5): p. 2339-47.
135. Kolb, H., The organization of the outer plexiform layer in the retina of the cat: electron microscopic observations. J Neurocytol, 1977. 6(2): p. 131-53. [PubMed]
136. Nelson, R., Cat cones have rod input: a comparison of the response properties of cones and horizontal cell bodies in the retina of the cat. J Comp Neurol, 1977. 172(1): p. 109-35. [PubMed]
137. Raviola, E. and N.B. Gilula, Gap junctions between photoreceptor cells in the vertebrate retina. Proc Natl Acad Sci U S A, 1973. 70(6): p. 1677-81. [PubMed] [Free Full text in PMC]
138. Smith, R.G., M.A. Freed, and P. Sterling, Microcircuitry of the dark-adapted cat retina: functional architecture of the rod-cone network. J Neurosci, 1986. 6(12): p. 3505-17. [PubMed]
139. Tsukamoto, Y., et al., Microcircuits for night vision in mouse retina. J Neurosci, 2001. 21(21): p. 8616-23. [PubMed]
140. Lasansky, A., Organization of the outer synaptic layer in the retina of the larval tiger salamander. Philos Trans R Soc Lond B Biol Sci, 1973. 265(872): p. 471-89. [PubMed]
141. Stell, W.K., A.T. Ishida, and D.O. Lightfoot, Structural basis for on-and off-center responses in retinal bipolar cells. Science, 1977. 198(4323): p. 1269-71. [PubMed]
142. Hack, I. and L. Peichl, Horizontal cells of the rabbit retina are non-selectively connected to the cones. Eur J Neurosci, 1999. 11(7): p. 2261-74.
143. Li, W., S. Chen, and S.H. DeVries, A fast rod photoreceptor signaling pathway in the mammalian retina. Nat Neurosci, 2010. 13(4): p. 414-6.
144. Tsukamoto, Y., et al., A novel connection between rods and ON cone bipolar cells revealed by ectopic metabotropic glutamate receptor 7 (mGluR7) in mGluR6-deficient mouse retinas. J Neurosci, 2007. 27(23): p. 6261-7.
145. Sharpe, L.T. and A. Stockman, Rod pathways: the importance of seeing nothing. Trends Neurosci, 1999. 22(11): p. 497-504.
146. Connaughton, V.P. and G. Maguire, Differential expression of voltage-gated K+ and Ca2+ currents in bipolar cells in the zebrafish retinal slice. Eur J Neurosci, 1998. 10(4): p. 1350-62. [PubMed]
147. Karschin, A. and H. Wässle, Voltage- and transmitter-gated currents in isolated rod bipolar cells of rat retina. J Neurophysiol, 1990. 63(4): p. 860-76. [PubMed]
148. Lasater, E.M., Membrane currents of retinal bipolar cells in culture. J Neurophysiol, 1988. 60(4): p. 1460-80. [PubMed]
149. Pan, Z.H. and H.J. Hu, Voltage-dependent Na+ currents in mammalian retinal cone bipolar cells. J Neurophysiol, 2000. 84(5): p. 2564-71. [PubMed]
150. Ma, Y.P., J. Cui, and Z.H. Pan, Heterogeneous expression of voltage-dependent Na+ and K+ channels in mammalian retinal bipolar cells. Vis Neurosci, 2005. 22(2): p. 119-33.
151. Cui, J. and Z.H. Pan, Two types of cone bipolar cells express voltage-gated Na+ channels in the rat retina. Vis Neurosci, 2008. 25(5-6): p. 635-45.
152. Zenisek, D., et al., Voltage-dependent sodium channels are expressed in nonspiking retinal bipolar neurons. J Neurosci, 2001. 21(13): p. 4543-50. [PubMed]
153. Protti, D.A. and I. Llano, Calcium currents and calcium signaling in rod bipolar cells of rat retinal slices. J Neurosci, 1998. 18(10): p. 3715-24. [PubMed]
154. Zenisek, D. and G. Matthews, Calcium action potentials in retinal bipolar neurons. Vis Neurosci, 1998. 15(1): p. 69-75. [PubMed]
155. Heidelberger, R. and G. Matthews, Calcium influx and calcium current in single synaptic terminals of goldfish retinal bipolar neurons. J Physiol, 1992. 447: p. 235-56. [PubMed] [Free Full text in PMC]
156. Kaneko, A., et al., Membrane currents and pharmacology of retinal bipolar cells: a comparative study on goldfish and mouse. Comp Biochem Physiol C, 1991. 98(1): p. 115-27. [PubMed]
157. Kaneko, A., L.H. Pinto, and M. Tachibana, Transient calcium current of retinal bipolar cells of the mouse. J Physiol, 1989. 410: p. 613-29. [PubMed] [Free Full text in PMC]
158. Maguire, G., et al., Gamma-aminobutyrate type B receptor modulation of L-type calcium channel current at bipolar cell terminals in the retina of the tiger salamander. Proc Natl Acad Sci U S A, 1989. 86(24): p. 10144-7. [PubMed] [Free Full text in PMC]
159. Kaneko, A. and M. Tachibana, A voltage-clamp analysis of membrane currents in solitary bipolar cells dissociated from Carassius auratus. J Physiol, 1985. 358: p. 131-52. [PubMed] [Free Full text in PMC]
160. Fan, S.F. and S. Yazulla, Dopamine depletion with 6-OHDA enhances dopamine D1-receptor modulation of potassium currents in retinal bipolar cells. Vis Neurosci, 2001. 18(2): p. 327-37. [PubMed]
161. Tessier-Lavigne, M., et al., Membrane currents in retinal bipolar cells of the axolotl. J Gen Physiol, 1988. 91(1): p. 49-72. [PubMed]
162. Kamermans, M., et al., Hemichannel-mediated inhibition in the outer retina. Science, 2001. 292(5519): p. 1178-80. [PubMed]
163. Vardi, N., et al., Evidence that different cation chloride cotransporters in retinal neurons allow opposite responses to GABA. J Neurosci, 2000. 20(20): p. 7657-63. [PubMed]
164. Thoreson, W.B. and R.F. Miller, Membrane currents evoked by excitatory amino acid agonists in ON bipolar cells of the mudpuppy retina. J Neurophysiol, 1993. 70(4): p. 1326-38. [PubMed]
165. Connaughton, V.P., et al., Immunocytochemical localization of excitatory and inhibitory neurotransmitters in the zebrafish retina. Vis Neurosci, 1999. 16(3): p. 483-90. [PubMed]
166. Freed, M.A., Y. Nakamura, and P. Sterling, Four types of amacrine in the cat retina that accumulate GABA. J Comp Neurol, 1983. 219(3): p. 295-304. [PubMed]
167. Freed, M.A., R.G. Smith, and P. Sterling, Rod bipolar array in the cat retina: pattern of input from rods and GABA-accumulating amacrine cells. J Comp Neurol, 1987. 266(3): p. 445-55. [PubMed]
168. Marc, R.E. and W. Liu, Fundamental GABAergic amacrine cell circuitries in the retina: nested feedback, concatenated inhibition, and axosomatic synapses. J Comp Neurol, 2000. 425(4): p. 560-82. [PubMed]
169. Marc, R.E., et al., GABA-ergic pathways in the goldfish retina. J Comp Neurol, 1978. 182(2): p. 221-44. [PubMed]
170. Pourcho, R.G. and D.J. Goebel, Neuronal subpopulations in cat retina which accumulate the GABA agonist, (3H)muscimol: a combined Golgi and autoradiographic study. J Comp Neurol, 1983. 219(1): p. 25-35. [PubMed]
171. Connaughton, V.P., R. Nelson, and A.M. Bender, Electrophysiological evidence of GABAA and GABAC receptors on zebrafish retinal bipolar cells. Vis Neurosci, 2008. 25(2): p. 139-53.
172. Lukasiewicz, P.D., B.R. Maple, and F.S. Werblin, A novel GABA receptor on bipolar cell terminals in the tiger salamander retina. J Neurosci, 1994. 14(3 Pt 1): p. 1202-12. [PubMed]
173. Tachibana, M. and A. Kaneko, gamma-Aminobutyric acid exerts a local inhibitory action on the axon terminal of bipolar cells: evidence for negative feedback from amacrine cells. Proc Natl Acad Sci U S A, 1987. 84(10): p. 3501-5. [PubMed] [Free Full text in PMC]
174. Tachibana, M. and A. Kaneko, Retinal bipolar cells receive negative feedback input from GABAergic amacrine cells. Vis Neurosci, 1988. 1(3): p. 297-305. [PubMed]
175. Lukasiewicz, P.D. and F.S. Werblin, A novel GABA receptor modulates synaptic transmission from bipolar to ganglion and amacrine cells in the tiger salamander retina. J Neurosci, 1994. 14(3 Pt 1): p. 1213-23. [PubMed]
176. Burkhardt, D.A., Responses and receptive-field organization of cones in perch retinas. J Neurophysiol, 1977. 40(1): p. 53-62. [PubMed]
177. Kondo, H. and J. Toyoda, GABA and glycine effects on the bipolar cells of the carp retina. Vision Res, 1983. 23(11): p. 1259-64. [PubMed]
178. Wu, S.M., Effects of gamma-aminobutyric acid on cones and bipolar cells of the tiger salamander retina. Brain Res, 1986. 365(1): p. 70-7. [PubMed]
179. Wu, S.M. and B.R. Maple, Amino acid neurotransmitters in the retina: a functional overview. Vision Res, 1998. 38(10): p. 1371-84. [PubMed]
180. Feigenspan, A., H. Wässle, and J. Bormann, Pharmacology of GABA receptor Cl– channels in rat retinal bipolar cells. Nature, 1993. 361(6408): p. 159-62.
181. Lukasiewicz, P.D. and C.R. Shields, Different combinations of GABAA and GABAC receptors confer distinct temporal properties to retinal synaptic responses. J Neurophysiol, 1998. 79(6): p. 3157-67. [PubMed]
182. Lukasiewicz, P.D. and C.R. Shields, A diversity of GABA receptors in the retina. Semin Cell Dev Biol, 1998. 9(3): p. 293-9. [PubMed]
183. Lukasiewicz, P.D. and R.O. Wong, GABAC receptors on ferret retinal bipolar cells: a diversity of subtypes in mammals? Vis Neurosci, 1997. 14(5): p. 989-94. [PubMed]
184. McGillem, G.S., T.C. Rotolo, and R.F. Dacheux, GABA responses of rod bipolar cells in rabbit retinal slices. Vis Neurosci, 2000. 17(3): p. 381-9. [PubMed]
185. Qian, H. and J.E. Dowling, Novel GABA responses from rod-driven retinal horizontal cells. Nature, 1993. 361(6408): p. 162-4. [PubMed]
186. Qian, H. and J.E. Dowling, GABAA and GABAC receptors on hybrid bass retinal bipolar cells. J Neurophysiol, 1995. 74(5): p. 1920-8. [PubMed]
187. Vaquero, C.F. and P. de la Villa, Localisation of the GABAC receptors at the axon terminal of the rod bipolar cells of the mouse retina. Neurosci Res, 1999. 35(1): p. 1-7. [PubMed]
188. Nelson, R., V.P. Connaughton, and A.E. Schaffner, Voltage probe measurements of glutamate responses form acutely dissociated zebrafish retinal neurons. Invest Ophthalmol Vis Sci, 1999. 40: p. S242.
189. Eggers, E.D. and P.D. Lukasiewicz, GABAA, GABAC and glycine receptor-mediated inhibition differentially affects light-evoked signalling from mouse retinal rod bipolar cells. J Physiol, 2006. 572(Pt 1): p. 215-25.
190. Heidelberger, R. and G. Matthews, Inhibition of calcium influx and calcium current by gamma-aminobutyric acid in single synaptic terminals. Proc Natl Acad Sci U S A, 1991. 88(16): p. 7135-9. [PubMed] [Free Full text in PMC]
191. Eggers, E.D. and P.D. Lukasiewicz, Receptor and transmitter release properties set the time course of retinal inhibition. J Neurosci, 2006. 26(37): p. 9413-25.
192. Frumkes, T.E. and R. Nelson, Functional role of GABA in cat retina: I. Effects of GABAA agonists. Vis Neurosci, 1995. 12(4): p. 641-50. [PubMed]
193. Frumkes, T.E., R. Nelson, and R. Pflug, Functional role of GABA in cat retina: II. Effects of GABAA antagonists. Vis Neurosci, 1995. 12(4): p. 651-61. [PubMed]
194. Freed, M.A., GABAergic circuits in the mammalian retina, in GABA in the retina and central visual system. 1992, Elsevier: Amsterdam.
195. Zhang, D.Q. and X.L. Yang, OFF pathway is preferentially suppressed by the activation of GABA(A) receptors in carp retina. Brain Res, 1997. 759(1): p. 160-2.
196. Matthews, G., G.S. Ayoub, and R. Heidelberger, Presynaptic inhibition by GABA is mediated via two distinct GABA receptors with novel pharmacology. J Neurosci, 1994. 14(3 Pt 1): p. 1079-90. [PubMed]
197. Mack, A.F., U.D. Behrens, and H.J. Wagner, Inhibitory control of synaptic activity in goldfish Mb bipolar cell terminals visualized by FM1-43. Vis Neurosci, 2000. 17(6): p. 823-9. [PubMed]
198. Pang, J.J., F. Gao, and S.M. Wu, Relative contributions of bipolar cell and amacrine cell inputs to light responses of ON, OFF and ON-OFF retinal ganglion cells. Vision Res, 2002. 42(1): p. 19-27. [PubMed]
199. Singer, J.H. and J.S. Diamond, Sustained Ca2+ entry elicits transient postsynaptic currents at a retinal ribbon synapse. J Neurosci, 2003. 23(34): p. 10923-33.
200. Chavez, A.E., W.N. Grimes, and J.S. Diamond, Mechanisms underlying lateral GABAergic feedback onto rod bipolar cells in rat retina. J Neurosci, 2010. 30(6): p. 2330-9.
201. Chavez, A.E., J.H. Singer, and J.S. Diamond, Fast neurotransmitter release triggered by Ca influx through AMPA-type glutamate receptors. Nature, 2006. 443(7112): p. 705-8.
202. Suzuki, S., M. Tachibana, and A. Kaneko, Effects of glycine and GABA on isolated bipolar cells of the mouse retina. J Physiol, 1990. 421: p. 645-62. [PubMed] [Free Full text in PMC]
203. Maple, B.R. and S.M. Wu, Glycinergic synaptic inputs to bipolar cells in the salamander retina. J Physiol, 1998. 506 ( Pt 3): p. 731-44. [PubMed]
204. Cunningham, R. and R.F. Miller, Electrophysiological analysis of taurine and glycine action on neurons of the midpuppy retina. I. Intracellular recording. Brain Res, 1980. 197(1): p. 123-38. [PubMed]
205. Műller, F., H. Wässle, and T. Voigt, Pharmacological modulation of the rod pathway in the cat retina. J Neurophysiol, 1988. 59(6): p. 1657-72. [PubMed]
206. Pourcho, R.G. and D.J. Goebel, A combined Golgi and autoradiographic study of (3H)glycine-accumulating amacrine cells in the cat retina. J Comp Neurol, 1985. 233(4): p. 473-80. [PubMed]
207. Wässle, H., I. Schafer-Trenkler, and T. Voigt, Analysis of a glycinergic inhibitory pathway in the cat retina. J Neurosci, 1986. 6(2): p. 594-604. [PubMed]
208. Eggers, E.D. and P.D. Lukasiewicz, Multiple pathways of inhibition shape bipolar cell responses in the retina. Vis Neurosci, 2011. 28(1): p. 95-108.
209. Chavez, A.E. and J.S. Diamond, Diverse mechanisms underlie glycinergic feedback transmission onto rod bipolar cells in rat retina. J Neurosci, 2008. 28(31): p. 7919-28.
210. Du, J.L. and X.L. Yang, Glycinergic synaptic transmission to bullfrog retinal bipolar cells is input-specific. Neuroscience, 2002. 113(4): p. 779-84. [PubMed]
211. Smiley, J.F. and S. Yazulla, Glycinergic contacts in the outer plexiform layer of the Xenopus laevis retina characterized by antibodies to glycine, GABA and glycine receptors. J Comp Neurol, 1990. 299(3): p. 375-88. [PubMed]
212. Stone, S. and M. Schutte, Physiological and morphological properties of off- and on-center bipolar cells in the Xenopus retina: effects of glycine and GABA. Vis Neurosci, 1991. 7(4): p. 363-76. [PubMed]
213. Hare, W.A. and W.G. Owen, Receptive field of the retinal bipolar cell: a pharmacological study in the tiger salamander. J Neurophysiol, 1996. 76(3): p. 2005-19. [PubMed]
214. Qian, H., et al., Responses of small- and large-field bipolar cells to GABA and glycine. Brain Res, 2001. 893(1-2): p. 273-7. [PubMed]
215. Merighi, A., E. Raviola, and R.F. Dacheux, Connections of two types of flat cone bipolars in the rabbit retina. J Comp Neurol, 1996. 371(1): p. 164-78. [PubMed]
216. von Gersdorff, H., et al., Evidence that vesicles on the synaptic ribbon of retinal bipolar neurons can be rapidly released. Neuron, 1996. 16(6): p. 1221-7. [PubMed]
217. Lagnado, L., A. Gomis, and C. Job, Continuous vesicle cycling in the synaptic terminal of retinal bipolar cells. Neuron, 1996. 17(5): p. 957-67. [PubMed]
218. Zenisek, D., J.A. Steyer, and W. Almers, Transport, capture and exocytosis of single synaptic vesicles at active zones. Nature, 2000. 406(6798): p. 849-54. [PubMed]
219. Dowling, J.E., The retina: an approachable part of the brain. 1987, Cambridge (MA): Belknap Press of Harvard University Press.
220. Dowling, J.E. and B.B. Boycott, Organization of the primate retina: electron microscopy. Proc R Soc Lond B Biol Sci, 1966. 166(2): p. 80-111. [PubMed]
221. Miller, R.F., et al., Pre- and postsynaptic mechanisms of spontaneous, excitatory postsynaptic currents in the salamander retina. Prog Brain Res, 2001. 131: p. 241-53.
222. Wong-Riley, M.T., Synaptic orgnization of the inner plexiform layer in the retina of the tiger salamander. J Neurocytol, 1974. 3(1): p. 1-33.
223. Gottesman, J. and R.F. Miller, Pharmacological properties of N-methyl-D-aspartate receptors on ganglion cells of an amphibian retina. J Neurophysiol, 1992. 68(2): p. 596-604. [PubMed]
224. Heidelberger, R. and G. Matthews, Dopamine enhances Ca2+ responses in synaptic terminals of retinal bipolar neurons. Neuroreport, 1994. 5(6): p. 729-32.
225. Morgans, C.W., Neurotransmitter release at ribbon synapses in the retina. Immunol Cell Biol, 2000. 78(4): p. 442-6. [PubMed]
226. von Gersdorff, H. and G. Matthews, Inhibition of endocytosis by elevated internal calcium in a synaptic terminal. Nature, 1994. 370(6491): p. 652-5.
227. Neves, G., A. Neef, and L. Lagnado, The actions of barium and strontium on exocytosis and endocytosis in the synaptic terminal of goldfish bipolar cells. J Physiol, 2001. 535(Pt 3): p. 809-24. [PubMed]
228. Pan, Z.H., Voltage-activated Ca2+ channels and ionotropic GABA receptors localized at axon terminals of mammalian retinal bipolar cells. Vis Neurosci, 2001. 18(2): p. 279-88.
229. Burrone, J. and L. Lagnado, Synaptic depression and the kinetics of exocytosis in retinal bipolar cells. J Neurosci, 2000. 20(2): p. 568-78. [PubMed]
230. Mennerick, S. and G. Matthews, Ultrafast exocytosis elicited by calcium current in synaptic terminals of retinal bipolar neurons. Neuron, 1996. 17(6): p. 1241-9. [PubMed]
231. von Gersdorff, H. and G. Matthews, Depletion and replenishment of vesicle pools at a ribbon-type synaptic terminal. J Neurosci, 1997. 17(6): p. 1919-27. [PubMed]
232. Rouze, N.C. and E.A. Schwartz, Continuous and transient vesicle cycling at a ribbon synapse. J Neurosci, 1998. 18(21): p. 8614-24. [PubMed]
233. Veruki, M.L., S.H. Morkve, and E. Hartveit, Functional properties of spontaneous EPSCs and non-NMDA receptors in rod amacrine (AII) cells in the rat retina. J Physiol, 2003. 549(Pt 3): p. 759-74.
234. Singer, J.H., et al., Coordinated multivesicular release at a mammalian ribbon synapse. Nat Neurosci, 2004. 7(8): p. 826-33.
235. Singer, J.H. and J.S. Diamond, Vesicle depletion and synaptic depression at a mammalian ribbon synapse. J Neurophysiol, 2006. 95(5): p. 3191-8.
236. Pinto, L.H., et al., Generation, identification and functional characterization of the nob4 mutation of Grm6 in the mouse. Vis Neurosci, 2007. 24(1): p. 111-23. [PubMed]
237. Takao, M., et al., Impaired behavioral suppression by light in metabotropic glutamate receptor subtype 6-deficient mice. Neuroscience, 2000. 97(4): p. 779-87. [PubMed]
238. Thompson, S., et al., Different inner retinal pathways mediate rod-cone input in irradiance detection for the pupillary light reflex and regulation of behavioral state in mice. Invest Ophthalmol Vis Sci, 2011. 52(1): p. 618-23. [PubMed]
239. Iwakabe, H., et al., Impairment of pupillary responses and optokinetic nystagmus in the mGluR6-deficient mouse. Neuropharmacology, 1997. 36(2): p. 135-43. [PubMed]
240. Tagawa, Y., et al., Immunohistological studies of metabotropic glutamate receptor subtype 6-deficient mice show no abnormality of retinal cell organization and ganglion cell maturation. J Neurosci, 1999. 19(7): p. 2568-79. [PubMed]
241. Berson, E.L. and S. Lessell, Paraneoplastic night blindness with malignant melanoma. Am J Ophthalmol, 1988. 106(3): p. 307-11. [PubMed]
242. Ripps, H., Night blindness revisited: from man to molecules. Proctor lecture. Invest Ophthalmol Vis Sci, 1982. 23(5): p. 588-609. [PubMed]
243. Alexander, K.R., et al., ‘On’ response defect in paraneoplastic night blindness with cutaneous malignant melanoma. Invest Ophthalmol Vis Sci, 1992. 33(3): p. 477-83. [PubMed]
244. Alexander, K.R., et al., Contrast-processing deficits in melanoma-associated retinopathy. Invest Ophthalmol Vis Sci, 2004. 45(1): p. 305-10.
245. Lei, B., et al., Human melanoma-associated retinopathy (MAR) antibodies alter the retinal ON-response of the monkey ERG in vivo. Invest Ophthalmol Vis Sci, 2000. 41(1): p. 262-6. [PubMed]
246. Dhingra, A., et al., Autoantibodies in melanoma-associated retinopathy target TRPM1 cation channels of retinal ON bipolar cells. J Neurosci, 2011. 31(11): p. 3962-7.
247. Kondo, M., et al., Identification of autoantibodies against TRPM1 in patients with paraneoplastic retinopathy associated with ON bipolar cell dysfunction. PLoS One, 2011. 6(5): p. e19911.
248. Schubert, G. and H. Bornschein, [Analysis of the human electroretinogram]. Ophthalmologica, 1952. 123(6): p. 396-413. [PubMed]
249. Goodman, G. and H. Bornschein, Comparative electroretinographic studies in congenital night blindness and total color blindness. AMA Arch Ophthalmol, 1957. 58(2): p. 174-82. [PubMed]
250. Miyake, Y., et al., Congenital stationary night blindness with negative electroretinogram. A new classification. Arch Ophthalmol, 1986. 104(7): p. 1013-20. [PubMed]
251. Miyake, Y., [Establishment of the concept of new clinical entities–complete and incomplete form of congenital stationary night blindness]. Nihon Ganka Gakkai Zasshi, 2002. 106(12): p. 737-55; discussion 756.
252. Bech-Hansen, N.T., et al., Mutations in NYX, encoding the leucine-rich proteoglycan nyctalopin, cause X-linked complete congenital stationary night blindness. Nat Genet, 2000. 26(3): p. 319-23. [PubMed]
253. Bech-Hansen, N.T., et al., Loss-of-function mutations in a calcium-channel alpha1-subunit gene in Xp11.23 cause incomplete X-linked congenital stationary night blindness. Nat Genet, 1998. 19(3): p. 264-7. [PubMed]
254. Zeitz, C., et al., Mutations in GRM6 cause autosomal recessive congenital stationary night blindness with a distinctive scotopic 15-Hz flicker electroretinogram. Invest Ophthalmol Vis Sci, 2005. 46(11): p. 4328-35.
255. Schiller, P.H., Central connections of the retinal ON and OFF pathways. Nature, 1982. 297(5867): p. 580-3. [PubMed]
256. Schiller, P.H., The connections of the retinal on and off pathways to the lateral geniculate nucleus of the monkey. Vision Res, 1984. 24(9): p. 923-32. [PubMed]
257. Knapp, A.G. and L.A. Mistler, Response properties of cells in rabbit’s lateral geniculate nucleus during reversible blockade of retinal on-center channel. J Neurophysiol, 1983. 50(5): p. 1236-45. [PubMed]
258. Cleland, B.G., M.W. Dubin, and W.R. Levick, Sustained and transient neurones in the cat’s retina and lateral geniculate nucleus. J Physiol, 1971. 217(2): p. 473-96.
259. Dolan, R.P. and P.H. Schiller, Effects of ON channel blockade with 2-amino-4-phosphonobutyrate (APB) on brightness and contrast perception in monkeys. Vis Neurosci, 1994. 11(1): p. 23-32. [PubMed]
260. Dolan, R.P. and P.H. Schiller, Evidence for only depolarizing rod bipolar cells in the primate retina. Vis Neurosci, 1989. 2(5): p. 421-4. [PubMed]
261. Qin, P. and R.G. Pourcho, Localization of AMPA-selective glutamate receptor subunits in the cat retina: a light- and electron-microscopic study. Vis Neurosci, 1999. 16(1): p. 169-77. [PubMed]
Ralph Nelson and Victoria Connaughton
Last Updated: February 29, 2012.
About the Authors
The biosketch for Ralph Nelson can be found in the About the Editors section of Webvision.
Victoria Connaughton, Ph.D. is an Associate Professor of Biology at American University in Washington DC. Born in Sellersville, Pennsylvania, Vikki earned a B. S. degree from Bucknell University and Ph. D in Marine Science from the University of Delaware, Lewes. She has been a postdoctoral fellow at the University of Texas, Houston in the Department of Neurobiology and Anatomy, and also at the National Institutes of Health where she continued studies of zebrafish retinal neuroanatomy and neurophysiology with co-author Ralph Nelson. Dr. Connaughton’s current research interests encompass the disciplines of developmental biology (nervous system development) and neurobiology. Specifically, she is interested in examining the relation between visually-guided behaviors in larval teleosts and maturation of retinal neurons, circuits, and receptor mechanisms. She is also interested in examining how the development of neural connections can be altered due to mutations or drugs. She has performed experiments that address behavioral/ecological questions, as well as those that employ cell biology techniques to examine retinal circuitry in both developing and adult retinal tissue.